Abstract
Background: Despite positive outcomes associated with specialist palliative care (PC) in diverse medical populations, little research has investigated specialist PC in surgical ones. Although cancer surgery is predominantly safe, operations can be extensive and unpredictable perioperative morbidity and mortality persist, particularly for patients with upper gastrointestinal (GI) cancers.
Objectives and Hypotheses: Our objective is to complete a multicenter, randomized controlled trial comparing surgeon-PC co-management with surgeon-alone management among patients pursuing curative-intent surgery for upper GI cancers. We hypothesize that perioperative PC will improve patient postsurgical quality of life. This study and design are based on >8 years of engagement and research with patients, family members, and clinicians surrounding major cancer surgery and advance care planning/PC for surgical patients.
Methods: Randomized controlled superiority trial with two study arms (surgeon-PC team co-management and surgeon-alone management) and five data collection points over six months. The principal investigator and analysts are blinded to randomization.
Setting: Four, geographically diverse, academic tertiary care hospitals. Data collection began December 20, 2018 and continues to December 2020.
Participants: Patients recruited from surgical oncology clinics who are undergoing curative-intent surgery for an upper GI cancer.
Interventions: In the intervention arm, patients receive care from both their surgical team and a specialist PC team; the PC is provided before surgery, immediately after surgery, and at least monthly until three months postsurgery. Patients randomized to the usual care arm receive care from only the surgical team.
Main Outcomes and Measures: Primary outcome: patient quality of life. Secondary outcomes: patient: symptom experience, spiritual distress, prognostic awareness, health care utilization, and mortality. Caregiver: quality of life, caregiver burden, spiritual distress, and prognostic awareness. Intent-to-treat analysis will be used.
Ethics and Dissemination: This study has been approved by the institutional review boards of all study sites and is registered on clinicaltrials.gov (NCT03611309, First received: August 2, 2018).
Keywords: palliative care for surgery, patient-centered outcomes research, perioperative palliative care
Introduction
In 2009, there were ∼48 million operations performed in the United States,1 and surgeons now operate on sicker and older patients than those who underwent similar operations just two decades ago.2,3 Although major surgery is more-often-than-not safe and relatively uneventful, significant patient morbidity and mortality persist.4–10 Moreover, although patients can be risk stratified for perioperative complications,6,7,11 it is impossible to prospectively predict exactly which patient will die or suffer a major perioperative complication.
Upper gastrointestinal cancer morbidity and mortality
Unfortunately, upper gastrointestinal (GI) cancers remain some of the highest morbidity, highest mortality, and poorest prognosis cancers.12 In 2015, in the United States, there were >140,000 diagnoses of upper GI cancers with ∼97,000 deaths (Ref.12, Table 1) and not infrequent severe cancer-related morbidity. A Spanish study of pancreatic cancer patients at the time of diagnosis found high symptom prevalence with noted symptoms being severe fatigue (86%), anorexia (83%), weight loss (85%), abdominal pain (79%), epigastric pain (71%), back pain (49%), persistent nausea (51%), chronic diarrhea (44%), intermittent vomiting (33%), and severe itching (32%).13 At the time of diagnosis, patients with gastric cancer note frequent and significant anorexia, nausea, abdominal pain, early satiety, and/or dysphagia14 and more than 90% of patients with esophageal cancer report at least one burdensome symptom with common complaints, including dysphagia, anorexia, weight loss, and odynophagia.15
Table 1.
| Type | Diagnoses | Deaths |
|---|---|---|
| Pancreatic | 48,960 | 40,560 |
| Liver | 39,230 | 27,170 |
| Esophageal | 16,980 | 15,590 |
| Stomach | 24,590 | 10,720 |
| Gall bladder | 10,910 | 3700 |
Upper GI cancer surgery-related morbidity and mortality
Surgery is currently the only treatment offering potential long-term survival for upper GI cancer patients and if a tumor is resectable, standard of care is to surgically remove it shortly after diagnosis.16–21 Treatment of pancreatic adenocarcinoma (PA) provides a useful example of surgical management of an upper GI cancer. Although surgery offers the only chance of cure or long-term survival,16 ∼80% of PA patients at the time of diagnosis have a disease that is already too advanced for curative-intent surgery.16,21 By definition, patients pursuing curative-intent surgery for PA must have stage I (a or b) or stage IIa disease; if patients have later stage (stage III or IV) disease, they are not offered curative-intent surgery.16,20 Curative-intent pancreatic cancer operations are extensive, most often necessitating a pancreaticoduodenectomy (Whipple surgery) or a distal pancreatectomy and splenectomy.16,21 Although the largest existing case series of these operations supports a 30-day perioperative mortality of only 1.4%, ∼40% of these patients still sustain surgery-related morbidity, including delayed gastric emptying, pancreatic fistulae, bilomas, and biliary strictures.22,23 Moreover, even after curative-intent surgery for PA and though new chemotherapeutics may offer prolonged survival,24,25 a majority of PA patients undergoing curative-intent surgery still ultimately die of PA; historical data suggest that approximately four out of five of these patients have eventual progression of their cancer to stage IV disease, with a median postoperative survival of two years.23,26
Beyond management of PA, morbidity and mortality for other upper GI cancers is similarly poor. For patients with localized hepatocellular carcinoma, the five-year mortality is 72%.12 Among patients who have undergone surgery for esophageal cancer or gastric cancer, 45% or 36%, respectively, note at least one significant postoperative symptom—including diarrhea, reflux, nausea, dysphagia, fatigue, loss of appetite, pain, or dyspepsia—and some symptoms persist for months after surgery.27 The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) data suggest that major morbidity for esophagectomy cancer surgery—including postoperative bleeding, anastomotic leak, pneumonia, and/or prolonged postoperative intubation—occurs in 24% of patients28 and five-year survival for patients with stage II or higher disease is only 15–30%.12
Palliative care for patients undergoing curative-intent surgery for upper GI cancers
It is our experience that every patient pursuing curative-intent surgery for an upper GI cancer hopes for long term survival. However, it is exactly because of the just-described, poor long-term prognoses and high symptom burden that we hypothesize that proactive, specialist-delivered palliative care (PC)29 is both reasonable and potentially beneficial for these surgical oncology patients and their family members. PC is patient- and family-centered care that symptomatically and psychosocially supports seriously ill patients and their families and optimizes quality of life, regardless of diagnosis, prognosis, or care goals.29 Studies among medical oncology patients support that proactive PC: improves quality of life,30–34 improves physical and psychological symptom management,30–35 enhances understanding of prognosis,36 lessens spiritual distress,33 lessens caregiver burden,33 improves caregiver social well-being,37 decreases caregiver psychological distress,34,37 lowers care costs,35,38,39 decreases aggressive end-of-life care interventions,31,40,41 and may even prolong patient survival.31,41 Despite these benefits, there have been few studies translating proactive PC from a medical to a surgical population and none comparing surgeon-PC team co-management with surgeon-alone management across patient-reported outcomes (PROs).42 Indeed, multiple studies even document surgeon and surgical culture resistance to PC involvement, particularly any discussions that might concern end-of-life care.43,44–50
Informed by intense prior engagement and research with diverse patient, family, clinician, and researcher stakeholders,51–61 our Patient-Centered Outcome Research Institute (PCORI)-funded study builds on the paucity of research regarding the impact of specialist PC on surgical oncologic patients and their family members. Addressing this important topic, we initiated a randomized clinical trial (the PERIOP-PC) trial; clinicaltrials.gov Identifier NCT03611309; PCORI: IHS 1609-36518).
Objectives and Hypotheses
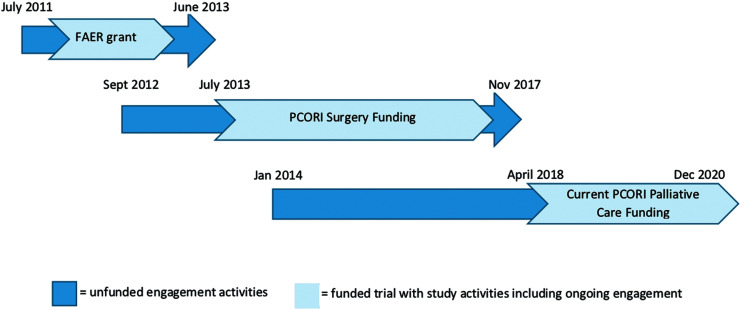
The objective of this study is to complete a multicenter, randomized controlled trial comparing the impact of surgeon-PC team co-management with surgeon-alone management among patients pursuing curative-intent surgery for upper GI cancers. The outcomes and study design were selected based on eight years of intense engagement with patients and family members, as well as other key stakeholders including surgeons, oncologists, anesthesiologists, surgical intensive care unit (SICU) nurses, PC clinicians, and health services researchers (Fig. 1). This project builds on our previous PCORI-funded research developing and integrating video-based, advance care planning into the perioperative period for patients and families preparing for major cancer surgery (Refs.51–61; clinicaltrials.gov Identifier NCT02489799; PCORI: CDR-12-11-4362). For PERIOP-PC, we hypothesize that surgeon-PC team co-management, as compared with surgeon-alone management, will improve quality of life for cancer patients at three months after the surgery (Hypothesis 1).
FIG. 1.
Timeline of engagement activities. FAER, Foundation for Anesthesia Education and Research; PCORI, Patient-Centered Outcomes Research Institute; PCORI Surgery, CDR-12-11-4362.
Our secondary aims explore multiple other patient and family member quantitative and qualitative outcomes and include the impact of surgeon-PC team co-management on:
- Patient mood symptoms, spiritual distress, prognostic awareness, and symptom burden;
- Caregiver mood symptoms, spiritual distress, prognostic awareness, and caregiver burden; and
- Patient, family member, surgeon, and PC clinician thoughts and beliefs about the perioperative experience.
We hypothesize that patients and family members in the surgeon-PC team co-management arm will have: improved mood symptoms, less spiritual distress, better prognostic awareness, decreased caregiver burden, and lower prevalence of symptoms (Hypotheses 2–6). We also hypothesize that surgeon-PC team co-management will be well tolerated and/or welcomed by patients, family members, surgeons, and PC clinicians (Hypothesis 7).
Methods
Study design
This study is a multicenter, randomized controlled trial comparing the impact of surgeon-PC team co-management with surgeon-alone management on PROs, including quality of life (primary outcome), mood symptoms, symptom score, caregiver burden, prognostic awareness, and spiritual distress.
Setting
This study is a multicenter study at four locations across the United States: The Johns Hopkins Hospital in Baltimore, MD; the Dana Farber Cancer Institute in Boston, MA; The University of New Mexico Hospital in Albuquerque, NM; and Stanford University Medical Center (SUMC) in Stanford, CA. The study is also being completed in partnership with the Palliative Care Research Cooperative (PCRC). Data collection began on December 20, 2018 and is planned to continue through December 2020.
Population
Our study sample includes patients pursuing nonemergent, upper GI cancer-related surgery with a goal of primary resection of the tumor (optimal surgical goal is cure, not merely disease palliation) at one of the four study sites. To further clarify, all study patients will be pursuing curative-intent surgical management for: pancreatic cancer (PA or neuroendocrine tumors), gastric cancer, cholangiocarcinoma, hepatocellular carcinoma, or esophageal cancer. Many patients are accompanied to the surgeon's clinic by a family member or friend (i.e., a “caregiver”). One caregiver for each patient is also invited to join the study. All participating surgeons and PC clinicians are comfortable with the two intervention arms and welcome participation in this clinical trial. Based on sample size calculations, explained in “Design Justification,” we aim to recruit 380 patients for the study.
Interventions/arms
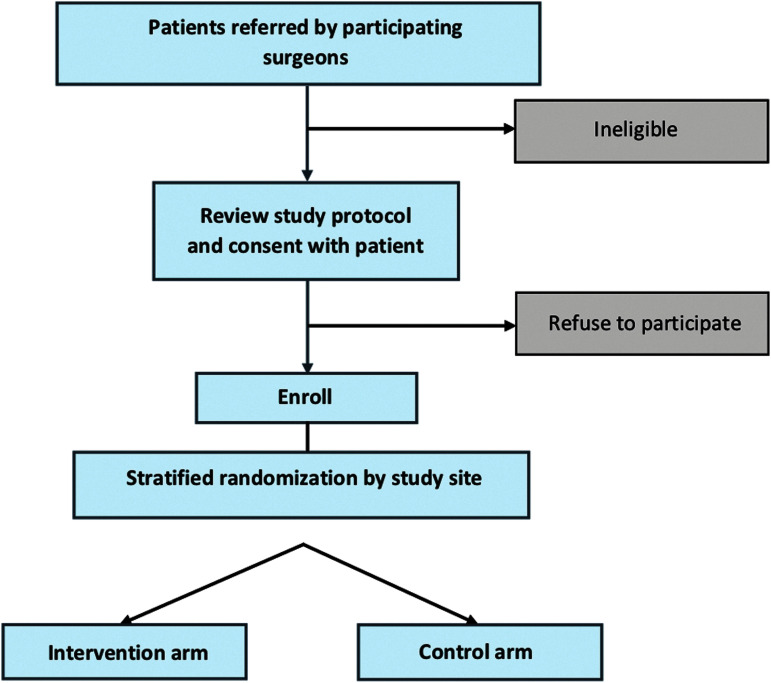
Patients are randomized into one of two arms: surgeon-alone management (enhanced usual care) or surgeon-PC team co-management (Fig. 2).
FIG. 2.
Trial enrollment.
Surgeon-alone management-enhanced usual care
Considered “enhanced usual care,” this involves surgeon and surgical team management of all perioperative care, including specific surgical care as well as management of perioperative symptoms, psychosocial support, and, if desired by the patient and/or caregiver, disease-related education and communication. The surgeon and surgical team care for the patient and their caregiver both before and after surgery. As consistent with standard practice, the surgeon may consult the PC team at any time, if desired. This “standard practice” reflects current surgical oncology care delivery at the four study sites and is “enhanced” by providing the National Comprehensive Cancer Network (NCCN)-recommended triggers for when to involve PC consultants (Ref.62, Table 2). Surgeons are encouraged to follow the NCCN guidelines in the Enhanced Usual Care arm as to when they should consider PC consultation, although, as consistent with standard practice, it is ultimately the surgeon and surgical team's decision about if and/or when to consult PC. This is an intention-to-treat study; if a surgeon consults PC for a patient in this arm, the consultation will proceed as per any standard consult with documentation and visits as per the patient, PC provider, and surgeon wishes.
Table 2.
Perioperative Palliative Care Trial Recommendations for Postoperative Consultation of Palliative Care Specialists for Patients in the Enhanced Usual Care Study Arm, Based on National Comprehensive Cancer Network Guidelines62
| Consider consultation of palliative care specialists in the following circumstances: |
| Patient/family or provider dissatisfaction with the care plan |
| Need of clarification of goals of care |
| Poor postoperative pain management and/or high nonpain symptom burden (i.e., distress, nausea, etc.) |
| Need for a palliative surgical procedure, such as palliative stenting, a venting gastrostomy, or a palliative-intent hepato-jenjunostomy |
| Frequent postoperative emergency room visits or hospitalizations |
| Prolonged postoperative ICU-level care |
| Communication barriers (i.e., language, literacy, physical barriers, cognitive impairment) |
| Patient or family request for hastened death |
| Difficult caregiver/family social circumstances and/or high risk for complicated bereavement issues |
| Compassion fatigue, moral distress, burnout, or distress related to complex care coordination among the clinician team |
Surgeon-PC team co-management—intervention
All patients receive the surgical care as described earlier in “Surgeon-alone management.” In addition to this care, PC is provided by a specialist team. The PC team's practices are summarized through the acronym TEAM—for Time, Education, Assessments, and Multidisciplinary (Table 3) and specifically comprise perioperative symptom management recommendations, psychosocial support, and, if desired by the patient and/or caregiver, disease-related education and communication. This specialist PC is consistent with that evaluated in previous medical oncologic clinical trials.31,41,63,64 In this study arm, patients and companions are seen by the PC team: (1) in an outpatient setting before surgery, (2) in the hospital during their postoperative hospitalization, and (3) on an at least monthly basis and/or as needed until 12 weeks after surgery (Fig. 3). Consistent with previous PC interventions,41,63,64 postoperative PC interactions after patient discharge from the hospital can be in person at the outpatient clinic or via telephone, Facetime, or Skype, whichever is preferred by the patient and caregiver.
Table 3.
Components of Surgeon Palliative Care Team Co-Management
| TEAM element | Description |
|---|---|
| T—Time | At least 60 minutes/month of time (per patient and caregiver preference) devoted to palliative care treatments for the patient and family. |
| E—Education | Patients and family members, per their desires and wishes, are counseled and educated about their disease, including self-management of symptoms, prognosis, and treatment options. |
| A—Assessment | Formal assessment of symptoms, including pain, dyspnea, constipation/diarrhea, anxiety/depression, fatigue, and nausea. |
| M—Multidisciplinary | Access to a multidisciplinary palliative care team composed of nurse, physician, social worker, pharmacist, and/or chaplain team members. |
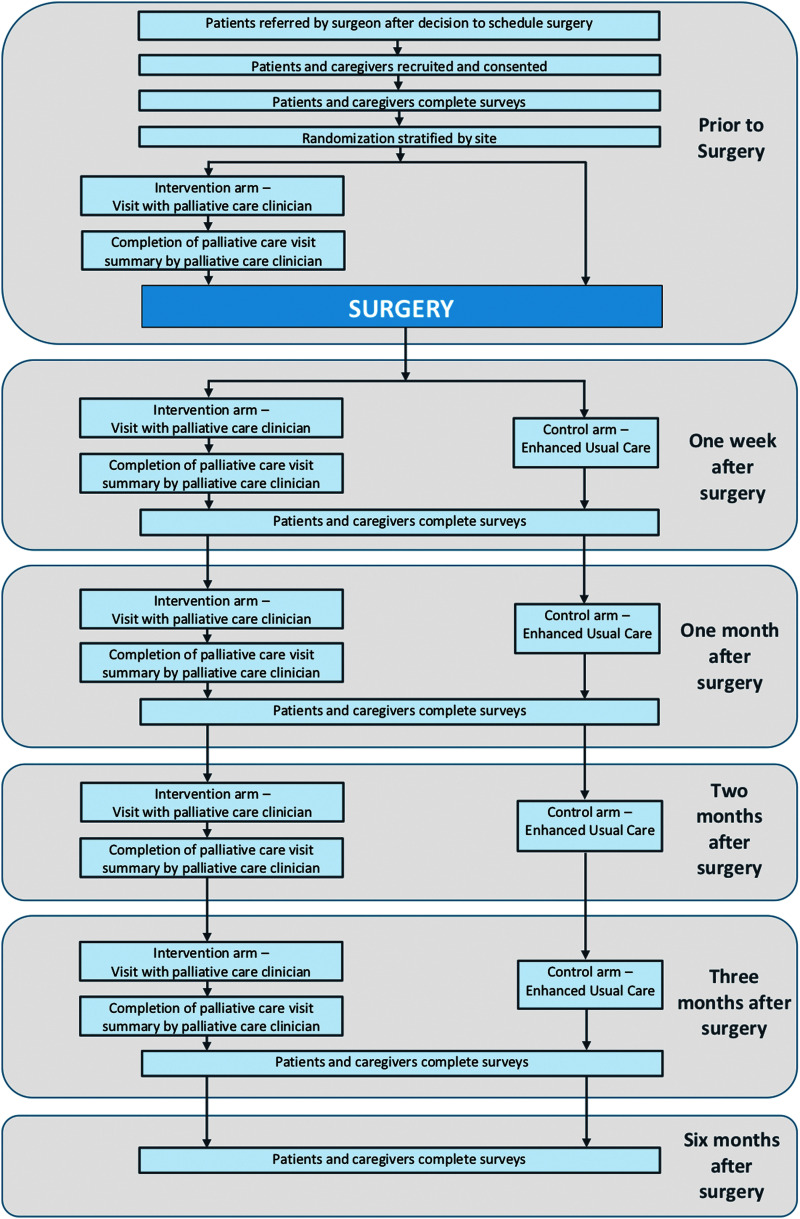
FIG. 3.
Trial data collection.
Randomization
Randomization is immediately after enrollment, stratified by study site, and completed via computer-generated random allocation with a block size of 6 by using the Research Electronic Data Capture (REDCap) database. Understandably, neither the patient, caregiver, nor surgeon can be blinded to the intervention. However, the principal investigator (PI) and analysis team are blinded to participant randomization and the research team acquiring outcome data is, whenever possible, blinded to participant randomization.
Outcome measures
Primary outcome
The primary outcome variable of this project is patient quality of life, measured by the Functional Assessment of Chronic Illness Therapy—Palliative Care (FACIT-PaL) Subscale.65 This subscale has not been previously used in a surgical population; however, FACIT-PaL includes all of the elements of Functional Assessment of Cancer Therapy-General (FACT-G), which have been used extensively as a quality-of-life outcome in cancer populations.65–67
Secondary outcomes
The secondary outcome variables of this project are (Table 4):
Table 4.
Instruments and Outcomes for Each Specific Aim
| SA1 (months 1–36) | All outcomes measured at approximately: | Patients | (1) Quality of life—Functional Assessment of Chronic Illness Therapy—Palliative Care (FACIT-Pal)a65 |
| Five days after surgery | Subscales: (i) Functional Assessment of Cancer Therapy-General (FACT-G) | ||
| One month after surgery | (ii) Trial Outcome Index (TOI) | ||
| (2) Demographics | |||
| Three months after surgery | (3) Edmonton Symptom Assessment Score (ESAS)b,69 | ||
| (4) Functional Assessment of Chronic Illness Therapy—Spiritual Well-being (FACIT-Sp-12)b,68 | |||
| Six months after surgery | |||
| (5) Mood symptoms including anxiety and depression subscales—PROMIS-2966,67 | |||
| (6) Health care utilizationb (No. of postoperative hospitalizations and/or emergency room visits) | |||
| (7) Presence of advance care planningb | |||
| (8) Prognostic understandingb,70 | |||
| (9) Mortalityb | |||
| Caregivers | (1) Demographics | ||
| (2) Zarit Caregiver Burden Scale (ZBI-12)b,71 | |||
| (3) Mood symptoms including anxiety and depression subscales—PROMIS-2967,68 | |||
| (3) Functional Assessment of Chronic Illness Therapy—Spiritual Well-being (FACIT-Sp-12) family/companion measureb,68 | |||
| (5) Prognostic understandingb | |||
| SA2 (months 8–16) | In-depth interviews (approx., n = 20 in each arm) | Patients | Patient experiences and beliefs regarding management of symptoms, prognostic clarity, and advance care planning practices and beliefs |
| In-depth interviews (approx., n = 20 in each arm) | Caregivers | Family member experiences and beliefs regarding prognostic clarity, caregiver experience, and patient experience of symptoms and advance care planning practices and beliefs | |
| In-depth interviews (approx., n = 8; all surgeons involved in the trial) | Surgeons | Surgeon experiences and beliefs regarding surgeon-alone vs. surgeon-palliative care team co-management of perioperative cancer patients and their families. | |
| In-depth interviews (approx., n = 10–15) | Palliative care clinicians | Palliative care team experiences and beliefs regarding surgeon-alone vs. surgeon-palliative care team co-management of perioperative cancer patients and their families. |
Primary outcome.
Secondary outcome.
Patient—mood symptoms (PROMIS-2968,69), spiritual distress (FACIT—Spiritual Well-being70), symptom experience (Edmonton Symptom Assessment Score71), prognostic awareness (questions adapted from the CANCORS study72), health care utilization, mortality, and self-described experiences and thoughts about surgeon-PC team co-management.
Caregiver—mood symptoms (PROMIS-29), spiritual distress (FACIT—Spiritual Well-being), prognostic awareness (questions adapted from the CANCORS study), caregiver burden (Zarit Caregiver Burden Scale—ZBI-1273), and self-described experiences and thoughts about surgeon-PC team co-management.
Surgeon—self-described experiences and thoughts about surgeon-PC team co-management.
PC Clinician—self-described experiences and thoughts about surgeon-PC team co-management.
Recruitment and Study Procedures
Eligibility criteria
Eligible patients are pursuing nonemergent, upper GI cancer-related surgery with a goal of primary resection of the tumor—optimal surgical goal is cure, not merely disease palliation—and must be diagnosed with one of the following cancers: pancreatic (adenocarcinoma or neuroendocrine tumors), hepatocellular, esophageal, gastric, or cholangiocarcinoma. They must have no previous involvement of PC providers in their care course. Potential study patients must also be able to give informed consent and be at least 18 years of age. As assessment for capacity for informed consent is a standard part of the surgical consent process, no patient is referred for the study without having been seen by the surgeon and deemed competent per the surgical team standard protocols.
One caregiver per patient is also asked to participate. In addition to being identified by the patient at being a key caregiver throughout the surgery period, these caregivers must be able to give informed consent and be at least 18 years of age.
Recruitment
Patients are identified through the treating surgeon's outpatient clinic. Each week at each study site, the research coordinator attends key meetings (new patient rounds, appropriate ambulatory clinics, case conferences) and reviews surgeon clinic schedules to identify potential participants. The research coordinator then confers with surgeon schedulers and/or clinical staff to verify eligibility. The research coordinator speaks with potentially eligible patients in the clinic or by phone. During this interaction, the patient is further screened for eligibility and consented. At the time of patient enrollment, caregivers of patients are also given the opportunity to participate, screened for eligibility, and consented.
Participants in both study arms receive $20 for each set of surveys completed. As this study includes five sets of surveys, total completion of study surveys results in $100. Participants selected for an in-depth follow-up interview receive another $20 compensation.
Patient trajectory
All participants—both patients and caregivers—in both study arms complete surveys at five time points (Fig. 3 and Table 4).
At recruitment (preoperative baseline)
Approximately five days after surgery
Approximately one month after surgery
Approximately three months after surgery (primary outcome time point)
Approximately six months after surgery
Participants in the intervention group also have PC visits at these time points:
Just after recruitment and before surgery
Approximately two to three days after surgery
Approximately one month after surgery
Approximately two months after surgery
Approximately three months after surgery
The surgeon may request for any patient in the enhanced usual care arm to be seen by the PC team; if this occurs, it is documented by the research team. Any patient in either study arm may choose to withdraw from the study at any time and/or a surgeon may choose to withdraw his or her patient from the study at any time. If patient withdrawal occurs, the patient will continue to receive the enhanced usual care and the patient and/or surgeon may choose to consult PC at any future time, as per standard practice.
All study participants are enrolled in the study from before surgery until six months after surgery. Participants randomized to the intervention arm have an additional 3+ study visits within this period. These visits, described earlier, are with the PC team: (1) in an outpatient setting before surgery, (2) in the hospital within 72 hours of their initial surgery, and (3) via phone or in-clinic (per patient preference) on an at least monthly basis and/or as needed until 12 weeks after surgery.
Some participants (either patients or caregivers) in the intervention arm are asked whether they wish to further participate in an additional in-depth interview to discuss their experiences and the impact of the PC team on their (or their family members') care. Study surgeons and PC clinicians are also requested to complete interviews. These interviews take ∼45–60 minutes and explore participant opinions and experiences with surgeon-PC team co-management. We expect to interview ∼20 patients and caregivers as well as 6–10 study surgeons and 6–10 study PC clinicians. Interviews can occur in-person or over the phone and will be audio-recorded. We will deliberately sample to represent a diversity of patient and caregiver ages, race, diagnoses, and geographic regions. As only approximately five to nine surgeons and PC clinicians will be heavily involved in the trial across the four sites, all of them will be interviewed.
Data Collection and Analysis Plan
Data collection: Surveys/medical records/administrative data
Study data are collected and managed by using REDCap,74 tools hosted at Stanford University. Outside the scheduled study visits, the study team abstract medical record information, which is incorporated as descriptive data on each patient. Information abstracted includes the patient's primary diagnosis, surgical procedure, active medical history (e.g., hypertension, coronary artery disease), hospital admission and discharge diagnoses (related to the major surgery they received), and any hospital readmission data collected within six months after the surgery.
Data management and security
The electronic dataset and recordings are stored on an encrypted computer that is password protected with a secure server. All paper copies of the consent form are stored in a locked filing cabinet. During the data collection period, only the study team has access to the Stanford-hosted REDCap database that contains protected health information.
We use standard processes to enhance data quality and reduce bias. We strive to have consistent recruitment staff at each study site, and all staff are required to follow the protocol document when interacting with patients. We monitor for data completeness on the REDCap data collection site to reduce missing or incomplete data, inaccuracies, and measurement bias and excessive variability. If we find missing data, we plan to run exploratory analyses to determine the missing data pattern, and then to run appropriate analyses to address the problem and account for it in our models.
The PC intervention
All study sites have experience in maintaining fidelity in multicenter trials. The PC teams at each site elected to use their own approach and their own standard usual notes with a project-related supplement section that is harmonized across sites (Table 5). The approach at each center includes a standardized symptom assessment, mood/depression/delirium assessment, spiritual assessment, coping assessment, and creation of an interdisciplinary action plan.
Table 5.
Palliative Care Intervention Arm Consult Recommended Components
| (1) Assessment of patient and family illness understanding |
| (2) Modified Edmonton Symptom Assessment Scale-Finances and Spirituality (ESAS-FS69) which assesses the following symptoms on a 0–10 point score, with a higher score indicating worsening symptom burden: pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, shortness of breath, constipation, financial distress, and spiritual pain |
| (3) Palliative Performance Scale80,81 |
| (4) Clarification of the patient's surrogate decision maker |
| (5) Discuss advance care planning or goals of care, if deemed appropriate at that time |
Statistical analysis plan
Statistical significance and software
We have set our overall level of statistical significance at p < 0.05. All statistical analyses will be performed in R statistical software.75
Intent-to-treat
Our study will use an intent-to-treat approach in which all data from study patients in both intervention and control arms are used, regardless of the level of adherence to the study arms.
Sample size and power
Temel et al.31 found that at 12 weeks, patients assigned to early PC (intervention) had a better quality of life than did patients assigned to standard care (control), indicated by mean score on the FACT—Lung (FACT-L) scale (in which scores range from 0 to 136, with higher scores indicating better quality of life). They observed an FACT-L mean score difference of 98.0 (SD 16.7, n = 74) in the intervention group to 91.5 (SD 16.5, n = 77) in the control group, resulting in an effect size of 0.42. The FACIT-Pal scale is a corollary of the FACT-L scale (which includes a specific PC subscale [PalS] instead of the lung cancer subscale); based on Temel's previous data but using FACIT-Pal as the primary outcome, we estimated our sample size (based on the unpaired two-sample t-test) to detect an anticipated small-to-moderate effect size of 0.4 at 12 weeks with 90% power and a probability of type I error of 0.05 (two-sided). Assuming that the nesting of patients within the four sites would introduce some within-site correlation that would decrease the efficiency of our estimators, we have incorporated a variance inflation factor of 20% for this consideration. In addition, to account for missing data due to patients dropping out of study or deaths (mortality rate will be separately analyzed) and based on past experience completing studies in this population and published perioperative mortality data,8,23 we assumed a patient completion rate of 0.86 (assuming dropout rate to be 11%,31 and mortality rate at 12 weeks to be 3%8,23). Under these assumptions, the estimated sample size needed is a total of 186 patients per arm. This results in a total of 186 × 2 = 372 patients. We plan to recruit 380.
Evaluation of hypotheses
Descriptive statistics will be calculated to summarize patients' characteristics and other baseline variables. Comparability of the intervention arm and the control arm will be assessed with regard to preintervention sociodemographic and health status measures derived from Medical Record Abstraction. Although randomization should account for such differences, a two-sample t-test/Mann–Whitney test will be performed to investigate the difference in two means or medians for continuous variables, and Fisher's exact test or chi-squared test will be used to investigate the difference in proportions for binary or categorical variables. We will identify and determine possible necessary adjustment for some baseline attributes. Historically, patient gender, age, race, education, and health status have been identified as important attributes and are usually adjusted for in the model. Surgeon attributes will be examined similarly.
Based on the type of the data, summary univariate (descriptive) statistics (mean, standard deviation, median, interquartile range, max, min, count, percentage) of all outcomes stratified by intervention assignment will be provided. Descriptive time trend plots (multiple visits) stratified by intervention assignment will be presented for outcomes that are measured at multiple visits. These plots will allow for the visual comparison of change patterns before and after the intervention in the two arms. Differences in outcomes between two arms at each visit will be tested by the two-sample t-test/Mann–Whitney test or Fisher's exact test/chi-squared test, based on the data types of the outcomes.
We will study the effect of intervention on the quality of life after accounting for various confounding variables, using a linear mixed model that accounts for within-subject variation due to repeated measures. We will study the effect of the intervention on the survival of patients after the surgery by using a Kaplan–Meier method and a Cox proportional hazards model. We will study the secondary outcomes such as physical symptom assessment, psychiatric symptom scores (measured through a subscale of PROMIS-29), spiritual distress assessment, and assessment of caregiver burden by using the linear mixed model. Sensitivity analyses will assess whether there are differential effects contingent on patient or study site characteristics.
Qualitative data will be transcribed, de-identified, and analyzed based on qualitative description.76,77 We will use NVivo software with an HIPPA-compatible, professional transcription service. A codebook will be determined by a three-person team with a single coder then analyzing the transcripts. Line-by-line, axial, and theoretical consensus coding will be used to organize and summarize findings, which will be validated through triangulation, member checking, and search for disconfirming data.
Ethics determination
The Stanford University Institutional Review Board (IRB), the Johns Hopkins Medicine IRB, the University of New Mexico IRB, and the Dana Farber Cancer Institute IRB have all reviewed and approved the study protocol and materials. All changes in study protocol, as needed, will be submitted and reviewed by each site IRB.
Study Implementation: Challenges and Contributions
Patient and key stakeholder engagement
Engagement activities and findings
This study builds on more than eight years of engagement and research surrounding the PC needs of surgical patients and their family members (Fig. 1). The PI (R.A.A.) completed two years of qualitative work with in-depth interviews with 26 critically ill surgical patients and their family members, including follow-up interviews with patients and family members 6- and 12 months after surgery and interviews with family members of decedent patients. Key team members (R.A.A., J.A.M., T.J.S.) also completed four years of PCORI-funded (CD-12-11-4362) work exploring perioperative advance care planning and PC issues among patients and family members preparing for major cancer surgery. This work included:
- An environmental scan with patient, family member, and clinician engagement to determine the “state of the science” for perioperative advance care planning61;
- Stakeholder summits with patients, family members, surgeons, PC clinicians, and researchers55,57;
- In-depth interviews and iterative engagement with perioperative patients, family members, the Johns Hopkins Hospital Patient and Family Advisory Council, and more than 70 different patients, family members, surgeons, PC clinicians, and other stakeholders55;
- A cross-sectional survey of 359 patients and/or family members from the lay public regarding perioperative PC issues and clarification of outcomes that patients and family members identify as important. A key outcome that they identified as important was that good perioperative care should include the opportunity for the patient and family to “have a meaningful discussion with their doctor about their goals and wishes for surgery,” which directly impacted our choice of perioperative PC in this clinical trial52,53;
- A systematic review of perioperative advance care planning aids59;
- Key informant interviews specifically about advance care planning and PC in surgery populations60;
- At-least monthly phone calls and co-publication with our patient/family co-investigators;
- Close collaboration and co-publication with our surgeon co-investigators;
- Development of a video-based advance care planning aid and testing of that aid in a clinical trial51,58;
- Clinical trial findings that patients and families found preoperative advance care planning “helpful” but that the video and patient/family activation did not change surgeon communication or behavior51; and
- Stakeholder summits after the clinical trial indicating that diverse stakeholders—particularly patients and family members—thought that preoperative advance care planning conversations should involve PC clinicians, rather than surgeons, and that PC practices (such as symptom management and psychosocial support) were likely to improve perioperative patient and family care.
Our current clinical trial is a direct response to patient, family, and clinician concerns that emerged from the engagement and research just described. Specifically throughout this work, we found that surgical patients, family members, and clinicians asked for physical and psychological support beyond the preoperative advance care planning that we developed and beyond what is typically currently provided by surgical clinicians. This was important to all respondents but especially important should either the patient have a major complication during the perioperative period or the cancer recur after surgery. Due to this input, we are conducting the current clinical trial exploring the effect of perioperative, specialist-driven PC that starts before surgery and continues for at least three months after surgery.
Patient and family voice
In our engagement work just described, the family members of patients who had a major surgical complication(s), particularly those who required a long SICU admission, clearly called for more physical and emotional support. This was best expressed by the wife of a patient who died from complications that evolved over four months after a major operation:
He just never recovered…he eventually deteriorated so they were doing cardioversion during the night, his kidneys died…I mean it was just one emergency after another. And while I will say that the medical staff was good and they talked to us, I was kind of surprised that they waited for me to say “why are you still doing this?” I actually had to call a family meeting and I asked for all of his physicians to be there and I brought all of my family and I said “I know that this is [hospital X] and you can do anything in the world, because you CAN…you are [hospital X]. If you can get it done anywhere, it can be done here twice. But my question is why are we doing this? I mean, WHY?” So, that got them to [think]…I don't know whether they wait for the family to bring these kind of things up or what…he was SO BAD. I mean, he couldn't maintain his blood pressure, his heart rhythm, his kidneys were gone…and they put diapers on his arms…they were weeping…I mean WHY? [sobbing]…it was just so awful…so I don't know whether they don't do it because there's always that one family that will say “you could've done more, you know…” but it is HARD when you're the one who has to bring it up.
—Wife of a patient who died after major surgery
As described to our team in our preliminary work as well as described in another major ethnographic study of SICU patients,45 family members told us that they think that the patient will either do well after surgery (the desired outcome) or, if “bad things” happen, then the patient would die on the operating room table. In reality, patients rarely die during the operation and rather, if “bad things” happen, then they happen in the SICU and usually unfold over a period of days to weeks to months after the surgery itself.45,78,79 Moreover, there is a wide spectrum of possible outcomes that exist between “doing well” and dying after surgery and different patients and families have widely variable beliefs on which short- or long-term outcomes they would or would not find acceptable. This concept was again best expressed by the husband of a patient with a complicated postoperative course:
We chose [this hospital] because we think it's the best. I think they've done everything they can. And basically what they're saying now, is that they've done everything… I brought in a woman who was vital and now she can't speak.… It took 9 hours, they took half her insides out…And now she's completely dependent…can't do anything anymore. She can't communicate. Of all the things we expected this is not one of them. They went down all the list of all the problems you could have. We didn't think this was on the list.
—Husband of a patient with a complicated postoperative course and long SICU stay
PC specialists help to identify and clarify patient and family priorities in complicated medical situations and thus, they are likely to be helpful in complicated postoperative courses. As described in the Introduction section to this article, poor perioperative outcomes shortly after cancer surgery are relatively rare, even for the extensive operations that are common in the treatment of upper GI cancer. However, in our engagement and research with patients and family members, they strongly believed that PC specialists were very likely to improve patient and family experience both during routine and uncomplicated perioperative care as well as during the management of patients with rare but poor perioperative outcomes and complications. If major perioperative complications arose, the PC team would be already involved and could proactively facilitate general and goals-related communication. Without the occurrence of perioperative complications, the PC team could still improve symptom management and psychosocial support of patients and family members throughout the hospitalization and postoperative care.
Our governance plan facilitates engagement throughout all study activities; Patient and family engagement is in the warp and weft of this trial design. First, our governance structure clearly identifies a lead patient/family partner for each of the four study sites (Table 6). The study PI and each site PI are paired with a patient/family partner who is unique to that site and role. The researcher communicates with their paired lead patient–family member on an at least monthly basis to discuss diverse topics such as: study progress, problem solving for study-related barriers or challenges, and for help “making sense” out of emerging study findings. The research team also had a kick-off stakeholder summit in Baltimore, MD in May 2018 where key members of the Executive Committee and the lead patient/family partner and site PI from each of the sites came together to plan for study implementation as well as to intensely engage over key study decisions, such as content and fidelity monitoring for the intervention arm activities.
Table 6.
Project Governance Structure
| Executive Committee (crossing all four sites): | |||
| Study PI—Rebecca Aslakson | |||
| Lead Patient/Family Partner—Judi Miller | |||
| Biostatistician—Suwei Wang | |||
| Outcomes Assessment and Quality Control Lead—Karl Lorenz | |||
| Lead Surgeon Partner—Arden Morris | |||
| Dana Farber Cancer Center/Brigham and Women's Hospital site | Johns Hopkins Hospital site | University of New Mexico site | Stanford University Medical Center site |
| Site PI: Elizabeth Rickerson | Site PI's: Thomas Smith and Fabian Johnston | Site PI: Bridget Fahy | Site PI: Rebecca Aslakson |
| Site Patient/Family Partner: Carole Siegel | Site Patient/Family Partner: Kenneth Kiedrowski | Site Patient/Family Partner: Candace Tellez | Site Patient/Family Partner: TBD |
| Site Surgeon Partner: Thomas E. Clancy | Site Surgeon Partner: Jinny Ha | Site Surgeon Partner: Victor H. Phuoc | Site Surgeon Partner: George Poultsides |
| Site Palliative Care Partner: James Tulsky | Site Palliative Care Partner: Marshall Gold | Site Palliative Care Partner: Esme Finlay | Site Palliative Care Partner: Shireen Heidari |
PI, principal investigator; TBD, to be determined.
Besides the integration of patients and family members in the governance plan of the core study, engagement is also facilitated through:
- Quarterly calls with a Key Stakeholder Panel with members representing key professional and patient advocacy organizations, and
- Study Specific Aim 2, which involves in-depth interviews with 58–63 study participants, including patients, family members, surgeons, and PC clinicians.
Fidelity Monitoring in the Context of Complex Interventions
Content of the intervention arm and fidelity of delivery of that content were determined through intense engagement between the research team and partner surgeons, PC clinicians, patients, and family members. More than half of our kick-off Stakeholder Summit in May 2018 was directly devoted to this topic. From study inception and based on previous influential PC specialist intervention trials,80 our team quickly agreed on the general content of the PC intervention arm (Table 3). However, the means to ensure fidelity of content delivery were hotly debated. Some team members felt strongly that intervention content should be protocolized, whereas others felt equally strongly that protocolization would unnaturally restrict PC specialist practice and that previous research suggests ineffectiveness, and even potential harm, when sensitive, goals-related communication is over-protocolized.81 After intense discussion both in-person and via iterative e-mails and conference calls, the diverse stakeholder team agreed that the PC specialists for intervention arm patients should: (1) approach these consults with the usual specialist PC practice of their own institution, (2) use their own institution's usual PC consult notes in the electronic health record, and (3) that all study patients also have a short protocolized addendum that addresses delivery and documentation of five recommended components (Table 6):
-
1.
Assessment of patient and family understanding of illness;
-
2.
Documentation of the patient's modified Edmonton Symptom Assessment Scale71;
-
3.
Documentation of the patient's Palliative Performance Score82,83;
-
4.
Clarification of the patient's surrogate decision maker; and
-
5.
If appropriate to the clinical situation as assessed by the PC specialist, discussion of advance care planning and/or goals of care.
To further assess fidelity and intervention arm content, PC specialists also complete a survey after every interaction with a study patient. This survey is based on one used in previous PC clinical trials and determines the content of that specific PC specialist–patient interaction.84
Response to Challenges and Barriers to Study Success
Since the funding of this study, four key challenges and barriers have been identified and addressed:
-
1.
PC specialist intervention arm content and fidelity monitoring—see earlier discussion
-
2.
Timely review by the IRBs across the study sites—Despite timely submission of the study protocol to each site's IRB within a month of study initiation, timeliness of review at each site was uncertain and varied in duration from 8 weeks to 10 months. Unfortunately, our team has prior experience with lengthy and unpredictable IRB review duration for previous PC-related clinical research and thus, this experience was neither unexpected nor reflective of any significant concerns or problems with this particular study.
-
3.
Change in study team composition—As is common in academic medicine, individuals periodically change institutions and study staff sometimes leave positions to pursue other activities. At one of the study sites (which also happened to be the site scheduled to first start enrollment), the site surgeon partner, the site PC clinician partner, and the site study coordinator all left that institution at different times within the first 10 months of study funding; consequently, study activities at that site were markedly delayed throughout that time.
-
4.
Enrollment—At the time of publication, all four sites are completely through IRB review and enrolling patients. At the first site that opened enrollment and that thus far has been enrolling for four months, enrollment has been unexpectedly slow. This is ultimately believed due to: changes in study team composition and inadequate access to patient enrollment from high-volume clinics for upper GI cancer surgeons. To address these enrollment concerns, a co-site PI was added to the team who is himself an upper GI surgeon and that new co-site PI recruited a new site surgeon partner from an active thoracic surgery clinic that had previously not been involved in the study; these activities immediately increased enrollment at that site. Finally, SUMC had initially been only a coordinating center for the clinical trial; when IRB approval was delayed at the three original study sites and enrollment started slowly for the first enrolling study site, SUMC was added as a fourth recruiting site so as to aid with meeting enrollment goals.
Plans for Dissemination and Spread
This trial is registered and described on clinicaltrials.gov (CT03611309), and results will be posted on that website. Results will also be presented and discussed at relevant professional society academic meetings and through publication in scientific journals. The full dataset will be available from the study PI, per reasonable request. In de-identified format, the dataset will also be available through the PCRC. In accordance with ethical publication practices, authorship related to any presentations or publications will be based on individuals having contributed substantial time and/or intellectual content (i.e., study design, analysis, project conceptualization, etc.) related to the results being presented. We have also developed a professional advisory board comprising members representing key patient, family members, and/or professional stakeholder groups, including: The National Coalition for Cancer Survivorship, The American College of Surgeons, The American Academy of Hospice and Palliative Medicine, The Palliative Care Research Cooperative, and The American Society of Anesthesiologists; these partner groups have also agreed to help us disseminate relevant study findings among their memberships.
Acknowledgments
This project is being supported by the Palliative Care Research Cooperative Group funded by the National Institute of Nursing Research U2CNR014637. This work is supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (IHS-1609-36518).
Author Disclosure Statement
No competing financial interests exist.
All statements in this article, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
This is a study protocol and thus does not contain or reflect any primary data.
References
- 1. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. 2010. Center for Disease Control and Prevention/National Center for Health Statistics. https://www.cdc.gov/nchs/4procedures/2010pro4_numberprocedureage.pdf (last accessed August6, 2019)
- 2. Schwarze ML, Shen Y, Hemmerich J, Dale W: Age-related trends in utilization and outcome of open and endovascular repair for abdominal aortic aneurysm in the United States, 2001–2006. J Vasc Surg 2009;50:722–729.e2. [DOI] [PubMed] [Google Scholar]
- 3. Finlayson E, Fan Z, Birkmeyer JD: Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J Am Coll Surg 2007;205:729–734 [DOI] [PubMed] [Google Scholar]
- 4. Radwan RW, Evans MD, Harris DA, et al. : Pelvic exenteration for advanced malignancy in elderly patients. Br J Surg 2016;103:e115–e119 [DOI] [PubMed] [Google Scholar]
- 5. Paul S, Altorki N: Outcomes in the management of esophageal cancer. J Surg Oncol 2014;110:599–610 [DOI] [PubMed] [Google Scholar]
- 6. Kozower BD, Sheng S, O'Brien SM, et al. : STS database risk models: Predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875–881; discussion 881–883. [DOI] [PubMed] [Google Scholar]
- 7. Ragulin-Coyne E, Carroll JE, Smith JK, et al. : Perioperative mortality after pancreatectomy: A risk score to aid decision-making. Surgery 2012;152(3 Suppl 1):S120–S127 [DOI] [PubMed] [Google Scholar]
- 8. Kneuertz PJ, Pitt HA, Bilimoria KY, et al. : Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg 2012;16:1727–1735 [DOI] [PubMed] [Google Scholar]
- 9. Pezzilli R, Falconi M, Zerbi A, et al. : Clinical and patient-reported outcomes after pancreatoduodenectomy for different diseases: A follow-up study. Pancreas 2011;40:938–945 [DOI] [PubMed] [Google Scholar]
- 10. Martin RC, Brown R, Puffer L, et al. : Readmission rates after abdominal surgery: The role of surgeon, primary caregiver, home health, and subacute rehab. Ann Surg 2011;254:591–597 [DOI] [PubMed] [Google Scholar]
- 11. Goldman L, Caldera DL, Nussbaum SR, et al. : Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977;297:845–850 [DOI] [PubMed] [Google Scholar]
- 12. American Cancer Society. 2019. https://www.cancer.org (last accessed August6, 2019)
- 13. Porta M, Fabregat X, Malats N, et al. : Exocrine pancreatic cancer: Symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189–197 [DOI] [PubMed] [Google Scholar]
- 14. Wanebo HJ, Kennedy BJ, Chmiel J, et al. : Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993;218:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cavallin F, Scarpa M, Cagol M, et al. : Esophageal Cancer Clinical Presentation: Trends in the last 3 decades in a large Italian series. Ann Surg 2018;267:99–104 [DOI] [PubMed] [Google Scholar]
- 16. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–1049 [DOI] [PubMed] [Google Scholar]
- 17. Miyazaki M, Yoshitomi H, Miyakawa S, et al. : Clinical practice guidelines for the management of biliary tract cancers 2015: The 2nd English edition. J Hepatobiliary Pancreat Sci 2015;22:249–273 [DOI] [PubMed] [Google Scholar]
- 18. Ajani JA, D'Amico TA, Almhanna K, et al. : Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194–227 [DOI] [PubMed] [Google Scholar]
- 19. Ajani JA, D'Amico TA, Almhanna K, et al. : Gastric cancer, version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286–1312 [DOI] [PubMed] [Google Scholar]
- 20. Benson AB, 3rd, D'Angelica MI, Abbott DE, et al. : NCCN guidelines insights: Hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw 2017;15:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tempero MA, Malafa MP, Al-Hawary M, et al. : Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028–1061 [DOI] [PubMed] [Google Scholar]
- 22. McEvoy SH, Lavelle LP, Hoare SM, et al. : Pancreaticoduodenectomy: Expected post-operative anatomy and complications. Br J Radiol 2014;87:20140050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cameron JL, He J: Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530–536 [DOI] [PubMed] [Google Scholar]
- 24. Suker M, Beumer BR, Sadot E, et al. : FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–2406 [DOI] [PubMed] [Google Scholar]
- 26. Winter JM, Brenna MF, Tang LH, et al. : Survival after resection of pancreatic adenocarcinoma: Results from a single institution over three decades. Ann Surg Oncol 2012;19:169–175 [DOI] [PubMed] [Google Scholar]
- 27. Bouras G, Markas SR, Burns EM, et al. : The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: A national linked database study. Eur J Surg Oncol 2017;43:454–460 [DOI] [PubMed] [Google Scholar]
- 28. Dhungel B, Diggs BS, Hunter JG., et al. : Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005–2008. J Gastrointest Surg 2010;14:1492–1501 [DOI] [PubMed] [Google Scholar]
- 29. Kelley AS, Morrison RS: Palliative care for the seriously ill. N Engl J Med 2015;373:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009;302:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 32. Zimmermann C, Swami N, Krzyzanowska M, et al. : Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014;383:1721–1730 [DOI] [PubMed] [Google Scholar]
- 33. Ferrell B, Sun V, Hurria A, et al. : Interdisciplinary palliative care for patients with lung cancer. J Pain Symptom Manage 2015;50:758–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Jawahri A, LeBlanc T, VanDusen H, et al. : Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: A randomized clinical trial. JAMA 2016;316:2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elsayem A, Swint K, Fisch MJ, et al. : Palliative care inpatient service in a comprehensive cancer center: Clinical and financial outcomes. J Clin Oncol 2004;22:2008–2014 [DOI] [PubMed] [Google Scholar]
- 36. Temel JS, Greer JA, Admane S, et al. : Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326 [DOI] [PubMed] [Google Scholar]
- 37. Sun V, Grant M, Koczywas M, et al. : Effectiveness of an interdisciplinary palliative care intervention for family caregivers in lung cancer. Cancer 2015;121:3737–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brumley R, Enguidanos S, Jamison P, et al. : Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. J Am Geriatr Soc 2007;55:993–1000 [DOI] [PubMed] [Google Scholar]
- 39. Smith TJ, Coyne P, Cassel B, et al. : A high-volume specialist palliative care unit and team may reduce in-hospital end-of-life care costs. J Palliat Med 2003;5:699–705 [DOI] [PubMed] [Google Scholar]
- 40. Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 2012;30:394–400 [DOI] [PubMed] [Google Scholar]
- 41. Bakitas MA, Tosteson TD, Li Z, et al. : Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lilley EJ, Khan KT, Johnston FM., et al. : Palliative care interventions for surgical patients: A systematic review. JAMA Surg 2016;151:172–183 [DOI] [PubMed] [Google Scholar]
- 43. Buchman TG, Cassell J, Ray SE., Wax ML: Who should manage the dying patient?: Rescue, shame, and the surgical ICU dilemma. J Am Coll Surg 2002;194:665–673 [DOI] [PubMed] [Google Scholar]
- 44. Cassell J, Buchman TG, Streat S, et al. : Surgeons, intensivists, and the covenant of care: Administrative models and values affecting care at the end of life—Updated… including commentary by Buchman TG and Stewart RM. Crit Care Med 2003;31:1551–1557; discussion 1557–1559. [PubMed] [Google Scholar]
- 45. Cassell J: Life and Death in Intensive Care. Philadelphia: Temple University Press, 2005 [Google Scholar]
- 46. Kruser JM, Pecanac KE, Brasel KJ, et al. : “And I think that we can fix it”: Mental models used in high-risk surgical decision making. Ann Surg 2015;261:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pecanac KE, Kehler JM, Brasel KJ, et al. : It's big surgery: Preoperative expressions of risk, responsibility, and commitment to treatment after high-risk operations. Ann Surg 2014;59:458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwarze ML, Redmann AJ, Alexander GC, et al. : Surgeons expect patients to buy-in to postoperative life support preoperatively: Results of a national survey. Crit Care Med 2013;41:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwarze ML, Bradley CT, Brasel KJ: Surgical “buy-in”: The contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med 2010;38:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bradley CT, Brasel KJ, Schwarze ML. Physician attitudes regarding advance directives for high-risk surgical patients: A qualitative analysis. Surgery 2010;148:209–216 [DOI] [PubMed] [Google Scholar]
- 51. Aslakson RA, Isenberg SR, Crossnohere NL, et al. : Integrating advance care planning videos into surgical oncologic care: A randomized controlled clinical trial. J Palliat Med 2019;22:764–772 [DOI] [PubMed] [Google Scholar]
- 52. Bridges JF., Crossnohere NL, Schuster AL, et al. : A patient and community-centered approach selecting endpoints for a randomized trial of a novel advance care planning tool. Patient Prefer Adherence 2018;12:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aslakson RA, Schuster ALR, Lynch TJ, et al. : Developing the storyline for an advance care planning video for surgery patients: Patient-centered outcomes research engagement from stakeholder summit to state fair. J Palliat Med 2018;21:89–94 [DOI] [PubMed] [Google Scholar]
- 54. Bridges JFP, Lynch T, Schuster ALR, et al. : A review of paper-based advance care planning aids. BMC Palliat Care 2018;17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Isenberg SR, Crossnohere NL, Patel MI, et al. : An advance care plan decision support video before major surgery: A patient- and family-centred approach. BMJ Support Palliat Care 2018;8:229–236 [DOI] [PubMed] [Google Scholar]
- 56. Isenberg SR, Aslakson RA, Dionne-Odom JN, et al. : Family companions' involvement during pre-surgical consent visits for major cancer surgery and its relationship to visit communication and satisfaction. Patient Educ Couns 2018;101:1066–1074 [DOI] [PubMed] [Google Scholar]
- 57. Mitchell IA, Schuster ALR, Lynch T, et al. : Why don't end-of-life conversations go viral? A review of videos on YouTube. BMJ Support Palliat Care 2017;7:197–204 [DOI] [PubMed] [Google Scholar]
- 58. Aslakson RA, Isenberg SR, Crossnohere NL, et al. : Utilising advance care planning videos to empower perioperative cancer patients and families: A study protocol of a randomised controlled trial. BMJ Open 2017;7:e016257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aslakson RA, Schuster AL, Reardon J, et al. : Promoting perioperative advance care planning: A systematic review of advance care planning decision aids. J Comp Eff Res 2015;4:615–650 [DOI] [PubMed] [Google Scholar]
- 60. Schuster AL, Aslakson RA, Bridges JF: Creating an advance-care-planning decision aid for high-risk surgery: A qualitative study. BMC Palliat Care 2014;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aslakson RA, Schuster AL, Miller J, et al. : An environmental scan of advance care planning decision aids for patients undergoing major surgery: A study protocol. Patient 2014;7:207–217 [DOI] [PubMed] [Google Scholar]
- 62. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Palliative Care. Version 1.2017–March 15 2017.: NCCN.org (last accessed August6, 2019)
- 63. Bakitas M, Lyons KD, Hegel MT, et al. : The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: Baseline findings, methodological challenges, and solutions. Palliat Support Care 2009;7:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bakitas M, Stevens M, Ahles T, et al. : Project ENABLE: A palliative care demonstration project for advanced cancer patients in three settings. J Palliat Med 2004;7:363–372 [DOI] [PubMed] [Google Scholar]
- 65. Lyons KD, Bakitas M, Hegel MT, et al. : Reliability and validity of the Functional Assessment of Chronic Illness Therapy-Palliative care (FACIT-Pal) scale. J Pain Symptom Manage 2009;37:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cella DF, Tulsky DS, Gray G, et al. : The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 1993;11:570–579 [DOI] [PubMed] [Google Scholar]
- 67. King MT, Agar M, Currow DC, et al. : Assessing quality of life in palliative care settings: Head-to-head comparison of four patient-reported outcome measures (EORTC QLQ-C15-PAL, FACT-Pal, FACT-Pal-14, FACT-G7). Support Care Cancer 2019 [Epub ahead of print.] [DOI] [PubMed]
- 68. Cella D, Riley W, Stone A, et al. : The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cella D, Yount S, Rothrock N, et al. : The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peterman AH, Fitchett G, Brady MJ, et al. : Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy—Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med 2002;24:49–58 [DOI] [PubMed] [Google Scholar]
- 71. Bruera E, Kuehn N, Miller MJ, et al. : The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9 [PubMed] [Google Scholar]
- 72. Ayanian JZ, Chrischilles EA, Fletcher RH, et al. : Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol 2004;22:2992–2996 [DOI] [PubMed] [Google Scholar]
- 73. Zarit SH, Reever KE, Bach-Peterson J: Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 1980;20:649–655 [DOI] [PubMed] [Google Scholar]
- 74. Harris PA, Taylor R, Theilke R, et al. : Research electronic data capture (REDCap)–A metadata-driven methadology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2016 [Google Scholar]
- 76. Sandelowski M: What's in a name? Qualitative description revisited. Res Nurs Health 2010;33:77–84 [DOI] [PubMed] [Google Scholar]
- 77. Sandelowski M: Whatever happened to qualitative description? Res Nurs Health 2000;23:334–340 [DOI] [PubMed] [Google Scholar]
- 78. Swoboda SM, Lipsett PA: Impact of a prolonged surgical critical illness on patients' families. Am J Crit Care 2002;11:459–466 [PubMed] [Google Scholar]
- 79. Lipsett PA, Swoboda SM, Dickerson J, et al. : Survival and functional outcome after prolonged intensive care unit stay. Ann Surg 2000;231:262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kavalieratos D, Corbelli J, Zhang D, et al. : Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA 2016;316:2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carson SS, Cox CE, Wallenstein S, et al. : Effect of palliative care-led meetings for families of patients with chronic critical illness: A randomized clinical trial. JAMA 2016;316:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ho F, Lau F, Downing MG, Lesperance M: A reliability and validity study of the Palliative Performance Scale. BMC Palliat Care 2008;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Anderson F, Downing GM, Hill J, et al. : Palliative performance scale (PPS): A new tool. J Palliat Care 1996;12:5–11 [PubMed] [Google Scholar]
- 84. Hoerger M, Greer JA, Jackson VA, et al. : Defining the elements of early palliative care that are associated with patient-reported outcomes and the delivery of end-of-life care. J Clin Oncol 2018;36:1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]