Abstract
Dysregulation of dopamine neurotransmission has been linked to the development of human immunodeficiency virus (HIV)-associated neurocognitive disorder (HAND). To investigate the mechanisms underlying this phenomenon, this study used an inducible HIV-1 transactivator of transcription (Tat) transgenic (iTat-tg) mouse model, which demonstrates brain-specific Tat expression induced by administration of doxycycline. We found that induction of Tat expression in the iTat-tg mice for either 7 or 14 days resulted in a decrease (∼30%) in the Vmax of [3H]dopamine uptake via both the dopamine transporter (DAT) and norepinephrine transporter (NET) in the prefrontal cortex (PFC), which was comparable to the magnitude (∼35%) of the decrease in Bmax for [3H]WIN 35,428 and [3H]nisoxetine binding to DAT and NET, respectively. The decreased Vmax was not accompanied by a reduction of total or plasma membrane expression of DAT and NET. Consistent with the decreased Vmax for DAT and NET in the PFC, the current study also found an increase in the tissue content of DA and dihydroxyphenylacetic acid in the PFC of iTat-tg mice after 7 days’ administration of doxycycline. Electrophysiological recordings in layer V pyramidal neurons of the prelimbic cortex from iTat-tg mice found a significant reduction in action potential firing, which was not sensitive to selective inhibitors for DAT and NET, respectively. These findings provide a molecular basis for using the iTat-tg mouse model in the studies of NeuroHIV. Determining the mechanistic basis underlying the interaction between Tat and DAT/NET may reveal novel therapeutic possibilities for preventing the increase in comorbid conditions as well as HAND.
SIGNIFICANCE STATEMENT
Human immunodeficiency virus (HIV)-1 infection disrupts dopaminergic neurotransmission, leading to HIV-associated neurocognitive disorders (HANDs). Based on our in vitro and in vivo studies, dopamine uptake via both dopamine and norepinephrine transporters is decreased in the prefrontal cortex of HIV-1 Tat transgenic mice, which is consistent with the increased dopamine and dihydroxyphenylacetic acid contents in this brain region. Thus, these plasma membrane transporters are an important potential target for therapeutic intervention for patients with HAND.
Introduction
Acquisition of the human immunodeficiency virus (HIV) leads to the development of acquired immunodeficiency syndrome (AIDS) and continues to be a global public health problem, with an estimated 37 million HIV-1–positive individuals worldwide. Despite the success of combinatorial antiretroviral therapy in controlling peripheral HIV infection and improving the lives of patients with HIV, roughly 50% of patients with HIV-1 develop a group of neurologic complications including cognitive dysfunction, motor deficits, and dementia, collectively referred to as HIV-associated neurocognitive disorders (HAND) (Heaton et al., 2010). Persistent viral replication and expression of HIV-1 viral proteins within the CNS are central to the development of HAND (Gaskill et al., 2009), particularly since most combinatorial antiretroviral therapy (cART) medications cannot cross the blood-brain barrier, whereas infected macrophages carrying the virus can (Buckner et al., 2006). Considering long-term viral protein exposure can accelerate damage to the mesocorticolimbic dopamine (DA) system (Nath et al., 1987; Berger and Arendt, 2000; Koutsilieri et al., 2002), there is a pressing need to define the molecular mechanism(s) by which HIV-1 infection impairs the DA system and affects the progression of HAND. HIV-1 viral proteins are associated with the persistence of HIV-related neuropathology and subsequent neurocognitive deficits (Frankel and Young, 1998; Power et al., 1998; Brack-Werner, 1999; Johnston et al., 2001). Specifically, the continuing presence of the trans-activator of transcription (Tat) protein in cART-treated patients with HIV (Johnson et al., 2013; Henderson et al., 2019) may play a crucial role in the neurotoxicity and cognitive impairment evident in neuroHIV (Rappaport et al., 1999; King et al., 2006), as the HIV-1 Tat protein has been detected in DA-rich brain areas (Del Valle et al., 2000; Hudson et al., 2000; Lamers et al., 2010) and in the sera (Westendorp et al., 1995; Xiao et al., 2000) of patients infected with HIV-1 (Johnson et al., 2013).
Maintaining a normal physiologic DA system is essential for a variety of brain activities involved in attention, learning, memory (Nieoullon, 2002; Cools, 2006), and motivation (Lammel et al., 2012; Tye et al., 2013). Converging lines of clinical observations, supported by imaging (Wang et al., 2004; Chang et al., 2008), neuropsychological performance testing (Kumar et al., 2011; Meade et al., 2011), and postmortem examinations (Gelman et al., 2012), have demonstrated that DA dysregulation is correlated with the abnormal neurocognitive function observed in HAND (Berger and Arendt, 2000; Purohit et al., 2011). Therapy-naïve patients with HIV demonstrate increased levels of DA and decreased DA turnover in the early stages of HIV infection (Scheller et al., 2010), which may initiate compensatory mechanisms that eventually result in decreased DA levels (Sardar et al., 1996; Kumar et al., 2009, 2011) and dopaminergic neuron damage (Wang et al., 2004; Chang et al., 2008) in the advanced stages of HIV infection.
DA transporter (DAT)-mediated DA reuptake is critical for maintaining normal DA homeostasis. Human DAT activity is strikingly reduced in patients infected with HIV-1, particularly those with a history of cocaine abuse, correlating with the severity of HIV-1–associated cognitive deficits (Wang et al., 2004; Chang et al., 2008). Our published in vitro work has demonstrated that exogenous application of recombinant Tat1–86 protein decreases DAT activity in cells (Midde et al., 2013, 2015; Quizon et al., 2016) and rat brain synaptosomes (Zhu et al., 2009b). This research raises a critical question of whether the in vitro Tat-dysregulated DAT function can be replicated with in vivo biologic expression of Tat protein in an animal model, which may contribute to the neurocognitive deficits observed in both these animals and the individuals infected with HIV. Moreover, the prefrontal cortex is an important brain region for higher cognitive function in which not only the DAT but also the norepinephrine transporter (NET) is capable of DA reuptake (Moron et al., 2002). For this reason, it is possible that Tat-induced dysfunction of the DA system could be mediated by inhibition of both DAT and NET. The inducible Tat transgenic (iTat-tg) mouse model recapitulates many aspects of the neuropathologies and neurocognitive impairments observed in HAND (Kim et al., 2003) and represents a clinically relevant model of symptomatic NeuroHIV. Thus, investigating the neuropathogenic role of DAT/NET-mediated dopaminergic transmission in the brain of iTat-tg mice may provide mechanistic insight into the development of cognitive deficits in the HIV-1–infected population. This iTat-tg mouse model allows for determination of how in vivo Tat protein expression influences dopaminergic neurotransmission by inhibiting DAT and NET, which may contribute to the development of HAND.
Materials and Methods
Animals.
Male iTat-tg and G-tg mouse breeding stock were provided by Dr. Jay P. McLaughlin at the University of Florida College of Pharmacy (Gainesville, FL). Mice in this colony were established from progenitors originally derived by Dr. Johnny J. He (Kim et al., 2003) at the Rosalind Franklin University of Medicine and Science (Chicago, IL). The iTat-tg mouse line genetically expresses a “tetracycline-on” system, which is integrated into the regulator for the astrocyte-specific glial fibrillary acidic protein promoter and coupled to the Tat1–86 coding gene, allowing astrocyte (brain)-specific Tat1–86 expression induced by doxycycline (Dox) administration (Kim et al., 2003). The iTat-tg mouse model facilitates the needed focus on Tat protein, allowing us to study Tat-mediated dysregulation of DAT and NET. In contrast, the G-tg mice possess the tetracycline-on system integrated into glial fibrillary acidic protein but lack the Tat1–86 coding gene, rendering them incapable of Tat expression but highly suitable as control subjects. Since the iTat-tg and G-tg mice are developed from the C57BL/6J mouse strain, C57BL/6J mice (obtained from the Jackson Laboratory, Bar Harbor, ME) were used as another control mouse line. Mice used for all experiments were between the ages of 9 and 14 weeks (see Supplemental Table 4). This age range was chosen based on the previous reports, which found both physiologic (Carey et al., 2013; Cirino et al., 2020) and behavioral deficits (Carey et al., 2012; Paris et al., 2014, 2015) in the iTat-tg mice using an identical age range. At the completion of all experiments, we conducted the correlation analysis on age with the respective experimental data values from individual animals after 7 or 14 days’ administration of Dox, which revealed no correlation between age and the respective experimental data values (see Supplemental Table 5). Mice were housed (four to five mice per cage) in a temperature-controlled (21 ± 2°C) and humidity-controlled (50% ± 10%) vivarium and were maintained on a 12-hour light/dark cycle (lights on at 07:00 hour) with ad libitum access to food and water. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals under the National Institutes of Health guidelines in Assessment and Accreditation of Laboratory Animal Care–accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of South Carolina, Columbia.
Drug Administration and Synaptosomal Preparation.
Male iTat-tg, G-tg, and C57BL/6J mice were administered either Dox (100 mg/kg per day; Sigma-Aldrich; St. Louis, MO) or saline (10 μl/g body weight) via intraperitoneal injection for 7 or 14 consecutive days. The optimized dose of Dox was chosen because it has been previously proven efficacious for induction of Tat (Zou et al., 2007; Carey et al., 2012; Paris et al., 2014b), and findings have shown that iTat so induced is biologically active during the period of behavioral testing (Zou et al., 2007). The 7- or 14-day Dox or saline treatment paradigm was chosen for the kinetic analysis of DA uptake based on the previous behavior studies (Carey et al., 2012, 2013; Paris et al., 2014a). In addition, based on the previous report that showed no significant difference in Tat immunoreactivity in whole brain of iTat-tg mice after 7 or 14 days’ administration of Dox (Paris et al., 2014b), and because no difference in the inhibitory effects of Tat on DA uptake were observed between these time points, only the 7-day administration paradigm was used in the subsequent studies. Selection of animals for saline or Dox treatment was made randomly among littermates.
All mice were rapidly decapitated 24 hours after the last saline or Dox injection. Brain tissue dissected from prefrontal cortex (PFC, prelimbic and infralimbic cortices combined), striatum, and hippocampus was pooled from a group of three mice (constituting a single sample for each region) and was used as a single replicate (n) for conducting independent experiments. Thus, “n” refers to the number of independent experiments conducted rather than the number of mice used. Synaptosomes were prepared using our published method (Zhu et al., 2004). The PFC region was a focus of the current study because it is a critical brain region for higher cognitive function (Miller and Cohen, 2001; Dalley et al., 2004; Ridderinkhof et al., 2004) and because the NET also plays a role in DA uptake in this region (Horn, 1973a; Raiteri et al., 1977). Given that DA uptake through DAT or NET is not identical throughout various brain regions, the DA uptake through DAT in striatum and NET in hippocampus was examined in addition to the PFC. The striatum and hippocampus were also selected because of their central role in DA neurotransmission (Horn, 1973b; Raiteri et al., 1977; Borgkvist et al., 2012). The tissue was homogenized immediately in 20 ml of ice-cold 0.32 M sucrose buffer containing 2.1 mM of NaH2PO4 and 7.3 mM of Na2HPO4 (pH 7.4), with 16 up-and-down strokes using a Teflon pestle homogenizer (clearance approximately 0.003 inches). Homogenates were centrifuged at 1,000g for 10 minutes at 4°C, and the resulting supernatants were then centrifuged at 12,000g for 20 minutes at 4°C. The resulting pellets were resuspended in the respective buffer for each individual assay as noted below.
Kinetic Analysis of Synaptosomal [3H]DA Uptake Assay.
To determine whether Dox-induced biologic Tat expression alters DA uptake via DAT or NET, the maximal velocity (Vmax) and Michaelis-Menten constant (Km) of synaptosomal [3H]DA uptake were examined using a previously described method (Zhu et al., 2016). In a pilot study, we measured the synaptosomal [3H]DA uptake via DAT, NET, or the serotonin transporter (SERT) in whole C57BL/6J mouse brain with the utilization of selective inhibitors for the individual transporters and found that the portion of DA uptake via DAT, NET, or SERT is 80%, 17%, and 3%, respectively (data not shown). Because of the minimal level of DA uptake via SERT, this transporter was not examined in the current study. Importantly, although NET density is overall lower in the whole mouse brain, in the PFC the NET is more concentrated than the DAT and plays a primary role in reuptake of DA (Moll et al., 2000; Moron et al., 2002), thus warranting the present investigation. Because DA is transported by DAT, NET, and SERT in the PFC (Moron et al., 2002; Williams and Steketee, 2004), kinetic analysis of [3H]DA uptake via DAT in the PFC was assessed in the presence of desipramine (1 μM) and paroxetine (5 nM) to prevent DA uptake into norepinephrine- and serotonin-containing nerve terminals, respectively, whereas [3H]DA uptake via NET in the PFC was assessed in the presence of GBR12909 (100 nM) and fluoxetine (100 nM) to prevent DA uptake into dopaminergic- and serotonin-containing nerve terminals, respectively. In brief, the resulting pellets described above were resuspended in Krebs-Ringer-HEPES assay buffer (125 mm NaCl, 5 mm KCl, 1.5 mm MgSO4, 1.25 mm CaCl2, 1.5 mm KH2PO4, 10 mm glucose, 25 mm HEPES, 0.1 mm EDTA, 0.1 mm pargyline, and 0.1 mm L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). Aliquots of synaptosomal tissue (50 μg/25 µl) were incubated with one of six mixed [3H]DA concentrations containing a range of DA (Sigma-Aldrich) concentrations (1 nM to 5 µM) and fixed [3H]DA (12 nM; Perkin Elmer, Waltham, MA) for 8 minutes at 37°C. Incubation was terminated by the addition of 3 ml of ice-cold assay buffer, followed by immediate filtration through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 hours). Filters were washed three times with 3 ml of ice-cold assay buffer using a Brandel cell harvester (model MP-43RS; Biomedical Research and Development Laboratories, Inc., Gaithersburg, MD). Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; Packard Corporation Inc., Meriden, CT). Bovine serum albumin (Sigma-Aldrich) was used as a standard (Bradford, 1976) to measure protein concentration for all samples. Nonspecific uptake of [3H]DA into DAT or NET was determined in the presence of 10 μM nomifensine (Sigma-Aldrich) or 10 μM desipramine, respectively.
[3H]WIN 35,428 and [3H]Nisoxetine Binding Assays.
[3H]WIN 35,428 and [3H]nisoxetine (both purchased from Perkin Elmer) represent substrate binding sites on the DAT and the NET, respectively. To determine whether biologic HIV-1 Tat expression alters these substrate binding sites, we performed the saturation binding of [3H]WIN 35,428 and [3H]nisoxetine for DAT and NET, respectively, using a previously described method (Reith et al., 2005; Zhu et al., 2009a). Saturation binding assays were conducted in duplicate in a final volume of 250 μl for PFC, striatum, and hippocampus. For [3H]WIN 35,428 binding, 50-μl aliquots (50 μg protein) of synaptosomes were incubated in 0.32 M sucrose buffer (pH 7.4) containing 2.1 mM NaH2PO4 and 7.3 mM Na2HPO4 (chemicals purchased from Sigma-Aldrich) with six concentrations of [3H]WIN 35,428 (1, 5, 10, 15, 25, 30 nM) on ice for 2 hours. Desipramine (1 µM) was included to inhibit [3H]WIN 35,428 binding to the NET in the PFC. Nonspecific binding was determined in the presence of 10 µM cocaine in the striatum and 10 µM nomifensine in the PFC. For [3H]nisoxetine binding, 50-μl aliquots (50 μg protein) of synaptosomes were incubated in assay buffer (150 mM Na2HPO4, 300 mM NaH2PO4, 1.22 M NaCl, 50 mM KCl, 12 mM MgSO4, 100 mM glucose, 10 mM CaCl, and 1 µM EDTA; pH 7.4) with one of six nisoxetine concentrations (0.5–30 nM) that was mixed with a fixed concentration of [3H]nisoxetine (3 nM) on ice for 2 hours. GBR 12909 (0.1 µM) was included to inhibit [3H]nisoxetine binding to the DAT. Nonspecific binding was determined in the presence of 10 µM desipramine. The reaction was terminated by rapid filtration onto Whatman GF/B glass filter filters and presoaked for 2 hours with assay buffer containing 10% polyethylenimine through a Brandel cell harvester (model MP-43RS). Filters were washed three times with 3 ml of ice-cold assay buffer. Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; Packard Corporation Inc.), and bovine serum albumin (Sigma-Aldrich) was used as a standard (Bradford, 1976) to measure protein concentration for all samples.
Biotinylation and Western Blot Assay.
Biotinylation was performed to determine alterations in plasmalemmal surface expression of DAT in the PFC and striatum and NET in the PFC and hippocampus after Dox-induced Tat expression. After 7 days’ administration of Dox or saline, synaptosomes were prepared as described above. The biotinylation assay was performed as described previously (Zhu et al., 2009a). Synaptosomes (800 µg per sample) prepared freshly were incubated in 500 µl of PBS Ca/Mg buffer (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, and 9.6 mM Na2HPO4; with 1 mM MgCl2 and 0.1 mM CaCl2; Sigma-Aldrich) containing 1.5 mg/ml EZ-link sulfo-NHS-biotin at 4°C for 1 hour. After incubation, samples were centrifuged at 8000g for 4 minutes at 4°C. To remove the free sulfo-NHS-biotin, the resulting pellets were resuspended and centrifugated three times with 1 ml of ice-cold 100 mM glycine in PBS/Ca/Mg buffer and centrifugated at 8000g for 4 minutes at 4°C. Final resulting pellets were resuspended in 1 ml of ice-cold 100 mM glycine in PBS/Ca/Mg buffer and incubated with continual shaking for 30 minutes at 4°C. Samples were centrifuged subsequently at 8000g for 4 minutes at 4°C, the resulting pellets were resuspended in 1 ml of ice-cold PBS/Ca/Mg buffer, and the resuspension and centrifugation step were repeated twice. Final pellets were lysed by sonication for 2–4 seconds in 500 µl of Triton X-100 buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µM pepstatin, 250 µM phenylmethysulfonyl fluoride, pH 7.4), followed by incubation and continual shaking for 20 minutes at 4°C. Lysates were centrifuged at 21,000g for 20 minutes at 4°C. Pellets were discarded, and 100 µl of supernatant was saved for assessing total DAT or NET expression. Monomeric avidin beads (100 µl; Thermo Scientific, Waltham, MA) were added to the remaining supernatant and incubated for 1 hour at room temperature. The samples were then centrifuged at 17,000g for 4 minutes, and 100 µl of the resulting supernatant was saved for the intracellular (nonbiotinylated) fraction. Resulting pellets containing the avidin-absorbed biotinylation proteins (cell surface fraction) were resuspended in 1 ml of 1.0% Triton X-100 buffer and centrifuged at 17,000g for 4 minutes at 4°C, which was repeated twice. Final pellets containing the biotinylated DAT and NET absorbed to monomeric avidin beads were eluted by addition of 50 µl of laemmeli buffer (Sigma-Aldrich) and incubated for 20 minutes at room temperature. The total, intracellular, and surface fractions were stored at −20°C until Western blot assay.
To obtain immunoreactive DAT and NET protein in total synaptosomal, intracellular, and cell surface fractions, samples were thawed and subjected to gel electrophoresis and Western blotting. Samples were separated by 10% SDS-polyacrylamide gel electrophoresis for ∼90 minutes at 125 V. Samples were then transferred to Immobilon-P transfer membranes (0.45-µm pore size; Millipore Co., Bedford, MA) in transfer buffer (50 mM Tris, 250 mM glycine, 3.5 mM SDS) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA) for 90 minutes at 75 V. The membranes were then incubated with blocking buffer (5% milk powder in PBS containing 0.5% Tween-20) for 1 hour at room temperature, followed by incubation with either goat anti-DAT (C-20 polyclonal antibody, diluted 1:500 in blocking buffer; Santa Cruz) or mouse anti-NET (MAb tech, 05-1 monoclonal antibody, diluted 1:5000 in blocking buffer) overnight at 4°C. Transfer membranes were then washed three times with blocking buffer at room temperature, followed by incubation with either anti-goat horseradish peroxidase (catalog number 305-035-045, diluted 1:10,000 in blocking buffer; Jackson Laboratory) or anti-mouse horseradish peroxidase (catalog number 7076S, diluted 1:15,000 in blocking buffer; Cell Signaling) for 1 hour at room temperature. Membranes were then washed an additional three times in PBS containing 0.5% Tween-20 (Sigma-Aldrich). Immunoreactive proteins on the transfer membranes were detected using Amersham enhanced chemiluminescence prime Western blotting detection reagent (GE life sciences, Chicago, IL) and developed on Hyperfilm (GE life sciences). After detection and quantification of DAT or NET, each blot was washed and reprobed with rabbit anti-calnexin (catalog number SC-11397 polyclonal antibody, diluted 1:10,000 in blocking buffer; Santa Cruz, Biotechnology), an endoplasmic reticular protein, to monitor protein loading between all groups. Multiple autoradiographs were obtained using different exposure times, and immunoreactive bands within the linear range of detection were quantified by densitometric scanning using Scion image software. Band density measurements, expressed as relative optical density, were used to determine levels of DAT and NET in the total synaptosomal fraction, the intracellular fraction (nonbiotinylated), and the cell surface fraction (biotinylated).
High-Performance Liquid Chromatography Analysis of DA and Dihydroxyphenylacetic Acid Tissue Contents in Brain Regions.
Concentrations of DA and dihydroxyphenylacetic acid (DOPAC) in PFC, nucleus accumbens, and striatum in iTat-tg and G-tg mice after 7 days’ administration of saline or Dox were determined using a high-performance liquid chromatography (HPLC) system coupled with electrochemical detection as described previously (Zhu et al., 2004). Twenty-four hours after the last injection of saline or Dox, whole brains from individual mice were removed, immediately stored in liquid nitrogen, and then stored at −80°C. To prepare samples for HPLC assay, whole brains were sliced on top of an ice-cold plate, and brain regions were dissected by biopsy punches with a plunger system (Miltex). Brain tissues were individually stored in 10 volume/tissue weight of 0.1 N perchloric acid at −80°C until assay. Upon assay, samples were thawed on ice and sonicated and centrifuged at 30,000g for 15 minutes at 4°C. For each sample, 20 µl of the resulting supernatant was injected onto the HPLC system. Chromatograms were recorded using EZ Chrom Elite software (Agilent, Santa Clara, CA). Retention times of DA and DOPAC standards were used to identify respective peaks. Peak heights were used to calculate the detected amounts of DA and DOPAC based on a standard curve generated from external standards. The HPLC system coupled with electrochemical detection (Coulochem III; ThermoFisher Scientific, Columbia, MD) consisted of a 582 solvent delivery system, an autosampler (model 542), a reverse phase HPLC column (MD-150, 3.2 × 150 mm, 3 µm, product number 70-0636), and electrochemical detector (cell 5014B). The mobile phase contained 124 mM citric acid monohydrate, 50 mM Na2HPO4, 10 mM NaCl, 0.1 mM EDTA, 0.2% octylsulfonic acid-sodium salt, and 5% methanol (pH 3.0), using a flow rate of 0.5 ml/min. Samples were kept at 4°C in a cooling tray in the autosampler.
Patch-Clamp Electrophysiology.
iTat-tg and C57 mice were decapitated after isoflurane anesthesia 24 hours after the last Dox or saline injection. Brains were rapidly removed, and coronal slices (300 μm) containing the PFC were cut using a Vibratome (VT1000S; Leica Microsystems) in an ice-cold artificial cerebrospinal fluid (aCSF, in millimolars: 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 1 MgCl2, and 2 CaCl2, pH 7.2–7.4, saturated with 95% O2 and 5% CO2) solution in which NaCl was replaced with an equiosmolar concentration of sucrose. Slices were incubated in aCSF at 32–34°C for 45 minutes and kept at 22–25°C thereafter until transfer to the recording chamber. All solutions had osmolarity between 305 and 315 mOsm. Slices were viewed under an upright microscope (Eclipse FN1; Nikon Instruments) with infrared differential interference contrast optics and a 40× water-immersion objective. For recordings, the chamber was continuously perfused at a rate of 1 to 2 ml/min with oxygenated aCSF heated to 32 ± 1°C using an automated temperature controller (Warner Instruments). Recording pipettes were pulled from borosilicate glass capillaries (World Precision Instruments) to a resistance of 4–7 MΩ when filled with the intracellular solution. The intracellular solution contained the following (in millimolars): 145 potassium gluconate, 2 MgCl2, 2.5 KCl, 2.5 NaCl, 0.1 BAPTA, 10 HEPES, 2 Mg-ATP, and 0.5 GTP-Tris, pH 7.2–7.3, with KOH, osmolarity 280–290 mOsm. Layer V pyramidal neurons of the prelimbic cortex were identified by their morphology. The layer V pyramidal neurons of the medial prefrontal cortex were selected for two reasons: first, dopamine (D1) receptor expression is enriched in deeper layers of the prefrontal cortex (Santana and Artigas, 2017), which are primarily affected by HIV-1 Tat protein (Brailoiu et al., 2017; Kesby et al., 2017); second, the neurons projecting to subcortical nuclei have been suggested to play a critical role in cognitive functioning (Douglas and Martin, 2004; Riga et al., 2014). Current step protocols (from −500 to +500 pA; 20-pA increments; 500-millisecond step duration) were run to determine action potential frequency versus current (f-I) relationship. Drugs were applied via the Y-tube perfusion system modified for optimal solution exchange in brain slices (Hevers and Luddens, 2002). Dopamine (10 nM, final concentration) was initially applied alone, followed by the combined application of dopamine (10 nM) and GBR-12909 (100 nM) and the combined application of dopamine (10 nM), GBR-12909 (100 nM), and desipramine (1 µM). All data were collected after a minimum of 2 minutes of drug exposure. Currents were low-pass filtered at 2 kHz and digitized at 20 kHz using a Digidata 1440A acquisition board (Molecular Devices) and pClamp10 software (Molecular Devices). Access resistance (10–30 MΩ) was monitored during recordings by injection of 10 mV hyperpolarizing pulses. All analyses were completed using Clampfit 10 (Molecular Devices).
Data Analysis.
Data are expressed as means ± S.E.M., and n refers to the number of individual experiments for each group. To analyze the kinetic parameters (Vmax/Km and Bmax/Kd) of [3H]DA uptake or [3H]WIN 35,428 and [3H]nisoxetine binding, best-fit nonlinear regression analysis using a single-site model was conducted for each individual experiment using GraphPad Prism 8 software. To determine whether a relationship existed between individual mouse age and the respective experimental data, a separate Pearson correlation analysis was conducted. The kinetic parameters were then compared between the saline- and Dox-treated groups for C57BL/6J or G-tg mice (to rule out any nonspecific effects of Dox administration) and subsequently for the iTat-tg mice using unpaired Student’s t tests, which were conducted using IBM SPSS statistics version 25. Analyses resulting in P < 0.05 were considered significant.
Results
Expression of HIV-1 Tat Produces a Decrease in Synaptosomal [3H]DA Uptake through Both DAT and NET in the PFC.
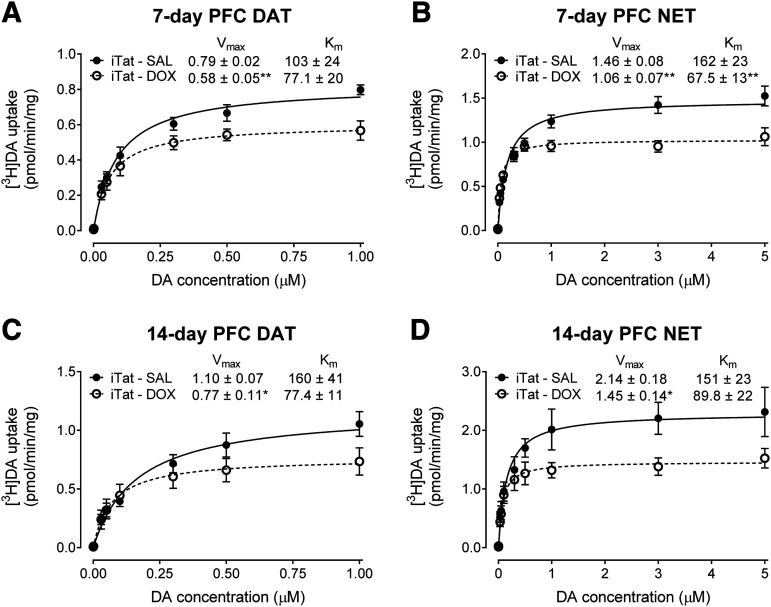
To determine the effects of Dox-induced Tat expression on DA uptake via DAT or NET, kinetic analyses of synaptosomal [3H]DA uptake were performed in iTat-tg mice after 7 or 14 days of Dox administration. After 7 days’ administration of Dox or saline, the Vmax of [3H]DA uptake via DAT and NET in the PFC was significantly reduced by 27% ± 4.8% [t(7.953) = 3.981, P < 0.01] and 27% ± 6.8% [t(11) = 3.752, P < 0.01], respectively, compared with the respective saline controls (Fig. 1, A and B). No changes in the Km values of [3H]DA uptake via DAT were observed (Fig. 1A); however, the Km values of [3H]DA uptake via NET of iTat-tg Dox-treated mice were significantly reduced by 58% ± 6.6% compared with saline-treated controls [t(11) = 3.708, P < 0.01] (Fig. 1B). After 14 days’ administration of Dox or saline, the Vmax values of [3H]DA uptake via DAT (Fig. 1C) and NET (Fig. 1D) in the PFC were significantly decreased by 30% ± 5.9% [t(9) = 2.356, P < 0.05] and 32% ± 3.1% [t(8) = 2.952, P < 0.05], respectively, compared with saline-treated controls. No significant differences in the Km of [3H]DA uptake via either DAT or NET were found (Fig. 1, C and D). We also determined the Vmax and Km values of [3H]DA uptake in the striatum and hippocampus, where DAT and NET are dominantly expressed. Results showed no observable difference in the Vmax and Km values of [3H]DA uptake via DAT in the striatum and NET in hippocampus between saline- and Dox-treated iTat-tg mice after either 7 or 14 days’ administration of Dox (Table 1). In the current study, in addition to iTat-tg mice treated with saline as a control for Dox treatment, C57BL/6J and G-tg mice were used as negative controls. No differences in the Vmax and Km values of [3H]DA uptake via DAT and NET in those brain regions were observed between saline- or Dox-treated mice (Supplemental Tables 1–3).
Fig. 1.
Kinetic analysis of synaptosomal [3H]DA uptake was determined in the PFC of iTat-tg mice after 7 or 14 days’ administration of saline or Dox. Synaptosomes were incubated with a range of mixed DA concentrations (0.1–5 µM, final concentration) containing a fixed concentration (12 nM) of [3H]DA. The Vmax and Km values for [3H]DA uptake via DAT (A and C) or NET (B and D) in the PFC of iTat-tg (iTat) mice after 7 (A and B) or 14 (C and D) days’ administration of saline or Dox were calculated using nonlinear regression analysis with a one-site binding parameter and represent the means from five to seven independent experiments ± S.E.M. *P < 0.05; **P < 0.01 compared with saline control group. SAL, saline.
TABLE 1.
Kinetic properties of [3H]DA uptake and [3H]WIN 35,428 binding or [3H]nisoxetine binding in iTat-tg mice
Data are expressed as means ± S.E.M. values from five to seven independent experiments performed in duplicate.
| Striatum (DAT) | Hippocampus (NET) | |||||
|---|---|---|---|---|---|---|
| Vmax (pmol/min per milligram) | Km (nM) | Vmax (pmol/min per milligram) | Km (nM) | |||
| Treatment duration | 7 days | iTat-tg saline | 15.6 ± 1.4 | 97.3 ± 6.6 | 0.898 ± 0.12 | 102 ± 23 |
| iTat-tg Dox | 15.6 ± 1.6 | 91.1 ± 3.4 | 0.926 ± 0.077 | 75.4 ± 14 | ||
| 14 days | iTat-tg saline | 67.6 ± 8.5 | 140 ± 11 | 1.96 ± 0.19 | 122 ± 18 | |
| iTat-tg Dox | 68.4 ± 8.9 | 141 ± 10 | 1.86 ± 0.26 | 115 ± 24 | ||
| Bmax pmol/mg | Kd (nM) | Bmax pmol/mg | Kd (nM) | |||
| 7 days | iTat-tg saline | 22.9 ± 3.7 | 20.34 ± 5.3 | 0.611 ± 0.10 | 13.4 ± 3.3 | |
| iTat-tg Dox | 25.4 ± 5.4 | 18.3 ± 1.8 | 0.552 ± 0.096 | 15.4 ± 2.0 | ||
Expression of HIV-1 Tat Does Not Alter Plasmalemmal Membrane Expression of DAT or NET in the PFC.
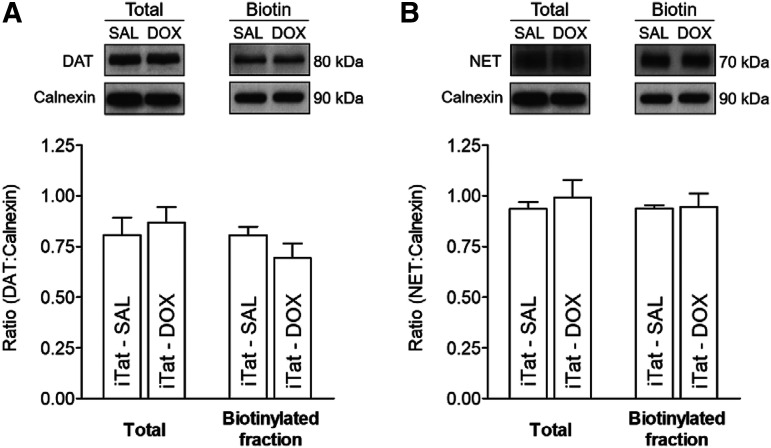
To determine whether the Tat expression–induced decrease in the Vmax of [3H]DA uptake via DAT or NET in PFC was associated with an alteration of the subcellular distribution of the transporters, biotinylation and immunoblot assays were performed to assess cell surface and intracellular transporter localization. Three subcellular fractions were prepared from the PFC, striatum, and hippocampus of iTat-tg mice after a 7-day Dox or saline administration, and DAT or NET immunoreactivity in both total fraction and cell surface fraction (biotinylated) were examined. No differences in DAT or NET immunoreactivity in PFC between saline- and Dox-treated iTat-tg mice were found in the ratio of total or surface transporters to calnexin (Fig. 2), indicating that the observed decrease in the Vmax for DAT or NET is not due to alteration of the available transporters on the cell surface. Moreover, no differences in the respective immunoreactivity of DAT in striatum or NET in hippocampus between saline- and Dox-treated iTat-tg mice were found in the ratio of total or surface transporters to calnexin (Supplemental Fig. 1). Additionally, no differences were observed in DAT or NET immunoreactivity in the PFC, striatum, or hippocampus between saline- and Dox-treated control mice in the ratio of total or surface transporters to calnexin (Supplemental Fig. 2).
Fig. 2.
Analysis of plasmalemmal surface expression of DAT and NET was determined in the PFC of iTat-tg mice after 7 days’ administration of saline or Dox. Synaptosomes were incubated with sulfo-NHS-biotin and Pierce monomeric avidin beads and washed multiple times to isolate the DAT or NET, which were present on the plasmalemmal membrane. Top panels: representative immunoblots of total and biotinylated (Biotin) fraction of DAT (A) or NET (B) from the PFC of iTat-tg (iTat) mice from Dox-treated and saline-treated (SAL) control groups. Calnexin was used as a control protein. Bottom panels: the ratio of total or biotinylated DAT (A) or NET (B) immunoreactivity to calnexin immunoreactivity expressed as means ± S.E.M. from five independent experiments.
Expression of HIV-1 Tat Produces a Decrease in [3H]WIN 35,428 and [3H]Nisoxetine Binding in the PFC.
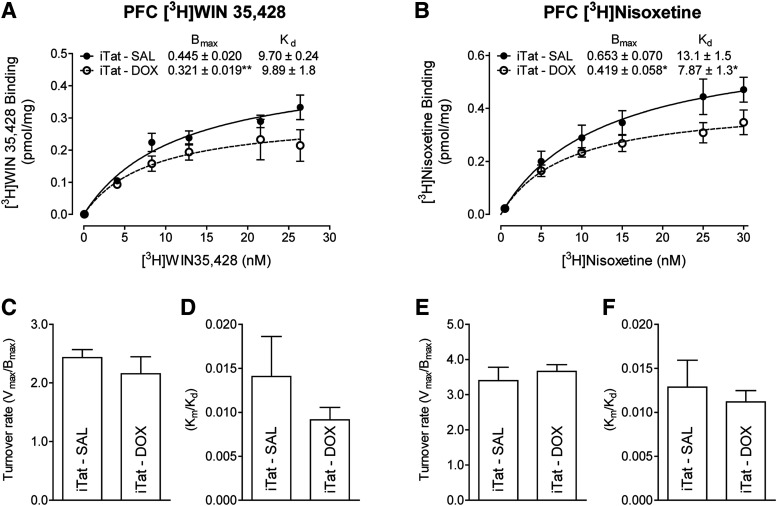
[3H]WIN 35,428 and [3H]nisoxetine are highly selective and potent reuptake inhibitors with high affinity for the DAT and NET, respectively (Tejani-Butt, 1992; Pristupa et al., 1994). To determine whether Tat-induced decreases in the Vmax of [3H]DA uptake via DAT or NET alter the respective substrate binding sites, kinetic analyses of [3H]WIN 35,428 and [3H]nisoxetine binding were performed in the brain regions of iTat-tg mice after 7 days of Dox or saline administration. For [3H]WIN 35,428 binding sites, in comparison with saline controls, the Bmax values in PFC of Dox-treated iTat-tg mice were decreased by 28% ± 2.7% [t(7) = 4.396, P < 0.01] (Fig. 3A), with no change in Kd values. However, no difference was observed in the Bmax values in striatum between saline-and Dox-treated iTat-tg mice (Table 1). For [3H]nisoxetine binding sites, the Bmax values in the PFC of Dox-treated iTat-tg mice were reduced by 36% ± 7.7% compared with saline-treated controls [t(7) = 2.479, P < 0.05] (Fig. 3B). The Kd value was reduced by 40% ± 10% compared with saline-treated controls [t(8) = 2.670, P < 0.05] (Fig. 3B). No difference in the Bmax values in hippocampus were found between saline-and Dox-treated iTat-tg mice (Table 1). Moreover, no differences were observed between saline- or Dox-treated control mice (Supplemental Tables 1 and 2).
Fig. 3.
Saturation binding of [3H]WIN 35,428 or [3H]nisoxetine in the PFC of iTat-tg mice after 7 days’ administration of saline (SAL) or Dox (DOX). For [3H]WIN 35,428 binding to DAT, synaptosomes were incubated in assay buffer with one of six concentrations of [3H]WIN 35,428 (1–30 nM, final concentration) and 1 µM desipramine on ice for 2 hours. Nonspecific binding was determined in the presence of 10 µM cocaine. For [3H]nisoxetine binding to NET, synaptosomes were incubated in assay buffer with one of six concentrations of nisoxetine (0.5–30 nM, final concentration) along with a fixed concentration of [3H]nisoxetine (3 nM) and 0.1 µM GBR12909 on ice for 2 hours. Nonspecific binding was determined in the presence of 10 µM desipramine. The Bmax and Kd values for [3H]WIN 35,428 (A) or [3H]nisoxetine (B) binding to DAT or NET in the PFC of iTat-tg (iTat) mice after 7 days’ administration of saline or Dox were calculated using nonlinear regression analysis with a one-site binding parameter and represent the means ± S.E.M. from five independent experiments. *P < 0.05; **P < 0.01 compared with saline control group. DA turnover rate values were determined for DAT from (C) the Vmax of [3H]DA uptake (Fig. 1A)/Bmax of [3H]WIN 35,428 binding and (D) the Km/Kd of [3H]DA uptake (Fig. 1A)/Bmax of [3H]WIN 35,428 binding and for NET from (E) the Vmax of [3H]DA uptake (Fig. 1B)/Bmax of [3H]nisoxetine binding and (F) the Km/Kd of [3H]DA uptake (Fig. 1B)/Bmax of [3H]nisoxetine binding.
Because of the comparable decreases observed in the Vmax of [3H]DA uptake and the Bmax of [3H]WIN 35,428 binding for the DAT in the PFC of Dox-treated iTat mice, comparison of the turnover rate (Vmax/Bmax) revealed no significant differences between saline-treated (2.43 ± 0.14) and Dox-treated (2.16 ± 0.29) groups (Fig. 3C). Analysis of the ratio of Km and Kd for Vmax of [3H]DA uptake and the Bmax of [3H]WIN 35,428 binding for the DAT in the PFC also revealed no significant differences between saline-treated (0.014 ± 0.005) and Dox-treated (0.009 ± 0.001) mice (Fig. 3D). No significant differences were found in turnover rate for [3H]DA uptake and [3H]nisoxetine binding for the NET in the PFC between saline-treated (3.40 ± 0.38) and Dox-treated (3.66 ± 0.19) mice (Fig. 3E) or for the ratio Km and Kd in the saline-treated (0.013 ± 0.003) and Dox-treated (0.011 ± 0.001) groups (Fig. 3F).
The Decreased DAT and NET Function in the PFC Is Consistent with Alterations in DA and DOPAC Tissue Content.
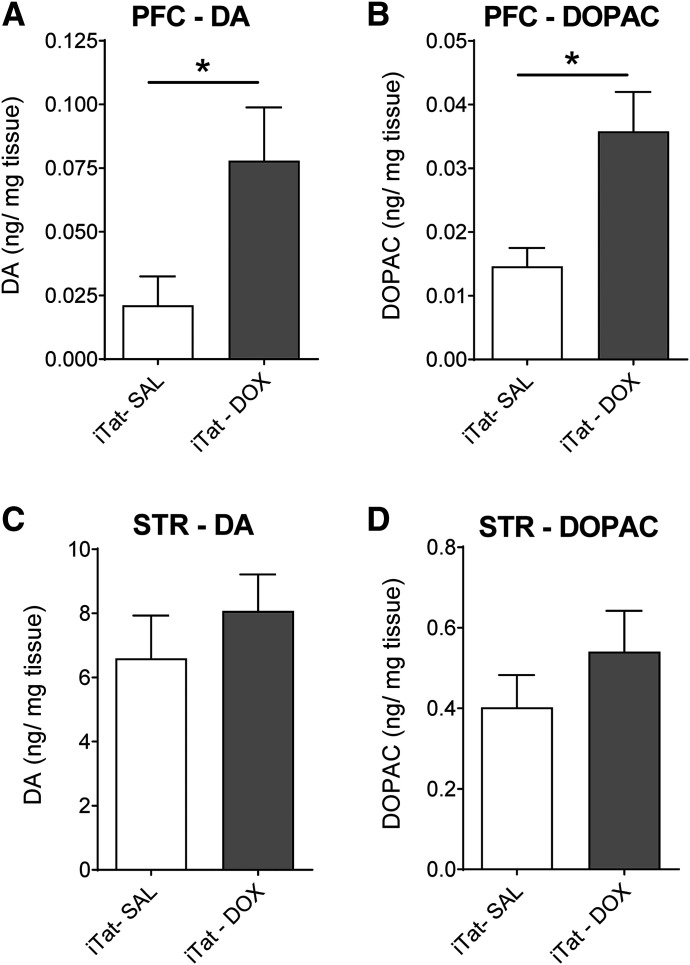
Given the critical role of the DAT and the NET in controlling DA homeostasis, a Tat-induced decrease in DA reuptake via DAT or NET in the PFC could result in alterations in the levels of DA and its primary metabolite DOPAC in the respective brain regions. DA and DOPAC content were determined in PFC and striatum from iTat-tg and G-tg mice after 7 days’ administration of saline or Dox. As shown in Fig. 4, DA tissue content in PFC from Dox-treated iTat-tg mice was increased by 268% (0.078 ± 0.021 ng/mg tissue) compared with saline-treated (0.021 ± 0.011 ng/mg tissue) controls [t(8) = 2.39, P < 0.05] (Fig. 4A). Similarly, DOPAC tissue content in the PFC of Dox-treated iTat-tg mice was increased by 144% (0.036 ± 0.006 ng/mg tissue) compared with saline-treated (0.015 ± 0.003 ng/mg tissue) controls [t(7) = 3.344, P < 0.05] (Fig. 4B). However, neither DA nor DOPAC content in striatum of iTat-tg mice differed significantly between saline- and Dox-treated groups (Fig. 4, C and D). We also examined the tissue levels of DA and DOPAC content in the PFC and striatum of G-tg mice, which showed no significant differences between saline- and Dox-treated groups (Supplemental Fig. 3).
Fig. 4.
DA and DOPAC tissue content in the PFC and striatum of iTat-tg mice after 7 days’ administration of saline or Dox. Top panels: DA (A) and DOPAC (B) tissue content in the PFC of iTat-tg (iTat) mice from Dox-treated (DOX) or saline-treated (SAL) control groups. Bottom panels: DA (C) and DOPAC (D) tissue content in striatum (STR) of iTat-tg mice from Dox-treated or saline-treated control groups. Data are expressed as nanograms per milligram tissue (mean ± S.E.M.) from five to six mice per group. *P < 0.05, compared with saline control group.
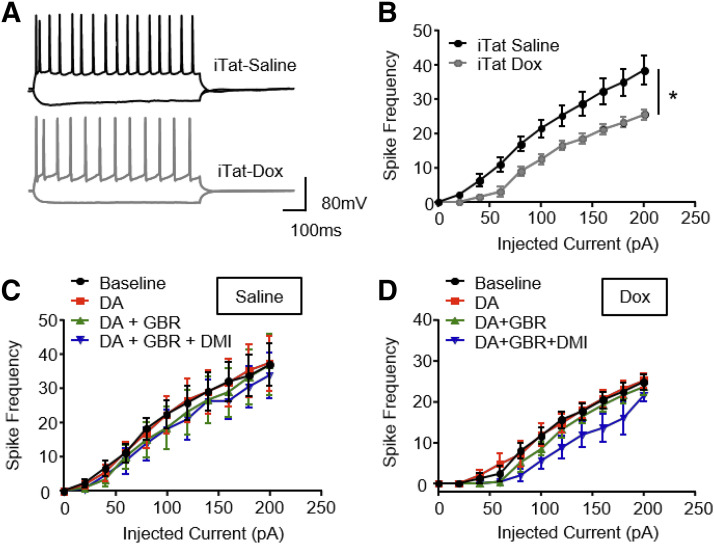
Action Potential Frequency Is Decreased in Layer V Pyramidal Neurons of the Prelimbic Cortex of iTat-Tg Mice.
To determine whether the Tat-induced decrease in DA uptake via DAT/NET in the PFC alters electrical firing signaling of neurons in this region, we performed whole-cell patch-clamp electrophysiology on layer V pyramidal neurons in the prelimbic region of PFC from iTat-tg mice after 7 days of saline or Dox treatment. As shown in Fig. 5B, ANOVA analysis revealed a significant main effect of current injection [F(10, 210) = 82.52, P < 0.0001] and a significant main effect of drug treatment (saline vs. dox) [F(1, 21) = 5.77, P = 0.025], as well as significant current × drug treatment interaction [F(10, 210) = 2.49, P = 0.008]. Dox-treated iTat-tg mice displayed a reduction of action potential output in this subset of neurons compared with the saline control group (Bonferroni P < 0.05). No differences were observed between saline- and Dox-treated C57BL/6J mice (Supplemental Fig. 4).
Fig. 5.
Whole-cell patch-clamp electrophysiology was performed in layer V pyramidal neurons of the prelimbic region of PFC in iTat-tg mice after 7 days’ administration of saline or Dox. Coronal slices (300 µm) containing the prelimbic cortex were cut with a Vibratome and incubated in an ice-cold aCSF solution at 32–34°C for 45 minutes and kept at 22–25°C thereafter until transfer to the recording chamber. (A) Representative traces from iTat-tg mice treated with saline (iTat-Saline) and Dox (iTat-Dox) after hyperpolarizing (−200 pA) and depolarizing (+200 pA) current steps. Summary of basal action potential frequency (B) and action potential frequency in response to acute application of DA (10 nM) or DA + GBR 12909 (100 nM) or DA + GBR 12909 + desipramine (DMI) (1 µM) of neurons from iTat-tg mice treated with saline (C) or Dox (D). *P < 0.05, n = 15 and n = 8 for saline- and Dox-treated mice, respectively.
Additionally, to assess the effects of inhibition of DAT or NET in the prelimbic region of PFC by selective inhibitors for the respective transporter on the observed decrease in action potential output, the basal action potentials from pyramidal neurons of iTat-tg mice with Dox or saline treatment were subsequently examined in the presence of DA (10 nM), DA + GBR-12909 (a potent DAT inhibitor; 100 nM), and DA + GBR-12909 + desipramine (a potent NET inhibitor, 1 µM). A two-way ANOVA with repeated measurement on action potential in iTat-tg mice treated with saline (Fig. 5C) and Dox (Fig. 5D) revealed a significant main effect of current injection [saline: F(10, 80) = 21.17, P < 0.0001; Dox: F(10, 40) = 145.4, P < 0.0001]; however, neither saline- nor Dox-treated mice displayed a significant main effect of drug exposure [saline: F(3, 24) = 1.005, P = 0.4; Dox: F(3, 12) = 1.945, P = 0.18]. Although the current injection × drug exposure interaction was not significant in saline-treated mice [F(30, 240) = 0.788, P = 0.78], Dox-treated mice displayed a significant interaction between current injection × drug exposure [F(30, 120) = 1.571, P = 0.05].
Discussion
The current study investigated the mechanism by which inducible Tat expression influences dopaminergic transmission in the PFC of iTat-tg mice. We have demonstrated that in vitro HIV-1 recombinant Tat1–86 protein reduces [3H]DA uptake through DAT in cells (Midde et al., 2013, 2015; Quizon et al., 2016; Sun et al., 2017) and rat striatal synaptosomes (Zhu et al., 2009b). Our current results show that the Vmax of [3H]DA uptake through both DAT and NET was decreased in the PFC of iTat-tg mice after 7- or 14-day Dox-induced Tat1–86 expression. We also observed corresponding decreases in the Bmax of [3H]WIN 35,428 and [3H]nisoxitine binding in this region, suggesting that the inhibitory effects of in vitro Tat on DAT and NET function can be replicated in the PFC of the iTat-tg mouse model with in vivo biologic Tat expression. Moreover, we also observed increased DA and DOPAC tissue content in the iTat-tg mice, which was again selective to the PFC, suggesting a neuroadaptive change in the DA system, perhaps in compensation for iTat protein-induced inhibition of DAT and NET function.
The most intriguing observation is that the Vmax for DA uptake via DAT or NET in the PFC was decreased (∼30%) in iTat-tg mice after 7 or 14 days’ administration of Dox, whereas no differences in Vmax were observed in the striatum or hippocampus of the iTat-tg mice between saline- and Dox-treated subjects. In addition, no differences in Vmax were found in these brain regions between saline- and Dox-treated subjects in the control G-tg and C57BL/6J mouse lines. These findings suggest that the Dox-induced Tat reduces DA transport in the PFC by inhibiting both DAT and NET in a region-specific manner. Moreover, after 7 days of Dox treatment, the Bmax value for [3H]WIN 35,428–labeled DAT binding sites in the PFC was decreased by 28% in iTat-tg mice, which is comparable to the magnitude of the decrease in Vmax for DAT, whereas the Bmax value for [3H]nisoxetine-labeled NET binding sites in the PFC was decreased by 35%, which was slightly more than the observed decrease in the Vmax for NET. Interestingly, DA uptake turnover (Vmax/Bmax), the efficacy of DA molecules being transported per second per uptake site (Lin et al., 2000), was not altered in the PFC of iTat-tg mice, which is consistent with our previous results showing no change in DA uptake turnover after in vitro exposure of rat synaptosomes to Tat (Midde et al., 2012). The efficacy of DA uptake largely depends on DAT expression in the plasma membrane, which is dynamically modulated by a trafficking mechanism (Zhu and Reith, 2008). Results from the surface biotinylation assay did not find any difference in total and plasma membrane expression of DAT or NET in the PFC in iTat-tg mice between Dox- and saline-treated groups, suggesting that the reductions in Vmax and Bmax are not due to Tat-induced transporter protein degradation or surface transporter trafficking. We have demonstrated that DA transport to cytoplasmic pool via DAT is a dynamic conversion of the transporter’s conformation between the three states (outward-open, outward-occluded, and inward-open) (Yuan et al., 2016) and that the Tat protein allosterically regulates DAT activity by binding to DAT in the outward-open state (Yuan et al., 2015; Zhu et al., 2018). Considering that DAT and NET share similar binding residues for Tat based on computational and homology modeling (Yuan et al., 2016), the Dox-induced Tat expression could reduce both Vmax and Bmax for DAT/NET by interfering with the Tat-DAT/NET binding sites and inducing a conformational change of DAT/NET from the outward-open state to the outward-occluded state.
The equivariant expression of inducible Tat protein across all brain regions in the iTat-tg mice subjected to 7 days of Dox treatment (100 mg/kg per day) has been confirmed by detecting Tat mRNA (Kim et al., 2003) and Tat immunoblotting (Carey et al., 2012). A caveat, however, is that it is unclear what the actual concentration of Dox-induced Tat expression is at or around the synaptic terminals, and how this effective concentration of Tat might be reflected in the brain of patients infected with HIV remains unclear, as most studies investigating the inhibitory effects of Tat on monoamine transporters are performed in vitro using recombinant Tat. For example, exposure to 140 nM recombinant Tat1–86 induces about 30% reduction of DA uptake in cells expressing wild-type human DAT (Midde et al., 2013, 2015; Quizon et al., 2016; Sun et al., 2017) and brain synaptosomes (Zhu et al., 2009b). One study reported that a detectable Tat concentration in frontal cortical brain samples from patients infected with HIV-1 is about 140 pmol (Hudson et al., 2000), which is 1000-fold lower than in vitro recombinant Tat used in reported studies. The Dox treatment of 100 mg/kg used in this study has been shown to induce ∼1 ng/ml Tat concentration in the iTat-tg mouse brain (Kim et al., 2003), which is comparable to Tat protein levels detected in sera of patients that are HIV+ (Westendorp et al., 1995; Xiao et al., 2000). Nevertheless, our studies with in vitro and in vivo Tat exposure provide molecular insight into the mechanisms underlying Tat-induced dysregulation of dopaminergic transmission via DAT and NET.
Consistent with the decreased Vmax for DAT and NET in PFC, the current study found an increase in the tissue content of DA and DOPAC in the PFC of iTat-tg mice 24 hours after 7 days’ administration of Dox. Similar studies have demonstrated increased DA content in the caudate putamen of iTat-tg mice 3 days after completing a 7-day Dox regimen (Kesby et al., 2016a) but decreased ratios of DOPAC/DA in the same region 10 days after completing a 7-day Dox regimen without changes in this ratio in PFC (Kesby et al., 2016b). Regarding these results, effects of Tat on DA tissue content in specific brain regions may be influenced by different Dox regimens and the timing of brain collection after Dox treatment. Levels of extracellular DA in the synaptic cleft are controlled by reuptake via plasma membrane transporters and presynaptic vesicle–mediated release. Indeed, we have demonstrated that in vitro exposure to Tat inhibits DA uptake via the vesicular monoamine transporter-2 (Midde et al., 2012), which is responsible for monoamine storage and the vesicle-mediated DA release. Therefore, a very complex influence of Tat protein on the DA system should be taken into consideration when we evaluate the actual DA levels in iTat-tg mice after Dox-induced Tat expression. Given that the DA content reflects the tissue levels of total DA rather than the specific extracellular DA levels, monitoring release and uptake dynamics of endogenous DA levels in iTat-tg mice by an electrochemical technique (e.g., fast scan cyclic voltammetry) is an interesting topic for future investigation.
Another important finding from the current study is that the inhibitory effects of Tat on the kinetic parameters of DA uptake through DAT/NET and the respective substrate binding sites are selective to the PFC. There are several possible explanations for our observations. First, compared with the PFC, the striatum and hippocampus exhibit a higher density of DAT and NET (Horn, 1973b; Raiteri et al., 1977; Borgkvist et al., 2012), which may provide protection from the acute inhibitory effects of HIV-1 Tat on transporter function. Second, HIV-1 Tat may be not expressed equally throughout different brain regions. Although a previous report showed that Tat immunoreactivity was expressed throughout brain of the Tat transgenic mice (Carey et al., 2012), the exact concentration of biologic Tat in these brain regions remains unclear. Indeed, our previous study has reported that HIV-1 Tat inhibits DAT function in a concentration-dependent manner (Zhu et al., 2009b). Third, with regard to HIV-1 Tat–induced decrease in DA transport through NET, the NET in the PFC is more concentrated than the DAT and plays a primary role in reuptake of DA (Moll et al., 2000; Moron et al., 2002). For these reasons, it is possible that HIV-1 Tat–induced dysfunction of the DA system in the PFC could be mediated by inhibition of both DAT and NET. In the early stages of HIV-1 infection, the cognitive impairments associated with the PFC are initially observed (Everall et al., 1991; Melrose et al., 2008), whereas severe damage such as degradation of the dopaminergic terminals is observed in the striatal area in the late stages of patients infected with HIV-1 (Wang et al., 2004; Chang et al., 2008). Moreover, volume loss and thinning of the PFC are found in patients with AIDS, which is associated with the severity of cognitive impairments (Thompson et al., 2005), suggesting that the PFC is more vulnerable to HIV infection compared with other regions. Thus, the current findings demonstrate that the inhibition of DAT/NET specifically in the PFC may play an important role in mediating Tat-induced neurocognitive impairment observed in HAND.
Whole-cell patch-clamp recordings show that the evoked basal action potential firing from layer V pyramidal neurons of the prelimbic cortex is reduced in iTat-tg mice treated with Dox compared with saline controls. This reduced neuronal firing is unlikely to have resulted from the Tat-induced decrease in DA uptake via DAT and NET since DA at the low concentration used in our experiments has no effect on action potentials, and previous studies indicate that higher concentrations of DA increase membrane excitability in striatal as well as prefrontocortical projection neurons (Hopf et al., 2003; Ortinski et al., 2015; Buchta et al., 2017; Lahiri and Bevan, 2020). Conversely, reduced neuronal firing is unlikely to have caused a deficit in DAT function via effects on membrane potential, since membrane hypoexcitability has been linked to surface expression of DAT (Richardson et al., 2016). Finally, DAT-mediated translocation of Na+ ions across the plasma membrane is not expected to have a pronounced impact on either the membrane potential or spike firing and a trend toward reduced action potential firing with desipramine of AMPA-mediated currents and voltage-gated Ca2+ channels (Koncz et al., 2014). Together, these observations suggest that decreased action potential firing of prelimbic cortex pyramidal neurons in iTat-tg mice is not directly related to Tat effects on DA transport. HIV-1 Tat may, however, affect action potential generation through mechanisms independent of DA uptake, with a variety of bidirectional effects reported by previous studies (Krogh et al., 2014; Ngwainmbi et al., 2014; Francesconi et al., 2018; Mohseni Ahooyi et al., 2018).
In conclusion, the current findings provide novel evidence that HIV-1 Tat protein dysregulates the dopaminergic system selectively in the PFC of iTat-tg mice. The inhibition of both DA uptake and substrate binding through both DAT and NET may have important implications for the role of the prefrontal DAT/NET in neurocognitive impairment is observed in HAND. Considering the HIV-1 Tat interaction with allosteric modulatory binding sites on these transporters (Zhu et al., 2011; Sun et al., 2017), the current study provides a biologic basis for developing allosteric modulators that specifically block Tat binding on these transporters with minimal effect on physiologic DA transport, which may have potential for therapeutic application in Tat-induced dysregulation of dopaminergic transmission and its associated cognitive deficits observed in patients infected with HIV-1.
Acknowledgments
We thank Wei-Lun Sun and Luyi Zhou for technical assistance.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- DA
dopamine
- DAT
dopamine transporter
- DOPAC
dihydroxyphenylacetic acid
- Dox
doxycycline
- GAP
transgenic
- HAND
HIV-associated neurocognitive disorder
- HIV
human immunodeficiency virus
- HPLC
high-performance liquid chromatography
- iTat-tg
inducible tat transgenic
- NET
norepinephrine transporter
- PFC
prefrontal cortex
- SERT
serotonin transporter
- Tat
transa-ctivator of transcription
Authorship Contributions
Participated in research design: Strauss, Ortinski, McLaughlin, Zhu.
Conducted experiments: Strauss, O’Donovan, Ma, Xiao, Lin, Bardo.
Performed data analysis: Strauss, Bardo, O’Donovan, Zhu.
Wrote or contributed to the writing and editing of the manuscript: Strauss, O’Donovan, Zhu.
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grants R01 DA035714 and R21 DA041932] (to J.Z.), [Grant R01 DA041513] (to P.I.O.), and [Grant P50 DA05312] (to M.T.B).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Berger JR, Arendt G. (2000) HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 14:214–221. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Malmlöf T, Feltmann K, Lindskog M, Schilström B. (2012) Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol 15:531–540. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. (1999) Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1–22. [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Deliu E, Barr JL, Console-Bram LM, Ciuciu AM, Abood ME, Unterwald EM, Brailoiu E. (2017) HIV Tat excites D1 receptor-like expressing neurons from rat nucleus accumbens. Drug Alcohol Depend 178:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta WC, Mahler SV, Harlan B, Aston-Jones GS, Riegel AC. (2017) Dopamine terminals from the ventral tegmental area gate intrinsic inhibition in the prefrontal cortex. Physiol Rep 5:e13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. (2006) Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol 1:160–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, Kaufman MJ. (2013) Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuropsychopharmacol Biol Psychiatry 43:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. (2012) Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. (2008) Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 42:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino THOMAS J, Harden SCOTT W, McLaughlin JAY P, Frazier CHARLES J. (2020) Region-specific effects of HIV-1 Tat on intrinsic electrophysiological properties of pyramidal neurons in mouse prefrontal cortex and hippocampus. J Neurophysiol 123 (4):1332–1341, doi: 10.1152/jn.00029.2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. (2006) Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev 30:1–23. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. (2000) Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol 6:221–228. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. (2004) Neuronal circuits of the neocortex. Annu Rev Neurosci 27:419–451. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. (1991) Neuronal loss in the frontal cortex in HIV infection. Lancet 337:1119–1121. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Marcondes MCG. (2018) HIV-1 Tat alters neuronal intrinsic excitability. BMC Res Notes 11:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel AD, Young JA. (1998) HIV-1: fifteen proteins and an RNA. Annu Rev Biochem 67:1–25. [DOI] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. (2009) Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol 175:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr., Soukup VM. (2012) Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol 7:686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, et al. CHARTER Group (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, Demarino C, Barclay RA, Snow J, Sacktor N, et al. (2019) Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 33 (Suppl 2):S145–S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Lüddens H. (2002) Pharmacological heterogeneity of gamma-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology 42:34–47. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. (2003) Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci 23:5079–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn AS. (1973a) Structure-activity relations for the inhibition of catecholamine uptake into synaptosomes from noradrenaline and dopaminergic neurones in rat brain homogenates. Br J Pharmacol 47:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn AS. (1973b) Structure activity relations for the inhibition of 5-HT uptake into rat hypothalamic homogenates by serotonin and tryptamine analogues. J Neurochem 21:883–888. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. (2000) Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol 6:145–155. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A. (2013) Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci USA 110:13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Zhang K, Silva C, Shalinsky DR, Conant K, Ni W, Corbett D, Yong VW, Power C. (2001) HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann Neurol 49:230–241. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S. (2016a) The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 109:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S, TMARC Group (2016b) Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neurotransmitter levels in mice. Behav Brain Res 311:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Najera JA, Romoli B, Fang Y, Basova L, Birmingham A, Marcondes MCG, Dulcis D, Semenova S. (2017) HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function. Brain Behav Immun 65:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. (2003) Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 162:1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. (2006) HIV tat and neurotoxicity. Microbes Infect 8:1347–1357. [DOI] [PubMed] [Google Scholar]
- Koncz I, Szász BK, Szabó SI, Kiss JP, Mike A, Lendvai B, Sylvester Vizi E, Zelles T. (2014) The tricyclic antidepressant desipramine inhibited the neurotoxic, kainate-induced [Ca(2+)]i increases in CA1 pyramidal cells in acute hippocampal slices. Brain Res Bull 104:42–51. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. (2002) Involvement of dopamine in the progression of AIDS Dementia Complex. J Neural Transm (Vienna) 109:399–410. [DOI] [PubMed] [Google Scholar]
- Krogh KA, Green MV, Thayer SA. (2014) HIV-1 Tat-induced changes in synaptically-driven network activity adapt during prolonged exposure. Curr HIV Res 12:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol 15:257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. (2011) Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol 17:26–40. [DOI] [PubMed] [Google Scholar]
- Lahiri AK, Bevan MD. (2020) Dopaminergic transmission rapidly and persistently enhances excitability of D1 receptor-expressing striatal projection neurons. Neuron 106:277–290.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Salemi M, Galligan DC, Morris A, Gray R, Fogel G, Zhao L, McGrath MS. (2010) Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J Neurovirol 16:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature 491:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Itokawa M, Uhl GR. (2000) Dopamine transporter proline mutations influence dopamine uptake, cocaine analog recognition, and expression. FASEB J 14:715–728. [DOI] [PubMed] [Google Scholar]
- Meade CS, Lowen SB, MacLean RR, Key MD, Lukas SE. (2011) fMRI brain activation during a delay discounting task in HIV-positive adults with and without cocaine dependence. Psychiatry Res 192:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. (2008) Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 188:337–347. [DOI] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J. (2012) HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol 7:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. (2013) Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol 8:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Yuan Y, Quizon PM, Sun WL, Huang X, Zhan CG, Zhu J. (2015) Mutations at tyrosine 88, lysine 92 and tyrosine 470 of human dopamine transporter result in an attenuation of HIV-1 Tat-induced inhibition of dopamine transport. J Neuroimmune Pharmacol 10:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Mohseni Ahooyi T, Shekarabi M, Decoppet EA, Langford D, Khalili K, Gordon J. (2018) Network analysis of hippocampal neurons by microelectrode array in the presence of HIV-1 Tat and cocaine. J Cell Physiol 233:9299–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. (2000) Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res 119:251–257. [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Jankovic J, Pettigrew LC. (1987) Movement disorders and AIDS. Neurology 37:37–41. [DOI] [PubMed] [Google Scholar]
- Ngwainmbi J, De DD, Smith TH, El-Hage N, Fitting S, Kang M, Dewey WL, Hauser KF, Akbarali HI. (2014) Effects of HIV-1 Tat on enteric neuropathogenesis. J Neurosci 34:14243–14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A. (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Briand LA, Pierce RC, Schmidt HD. (2015) Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology 92:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JASON J, Singh HARMINDER D, Carey AMANDA N, McLaughlin JAY P. (2015) Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res 291:209–218, doi: 10.1016/j.bbr.2015.05.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Fenwick J, McLaughlin JP. (2014a) Estrous cycle and HIV-1 Tat protein influence cocaine-conditioned place preference and induced locomotion of female mice. Curr HIV Res 12:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. (2014b) Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 231:2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, McArthur JC, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson RT, Chesebro B. (1998) Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol 72:9045–9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa ZB, Wilson JM, Hoffman BJ, Kish SJ, Niznik HB. (1994) Pharmacological heterogeneity of the cloned and native human dopamine transporter: disassociation of [3H]WIN 35,428 and [3H]GBR 12,935 binding. Mol Pharmacol 45:125–135. [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. (2011) Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol 44:102–110. [DOI] [PubMed] [Google Scholar]
- Quizon PM, Sun WL, Yuan Y, Midde NM, Zhan CG, Zhu J. (2016) Molecular mechanism: the human dopamine transporter histidine 547 regulates basal and HIV-1 Tat protein-inhibited dopamine transport. Sci Rep 6:39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. (1977) Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol 41:133–143. [DOI] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. (1999) Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol 65:458–465. [DOI] [PubMed] [Google Scholar]
- Reith ME, Wang LC, Dutta AK. (2005) Pharmacological profile of radioligand binding to the norepinephrine transporter: instances of poor indication of functional activity. J Neurosci Methods 143:87–94. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Saha K, Krout D, Cabrera E, Felts B, Henry LK, Swant J, Zou MF, Newman AH, Khoshbouei H. (2016) Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nat Commun 7:10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. (2004) Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56:129–140. [DOI] [PubMed] [Google Scholar]
- Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. (2014) Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci 8:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Artigas F. (2017) Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex. Front Neuroanat 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar AM, Czudek C, Reynolds GP. (1996) Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport 7:910–912. [DOI] [PubMed] [Google Scholar]
- Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, et al. (2010) Increased dopaminergic neurotransmission in therapy-naïve asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm (Vienna) 117:699–705. [DOI] [PubMed] [Google Scholar]
- Sun WL, Quizon PM, Yuan Y, Zhang W, Ananthan S, Zhan CG, Zhu J. (2017) Allosteric modulatory effects of SRI-20041 and SRI-30827 on cocaine and HIV-1 Tat protein binding to human dopamine transporter. Sci Rep 7:3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani-Butt SM. (1992) [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther 260:427–436. [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. (2005) Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci USA 102:15647–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, et al. (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. (2004) Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127:2452–2458. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. (1995) Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. (2004) Characterization of dopamine transport in crude synaptosomes prepared from rat medial prefrontal cortex. J Neurosci Methods 137:161–165. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. (2000) Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA 97:11466–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Huang X, Midde NM, Quizon PM, Sun WL, Zhu J, Zhan CG. (2015) Molecular mechanism of HIV-1 Tat interacting with human dopamine transporter. ACS Chem Neurosci 6:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Huang X, Zhu J, Zhan CG. (2016) Computational modeling of human dopamine transporter structures, mechanism and its interaction with HIV-1 transactivator of transcription. Future Med Chem 8:2077–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. (2011) Recombinant human immunodeficiency virus-1 transactivator of transcription1-86 allosterically modulates dopamine transporter activity. Synapse 65:1251–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Zhan CG. (2018) The role of human dopamine transporter in NeuroAIDS. Pharmacol Ther 183:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Dwoskin LP. (2009a) Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther 328:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. (2004) Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res 148:107–117. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. (2009b) HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther 329:1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Reith ME. (2008) Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets 7:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yuan Y, Midde NM, Gomez AM, Sun WL, Quizon PM, Zhan CG. (2016) HIV-1 transgenic rats display an increase in [(3)H]dopamine uptake in the prefrontal cortex and striatum. J Neurovirol 22:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Kim BO, Zhou BY, Liu Y, Messing A, He JJ. (2007) Protection against human immunodeficiency virus type 1 Tat neurotoxicity by Ginkgo biloba extract EGb 761 involving glial fibrillary acidic protein. Am J Pathol 171:1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]