Abstract
Background: Systematic symptom assessment is not a standard of care in children with cancer. Many well-known symptom assessment tools are lengthy or difficult to integrate into a daily pediatric palliative care practice. We created a series of brief and simple questions to be systematically given to children and their caregivers.
Objective: The primary objective was to determine the percentage of eligible children and caregivers exposed to the questions that were able to complete the assessment. Secondary objectives included documenting the symptom burden at the time of consultation, evaluating the level of agreement in symptom reporting between children and caregivers, as well as between children/caregivers and the referring medical team.
Design: A series of systematic questions were presented to all caregivers (if present) and children who were seven years of age or older at the time of initial consultation with pediatric palliative care.
Results: One hundred twenty-two consecutive children/caregiver dyads were given the survey. One hundred seven of 108 (99%) eligible caregivers and 83 of 97 (86%) eligible children completed the survey. Lack of appetite (child—72/83, 87%; caregiver—89/107, 83%) and pain (child—71/83, 86%; caregiver—86/107, 80%) were the most commonly reported symptoms. Caregivers reported irritability (p = 0.005) and nervousness (p < 0.001) more frequently than children. Referring medical teams significantly underdiagnosed psychological and other less clinically evident symptoms such as lack of appetite, fatigue, and sleep disturbance (p < 0.001).
Conclusions: Our series of questions is easy to complete by children and caregivers. Systematic symptom assessment of children with cancer referred to palliative care should become a true standard of care.
Keywords: palliative care, patient reported outcomes, pediatric oncology, pediatric, symptom assessment
Introduction
Cancer remains a leading cause of disease-related death among children in the United States.1,2 Despite the overall survival rate exceeding 80%, the short- and long-term implications of cancer treatment on a child's physical, psychological, social, and cognitive functioning are evident.1,3,4 Physical and psychological suffering from the natural course of the disease or the treatment of the disease significantly impacts the quality of life of children and the families that care for them.5–8
Systematic symptom assessment in children with cancer is not routinely performed despite evidence that there is a high symptom burden.5 We surveyed 116 pediatric palliative care physicians who are part of the American Academy of Pediatrics Section on Hospice and Palliative Medicine electronic mail listserv. Of the 51 respondents, only 1 program (2%) performs systematic symptom assessment at each patient encounter. The use of patient-reported outcomes (PRO) is the gold standard for evaluation and treatment of physical and psychological symptoms, but many existing tools are not designed for use in a busy clinical setting. The Memorial Symptom Assessment (MSAS) is the most well-known validated tool for child symptom screening.9,10
The primary disadvantages are the time needed to complete (6–11 minutes), the time frame of symptoms assessed (48 hours to 7 days), the number of items to finish (8–30), and the complexity of rating each symptom in a variety of domains (presence or absence, severity, and how bothersome). Other tools have been described, focusing on specific age groups11 or a particular time in the course of the disease12; these tools are administered in long intervals,13 limiting their applicability for use in daily clinical decision making.
Simplified systematic symptom assessment tools such as the Edmonton Symptom Assessment System (ESAS) are widely used in adults with cancer.14 Some benefits of these tools include the ease and quickness of administration,15 the ability to monitor symptoms longitudinally,16 and it can be completed in the inpatient and outpatient setting.
We developed a set of systematic questions regarding symptoms that could be integrated into the daily practice of pediatric palliative care. The primary objective of this retrospective study was to determine the percentage of children and primary caregivers seen during the study period exposed to the questions who were able to complete the assessment.
The secondary objectives of this study were to determine the frequency and level of physical and psychological symptom distress at the time of consultation, evaluate the level of agreement between child and caregiver reports, and to report the percentage of patients who reported symptoms not diagnosed at the time of consultation. Exploratory aims of the study were to examine factors associated with higher symptom expression including age, gender, and type of cancer.
Methods
This study was conducted at MD Anderson Cancer Center (MDACC). The MDACC Institutional Review Board reviewed and approved this retrospective study (PA17-0798).
Subjects
Inclusion criteria
All children with cancer (and their caregiver) referred to the pediatric palliative care service were eligible to complete the symptom assessment questions. Eligible caregivers were defined as parents, legal guardians, or grandparents identified as the primary caregiver to the child. A professional medical translator translated English-language versions into Spanish. Other languages utilized a professional interpreter (in person, audio, or video).
Exclusion criteria
Children less than seven years of age and those with underlying developmental delay [autism, autism-spectrum disorders, and impaired cognitive abilities because of central nervous system (CNS) tumors] were not eligible to complete the survey.
Symptom assessment questions
We created the symptom assessment questions based on the validated ESAS,17 validated MSAS for children 7–12 years of age and 10–18 years of age,9,10,18 and PediQUEST PQ-MSAS19 PRO tools. The primary goal was to balance comprehensiveness with efficiency. Each PRO was analyzed to determine the frequency and level of distress for each symptom. A group including a board-certified child psychiatrist and two board-certified hospice and palliative medicine specialists selected 11 symptoms in a consensus decision-making process based on a literature review and clinical experience identifying symptoms that could be amenable to assessment and treatment by a pediatric palliative care physician.
The symptom assessment questions assess 11 physical and psychological symptoms. The time frame of symptom assessed is the previous 24 hours. Participants were asked to use a 5-point Likert type scale to describe how bothersome each symptom was (0 = not at all, 1 = a little bit, 2 = kind of, 3 = quite a bit, and 4 = a lot) in the previous 24 hours. A reassuring aspect of the symptom assessment questions is that the 0–4 level of symptom distress scoring system has been validated in the MSAS. Readability was tested before distributing the questions by utilizing Grammarly®, an online English-language writing-enhancement platform; the series of questions were determined to have a Flesch–Kincaid Grade Level of 2.5.
Administration of the questions
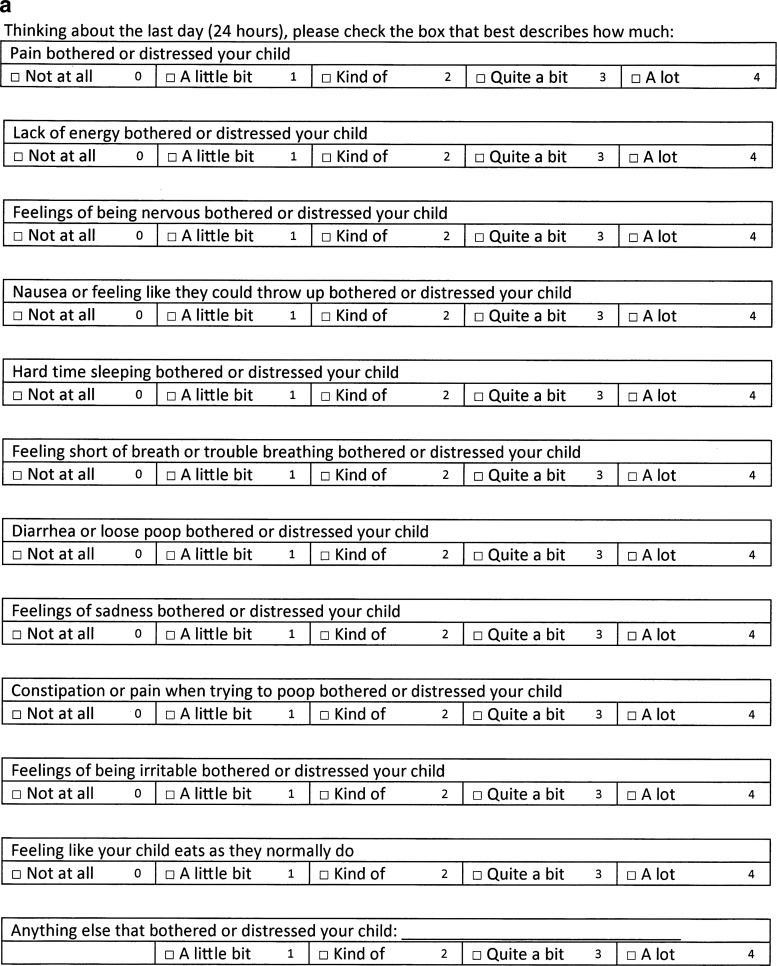
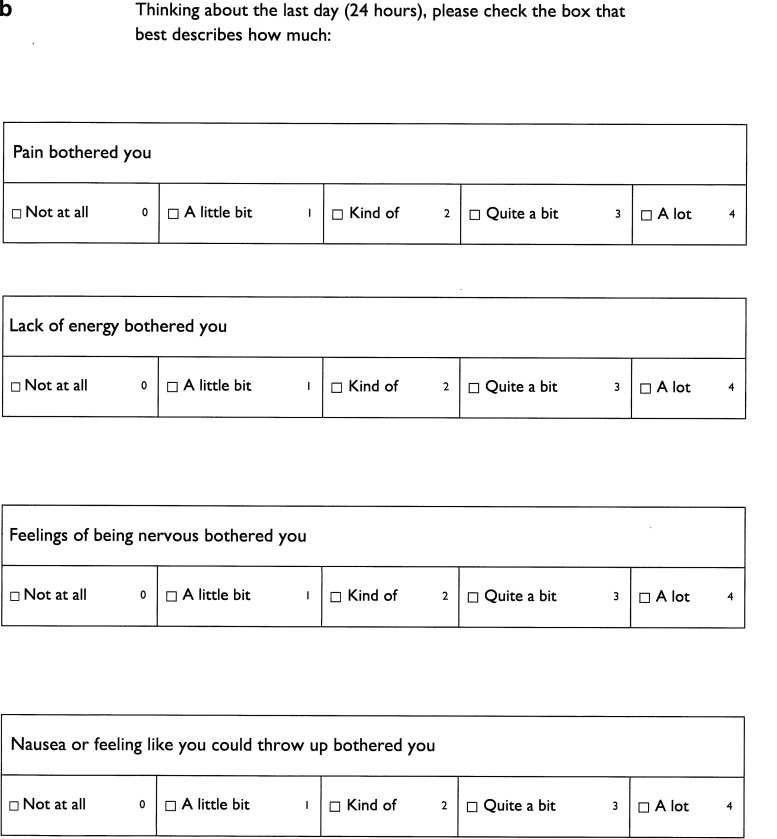
The questions were printed on a one-page document for caregivers (Fig. 1a) and a three-page document (Fig. 1b) for the child. To not visually overwhelm the child, the child handout had a larger font size and the font style was Gill Sans, one of the most commonly used fonts for children's books and school textbooks. Inpatients were given instructions for answering the questions by the single pediatric palliative care physician, and outpatients were given instructions by one of three nurses who regularly work in the pediatric palliative care clinic. Caregivers and children were instructed to fill out the forms separately. Both the physician and nurses followed a simple script that read, “Thinking about the last day or 24 hours, please check the box that best describes on average how much each symptom bothered the child/(you).” If the child expressed that they did not understand the word for the symptom they could ask the physician, nurse, or caregiver for further clarification but caregivers were reminded not to assign a number or coach the child toward a number. The symptom scoring was reviewed with the caregiver and child afterward.
FIG. 1.
(a) Caregiver symptom assessment questions. (b) Child symptom assessment questions.
Time to complete the survey was performed using an electronic stopwatch in a pilot group of 30 randomly selected caregivers and children. The start time was defined as when the participant began reading the questions and the stop time was defined as when the participant handed the questions back to the physician.
Statistical analysis
Demographics, disease characteristics, symptom frequency reported by the child and by the caregiver, and level of symptom distress score are summarized by descriptive statistics that included frequencies and proportions for categorical and mean ± standard deviation (SD) and median (range) for continuous variables. Frequency and proportion of symptom assessment question completion and reasons for not completing the series of question are reported. Spearman correlation evaluates the correlation between child and caregiver symptom score. We determined the frequency and proportion of each symptom being reported by the child or caregiver while not being diagnosed by the referring medical service. Documentation of the presence of a symptom by the referring service was performed by manual analysis of the three antecedent notes of the referring service before initial consultation with pediatric palliative care. A symptom counted as being diagnosed by the referring team if (1) it was described in a clinical note and treated, (2) described in a clinical note but not treated, or (3) not described in a clinical note but treated as evidenced by the presence of a medication prescribed to address the specific symptom or referral to another service for management of the particular symptom (i.e., psychology for nervousness, irritability, or sadness). The proportion of positive symptoms from a physician's diagnosis is compared with that of the child/caregivers' report by McNemar test. Kappa coefficient is also reported to further evaluate the agreement between physicians' diagnosis and child/caregiver's symptom assessment reports. A symptom is considered positive if either a child or caregiver reported a score of >0. The Wilcoxon rank sum test evaluated the association of patients' symptom expression with gender, the age of the child, and tumor type. For variables that indicated a significant association with a symptom, a univariate logistic regression model was then applied to investigate this association further. The logistic model evaluates the effect of a variable on the probability of a positive symptom reported by children. Estimates of odds ratios (ORs) and their 95% confidence intervals are presented. An OR >1 indicates a tendency toward a positive symptom report (score >0).
Results
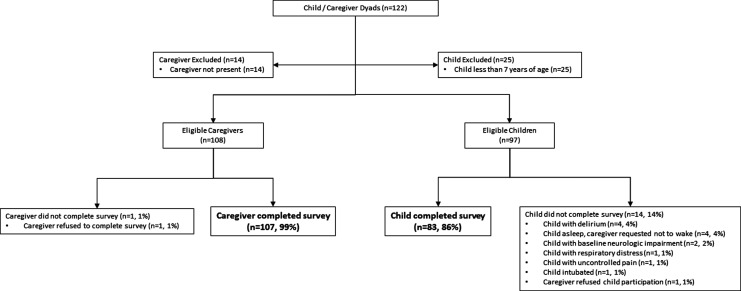
A single physician who is board certified in both General Pediatrics and Hospice and Palliative Medicine saw 122 consecutive children with cancer referred to the pediatric palliative care service. Fourteen caregivers were not present at the time of consultation, and 25 children were under the age of 7; these participants were not eligible to be part of the study. As given in Figure 2 and Table 1, virtually all (107/108, 99%) caregivers presented with the questions completed it; only one caregiver (1%) refused to complete the questions. The majority (83/97, 86%) of eligible children who received the survey completed it. Delirium (4, 4%) and the caregiver requesting the child not be awoken (4, 4%) were the most common reasons children did not complete the questions. The time to complete the survey of the pilot group (mean ± SD) was 59 (±11) seconds for caregivers and 73 (±8) seconds for children.
FIG. 2.
Caregiver and child participant accrual.
Table 1.
Questionnaire Completion Characteristics and Pediatric Palliative Care Patient Demographics
| n (%) | |
|---|---|
| Caregiver, n = 108 | |
| Caregiver completed survey | 107 (99.1) |
| Caregiver refused to complete | 1 (0.9) |
| Child, n = 97 | |
| Child completed survey | 83 (85.6) |
| Child did not complete survey | 14 (14.4) |
| Delirium | 4 (4.1) |
| Asleep, caregiver request not to wake | 4 (4.1) |
| Baseline neurologic impairment | 2 (2.1) |
| Respiratory distress | 1 (1.0) |
| Uncontrolled pain | 1 (1.0) |
| Intubated | 1 (1.0) |
| Caregiver refused child to answer | 1 (1.0) |
| Age, n = 122 | |
| Mean ± SD (years) | 12.7 ± 6.2 |
| Gender, n = 122 | |
| Male | 72 (59.0) |
| Diagnosis, n = 122 | |
| Solid tumor | 65 (53.3) |
| Hematological malignancy | 38 (31.1) |
| Central nervous system | 19 (15.6) |
| Referring specialty, n = 122 | |
| Pediatric oncology | 101 (82.8) |
| Pediatric critical care medicine | 7 (5.7) |
| Pediatric surgery/surgical subspecialties | 5 (4.1) |
| Pediatric stem cell transplant | 4 (3.3) |
| Pediatric neurology | 3 (2.5) |
| Pediatric endocrinology | 2 (1.6) |
SD, standard deviation.
Demographic characteristics of the children are summarized in Table 1. The mean age was 12.7 years and the majority were male (59.0%) patients and had a solid tumor (53.3%). The oncology service referred the most children (101/122, 82.8%) but referrals came from a broad range of pediatric clinical services.
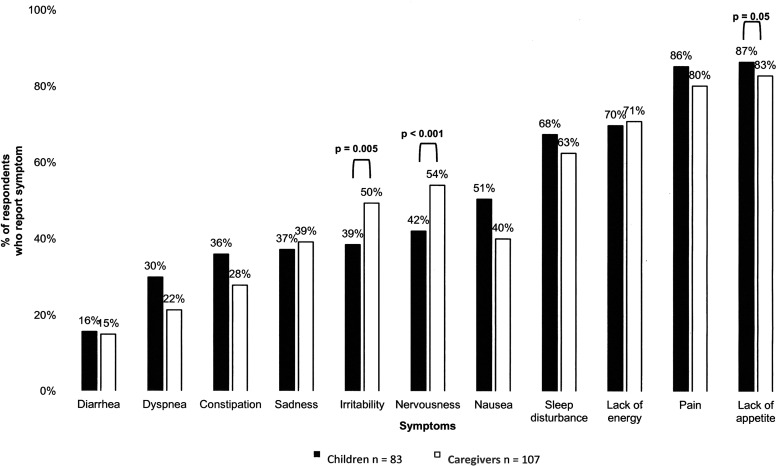
The frequency of symptoms as reported by the child and caregiver is given in Figure 3. More than half of children reported the presence of 5 of 11 symptoms, whereas more than half of caregivers reported the presence of 6 of 11 symptoms. Lack of appetite (child—72/83, 86.7%; caregiver—89/107, 83.2%) and pain (child—71/83, 85.5%; caregiver—86/107, 80.4%) were the most commonly reported symptoms. Caregivers reported irritability (p = 0.005) and nervousness (p < 0.001) significantly more frequently than children.
FIG. 3.
Percentage of children and caregiver reporting the presence of a symptom at the time of consultation with pediatric palliative care.
The level of symptom distress as reported by the child and caregiver is given in Table 2, as is the correlation between child and caregiver symptom score. Both children and caregivers rated pain and lack of appetite as the most bothersome symptoms. There was a very strong correlation for pain (rs > 0.8) and a strong correlation for all other symptoms (rs > 0.6). It should be noted that the psychological symptoms of sadness (rs = 0.65) nervousness (rs = 0.60), and irritability (rs = 0.60) had the lowest correlation between child and caregiver ratings. Spearman's correlation found a significant correlation between the child and caregiver symptom rating for all symptoms (p < 0.001).
Table 2.
Level of Symptom Distress Reported by Children and Caregiver at the Time of Consultation with Pediatric Palliative Care and Correlation between Child and Caregiver Rating
| Symptom | Child, mean (±SD), n = 83 | Caregiver, mean (±SD), n = 107 | Correlation (rs) | p |
|---|---|---|---|---|
| Pain | 2.54 ± 1.36 | 2.21 ± 1.49 | 0.83 | <0.001 |
| Lack of appetite | 2.39 ± 1.33 | 2.36 ± 1.42 | 0.7 | <0.001 |
| Sleep disturbance | 1.55 ± 1.40 | 1.60 ± 1.50 | 0.71 | <0.001 |
| Lack of energy | 1.54 ± 1.34 | 1.67 ± 1.34 | 0.74 | <0.001 |
| Nausea | 1.00 ± 1.28 | 0.85 ± 1.27 | 0.67 | <0.001 |
| Irritability | 0.77 ± 1.14 | 0.99 ± 1.23 | 0.6 | <0.001 |
| Nervousness | 0.78 ± 1.12 | 1.11 ± 1.27 | 0.6 | <0.001 |
| Constipation | 0.69 ± 1.10 | 0.58 ± 1.07 | 0.79 | <0.001 |
| Dyspnea | 0.57 ± 1.01 | 0.48 ± 1.05 | 0.78 | <0.001 |
| Sadness | 0.58 ± 0.91 | 0.74 ± 1.16 | 0.65 | <0.001 |
| Diarrhea | 0.35 ± 0.93 | 0.38 ± 1.01 | 0.79 | <0.001 |
p < 0.05 indicates significant correlation between child and caregiver symptom rating.
Table 3 provides the frequency of symptoms reported by the child or caregiver at the time of consultation not previously diagnosed by the referring medical service. Lack of appetite (74, 61.2%), sleep disturbance (68, 55.7%), lack of energy (60, 49.2%), irritability (56, 45.9%), sadness (47, 38.5%), and nervousness (43, 35.3%) were all reported by the child or caregiver but not diagnosed by the referring service at the time of consultation (McNemar's test, p < 0.001). These symptoms also had low Kappa coefficients. Pain had the highest Kappa coefficient (0.94), which indicates a strong agreement between physicians' diagnosis and children/caregiver reports.
Table 3.
Frequency of Symptoms Reported by the Child/Caregiver at the Time of Consultation Not Previously Diagnosed by the Referring Medical Service
| Symptom | Reported by child/caregiver but not diagnosedaby referring medical service | pb | Kappa coefficientc | |
|---|---|---|---|---|
| n | % | |||
| Lack of appetite | 74 | 61.2 | <0.001 | 0.02 |
| Sleep disturbance | 68 | 55.7 | <0.001 | −0.06 |
| Lack of energy | 60 | 49.2 | <0.001 | 0.09 |
| Irritability | 56 | 45.9 | <0.001 | 0.04 |
| Sadness | 47 | 38.5 | <0.001 | 0.01 |
| Nervousness | 43 | 35.3 | <0.001 | 0.26 |
| Dyspnea | 26 | 21.3 | 0.30 | 0.06 |
| Nausea | 25 | 20.5 | 0.89 | 0.20 |
| Constipation | 17 | 13.9 | 0.13 | 0.24 |
| Diarrhea | 10 | 8.2 | 1.00 | 0.38 |
| Pain | 2 | 1.9 | 0.16 | 0.94 |
“Diagnosis” is defined as (1) described in a clinical note and treated, (2) described in a clinical note but not treated, or (3) not described in a clinical note but treated as evidenced by the presence of a medication prescribed to treat the specific symptom or referral to another service for management of the specific symptom.
p < 0.05 indicates significant difference in symptom reporting between child/caregiver and referring medical team (by McNemar's test).
The Kappa coefficient measures the agreement between the child/caregiver symptom report and the referring medical service documentation of symptoms in the three antecedent notes.
In our exploratory analysis, there was no significant difference in the level of symptom distress between male and female patients or when comparing 7–11 years of age and >11 years of age; the only exception was that sleep disturbance was more significant in female (1.97 ± 1.40) than male (1.32 ± 1.36) patients (p = 0.04). Children with CNS tumors reported significantly higher mean scores for lack of energy (2.44 ± 1.42) and irritability (1.67 ± 1.32) compared with children with hematologic malignancies or solid tumors (p < 0.05); they were also almost 10 times more likely to report irritability (p = 0.05) and eight times more likely to report lack of energy (p = 0.03). Children with CNS and solid tumors were significantly more likely to report constipation (p = 0.01) compared with those with hematologic malignancy.
Discussion
The primary objective of this retrospective study was to determine the feasibility of using a set of questions that assessed how bothersome common physical and psychological symptoms were in children with cancer referred to a pediatric palliative care service. With the very high completion percentage rates (99% for caregivers and 86% for children), our study demonstrates that this survey is easy and quick to complete, with most caregivers and children completing it in <80 seconds.
We found similar results to other studies in the symptom burden reported by the child and caregivers,13 and that caregivers rated psychological symptoms more frequently and with greater intensity than their child.20–23 These findings should provide reassurance that the questions accurately capture the symptom burden of children and that children report symptoms differently from their caregivers. This discrepancy raises an interesting problem for clinicians, as a child PRO is considered to be the gold standard for symptom assessment. What is the best course of action when discrepant reports by the child and caregiver about psychological symptoms are encountered? Who does one place more confidence in—the child who denies sadness or irritability, or the caregiver who perceives the child to be suffering from these symptoms? One must consider the possibility that a child may not have the developmental capacity to recognize, acknowledge, and accurately report psychological symptoms. The importance of obtaining both the child and caregiver perspective underscores the real value of standardized screening in pediatric palliative care—to allow entry points for the clinician to inquire, explore, and learn the essential details about what the child and caregiver are perceiving and experiencing.
Our study found that psychological and other less clinically evident symptoms such as lack of appetite, fatigue, and sleep disturbance were significantly underdiagnosed by the referring team as has been reported elsewhere.23,24 The discrepancy may be potentially explained if the child developed new symptoms between the last referral team visit and first pediatric palliative care consultation. However, it may be reflective of undiagnosed symptoms, and so systematic symptom screening should play a considerable role for all children irrespective of where they may be in their treatment course. Pediatric oncology has a rich tradition of recognizing the importance of psychosocial support and dedicates substantial financial resources to social workers, psychologists, and neuropsychologists to help address these issues. Our study suggests that a more robust standardized screening will help identify children who need augmented psychological support and treatment for their less clinically evident symptoms before they are referred to pediatric palliative care. Furthermore, symptom severity scoring in children that shows congruence between a caregiver and a health care provider is associated with improved satisfaction in the care received and parental quality of life,25 speaking of the importance of identifying symptoms in a systematic manner.
Our findings show that pediatric palliative care patients have a very high frequency of distressing symptoms and therefore continuous monitoring of those symptoms is crucial. The main barriers to the use of existing validated tools are the length of time to complete,9,10 complexity of implementing the tools,19 focus on a single symptom such as fatigue, nausea, depression, and anxiety,26–29 focus on a multitude of symptoms,30–32 or have different versions for different age groups.31–33 All these factors make a majority of the existing validated tools cumbersome and difficult to implement on a consistent basis. Our survey shows very few groups perform systematic symptom assessment. Our findings justify conducting validation of the psychometric properties of these questions including validity and reliability using some of the existing gold standards. Ideally, these future studies should be performed in multiple clinical settings including children with different life-threatening illnesses.
There are many strengths to this study. First, it is an extensive consecutive series of children with cancer referred to pediatric palliative care. Second, the survey is quick and easy to complete as evidenced by the high completion rate by the child and the caregiver. Third, we measured symptom burden from the child and caregiver simultaneously. Fourth, children and caregivers were evaluated in both the outpatient and inpatient setting that captures a much wider range of symptom burden. Fifth, the numeric rating scale for each symptom is similar to validated PRO tools.9,10 Finally, the results are consistent with previous studies that assessed symptom burden in children with cancer.9,10,13
There are several limitations to this study. First, our study was conducted in a single National Cancer Institute-designated comprehensive care center, and the children may not be representative of patient populations found at other pediatric oncology centers. Second, we did not differentiate between parental and nonparental caregivers. Nonparental caregivers may assess symptoms differently from a parent, and this should be tested in future research. Third, and most importantly, although similar to other PRO tools, our survey was not validated before we conducted the study. The primary objective was to test the feasibility. With a high completion rate and a short amount of time spent answering the questions, we can confidently say the task of completing is minimal. The obvious next critical task is to validate the questions.
Conclusions
By quickly completing our series of questions, the child and caregiver set the agenda for consultation with pediatric palliative care. This is essential, as the association between psychological symptoms reported by the child or caregiver and a previous diagnosis by the referring team is low. Many sources of suffering in the child are overlooked until they are evaluated systematically by pediatric palliative care; although this study was conducted exclusively in children with cancer, the value of systematic screening of all patients referred to pediatric palliative care cannot be understated and should be a standard of care. More research is needed to validate the questions we developed.
Acknowledgments
The authors acknowledge the staff at the Child and Adolescent Center at MD Anderson for their assistance in the distribution of the sample questions.
Author Disclosure Statement
Dr. Madden, Dr. Charone, Dr. Mills, Dr. Bruera, Dr. Dibaj, Ms. Liu, and Ms. Williams have no financial conflicts of interest, or disclosures to make regarding this study.
References
- 1. Siegel RL, Miller KD, Jemal A: Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30 [DOI] [PubMed] [Google Scholar]
- 2. Society AC: Cancer facts & figures 2017. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf 2017. (Last accessed January30, 2018)
- 3. Hudson MM, Ness KK, Gurney JG, et al. : Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–1582 [DOI] [PubMed] [Google Scholar]
- 5. Wolfe J, Grier HE, Klar N, et al. : Symptoms and suffering at the end of life in children with cancer. N Engl J Med 2000;342:326–333 [DOI] [PubMed] [Google Scholar]
- 6. Olagunju AT, Sarimiye FO, Olagunju TO, et al. : Child's symptom burden and depressive symptoms among caregivers of children with cancers: An argument for early integration of pediatric palliative care. Ann Palliat Med 2016;5:157–165 [DOI] [PubMed] [Google Scholar]
- 7. Waldman E, Wolfe J: High symptom burden in children with cancer and high parental satisfaction: Why the disconnect? Ann Palliat Med 2013;2:54–55 [DOI] [PubMed] [Google Scholar]
- 8. Linder LA: Developmental diversity in symptom research involving children and adolescents with cancer. J Pediatr Nurs 2008;23:296–309 [DOI] [PubMed] [Google Scholar]
- 9. Collins JJ, Devine TD, Dick GS, et al. : The measurement of symptoms in young children with cancer: The validation of the Memorial Symptom Assessment Scale in children aged 7–12. J Pain Symptom Manage 2002;23:10–16 [DOI] [PubMed] [Google Scholar]
- 10. Collins JJ, Byrnes ME, Dunkel IJ, et al. : The measurement of symptoms in children with cancer. J Pain Symptom Manage 2000;19:363–377 [DOI] [PubMed] [Google Scholar]
- 11. Lyon ME, Jacobs S, Briggs L, et al. : A longitudinal, randomized, controlled trial of advance care planning for teens with cancer: Anxiety, depression, quality of life, advance directives, spirituality. J Adolesc Health 2014;54:710–717 [DOI] [PubMed] [Google Scholar]
- 12. Patel SK, Fernandez N, Wong AL, et al. : Changes in self-reported distress in end-of-life pediatric cancer patients and their parents using the pediatric distress thermometer. Psychooncology 2014;23:592–596 [DOI] [PubMed] [Google Scholar]
- 13. Wolfe J, Orellana L, Ullrich C, et al. : Symptoms and distress in children with advanced cancer: Prospective patient-reported outcomes from the PediQUEST study. J Clin Oncol 2015;33:1928–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruera E, Kuehn N, Miller MJ, et al. : The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9 [PubMed] [Google Scholar]
- 15. Wong A, Rodriguez-Nunez A, Tayjasanant S, et al. : Edmonton Symptom Assessment Scale (ESAS): Time duration of self-completion versus assisted-completion in palliative care patients—A randomized controlled trial. J Clin Oncol 2016;34:67 [Google Scholar]
- 16. Kang JH, Kwon JH, Hui D, et al. : Changes in symptom intensity among cancer patients receiving outpatient palliative care. J Pain Symptom Manage 2013;46:652–660 [DOI] [PubMed] [Google Scholar]
- 17. Chang VT, Hwang SS, Feuerman M: Validation of the edmonton symptom assessment scale. Cancer 2000;88:2164–2171 [DOI] [PubMed] [Google Scholar]
- 18. Drake R, Frost J, Collins JJ: The symptoms of dying children. J Pain Symptom Manage 2003;26:594–603 [DOI] [PubMed] [Google Scholar]
- 19. Wolfe J, Orellana L, Cook EF, et al. : Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: Results from the PediQUEST randomized controlled trial. J Clin Oncol 2014;32:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eiser C, Morse R: Can parents rate their child's health-related quality of life? Results of a systematic review. Qual Life Res 2001;10:347–357 [DOI] [PubMed] [Google Scholar]
- 21. Rajmil L, Lopez AR, Lopez-Aguila S, Alonso J: Parent-child agreement on health-related quality of life (HRQOL): A longitudinal study. Health Qual Life Outcomes 2013;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baggott C, Cooper BA, Marina N, et al. : Symptom assessment in pediatric oncology: How should concordance between children's and parents' reports be evaluated? Cancer Nurs 2014;37:252–262 [DOI] [PubMed] [Google Scholar]
- 23. Zhukovsky DS, Rozmus CL, Robert RS, et al. : Symptom profiles in children with advanced cancer: Patient, family caregiver, and oncologist ratings. Cancer 2015;121:4080–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldman A, Hewitt M, Collins GS, et al. : Symptoms in children/young people with progressive malignant disease: United Kingdom Children's Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics 2006;117:e1179–e1186 [DOI] [PubMed] [Google Scholar]
- 25. Vollenbroich R, Borasio GD, Duroux A, et al. : Listening to parents: The role of symptom perception in pediatric palliative home care. Palliat Support Care 2016;14:13–19 [DOI] [PubMed] [Google Scholar]
- 26. Hinds PS, Yang J, Gattuso JS, et al. : Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. J Pain Symptom Manage 2010;39:572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dupuis LL, Taddio A, Kerr EN, et al. : Development and validation of the pediatric nausea assessment tool for use in children receiving antineoplastic agents. Pharmacotherapy 2006;26:1221–1231 [DOI] [PubMed] [Google Scholar]
- 28. Saylor CF, Finch AJ, Jr, Spirito A, Bennett B: The children's depression inventory: A systematic evaluation of psychometric properties. J Consult Clin Psychol 1984;52:955–967 [DOI] [PubMed] [Google Scholar]
- 29. Deacy AD, Gayes LA, De Lurgio S, Wallace DP: Adaptation of the state-trait inventory for cognitive and somatic anxiety for use in children: A preliminary analysis. J Pediatr Psychol 2016;41:1033–1043 [DOI] [PubMed] [Google Scholar]
- 30. Nixon Speechley K, Maunsell E, Desmeules M, et al. : Mutual concurrent validity of the child health questionnaire and the health utilities index: An exploratory analysis using survivors of childhood cancer. Int J Cancer Suppl 1999;12:95–105 [DOI] [PubMed] [Google Scholar]
- 31. Bhatia S, Jenney ME, Bogue MK, et al. : The Minneapolis-Manchester Quality of Life instrument: Reliability and validity of the Adolescent Form. J Clin Oncol 2002;20:4692–4698 [DOI] [PubMed] [Google Scholar]
- 32. Bhatia S, Jenney ME, Wu E, et al. The Minneapolis-Manchester Quality of Life instrument: Reliability and validity of the Youth Form. J Pediatr 2004;145:39–46 [DOI] [PubMed] [Google Scholar]
- 33. Varni JW, Burwinkle TM, Katz ER, et al. : The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002;94:2090–2106 [DOI] [PubMed] [Google Scholar]