Key Points
Question
Does BRAF V600E/K mutation status or previous BRAF inhibitor (BRAFi) with or without MEK inhibitor (MEKi) therapy affect response to pembrolizumab in patients with advanced melanoma?
Findings
This post hoc analysis of 3 randomized clinical trials (KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006) involved 1558 patients with advanced melanoma and known BRAF tumor status (BRAF wild-type or BRAF V600E/K-mutant melanoma) who had all been treated with pembrolizumab and some of whom had undergone prior treatment with BRAF inhibitors with or without MEK inhibitors. Patients with BRAF wild-type and BRAF V600E/K–mutant melanoma had objective response rates (ORRs) of 39.8% and 34.3%, respectively, and similar respective rates of 4-year progression-free survival (PFS; 22.9% and 19.8%) and overall survival (OS; 37.5% and 35.1%); patients with BRAF V600E/K–mutant melanoma who had vs had not received previous BRAFi with or without MEKi had baseline characteristics with worse prognosis: lower ORR (28.4% vs 44.2%), 4-year PFS (15.2% vs 27.8%), and OS (26.9% vs 49.3%).
Meaning
The results of this study support the use of pembrolizumab for the treatment of advanced melanoma regardless of BRAF V600E/K mutation status or prior BRAF inhibitor with or without MEK inhibitor therapy.
This post hoc subgroup analysis examines the results of 3 randomized clinical trials to assess the association of BRAF V600E/K mutation status and previous BRAF inhibitor and MEK inhibitor therapy with response to treatment with pembrolizumab in patients with advanced melanoma.
Abstract
Importance
The optimal sequencing of immune checkpoint inhibitors and targeted therapy for BRAF V600E/K-mutant melanoma is not well established.
Objective
To assess the association of BRAF wild-type (WT) or BRAF V600E/K-mutant status and BRAF inhibitor (BRAFi) with or without MEK inhibitor (MEKi) therapy with response to pembrolizumab.
Design, Setting, and Participants
This study is a post hoc subgroup analysis of pooled data from 3 multinational, multisite studies: KEYNOTE-001 (data cutoff September 1, 2017), KEYNOTE-002 (data cutoff May 30, 2018), and KEYNOTE-006 (data cutoff December 4, 2017). Patients included in this analysis were adults with advanced melanoma and known BRAF V600E/K tumor status who had received pembrolizumab.
Interventions
Patients received pembrolizumab in dosages of 2 mg/kg every 3 weeks, 10 mg/kg every 2 weeks, or 10 mg/kg every 3 weeks.
Main Outcomes and Measures
End points were objective response rate (ORR) and progression-free survival (PFS) assessed by Response Evaluation Criteria in Solid Tumors, version 1.1, and overall survival (OS). Objective response rates, 4-year PFS, and OS rates were compared in the following patient subgroups: BRAF WT vs BRAF V600E/K-mutant melanoma and BRAF V600E/K-mutant melanoma with vs without previous treatment with BRAFi with or without MEKi therapy.
Results
The overall study population (N = 1558) included 944 men (60.6%) and 614 women (39.4%). The mean (SD) age was 60.0 years (14.0). The ORR was 38.3% (596/1558), 4-year PFS rate was 22.0%, and 4-year OS rate was 36.9%. For patients with BRAF WT (n = 1124) and BRAF V600E/K-mutant melanoma (n = 434), ORR was 39.8% (n = 447) and 34.3% (n = 149), 4-year PFS rate was 22.9% and 19.8%, and 4-year OS rate was 37.5% and 35.1%, respectively. Patients with BRAF V600E/K-mutant melanoma who had (n = 271) vs had not (n = 163) previously received BRAFi with or without MEKi therapy had baseline characteristics with worse prognosis; ORR was 28.4% (n = 77) and 44.2% (n = 72), 4-year PFS rate was 15.2% and 27.8%, and 4-year OS rate was 26.9% and 49.3%, respectively.
Conclusions and Relevance
Results of this subgroup analysis support the use of pembrolizumab for treatment of advanced melanoma regardless of BRAF V600E/K mutation status or receipt of prior BRAFi with or without MEKi therapy.
Introduction
The outlook for patients with metastatic melanoma has improved considerably with the availability of targeted agents and immune checkpoint inhibitors. For patients with metastatic BRAF wild-type (WT) tumors, current guidelines recommend anti–programmed death 1 (PD-1) monotherapy or anti–PD-1/anti–cytotoxic T-lymphocyte antigen 4 (CTLA-4) combination therapy.1 In approximately 40% of patients with metastatic melanoma, the melanoma contains a BRAF mutation, and more than 90% of those have an activating BRAF V600E/K mutation.2 The standard of care for BRAF V600-mutant melanoma includes the combination of a BRAF inhibitor and a MEK inhibitor (BRAFi and MEKi) as well as immunotherapy.1 Because these regimens have distinct mechanisms and toxic effects, the therapy sequence that achieves optimal efficacy and tolerability in patients with BRAF V600-mutant melanoma remains unknown.3
Pembrolizumab is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2). Pembrolizumab is approved in many countries for 1 or more advanced cancers, including unresectable or metastatic melanoma.4,5 The efficacy of pembrolizumab in metastatic melanoma was initially established in the KEYNOTE-001 trial.6,7 In this study involving treatment-naive and previously treated patients, a lower objective response rate (ORR) was seen in patients with BRAF V600E/K-mutant melanoma (n = 133) than in those with WT (n = 442) melanoma (26% vs 36%).8 However, a similar response rate was observed in patients who were treatment naive regardless of BRAF V600E/K mutation status (50% vs 45% in BRAF V600E/K-mutant vs WT), suggesting that the reduced response rate seen in the overall population with BRAF V600E/K mutation might have been attributed to prior BRAFi with or without MEKi (BRAFi and MEKi or BRAFi alone) therapy rather than mutation status.8 In line with these observations, several retrospective analyses suggest that treatment with the anti–CTLA-4 antibody ipilimumab after BRAFi with or without MEKi therapy may be associated with poor outcomes, and translational studies suggest that most biopsied mitogen-activated protein kinase (MAPK) inhibitor-resistant melanoma metastases have a poorly immunogenic tumor microenvironment,9,10 raising questions as to the most effective therapy sequence for patients with BRAF V600-mutant melanoma.11,12 This post hoc subgroup analysis was conducted to assess the effect of BRAF V600E/K mutation status and BRAFi with or without MEKi therapy on response to pembrolizumab in patients with ipilimumab-refractory and ipilimumab-naive advanced melanoma enrolled in the KEYNOTE-001,6,7 KEYNOTE-002,13,14 or KEYNOTE-00615,16 studies.
Methods
Study Design and Patients
Results of the KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006 trials have been published.6,7,13,14,15,16 There were important differences in inclusion criteria regarding prior treatments and baseline lactate dehydrogenase (LDH) levels in these 3 studies (eMethods in the Supplement).
Briefly, in the open-label, phase 1 KEYNOTE-001 study of pembrolizumab, eligible patients were ipilimumab naive, ipilimumab treated, or ipilimumab refractory. Ipilimumab-naive patients with BRAF V600E/K mutation could have previously received BRAFi with or without MEKi therapy, and ipilimumab-refractory patients with BRAF V600E/K mutation were required to have received prior BRAFi with or without MEKi therapy.
The randomized, double-blind, phase 2 KEYNOTE-002 study compared pembrolizumab with chemotherapy in patients with ipilimumab-refractory advanced melanoma. Patients with BRAF V600E/K mutation were required to have previously received BRAFi with or without MEKi therapy.
The randomized, open-label, phase 3 KEYNOTE-006 study compared pembrolizumab with ipilimumab in patients with ipilimumab-naive melanoma who had received no or 1 prior systemic therapy for advanced/metastatic disease. Patients with BRAF V600E/K mutation might have previously received prior BRAFi with or without MEKi therapy as first-line systemic therapy; however, BRAFi with or without MEKi therapy was not required for patients with normal LDH levels and no clinically significant tumor-related symptoms or evidence of rapid disease progression.
All studies were sponsored by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, New Jersey, and were conducted in accordance with the Declaration of Helsinki17 and the International Council for Harmonisation and Good Clinical Practice guidelines. All protocols and amendments were approved by the appropriate institutional review boards or ethics committees. All patients provided written informed consent.
Treatments, Assessments, and End Points
Patients received pembrolizumab in 1 of the following regimens: 2 mg/kg every 3 weeks, 10 mg/kg every 2 weeks, or 10 mg/kg every 3 weeks. As previously described, efficacy and safety profiles were similar across pembrolizumab regimens6,7,13,15,16,18; therefore, pembrolizumab dose groups were pooled across studies for this analysis.
Best overall response in all studies was assessed per Response Evaluation Criteria in Solid Tumors, version 1.1, by investigator review.6,13,15 In the KEYNOTE-001 study, response was assessed every 12 weeks, and in KEYNOTE-002 and KEYNOTE-006, response was assessed at week 12, every 6 weeks until week 48, and then every 12 weeks thereafter.13,14,15,16 End points examined in this pooled analysis were ORR, progression-free survival (PFS), and overall survival (OS).
Statistical Analysis
Data from the intention-to-treat (ITT) populations were pooled from all the studies. Data cutoffs were as follows: September 1, 2017 (KEYNOTE-001), May 30, 2018 (KEYNOTE-002), and December 4, 2017 (KEYNOTE-006). Summary statistics for ORR are provided as percentages. For PFS and OS, 4-year rates are provided. Comparisons between the following patient subgroups were performed: BRAF V600 WT vs BRAF V600E/K-mutant and, among those with mutant disease, previous BRAFi with or without MEKi vs no previous BRAFi with or without MEKi treatment. The Miettinen and Nurminen method was used to determine 95% CIs and P values. The Kaplan-Meier method was used to estimate median PFS and OS. For patients lost to follow-up, data from the date of last contact were used. To determine baseline risk factors associated with best overall response, 1-tailed univariable analysis of each independent variable was conducted; factors for which the univariable test had a P value of less than 0.05 and those factors that had clinical relevance were selected for the multivariable logistic regression model (eMethods in the Supplement). A P value less than 0.05 was considered as significant.
Results
Patients and Efficacy
Data from 1558 pembrolizumab-treated patients with known BRAF V600E/K mutation status across studies (647 patients from KEYNOTE-001, 361 from KEYNOTE-002, and 550 from KEYNOTE-006) were pooled for this subgroup analysis. Overall, 944 (60.6%) were male and 614 (39.4%) were female; the mean (SD) age of participants was 60.0 (14.0) years. Of these, 1124 (72.1%) had BRAF WT and 434 (27.9%) had BRAF V600E/K-mutant melanoma; 271 (62.4%) were treated previously with BRAFi with or without MEKi and 163 (37.6%) were BRAFi with or without MEKi therapy naive. A total of 29 patients (1.9%) were lost to follow-up. For the overall analysis population (N = 1558), the ORR was 38.3% (596/1558), the 4-year PFS rate was 22.0%, and the 4-year OS rate was 36.9%.
BRAF Wild-Type vs BRAF V600E/K-Mutant Melanoma
Although baseline characteristics were generally similar between the BRAF WT and BRAF V600E/K-mutant subgroups, because of differences in inclusion criteria across the 3 studies, there were some key distinctions among patients included. Most patients (271 of 434 [62.4%]) in the mutant subgroup had previously received BRAFi with or without MEKi therapy (Table 1). Additionally, more patients with BRAF WT than mutant disease were older (≥65 years, 552/1125 [49.1%] vs 106/434 [24.4%]) and had previously received ipilimumab treatment (554/1124 [49.3%] vs 148/434 [34.1%]). Univariate analysis revealed that patients with BRAF WT melanoma had a higher ORR than those with BRAF V600E/K-mutant melanoma (447/1124 [39.8%] vs 149/434 [34.3%]; 5.4% difference [95% CI, 0.1-10.6]) (eFigure 1 in the Supplement) and a similar 4-year PFS rate (22.9% vs 19.8% of patients) (eFigure 2A in the Supplement). Median PFS was numerically longer (hazard ratio, 0.84; 95% CI, 0.74-0.96) for patients with BRAF WT (7.9 months [95% CI, 6.4-9.7]) than for patients with BRAF V600E/K-mutant melanoma (5.6 months [95% CI, 4.2-6.7]) (Figure, A), as was the 4-year OS rate (37.5% vs 35.1%) (eFigure 2B in the Supplement) and median OS (23.0 months [95% CI, 20.2-26.7] vs 21.8 months [95% CI, 17.1-27.3]) (eFigure 3A in the Supplement). The ORR in patients with BRAF WT melanoma (447/1124 [39.8%]) was similar to that in patients with BRAF V600E/K-mutant disease (72/163 [44.2%]) not previously treated with BRAFi with or without MEKi.
Table 1. Patient Baseline Demographics and Disease Characteristics.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| BRAF WT (n = 1124) | BRAF V600E/K-mutant (n = 434) | BRAF V600E/K-mutant with prior BRAFi with or without MEKi treatment (n = 271) | BRAF V600E/K-mutant with no prior BRAFi with or without MEKi treatment (n = 163) | |
| Age, mean (SD), y | 62.4 (13.2) | 53.8 (14.2) | 53.4 (13.6) | 54.5 (15.1) |
| <65 | 572 (50.9) | 328 (75.6) | 213 (78.6) | 115 (70.6) |
| ≥65 | 552 (49.1) | 106 (24.4) | 58 (21.4) | 48 (29.4) |
| Male | 691 (61.5) | 253 (58.3) | 161 (59.4) | 92 (56.4) |
| ECOG PS | ||||
| 0 | 721 (64.1) | 296 (68.2) | 168 (62.0) | 128 (78.5) |
| 1 | 400 (35.6) | 138 (31.8) | 103 (38.0) | 35 (21.5) |
| Missing | 3 (0.3) | 0 | 0 | 0 |
| PD-L1 status | ||||
| Positive | 603 (53.6) | 243 (56.0) | 136 (50.2) | 107 (65.6) |
| Negative | 177 (15.7) | 82 (18.9) | 63 (23.2) | 19 (11.7) |
| Unknown | 151 (13.4) | 46 (10.6) | 31 (11.4) | 15 (9.2) |
| Missing | 193 (17.2) | 63 (14.5) | 41 (15.1) | 22 (13.5) |
| Metastatic stage | ||||
| M0/M1a/M1b | 290 (25.8) | 106 (24.4) | 49 (18.1) | 57 (35.0) |
| M1c | 834 (74.2) | 328 (75.6) | 222 (81.9) | 106 (65.0) |
| Brain metastasis | ||||
| No | 1022 (90.9) | 373 (85.9) | 224 (82.7) | 149 (91.4) |
| Yes | 98 (8.7) | 59 (13.6) | 46 (17.0) | 13 (8.0) |
| Missing | 4 (0.4) | 2 (0.5) | 1 (0.4) | 1 (0.6) |
| LDH level | ||||
| Elevated | 421 (37.5) | 153 (35.3) | 126 (46.5) | 27 (16.6) |
| Normal | 684 (60.9) | 275 (63.4) | 142 (52.4) | 133 (81.6) |
| Missing | 19 (1.7) | 6 (1.4) | 3 (1.1) | 3 (1.8) |
| Prior lines of therapy | ||||
| 0 | 154 (13.7) | 41 (9.4) | 0 | 41 (25.2) |
| 1 | 346 (30.8) | 139 (32.0) | 111 (41.0) | 28 (17.2) |
| 2 | 232 (20.6) | 89 (20.5) | 79 (29.2) | 10 (6.1) |
| ≥3 | 151 (13.4) | 83 (19.1) | 81 (29.9) | 2 (1.2) |
| Missing | 241 (21.4) | 82 (18.9) | 0 (0) | 82 (50.3) |
| Prior ipilimumab exposure | ||||
| Exposed | 554 (49.3) | 148 (34.1) | 138 (50.9) | 10 (6.1) |
| Naive | 570 (50.7) | 285 (65.7) | 132 (48.7) | 153 (93.9) |
| Missing | 0 | 1 (0.2) | 1 (0.4) | 0 |
| Baseline tumor size, mm | ||||
| ≤93 | 543 (48.3) | 220 (50.7) | 117 (43.2) | 103 (63.2) |
| >93 | 460 (40.9) | 155 (35.7) | 124 (45.8) | 31 (19.0) |
| Missing | 121 (10.8) | 59 (13.6) | 30 (11.1) | 29 (17.8) |
| Albumin | ||||
| AUC ≤0.834 | 717 (63.8) | 256 (59.0) | 182 (67.2) | 74 (45.4) |
| AUC >0.834 | 393 (35.0) | 169 (38.9) | 84 (31.0) | 85 (52.1) |
| Missing | 14 (1.2) | 9 (2.1) | 5 (1.8) | 4 (2.5) |
| Prior systemic BRAFi therapy | ||||
| No | 1101 (98.0) | 163 (37.6) | 0 | 163 (100) |
| Yes | 23 (2.0) | 271 (62.4) | 271 (100) | 0 |
Abbreviations: AUC, area under the receiver operating characteristics curve; BRAFi, BRAF inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; MEKi, MEK inhibitor; PD-L1, programmed death ligand 1; WT, wild type.
Figure. Kaplan-Meier Estimates of Progression-Free Survival.
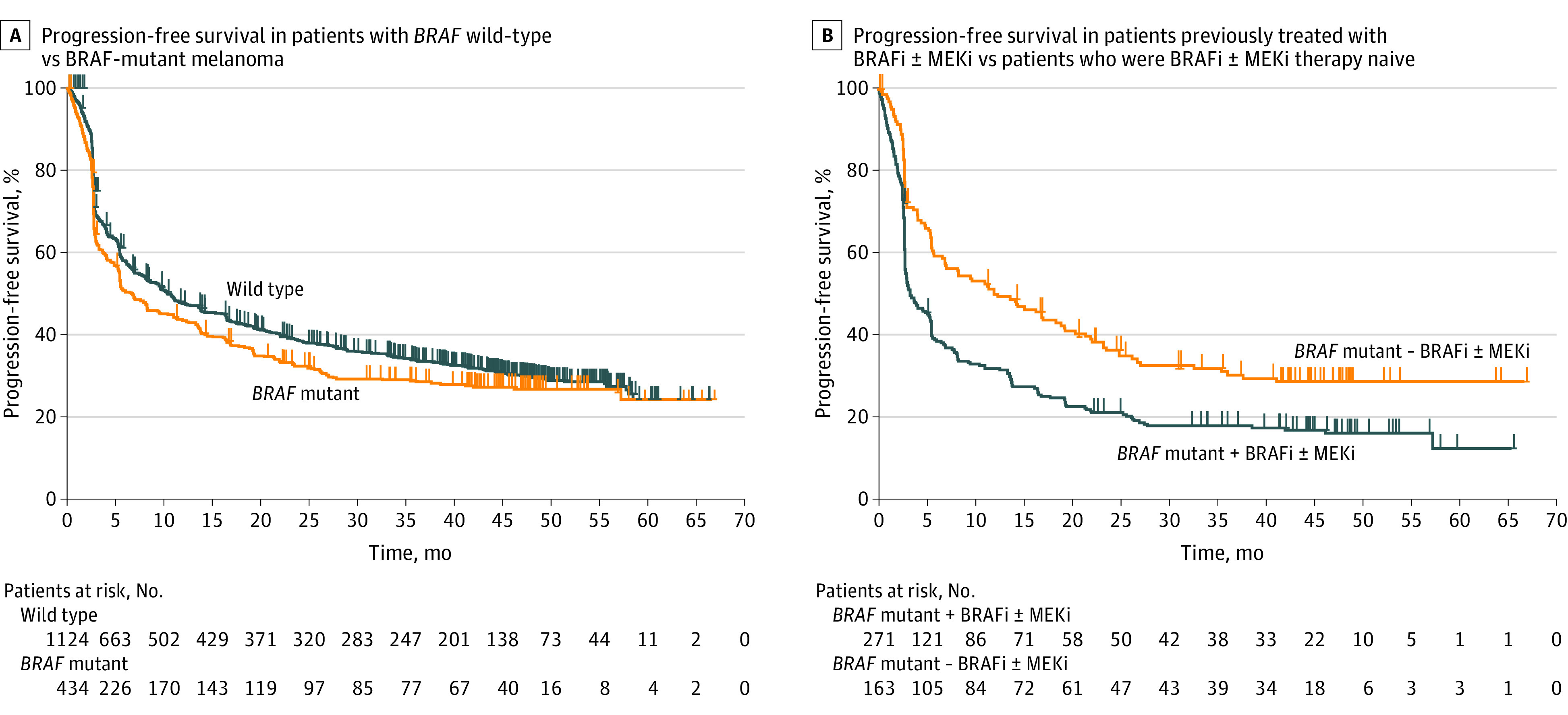
BRAFi indicates BRAF inhibitor; MEKi, MEK inhibitor.
Patients With BRAF V600E/K-Mutant Melanoma Previously Treated With a BRAFi With or Without MEKi vs Patients With BRAFi With or Without MEKi Therapy–Naive Melanoma
Baseline characteristics were different between patients with BRAF V600E/K-mutant disease previously treated with a BRAFi with or without MEKi and those who were BRAFi with or without MEKi therapy naive (Table 1). A greater proportion of patients previously treated with BRAFi with or without MEKi had a higher Eastern Cooperative Oncology Group performance status (103/271 [38.0%] vs 35/163 [21.5%]), PD-L1–negative tumors (63/271 [23.2%] vs 19/163 [11.7%]), M1c disease (222/271 [81.9%] vs 106/163 [65.0%]), stable brain metastases (46/271 [17.0%] vs 13/163 [8.0%]), elevated serum LDH levels (126/271 [46.5%] vs 27/163 [16.6%]), undergone more prior lines of therapy (≥3 lines, 81/271 [29.9%] vs 2/163 [1.2%]), undergone prior ipilimumab treatment (138/271 [50.9%] vs 10/163 [6.1%]), larger tumors (>93 mm, 124/271 [45.8%] vs 31/163 [19.0%]), and lower albumin levels (area under the receiver operating characteristic curve ≤0.834, 182/271 [67.2%] vs 74/163 [45.4%]).
BRAFi with or without MEKi–treated patients had a lower ORR with pembrolizumab than those who were BRAFi with or without MEKi therapy naive (77/271 [28.4%] vs 72/163 [44.2%]; −15.8% difference [95% CI, −25.0 to −6.5]) (eFigure 1 in the Supplement). In subgroup analysis by baseline characteristics, lower ORR to pembrolizumab was observed in BRAFi with or without MEKi–treated patients vs BRAFi with or without MEKi therapy–naive patients across subgroups. The greatest differences were observed for the following factors: age 65 years and older (21/58 [36.2%] vs 27/48 [56.3%]; difference, −20.0%), PD-L1 positive status (36/136 [26.5%] vs 50/107 [46.7%]; −20.3%), metastatic stage (M0/M1a/M1b) (13/49 [26.5%] vs 29/57 [50.7%]; −24.3%), and elevated LDH level (23/126 [18.3%] vs 15/27 [55.6%]; −37.3%) (eFigure 1 in the Supplement).
Patients who were previously treated with BRAFi with or without MEKi therapy had lower 4-year PFS rates (15.2% vs 27.8%) (eFigure 2A in the Supplement) and shorter median PFS (3.4 months [95% CI, 2.9-5.3] vs 12.0 months [95% CI, 7.0-18.4]) (hazard ratio 1.64 [95% CI, 1.31-2.06]) (Figure, B) than those who were BRAFi with or without MEKi therapy naive. Patients who were previously treated with BRAFi with or without MEKi therapy had lower 4-year OS (26.9% vs 49.3%) (eFigure 2B in the Supplement) and shorter median OS (13.8 months [95% CI, 11.0-17.1] vs 45.4 months [95% CI, 33.9 to not reached]) (eFigure 3B in the Supplement) than those who were BRAFi with or without MEKi therapy naive. Similar to the trend with ORR, lower 4-year PFS rates and 4-year OS rates were observed with pembrolizumab treatment in BRAFi with or without MEKi–treated patients vs BRAFi with or without MEKi therapy–naive patients across subgroups. Patients who were previously treated with BRAFi with or without MEKi therapy and were 65 years and older (4-year PFS rate, 15.7% vs 37.3%; difference, −21.6%), had disease with metastatic stage M0/M1a/M1b (11.9% vs 36.9%; difference, −24.9%), were PD-L1 positive (12.4% vs 30.8%; difference −18.4%), or had elevated LDH level (9.3% vs 37.8%; difference, −28.4%) had lower 4-year PFS rates than those who were not previously treated with BRAFi with or without MEKi therapy (eFigure 2A in the Supplement). Patients who were previously treated with BRAFi with or without MEKi therapy and 65 years and older (4-year OS rate, 27.6% vs 56.8%; difference, −29.2%), PD-L1-positive (24.7% vs 53.0%, difference, −28.3%), had disease with metastatic stage (M0/M1a/M1b) (37.8% vs 63.6%, difference, −25.7%), or had elevated LDH (14.2% vs 49.7%; difference, −35.5%) had lower 4-year OS rates than those who were BRAFi with or without MEKi therapy naive.
Factors Associated With Response
Although many baseline characteristics were associated with worse overall response in subgroup analysis (eTable 1 in the Supplement), multivariate analysis confirmed that PD-L1−negative tumors, elevated LDH levels, lower albumin levels, larger baseline tumors, and prior ipilimumab exposure were associated with worse overall response (Table 2). Multivariate analysis accounting for patients with missing baseline tumor size (n = 178) further corroborated these findings (Table 2; eTable 2 in the Supplement). Multivariate analysis of baseline factors associated with worse PFS identified PD-L1–negative tumors, elevated LDH levels, prior ipilimumab exposure, larger baseline tumors, and prior exposure to BRAFi therapy (Table 3). Multivariate analysis with study as a covariate supported these findings (eTable 3 in the Supplement).
Table 2. Multivariate Analysis of Factors Associated With Best Overall Responsea.
| Risk factor at baseline | Effect | OR (95% CI) | P value |
|---|---|---|---|
| Baseline albumin/albumin ULN | Units = −1b | 0.16 (0.05-0.49) | .001 |
| LDH level | Elevated vs normal | 0.61 (0.47-0.79) | <.001 |
| Tumor size | >93 mm vs ≤93 mmc | 0.47 (0.36-0.61) | <.001 |
| Ipilimumab exposure | Ipilimumab exposed vs ipilimumab naive | 0.71 (0.56-0.91) | .006 |
| Prior systemic BRAFi therapy | Yes vs no | 0.71 (0.52-0.97) | .030 |
| PD-L1 status | Negative vs positive | 0.52 (0.37-0.73) | <.001 |
| Sex | Female vs male | 0.64 (0.50-0.81) | <.001 |
Abbreviations: BRAFi, BRAF inhibitor; LDH, lactate dehydrogenase; OR, odds ratio; PD-L1, programmed death ligand 1; ULN, upper limit of normal.
Analysis included all response-evaluable patients, regardless of BRAF V600E/K mutation status. Overall response was determined by Response Evaluation Criteria in Solid Tumors, version 1.1, per investigator review.
A 1-unit decrease was used to ensure the OR direction for all risk factors was the same.
Cutoff chosen based on the value that showed the most significant difference in response.
Table 3. Multivariate Analysis of Factors Associated With Progression-Free Survivala.
| Risk factor | Effect | HR (95% CI) | P value |
|---|---|---|---|
| Baseline LDH level | Elevated vs normal | 1.45 (1.27-1.65) | <.001 |
| ECOG PS at screening | 1 vs 0 | 1.20 (1.06-1.36) | .004 |
| Ipilimumab exposureb | Ipilimumab exposed vs ipilimumab naive | 1.13 (1.00-1.28) | .042 |
| PD-L1 status | Negative vs positive | 1.58 (1.35-1.84) | <.001 |
| Baseline tumor size | >93 mm vs ≤93 mmc | 1.44 (1.26-1.65) | <.001 |
| Prior systemic BRAFi therapy | Yes vs no | 1.31 (1.14-1.52) | <.001 |
| Sex | Female vs male | 1.23 (1.09-1.38) | <.001 |
| Albumin | ≤0.834 vs >0.834 | 1.23 (1.08-1.40) | <.001 |
Abbreviations: BRAFi, BRAF inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; PD-L1, programmed death ligand 1.
Analysis included all patients, regardless of BRAF mutation status. Progression-free survival was determined by Response Evaluation Criteria in Solid Tumors, version 1.1, per investigator review.
The clinical study and prior ipilimumab exposure were highly correlated, and only 1 could be included in the model. See eTable 3 in the Supplement for multivariate analysis with study included as a covariate instead of ipilimumab exposure.
Cutoff chosen based on the value that showed the most significant difference in response.
Safety
Any-grade treatment-related adverse events were similar across all subgroups and ranged from 194 of 271 patients (71.6%) to 139 of 164 patients (84.7%) (eTable 4 in the Supplement). The safety profile of pembrolizumab was generally similar regardless of BRAF V600E/K mutation status or receipt of prior BRAFi with or without MEKi therapy.
Discussion
Nearly half of melanomas harbor activating BRAF mutations,19,20 which lead to constitutive activation of the MAPK pathway21 and provide a target for BRAFi and MEKi therapy.22 Limited clinical data are available on the association of BRAF mutation status or exposure to prior BRAFi with or without MEKi therapy with response to immunotherapies.12,20 The results of this of this post hoc pooled subgroup analysis of data from 3 clinical trials show that pembrolizumab may provide clinical benefit in patients with BRAF WT and BRAF V600E/K-mutant advanced melanoma and in patients who were or were not previously treated with BRAFi with or without MEKi therapy. There were no meaningful differences in the safety profile among pembrolizumab-treated patients in the 4 subgroups. The 1558 patients pooled from 3 pivotal trials form, to our knowledge, the largest data set reported for this critical analysis of the association of BRAF V600E/K mutation and prior BRAF-directed therapy with therapeutic outcomes of single-agent PD-1 blockade. Similar to our findings, in a retrospective post hoc analysis of pooled data from 4 nivolumab clinical trials, patients with BRAF WT melanoma had a response to treatment and a safety profile similar to those of patients with mutant disease; however, that report did not evaluate prior exposure to combined BRAFi and MEKi therapy.23
As mentioned previously, because of differences in inclusion criteria regarding patients with BRAF V600E/K-mutant disease, a greater proportion of BRAFi with or without MEKi–treated vs BRAFi with or without MEKi therapy–naive patients in the current pooled analysis had baseline characteristics known to be associated with worse outcomes in patients with melanoma, such as PD-L1−negative tumors (63/271 [23.2%] vs 19/163 [11.7%]), elevated LDH levels (126/271 [46.5%] vs 27/163 [16.6%]), higher Eastern Cooperative Oncology Group performance status (103/271 [38.0%] vs 35/163 [21.5%]), larger baseline tumors (>93 mm, 124/271 [45.8%] vs 31/163 [19.0%]), and lower albumin levels (area under receiver operating curve ≤0.834, 182/271 [67.2%] vs 74/163 [45.4%]) (Table 1). For example, low serum LDH level has been associated with favorable OS in patients with melanoma24 and PD-L1 expression correlates with pembrolizumab response.25 Worse outcomes—namely, lower ORR and 4-year PFS and OS rates in patients with BRAF V600E/K-mutant melanoma treated with BRAFi with or without MEKi therapy than those who were BRAFi with or without MEKi therapy naive—are likely to have resulted because of the aforementioned imbalance in baseline characteristics.
Another explanation for the lower ORR in patients previously treated with BRAFi with or without MEKi therapy vs those who were naive to BRAFi with or without MEKi therapy is that prior BRAFi with or without MEKi treatment may have led to long-term response in patients with favorable baseline characteristics26 (normal LDH levels, fewer than 3 sites with metastatic disease) and therefore selection of patients with less favorable characteristics or pembrolizumab resistance. Inhibition of the MAPK pathway in advanced melanoma has been associated with transcriptional induction of the innate anti–PD-1 resistance signature, involving mesenchymal transition, angiogenesis, hypoxia, and wound-healing genes.27
Lower response rates were observed in patients with melanoma and brain metastases treated with nivolumab and ipilimumab after progression on BRAFi and MEKi therapy compared with patients who were treatment naive.28 Simeone et al29 reported similar findings from a retrospective study of patients with metastatic melanoma who had previously received ipilimumab when response to pembrolizumab was affected by prior BRAFi treatment; patients with BRAF V600-mutant melanoma previously treated with a BRAFi had lower median PFS and disease control rate with pembrolizumab than patients with WT disease.29 This observation may have important ramifications for therapy sequencing for BRAF V600-mutant melanoma and is consistent with observations of earlier retrospective reports that outcomes with ipilimumab were inferior in patients with BRAF V600-mutant melanoma previously treated with BRAFi compared with outcomes with ipilimumab as first-line therapy.12,20
Ongoing randomized clinical trials will allow these outcomes to be better understood and analyzed for correlative, prognostic, and predictive factors. Results of a 2017 retrospective study3 to investigate the sequencing of BRAFi with or without MEKi and anti–PD-1 therapy for the treatment of BRAF V600-mutant melanoma indicated that BRAFi with or without MEKi and anti–PD-1 therapies were effective when used as front-line therapy, whereas anti–PD-1 therapy was only moderately effective after progression with BRAFi with or without MEKi therapy, and BRAFi therapy resulted in a poor response when used as salvage therapy after anti–PD-1 therapy.3 Perhaps a subgroup of patients is likely to benefit from both classes of therapies, whereas those with aggressive disease may exhibit poor response to both classes of therapies.30 The definitive answer to the question regarding the optimal sequence of immunotherapy and targeted therapy for patients with BRAF V600E/K-mutated melanoma will come from randomized trials including EA6134,31 SECOMBIT,32 EBIN,33 COWBOY,34 NeoTrio,35 and ImmunoCobiVem.36
An alternative approach involving a combination of PD-1 inhibitors with BRAFi and MEKi is currently being tested in clinical trials for patients with BRAF V600-mutant melanoma.37,38 This combination strategy is based on the immune-modulating effects of BRAF and MEK inhibition. The phase 2 KEYNOTE-022 trial of pembrolizumab plus dabrafenib and trametinib (triplet therapy) in patients with treatment-naive BRAF V600E/K-mutant advanced melanoma showed numerically longer PFS and duration of response with triplet therapy compared with dabrafenib and trametinib alone.38 Initial results from the phase 3 COMBI-i trial of spartalizumab plus dabrafenib and trametinib, and the phase 2 TRIDeNT trial of nivolumab with dabrafenib and trametinib, reported promising preliminary efficacy and manageable safety in BRAF V600-mutant melanoma.37,39 However, the optimal regimen (PD-1 inhibitor and BRAFi and MEKi) and sequence of these therapies remains to be established.
Limitations
The primary limitations of this analysis were its retrospective nature and the pooling of data from 3 trials with different study designs and patient populations. Of particular note were the different inclusion criteria, particularly criteria pertaining to patients with BRAF V600E/K-mutant melanoma, across the 3 studies. Patients with BRAF V600E/K-mutant melanoma had to be previously treated with BRAFi with or without MEKi therapy in KEYNOTE-002 and in KEYNOTE-001 if they were ipilimumab refractory. In contrast, in KEYNOTE-006, patients with BRAF V600E/K-mutant melanoma who were BRAFi naive were eligible if they had a normal LDH level and no clinically significant tumor-related symptoms or evidence of rapidly progressing melanoma (thereby excluding BRAFi-naive patients with high LDH level and poorer prognosis), whereas those who had received prior BRAFi were eligible regardless of LDH level. Thus, patients with BRAF V600E/K-mutant melanoma who received pembrolizumab followed by BRAFi with or without MEKi might have had a better prognosis than those who received the reverse sequence. As in most immunotherapy clinical trials, patients with Eastern Cooperative Oncology Group performance status (0 or 1) were enrolled, which may not reflect a real-world population.40 Furthermore, in countries where targeted therapies are available for BRAF V600E/K-mutant melanoma, patients with BRAF WT tumors may be more likely to enroll in clinical studies than patients with BRAF V600E/K-mutant melanoma due to a lack of alternative treatment options. Owing to the retrospective nature of the current analysis, definitive conclusions cannot be made regarding sequencing. In addition, the small sample size for some of the subgroups also limits the interpretation.
Conclusions
In conclusion, results of this post hoc pooled subgroup analysis support the use of pembrolizumab for the treatment of patients with advanced melanoma regardless of BRAF V600E/K mutation status or use of BRAFi with or without MEKi therapy. Results of well-designed randomized studies that evaluate potential determinants of outcome will help to determine optimal first-line agents for defined subgroups of patients with advanced melanoma.
eMethods
eTable 1. Univariate Analysis of Factors Associated With Best Overall Response per RECIST v 1.1 per Investigator Review
eTable 2 Multivariate Analysis of Factors Associated With Best Overall Response per RECIST v 1.1 per Investigator Review, Accounting for Patients With Missing Baseline Tumor Size
eTable 3. Multivariate Analysis of Factors Associated With Progression-Free Survival per RECIST v1.1 per Investigator Review With Study as a Covariate
eTable 4. Any-Grade Treatment-Related Adverse Events That Occurred in ≥5% of Patients
eFigure 1. Overall Response Rate in Evaluable Patients (N = 1558) With BRAF Wild-Type Versus Mutant Melanoma
eFigure 2. Four-Year Progression-Free Survival (PFS) and Overall Survival (OS) in Patients With BRAF Wild-Type Versus Mutant Melanoma
eFigure 3. Kaplan-Meier Estimates of Overall Survival
References
- 1.National Comprehensive Cancer Network NCCN clinical practice guidelines: cutaneous melanoma (version 1, 2020). Accessed January 13, 2020. https://www.nccn.org/professionals/physician_gls/default.aspx
- 2.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239-1246. doi: 10.1200/JCO.2010.32.4327 [DOI] [PubMed] [Google Scholar]
- 3.Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-PD-1 before and after BRAF inhibition. J Immunother. 2017;40(1):31-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KEYTRUDA (pembrolizumab) 50 mg powder for concentrate for solution for infusion (summary of product characteristics). Hoddesdon, UK: Merck Sharp & Dohme Limited; November 2019.
- 5.KEYTRUDA (pembrolizumab) injection, for intravenous use (prescribing information). Whitehouse Station, NJ: Merck Sharp & Dohme Corp. June 2020.
- 6.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134-144. doi: 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109-1117. doi: 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 8.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600-1609. doi: 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- 9.Hugo W, Shi H, Sun L, et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell. 2015;162(6):1271-1285. doi: 10.1016/j.cell.2015.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakavand H, Vilain RE, Wilmott JS, et al. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015;28(12):1535-1544. doi: 10.1038/modpathol.2015.110 [DOI] [PubMed] [Google Scholar]
- 11.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med. 2012;10:107. doi: 10.1186/1479-5876-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackerman A, Klein O, McDermott DF, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695-1701. doi: 10.1002/cncr.28620 [DOI] [PubMed] [Google Scholar]
- 13.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-918. doi: 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45. doi: 10.1016/j.ejca.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 15.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 16.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853-1862. doi: 10.1016/S0140-6736(17)31601-X [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Joshua AM, Weber JS, et al. Pembrolizumab (pembro; MK-3475) for advanced melanoma (MEL): randomized comparison of two dosing schedules. Ann Oncol. 2014;25(suppl 4):1-41. doi: 10.1093/annonc/mdu438.42 [DOI] [Google Scholar]
- 19.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-954. doi: 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 20.Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63(4):756-759. [PubMed] [Google Scholar]
- 22.Eroglu Z, Ribas A. Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Ther Adv Med Oncol. 2016;8(1):48-56. doi: 10.1177/1758834015616934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440. doi: 10.1001/jamaoncol.2015.1184 [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-6206. doi: 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102-4109. doi: 10.1200/JCO.2016.67.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743-1754. doi: 10.1016/S1470-2045(16)30578-2 [DOI] [PubMed] [Google Scholar]
- 27.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35-44. doi: 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681. doi: 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 29.Simeone E, Grimaldi AM, Festino L, et al. Correlation between previous treatment with BRAF inhibitors and clinical response to pembrolizumab in patients with advanced melanoma. Oncoimmunology. 2017;6(3):e1283462. doi: 10.1080/2162402X.2017.1283462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long GV, Eroglu Z, Infante J, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2018;36(7):667-673. doi: 10.1200/JCO.2017.74.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabrafenib and trametinib followed by ipilimumab and nivolumab or ipilimumab and nivolumab followed by dabrafenib and trametinib in treating patients with stage III-IV BRAFV600 melanoma. ClinicalTrials.gov identifier: NCT02224781. Updated April 3, 2020. Accessed April 3, 2020. https://clinicaltrials.gov/ct2/show/NCT02224781
- 32.Sequential combo immuno and target therapy (SECOMBIT) study (SECOMBIT). ClinicalTrials.gov identifier: NCT02631447. Updated June 10, 2019. Accessed April 6, 2020. https://clinicaltrials.gov/ct2/show/NCT02631447
- 33.Immunotherapy with ipilimumab and nivolumab preceded or not by a targeted therapy with encorafenib and binimetinib (EBIN). ClinicalTrials.gov identifier: NCT03235245. Updated April 6, 2020. Accessed April 6, 2020. https://clinicaltrials.gov/ct2/show/NCT03235245
- 34.Induction therapy with vemurafenib and cobimetinib to optimize nivolumab and ipilimumab therapy (COWBOY). ClinicalTrials.gov identifier: NCT02968303. Updated April 18, 2019. Accessed April 6, 2020. https://clinicaltrials.gov/ct2/show/NCT02968303
- 35.Neoadjuvant dabrafenib, trametinib with or without pembrolizumab in BRAF mutant resectable stage III melanoma (NeoTrio).ClinicalTrials.gov identifier: NCT02858921. Updated December 16, 2019. Accessed April 6, 2020. https://clinicaltrials.gov/ct2/show/NCT02858921
- 36.Evaluating the efficacy and safety of a sequencing schedule of cobimetinib plus vemurafenib followed by immunotherapy with an anti-PD-L1 antibody in patients with unresectable or metastatic BRAF V600 mutant melanoma (ImmunoCobiVem). ClinicalTrials.gov identifier: NCT02902029. Updated January 31, 2020. Accessed April 6, 2020. https://clinicaltrials.gov/ct2/show/NCT02902029
- 37.Long GV, Lebbe C, Atkinson V, et al. The anti–PD-1 antibody spartalizumab (S) in combination with dabrafenib (D) and trametinib (T) in previously untreated patients (pts) with advanced BRAF V600–mutant melanoma: updated efficacy and safety from parts 1 and 2 of COMBI-i. J Clin Oncol. 2019;37(15, suppl):9531. doi: 10.1200/JCO.2019.37.15_suppl.9531 [DOI] [Google Scholar]
- 38.Ascierto PA, Ferrucci PF, Fisher R, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. 2019;25(6):941-946. doi: 10.1038/s41591-019-0448-9 [DOI] [PubMed] [Google Scholar]
- 39.Tawbi HAH, Amaria RN, Glitza IC, et al. Safety and preliminary activity data from a single center phase II study of triplet combination of nivolumab (N) with dabrafenib (D) and trametinib (T) [trident] in patients (Pts) with BRAF-mutated metastatic melanoma (MM). J Clin Oncol. 2018;36(15, suppl):9560. [Google Scholar]
- 40.Baik CS, Rubin EH, Forde PM, et al. Immuno-oncology clinical trial design: limitations, challenges, and opportunities. Clin Cancer Res. 2017;23(17):4992-5002. doi: 10.1158/1078-0432.CCR-16-3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Univariate Analysis of Factors Associated With Best Overall Response per RECIST v 1.1 per Investigator Review
eTable 2 Multivariate Analysis of Factors Associated With Best Overall Response per RECIST v 1.1 per Investigator Review, Accounting for Patients With Missing Baseline Tumor Size
eTable 3. Multivariate Analysis of Factors Associated With Progression-Free Survival per RECIST v1.1 per Investigator Review With Study as a Covariate
eTable 4. Any-Grade Treatment-Related Adverse Events That Occurred in ≥5% of Patients
eFigure 1. Overall Response Rate in Evaluable Patients (N = 1558) With BRAF Wild-Type Versus Mutant Melanoma
eFigure 2. Four-Year Progression-Free Survival (PFS) and Overall Survival (OS) in Patients With BRAF Wild-Type Versus Mutant Melanoma
eFigure 3. Kaplan-Meier Estimates of Overall Survival


