Abstract
Using advanced gene editing technologies, xenotransplantation from multi-transgenic alpha-1,3-galacto-syltransferase knockout pigs has demonstrated marked prolongation of renal xenograft survival, ranging from days to greater than several months for life-supporting kidneys and >2 years in a heterotopic non-life-supporting cardiac xenograft model. However, continuous administration of multiple immunosuppressive drugs continues to be required, and attempts to taper immunosuppression have been unsuccessful. These data are consistent with previous reports indicating that the human-anti-porcine T cell response is similar or stronger than that across allogeneic barriers. Due to the strength of both the innate and adaptive immune responses in xenotransplantation, the level of continuous immunosuppression needed to control these responses and prolong xenograft survival has been associated with prohibitive morbidity and mortality. These facts provide compelling rationale to pursue a clinically applicable strategy for the induction of tolerance.
Mixed chimerism and thymic tissue transplantation have both achieved xenogeneic tolerance in pig-to-mouse models, and both have recently been extended to pig-to-baboon models. Although these strategies are promising in small animal models, neither direct intravenous injection of porcine bone marrow cells nor direct fetal thymic tissue transplantation into recipients was able to achieve >2 days chimerism following BM Tx or the engraftment of thymic tissues across xenogeneic barriers in pig-to-nonhuman primate models. Several innovative procedures have been largely developed by Kazuhiko Yamada to overcome these failures. These include vascularized thymic transplantation, combined with either thymokidney (TK) or vascularized thymic lobe (VTL) transplantation. Utilizing the strategy of transplanting vascularized thymic grafts with kidney from the same GalT-KO donor without further gene modification, we have achieved longer than 6 months survival of life-supporting kidneys in a baboon. Notably, the recipient became donor specific unresponsive and developed new thymic emigrants. In this chapter, we introduce a brief summary of our achievements to date toward the successful induction of tolerance by utilizing our novel strategy of vascularized thymic transplantation (including thymokidney transplantation), as well as describe the step-by-step methodology of surgical and in vitro procedures which are required for this experiment.
Keywords: Xenotransplantation, Transplantation, Kidney, Vascularized thymic transplantation, Tolerance
1. Introduction
1.1. Xenogeneic T Cell Responses
Due to the intensity of antibody-derived rejection across xenogeneic barriers, researchers have not paid much attention to xenogeneic anti-donor T cell responses to vascularized donor organs. Early investigations into T cell responses exploited the mouse antihuman or anti-pig models that revealed a defective mouse CD4 T cell antistimulator MHC class II molecular interaction, effectively eliminating T cell activation via the direct pathway [1, 2], and suggested that if humoral mechanisms were overcome, cell-mediated rejection would prove to be a minor obstacle. However, we have clearly demonstrated in the early 1990s [3, 4] that (1) human T cells respond at least as well to xeno-MHC antigens (Ags) as they do to allo-MHC Ags in MLR. In addition, human-anti-pig T cell responses appear to share similar antigen-presenting cell (APC) requirements for stimulator (direct pathway) or responder (indirect pathway), and (2) the majority of the primary human-anti-pig xeno-response is directed toward porcine MHC class II Ags and involves interactions with human CD4 accessory molecules. These data taken together indicate that the human-anti-porcine T cell response is similar in strength and specificity to the allogeneic response [4].
1.2. The Importance of Inducing T Cell
Efforts to avoid humoral rejection have included the use of transgenic donors expressing complement-inhibitory proteins, such as human decay-accelerating factor (hDAF, hCD55) [5], CD59 (membrane attack complex inhibitor) [6], and human membrane cofactor protein (hMCP, hCD46) [7] as well as the elimination of Gal expression on pig cells. Despite the early success of these efforts to control hyperacute rejection (HAR), both severe delayed xenograft rejection (DXR) and acute cellular xenograft rejection (ACXR) have remained obstacles to reach long-term survival of solid organ xenografts. We have achieved life-supporting hDAF-to-baboon renal survival for up to 29 days using chronic immunosuppression [8]. We hoped that GalT-KO pigs [9] would further prolong the survival of life-supporting renal xenografts; however, GalT-KO pigs only led to minimal improvement in survival (34 days) using immunosuppressive regimens that included ATG and anti-CD154 mAb [10]. Additionally, Zhong and his colleagues reported maximum survivals of only 16 days [11] using GalT-KO kidneys. Even with the use of heavy immunosuppression in these protocols, rejected GalT-KO grafts showed severe cellular and humoral rejection. The glomeruli and peritubular capillaries of these rejected xenografts stained brightly for IgG. Additionally, investigators observed consistent aberrations in the complement cascade.
The development of cellular rejection in this model despite heavy chronic immunosuppression suggests that small numbers of T cells can initiate both cellular and humoral responses. These data are consistent with reports by Korsgren and colleagues which indicate that even very small numbers of T cells suffice to initiate rejection of porcine islets by macrophages in T cell-deficient rodents [12, 13]. The level of immunosuppression needed to control these T cell responses and prolong xenograft survival has been associated with prohibitive morbidity and mortality. More recently, Shin et al. reported that pig islets, engrafted >500 days in nonhuman primates, were fully rejected by activated immune cells, particularly CD4+ and CD8+ T cells, when immunosuppressive maintenance drugs were discontinued [14, 15]. Therefore, strategies directed at inhibition of the direct and indirect pathways must be included for successful xenotransplantation between pigs and primates.
1.3. Yamada’s Strategy to Overcome Xenogeneic T Cell Responses
Thymic tissue, as opposed to vascularized thymic grafts, was first demonstrated to induce xenograft tolerance in a pig-to-mouse transplant model by Sykes et al. Fetal thymic tissue was transplanted directly under the renal capsule in T cell-depleted thymectomized mice [16]. Their results in the pig-to-mouse model showed that murine recipients of porcine thymic tissue develop donor-specific unresponsiveness in mixed lymphocyte reaction (MLR) assays and subsequently accept skin grafts from the thymic donors [17,18]. In order to extend these results to our pig-to-primate model, it was first necessary to demonsttate that thymic transplantation was possible in the pig. However, our initial attempts to implant minced thymic tissue as in the mouse model failed, with evidence of graft rejection within 15–30 days in an allogeneic pig model [19]. We hypothesized that ischemic damage to the transplanted thymus during revascularization led to an active immunologic response and that thymic tissue should be transplanted as a vascularized organ. Furthermore, during the tenuous revascularization period, unless T cell depletion was complete (which is very difficult in large animals), thymic tissue was rejected before it had the ability to induce tolerance.
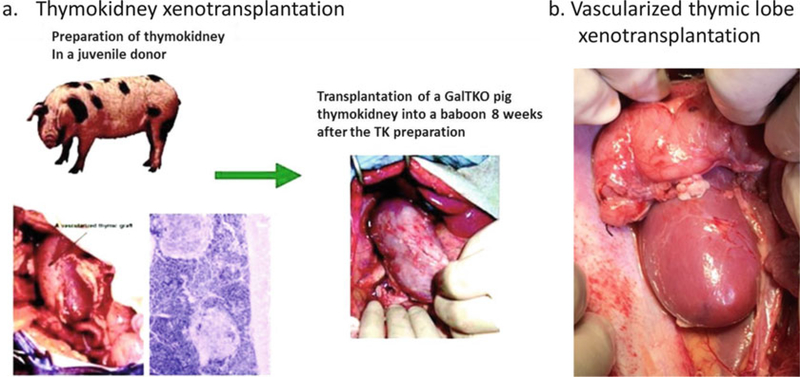
In order to eliminate the revascularization period following allogeneic or xenogeneic transplantation, Yamada developed two procedures for vascularized thymic transplantation in miniature swine. One involves the preparation of composite “thymokidneys” in which autologous thymic tissue is allowed to engraft for 1–2 months under the donor’s own kidney capsule before allogeneic transplantation [20–22] (see Fig. 1a) The other is the transplantation of an isolated vascularized thymic lobe (VTL) graft, or the so-called Yamada VTL procedure [23] (see Fig. 1b). Using these novel techniques, we demonstrated that vascularized thymic tissue induces tolerance and supports thymopoiesis across fully allogeneic barriers in MGH miniature swine [24]. In other experiments, when kidneys and non-vascularized thymic tissue from the same donor were transplanted as separate grafts, recipients rejected the non-vascularized allogeneic thymic grafts within 4 weeks, and kidney grafts were rejected within the second postoperative month. In contrast, recipients of thymus with simultaneous kidney (thymokidney), heart (heart plus VTL), or kidney following VTL transplant survived long term and demonstrated stable graft function and in vitro donor-specific unresponsiveness [21, 22, 24, 25]. Thus, the VTL-based approach can be used in combination with the described procedures for heart transplantation [26].
Fig. 1.
Schematic diagram of preparation of the thymokidney in a donor pig and the pig thymokidney xenotransplantation in a baboon (a). HE specimen and a gross thymokidney (left) at 12 weeks after preparation in a donor pig (left). Gross GalT-KO thymokidney transplanted in a baboon (right). Schematic diagram of preparation of the vascularized thymic lobe xenotransplantation (b)
Based on these encouraging results, our team has extended this vascularized thymic transplant strategy to a pig-to-baboon model of renal xenotransplantation. In initial studies, recipients of GalTKO kidneys and vascularized thymic grafts from the same donors maintained normal creatinine levels for up to 83 days, while GalT-KO kidneys without thymic grafts were rejected by 34 days [10], even with potent immunosuppression. In the past 5 years, further modification of the induction regimen facilitated survival of life-supporting thymokidneys for over 6 months [27, 28]. Notably, multiple recipients bearing functional thymokidneys for over 3 months developed T cells with the phenotype of new thymic emigrants (CD4+/CD31+/CD45+) as well as donor-specific unresponsiveness at the T and B cell levels. Because recipients were completely thymectomized prior to transplantation, these thymic emigrants most likely developed in the vascularized thymic grafts of the composite thymokidneys.
More recently, we have used hCD47/hCD55 transgenic GalT-KO pigs [29] as donors of VTL grafts. By expressing hCD47 on porcine thymic emigrants (which minimizes macrophage phagocytosis via the CD47-SIRP-alpha pathway), we found a CD4/CD8 double-positive population with a small number of CD4 or CD8 single positive cells in porcine VTL grafts in baboons, indicating active thymopoiesis as early as day 50 following VTL transplantation (Yamada K et al. manuscript in preparation).
The following section describes our method for kidney transplantation co-transplanted with a vascularized thymic graft in a pig-to-baboon model, in addition to helpful tips and pitfalls to avoid for this procedure.
2. Materials
2.1. Catheter Placement
A lightweight spandex/lycra jacket (Lomir Biomedical Inc., Malone, NY).
A rigid swivel that allows the animal to move freely in the cage without disturbing line connections.
A 1.0–1.5-m aluminum tether that attaches the swivel to the jacket.
Catheters, generally 1.5–2.0 m flexible, clear Tygon microbore tubing (Norton Performance Plastics, Akron, OH).
2.2. Thymectomy (Baboons) and VTL
Surgical telescopes (preferably × 3.5 or greater) for the primary assistant and the primary surgeon.
Surgical draping and instruments including scalpel, bone saw and/or heavy bone scissors, Weitlaner retractors, electrocautery, hemoclips, and bone wax as well as suture materials (PDS, Prolene, and silk ties).
Cotton swab applicators for gentle separation of the thymic tissue from the pericardium.
Anesthesia and mechanical ventilator.
Euro-Collins solution or UW solution.
2.3. Thymokidney, VTL, and Kidney
Surgical draping and surgical instruments are similar to those in clinical pediatric kidney transplantation, including scalpel, electrocautery, fine-tip/Castro needle drivers, ×2.5–4.5-magnification surgical loupes, 6-0 to 8-0 nonabsorbable surgical suture, Euro-Collins or UW solution, Balfour retractors, and sterile ice.
Anesthesia and mechanical ventilator.
2.4. Flow Cytometry to Assess T Cell and B Cell Depletion and Host Thymopoiesis in Porcine Thymic Grafts
Antihuman (cross-reactive to baboons) antibodies for CD3 (polyclonal rabbit antihuman CD3, A0452; Dako North America, Inc., Carpinteria, California, USA), CD4 (mouse antihuman CD4 mAb, 1F6; Invitrogen, Carlsbad, California, USA), CD8 (mouse antihuman CD8; BD Biosciences, Franklin Lakes, New Jersey, USA), CD20 (mouse antihuman CD20; BD Biosciences), and CD25 (mouse antihuman, BD Biosciences). All conjugated to a fluorochrome.
Anti-pig monoclonal antibodies (mAb, available in our research center): Anti-porcine CD1, CD2, CD3, CD4, CD8, CD25, CD45RA, Class I, Class II, PAA (produced internally). All conjugated to fluorochrome.
Conjugated anti-mouse IgG1 and anti-mouse IgG2a to be used as controls.
Cold FACS media: 1 L of Hanks’ balanced salt solution, 1 g of bovine serum albumin (BSA), 1 g of sodium azide.
FACS tubes.
2.5. Baboon Mixed Lymphocyte Reaction
Reagents for peripheral blood monocytes (PBMC) isolation: Hanks’ balanced salt solution (Life Technologies, Grand Island, NY, USA), Histopaque 1077 (Sigma-Aldrich, St. Louis, MO, USA), ACK lysing buffer (BioWhittaker, Maryland, MD, USA).
MLR medium: AIMV media (see Subheading 2 and Note 1).
MLR plate: 96-well flat-bottom plate (Costar, Cambridge, MA, USA).
Cell Proliferation Dye eFluor 670 (Invitrogen, Carlsbad, California, USA).
Antihuman (cross-reactive to baboons) antibodies, the same as was previously described (see Subheading 2.4).
FACS tubes.
Cold FACS media: 1 L of Hanks’ balanced salt solution, 1 g of BSA, 1 g of sodium azide.
2.6. Antibody/Complement-Mediated Cytotoxicity Assay
Target pig PBMC.
Medium 199 with 2% fetal bovine serum (FBS).
Decomplemented serum samples (56 °C for 30 min).
7-AAD (Sigma) diluted to 5 μg/mL in FACS media.
Rabbit complement (at a predetermined working dilution).
96-Well U-bottom plates and lids, non-sterile.
Combitips, combi-pipettor, micropipette tips, multichannel pipettor, Pasteur pipettes, micropipettor, truncated pipette tip, plastic trough.
37 °C, CO2 incubator.
Centrifuge.
FACS tubes.
Cold FACS media: 1 L of Hanks’ balanced salt solution, 1 g of BSA, 1 g of sodium azide.
Ice and ice bucket.
3. Methods
Though significant academic progress has been made, xenotransplantation has not yet become a clinical reality. Below is a brief introduction to the surgical and immunologic procedures compiled from the most successful pig-to-nonhuman primate renal transplantation protocol developed by Yamada.
3.1. Catheter Placement
At the authors’ institution, kidney xenotransplantation is conducted in life-supporting models, and a bilateral native nephrectomy is typically performed at the time of transplantation. Thus, daily laboratory values are required to monitor the animal’s health and graft function. For these reasons, the placement of central venous and arterial catheters or “lines” is required for animal care during the induction, peri-transplantation, and post-transplantation periods. Lines provide the caregiver with the ability to both deliver drug therapy and draw blood for diagnostic testing. This is of particular importance in baboons or monkeys because it is difficult to perform a physical examination beyond simple observation. Although central catheters confer a risk of infection, there is greater risk associated with sedation for daily or twice-daily blood draws and drug-administration, especially in potentially fragile post-xenotransplantation recipients.
The lines are utilized for (1) continuous blood pressure monitoring, (2) blood samples for CBC, blood chemistry, and drug levels, and (3) the number of drugs administered (continuous and/or intermittent) and use of different lines due to drug compatibility. We used to place three lines (two venous, one arterial) 1 week prior to transplantation. The arterial line is used primarily for blood draws, and the two venous lines are used for drug delivery. However, we have recently started placing only two venous lines with success in order to lessen the risk of infection by minimizing indwelling lines. We have found that continuous blood pressure monitoring is generally not needed once the animal is recovered from anesthesia, and blood draws can be performed from one of the two venous lines. Importantly, soluble MMF must be placed on its own dedicated D5%W carrier line and not mixed with normal saline.
The following is our procedure for placing central lines in the jugular veins as well as the carotid.
Generally, central lines are placed in the great vessels of the neck, on the left side.
Make a 3-cm transverse incision sharply, one fingerbreadth above the clavicle, starting from the midline and extending across the anterior border of the sternocleidomastoid (SCM).
Electrocautery and blunt dissection should be used to transect the platysma and isolate the external jugular (EJ) vein, the internal jugular (IJ) vein, and the carotid artery.
Make a small incision in the animal’s back (between shoulder blades), and tunnel the lines through the subcutaneous tissue and into the open neck wound.
Dissect the sternocleidomastoid and retract medially or laterally for access to the carotid sheath. The easier approach is to retract laterally.
Open the carotid sheath and isolate the carotid, and then tie off distally.
Place a small bulldog clamp on the vessel, 1 cm proximally.
Create an arteriotomy using tenotomy scissors and then cannulate the vessel.
Release clamps and advance the arterial catheter (see Note 3).
A similar procedure is performed for the IJ followed by the EJ (see Note 4).
Close the skin with buried subcuticular sutures.
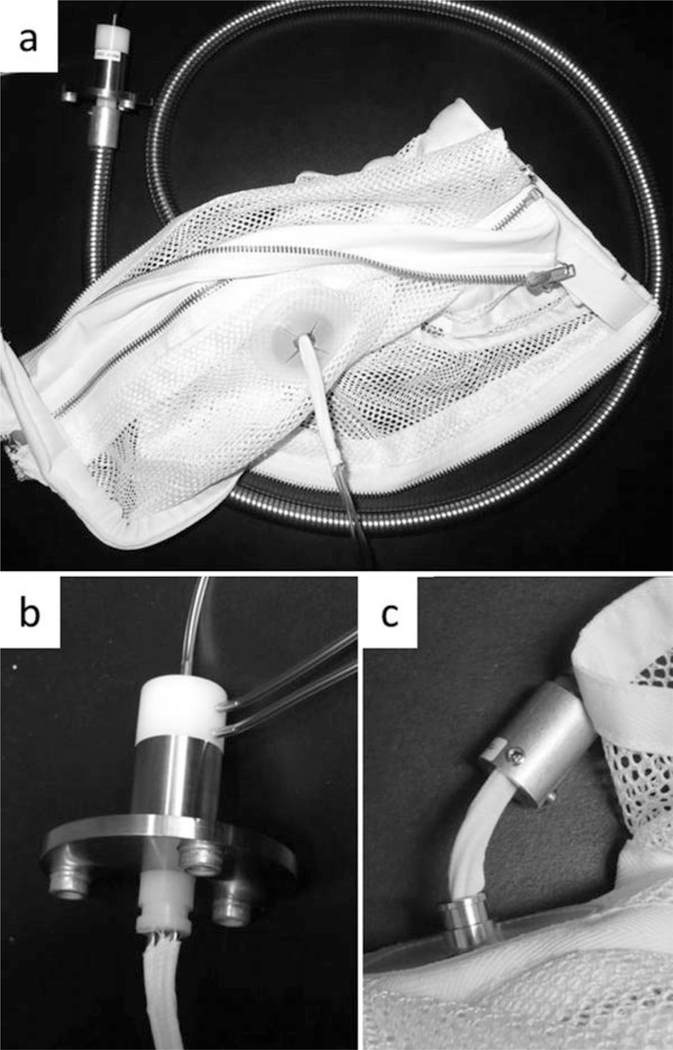
Place a protective line jacket on the animal in standard fashion (Fig. 2. See Note 5).
Fig. 2.
A picture of the line system used for baboon recipients of xenografting procedures. The mesh jacket is rigidly attached to an aluminum tether through which 1–3 catheters are placed. The tethers are 4–6 ft. in length. (b) A close-up view of the swivel shown in (a), which has been unattached from the aluminum tether. As many as three catheters are securely affixed to each of three ports on the swivel. This allows the animal to move within the cage without twisting the lines. (c) A close-up view of the connection between the jacket and the tether. In this image, the jacket has been unattached from the tether, showing the tapewrapped catheters traveling through the jacket and into the tethering system
3.2. Host Thymectomy and VTL Procurement from Donor Pigs (See Notes 6–9)
Our previous data in pig-to-mouse models have demonstrated that recipient thymectomy is required for the induction of xenogeneic tolerance [18, 24]. Three weeks prior to transplantation and 2 weeks prior to line placement, animals on the tolerance-induction (thymokidney) protocol undergo thymectomy.
3.2.1. Host Thymectomy
After intubating the donor pig, the animal is placed supine on the operating table.
First, expose the median pectoral groove using electrocautery, blunt dissection, and finger fracture.
Dissect the manubrium, exposing the jugular notch, and then perform a sternotomy (see Note 7).
Dissect and remove the thymic gland (see Note 8) with gentle traction using cotton swab applicators.
Weigh the excised thymus and take several pieces for histology and immunologic assays.
Test for air leak with approximately 5 mL of normal saline poured into the thorax. If no leak is observed, go to the next step. If an air leak is observed, repair with an absorbable suture.
Following meticulous hemostasis, close chest with interrupted 0-PDS sutures (see Note 9).
Before completely closing the sternum, ask the anesthesiologist to provide a sustained breath to fully expand the lungs. Again, assess for air leak with 3–5 mL of normal saline on the sternum to determine if a chest tube is required. If thymectomy is appropriately performed, it should be done in 60 min, and no chest tube is typically required.
3.2.2. VTL Procurement from Donor Pigs (Yamada’s Procedure) [23, 24]
The VTL harvest and VTL transplantation procedures are technically challenging. To improve the likelihood of success, the surgeon should have extensive experience with porcine thymectomy [30] as well as vascular surgical techniques.
To procure thymic lobe(s) from the donors, make a longitudinal cervical incision to expose the porcine cervical thymus [30].
Expose the pre-tracheal muscles and the connective tissue sheath overlying the thymus to provide access to the left and right lobes [23].
Carefully dissect the cervical thymus away from the connective tissue and ligate vessels that are not feeding or drainage vessels of the thymic lobes (see Note 10).
Excise thymic lobe with well-preserved feeding arteries and drainage veins and perfuse the excised thymic lobe with Euro-Collins solution.
3.3. Thymokidney, VTL, and Kidney Transplantations
3.3.1. Thymokidney Preparation in GalT-KO Pig Donors (Yamada’s Thymokidney Procedure) [20, 21]
The thymokidney model of xenotransplantation is the most successful life-supporting xenograft protocol for large animals [10]. In this model, donor-specific T cell tolerance to the donor graft is induced by co-transplantation of donor thymic tissue. The procedure for the preparation of a composite thymokidney was developed because transplantation of prevascularized thymic tissue helps to prevent revascularization-associated ischemia. For this reason, thymic tissue in this model is placed beneath the autologous native renal capsule in the donor (7–12-week-old juvenile miniature swine) to create a composite thymokidney 6–8 weeks prior to composite transplantation (see Note 11). During this time, the thymic tissue becomes vascularized by the autologous kidney. The prevascularized thymokidney graft is then transplanted as a composite kidney graft using the kidney transplantation procedure described below [20–22] (see Fig. 1a).
Excise cervical thymic lobe(s) (see Note 12) through a midline or 1-cm lateral neck incision [21].
Excise a cervical thymic lobe and mince the excised thymic tissue with Metzenbaum scissors or a scalpel on the back table (see Note 13), and then close cervical incision.
Expose the kidney using a retroperitoneal flank incision (right and left thymokidney preparation should be performed through two separate flank incisions to prevent abdominal adhesions as both kidneys will be procured in several weeks) and then place thymic tissue beneath the autologous renal capsule (Fig. 1a left. See Note 14).
Close the edge of the capsular window using electrocautery or with a single 7-0 Prolene suture.
Close flank incision in layers with 3-0 or 2-0 vicryl. Nylon can be used for pig skin.
3.3.2. Thymokidney Procurement from GalT-KO Pig Donors
The thymokidney procurement for pig-to-baboon xenotransplantation is similar to human kidney procurement. However, since the donor kidney contains thymus underneath the kidney capsule, it is necessary to preserve the donor’s perinephric fat. This is because local tissues may be adherent resulting from the thymokidney preparation. Careful manipulation and prudent use of electrocautery are required to avoid further damage to the fragile thymic tissue vascularized by the kidney.
A midline incision from xiphoid to pubis is used to provide access to the abdomen (see Note 15).
Expose thymokidneys (see above), and isolate the renal vein (RV) and artery (RA), transecting each vessel at the level of the aorta (Aa) (see Note 16).
Remove thymokidney(s) and flush via the RA on the back table using Euro-Collins or UW solution and immediately place on ice.
3.3.3. GalT-KO Thymokidney Transplantation to Baboons
Surgical thymokidney transplantation is generally performed using methods similar to those of pediatric kidney transplantation. Although primary kidney transplantation in pig-to-nonhuman primates may be described by other groups, the unique and complex nature of the procedures described in this chapter will help readers perform successful xenogeneic kidney transplantation. In addition, while many groups have experimented with xenotransplantation, the procedures developed by Dr. Yamada have resulted in both clinical (minimal acute tubular damages) and immunologic (extended graft survival) successes for our group. Because porcine kidneys are predisposed to vasoconstriction, surgeons should minimally manipulate the graft as described below (see Note 17). Each vascular anastomosis (renal vein and renal artery) should be done within 12–15 min, ideally under 10 min.
Make a midline abdominal incision from xiphoid to pubis.
Gain exposure using a Balfour retractor and retract the animal’s bowels to the left, allowing access to the right retroperitoneum.
For nephrectomy of the right kidney, focus interiorly and isolate the ureter. Ligate the ureter such that at least 50% of the native length remains attached to the bladder (this is done so that a future uretero-ureteral anastomosis can be performed if a technical complication arises).
Skeletonize the host renal vessels to the level of inferior vena cava (IVC) and the aorta (Aa). Then, ligate the RA using 3-0 silk ties followed by ligation of the RV.
Place hemoclips proximally.
For kidney transplantation (see Note 16), dissect the Aa and IVC such that the surgeon has sufficient access to allow placement of the vascular clamp and anastomosis (care is taken to avoid injuring the inferior mesenteric artery).
Heparinize the recipient with 70–100 IU/kg at least 2 min prior to beginning the transplant.
First, cross clamp recipient IVC with a Satinsky clamp (see Note 16). The venous anastomosis is done first because the RA will over lie the IVC following anastomosis (see Note 17).
Create venotomy with an 11-blade or Potts’ scissors.
Typically, place two stay sutures (one superiorly and one interiorly on the cava) and sew first the back wall, followed by the front wall with 7-0 Prolene (8-0 if baboons are <5 kg). Senior surgeons may prefer one running suture method. The venous anastomosis should be completed under 12 min.
Place a bulldog on the graft RV and gently release the Satinsky clamp from the recipient IVC, allowing venous blood to backfill the RV (see Note 18, important).
After clamping the Aa with a Satinsky clamp, use an 11-blade and Potts’ scissors to create an arteriotomy.
Perform the arterial anastomosis using 7-0 running Prolene (8-0 if baboons are <5 kg). The author highly recommends using a parachute (3 o’clock single knot) technique performed at least under 15 min as this minimizes manipulation of the graft and ischemic time (see Note 19).
Release the aortic Satinsky clamp. Obtain hemostasis using interrupted 7-0 Prolene sutures as needed if bleeding is encountered. In our experience with this technique, the kidney becomes pink and warm and produces urine within minutes of revascularization.
After confirming the graft is producing urine, perform a leftsided nephrectomy (see Note 20).
Perform the neo-ureterocystostomy using 2 or 3 running 7-0 PDS or Prolene sutures. Placement of a 3F or 4F stent is recommended (the stent can be removed after day 30).
Cystopexy can be performed if necessary (such as a short donor ureter).
Close the abdomen in 3–4 layers. Our practice is to close the peritoneum with absorbable suture, followed by closure of the fascia with a running permanent suture, and then closing the superficial fascia and skin with a running absorbable suture.
Wean off the ventilator and replace the jacket. The animal should be perched within 2 h of extubation. See Note 21 for animal care recommendations.
3.3.4. VTL Transplantation (Yamada’s VTL Procedure)
Like the VTL harvest, the VTL transplantation is technically challenging, and extensive experience with vascular surgical techniques will increase the likelihood of success. The team should practice with a pig-to-pig allogeneic model before applying these techniques to a pig-to-nonhuman primate model. The advantage of using the thymokidney is that the prevascularized thymus is transplanted simultaneously with the kidney. However, the VTL procedure can accompany the transplantation of any organ (including the kidney). In contrast to the prevascularized thymokidney, this procedure is more complex as the actual thymic lobe must be anastomosed to the recipient vessels. In theory, a co-transplanted thymic graft should confer tolerance to the recipient regardless of the transplanted organ (i.e., liver, heart, etc.). In an allogeneic model of VTL and simultaneous heart transplantation [25], the recipient becomes tolerant in a donor-specific manner. Although attempts to prepare thymohearts have been undertaken, these results have not been encouraging. One reason for failure of the thymoheart may relate to the anatomic challenges (limited space) beneath the pericardium. Thymoliver grafts have, as of yet, not been attempted. The VTL transplantation from the GalT-KO pig into baboons is typically completed using end-to-side anastomoses for the thymic artery and vein to the recipient animal Aa or IVC above the renal vessel anastomosis for simultaneous VTL and kidney transplantation (see Fig. 1b) or carotid artery and IJ for isolated VTL transplant, respectively [23, 24]. This is done in a similar manner to that of kidney transplantation (see above and Subheading 4). Either 7-0 or 8-0 Prolene should be used, and ×3.5 or greater (greater than ×4.5 for end-to-end) surgical loupes are recommended. See Note 21 for animal care recommendations.
3.4. Flow Cytometry to Assess for T Cell and B Cell Depletion and Host Thymopoiesis in Porcine Thymic Grafts
In order to assess T cell or B cell levels as well as host T cell development in the transplanted thymus (thymopoiesis), antihuman monoclonal antibodies (cross-reactive to baboon molecules) are used. Anti-porcine mAb are used to assess pig thymocytes in the thymic grafts after xenotransplantation. Some anti-porcine antibodies may not be commercially available.
Resuspend 1 × 106 PBMC or thymocytes in 100 μL of Hanks’ balanced salt solution containing 0.1% BSA and 0.05% sodium azide.
Incubate for 30 min at 4 °C with conjugated antibodies. Use conjugated anti-mouse IgG1 and anti-mouse IgG2a as controls for nonspecific binding.
After three washes, analyze cells by flow cytometry using the FSC-H/FSC-A Area Scaling to discriminate doublets.
3.5. Baboon Mixed Lymphocyte Reaction
The MLR assesses immunologic responses to the donor (mainly CD4 T cell responses against donor antigens on MHC class II) as compared to that against a third party to determine whether (1) the recipient is immunocompetent and (2) the recipient has developed a donor-specific response. Here, we describe bulk MLR assays using PBMC.
Isolate and resuspend responders and stimulators at 4 × 106 cells/mL in AIMV media (see Note 1).
For eFluor 670 labelling, prepare a 5 μM solution of eFluor 670 in room-temperature HBSS by adding 10 μL of 2.5 mM eFluor 670 stock to 5 mL of HBSS. This will be mixed 1:1 with the responder cell suspension.
After vortexing the cells, incubate for 10 min at 37 °C in the dark.
Stop the labeling by adding 1 mL of FBS and five times volume of cold AIMV media.
Incubate on ice for 5 min.
Wash twice with AIMV media.
Count the cells and resuspend labelling responder cells at 4 × 106 cells/mL in AIMV media.
Plate labelling responder cells at 4 × 105 cells/well with an equal volume of stimulators in a 96-well flat-bottom plate. All samples should be tested in triplicate.
Incubate in 5% CO for 5 days at 37 °C.
After 5 days of incubation, remove cells from wells by pipetting up and down into FACS tube.
Wash wells with HBSS and add to appropriate FACS tube.
Wash twice with FACS media.
Add conjugated niAb and incubate at 4 °C for 30 min.
Wash twice as previously described.
Resuspend cells in 400 μL of FACS media.
Acquire cells using a flow cytometer.
3.6. Antibody/Complement-Mediated Cytotoxicity Assay
The 7-AAD-dye-exclusion FACS procedure is designed for the titration of cytotoxic antibodies to cell surface antigens, using an antibody/complement reaction, followed by a dye exclusion assay and FACS acquisition. A similar procedure is used for SLA genotype testing of swine peripheral blood lymphocytes.
Process blood and count PBMC.
Prepare cell solutions of 5 × 106 cells/mL. The dilutions are made in Medium 199 (2% FBS, i.e., 10 mL of FBS and 490 mL of Medium 199). Keep the diluted suspensions on ice.
Identify the test panel and determine the appropriate dilutions for experimental serum samples. Also, decide which pig PBMC will be used as target cells. Typical setup involves serial dilutions of selected serum samples beginning at 1:2.
For sera serial dilutions, add 25 μL of Medium 199/2% FBS to each well of the 96-well round-bottom plate and 25 μL of each serum to the first well of each row, diluting the serum samples across the row using a multichannel pipette up to 1:256 dilution. Mix the dilutions of the samples by pipetting up and down five times.
You also need to prepare three control wells per target cell containing either media alone, the diluted rabbit complement, or both. These will act as the viability and complement background controls.
Add 25 μL of the appropriate cell suspension (pig target cells) to each well, including the complement and medium control wells.
Incubate the plate in the CO2 incubator for 15 min at 37 °C.
After incubation, add 125 μL of Medium 199/2% FBS to each well in the plate. Centrifuge at 260 × g for 5 min at 4 °C (brake on).
Dilute the complement to an appropriate (predetermined) dilution in Medium 199 (1:8, i.e., 0.5 mL of rabbit complement and 3.5 mL of M199).
Dispense 25 μL of the diluted complement into the appropriate wells.
Seal the plate with a plate sealer and shake the plate on the tray shaker for 30 s.
Incubate the plate in the CO2 incubator at 37 °C for 30 min.
Put the plate on ice.
Add 200 μL cold FACS media to all wells containing cells.
Transfer the contents of each well to the FACS tubes.
Add 5 μL of 7-AAD antibody to all tubes.
Place tubes either on ice or at 4 °C for 30 min.
Acquire cells using FACS machine looking at FSC vs. FL-3. The dead cell population will be stained with 7-AAD and be positive in FL-3.
Acknowledgments
We thank Ms. Haruna Shimizu for her editorial assistance. This research was supported by NIH grant (NIAID 5P01AI045897). All procedures and animal care were performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by Columbia University Medical Center.
Footnotes
For the MLR assay, use an AIM-V media which is serum-free. This is important because serum-based media are associated with higher levels of background stimulation, especially across a xenogeneic barrier.
The EJ should be tagged with a vessel loop. This is done because the vein is very small and frequently experiences vasospasm. To prevent visual obstruction, the EJ catheter is placed after the IJ vein and arterial catheters.
The arterial catheter should not be advanced more than 6 cm in a 6-kg baboon.
The IJ and EJ are low pressure vessels. After distal ligation, a proximal clamp is typically unnecessary prior to venotomy. Because anatomically, the EJ communicates with the subclavian vein at an acute angle, the EJ line is typically only advanced 1–2 cm. The catheters are tacked to the SCM, deep to the reapproximated platysma. This may help to avoid contamination if a wound infection (rare) occurs.
Catheter-related issues: If line infection occurs or the lines thrombose, they must be removed. In order to do so, the animal is taken to the OR for line removal through the same incision used for placement. Expect dense scar tissue around the lines, and extreme care must be taken to avoid puncture or damaging the lines during dissection, as this would introduce a potentially fatal air embolus or hemorrhage. If additional lines are required after removal, the femoral vessels can be used. In doing so, the femoral artery will need to be tied distally, which is tolerated remarkably well in baboons. Both ipsilateral femoral vessels can be used during a single line placement. In some cases, it may be necessary to subcutaneously tunnel a second venous line across the animal’s anterior pelvis. Two and sometimes three lines can be tunneled safely through the subcutaneous tissues over the animal’s back (at the level of L1). The longitudinal groin incisions, despite not being covered by the jacket, are rarely a problem. If needed, all four vessels (two femoral arteries and two femoral veins) can be ligated without causing clinically significant morbidity.
Thymectomy and VTL procurement are technically challenging and should be performed by an experienced surgeon.
A small finger (typically the fifth digit) is used to develop the plane between the sternum and the mediastinum, caudally. The same is then done from the xiphoid process, rostral. Once the plane from the manubrium to the xiphoid has been fully developed, electrocautery, a bone saw, and heavy scissors are used to perform a sternotomy. A heavy Weitlaner retractor is used to provide exposure.
In juvenile animals, the intact thymus is easily distinguished from fat by its texture and lighter color. Taking care to avoid damaging the pleura and pericardium, the thymus is gently dissected off the heart using cotton-swabbed applicators. Small but major vessels to the thymus are present at the superior aspect of the mediastinal thymus, which should be identified and clipped or ligated with 3-0 or 4-0 silks.
Insight following thymectomy: Prior to tying the last two sutures, the anesthesiologist is instructed to provide a sustained breath. If a lung injury is present, air bubbles will rise from the pooled mediastinal saline. Once no more bubbles are encountered, the chest closure is completed, and the animal is weaned from the ventilator as quickly as possible. This is important in case poor respiratory drive is encountered due to technical issues, allowing it to be dealt with prior to leaving the operating room. The chest is closed in three layers, and the skin is closed using an absorbable subcuticular suture.
The blood supply to the thymic lobes is variable through branches that typically arise from the subclavian artery opposite from the internal mammary artery. The thymic veins primarily drain into the native internal jugular vein. At the time of thymic lobe procurement, both the feeding arteries (subclavian or thymic) and the internal jugular vein are taken en bloc. In addition, the rostral thymus may have collateral blood flow from the carotid. In this case, the carotid artery is procured en bloc as well.
Fetal porcine thymic tissue has been shown to proliferate under mice renal capsules and subsequently induce tolerance. However, in our experience, when only several pieces of autologous porcine thymic tissue were placed subcapsularly, the tissue did not grow well. Because of poor fetal thymic availability, we chose to use juvenile pigs (7–12 weeks) for thymokidney preparation. In addition, we have observed poor growth of thymi from aged swine (>12 months old) when placed beneath the renal capsule. For this reason, juvenile swine (6–10 kg) are preferred [21].
Miniature swine, unlike baboons or humans, have a cervical thymic lobe in addition to the mediastinal thymus. The cervical thymus is ideal for thymokidney preparation or VTL transplantation because it is easily accessed using a longitudinal off-midline cervical incision. A single, fully excised cervical thymic lobe is typically required for the preparation of two thymokidneys. Thus, only half of a thymic lobe is sufficient for a single thymokidney.
Upon removal of the thymic tissue, the gland is minced prior to implantation. However, we have observed poor results when the thymus is minced too finely or when the thymus is injected subcapsularly using an angiocatheter. This is likely due to increased cell lysis and inflammatory changes.
The kidney is reached using a flank incision, and a capsular window is created using an 11-blade or angiocatheter. The tissue must be placed carefully under the renal capsule, taking care not to tear the capsule. The optimal amount of thymus to procure from the juvenile is typically related to the size of the kidney itself. Unpublished data from our laboratory has indicated that when only a few small pieces of thymus were implanted, subsequent growth of the transplanted thymus was insufficient. We have observed improved results when 40–60% of the superior surface of the kidney was covered with thymus [20].
If the recipient has a second kidney (or thymokidney) to be used at a later date, the animal must be recovered. Thus, in transecting the RA, care must be taken not to take much of a Carrel patch. Typically, 1–2 mm of aortic patch is sufficient for the recipient transplant. A small Carrel patch allows the surgeon to perform a primary longitudinal closure of the donor Aa using 6 or 7-0 Prolene suture. If the Carrel patch is larger, it will be necessary to close the Aa using a Dacron or Gore-Tex patch. Thereafter, the abdomen is closed in the standard threelayer fashion and the animal is recovered.
Transplantation should be performed by a skilled surgeon, and the vascular anastomosis should be completed within ideally 20 min, at most 30 min. If a young surgeon is performing the xenokidney transplant, an attending surgeon should be mindful of the anastomosis time and ensure that the graft is minimally manipulated.
It is important to note the blood pressure before and after clamping the IVC as hemodynamics may vary dramatically with clamping and unclamping the IVC. If blood pressure decreases, the distal Aa can be clamped using a bulldog to improve circulation.
Clamping the Aa will diagnose bleeding from the venous anastomosis. To avoid narrowing the venous anastomosis, sutures should not be tied prior to unclamping the vein. This will allow the RV to engorge prior to tying the final knot. If the surgeon is concerned about bleeding, sutures can be tied loosely prior to unclamping. The venous anastomosis should be completed quickly (under 15 min) and preferably within 10 min.
As porcine kidneys have a tendency to suffer vasoconstriction due to manipulation, the authors recommend an anastomotic approach that minimizes touching the xenograft. To do so, the surgeon should stand on the animal’s left side and be able to perform both the arterial and venous anastomoses quickly and skillfully. A large Carrel patch for the donor vessel is not recommended.
In our standard model of xenotransplantation, we generally remove both kidneys prior to xenograft transplantation. However, in some circumstances, if investigators are expecting an increased risk of graft rejection, the renal graft (pig) may be transplanted before or after the left kidney is removed. In some cases, such as the presence of high levels of preexisting anti-non-Gal antibody or if the transplant is expected to undergo early rejection, it may be beneficial to leave the contralateral native kidney and ligate the ureter. Removing the ligature will save the life of the animal to follow immunologic assessment even after removal of the rejected graft.
Animal care: Following line placement and prior to transplantation, the animals undergo a brief, but stringent induction using T cell and B cell depleting agents. These are generally monoclonal or polyclonal antibodies that are commercially available. Administration of any cellular depleting antibody should be preempted by flow-cytometric T and B cell subset phenotyping. T cell-depleting agents have been tested at multiple doses and at multiple time points; however, the general goal is to achieve near-complete T cell depletion (50–150 cells/μL) for the first 2 weeks which protects the transplanted thymic grafts in the induction period (Yamada, K. et al., manuscript in preparation). Because immunosuppression is extensive during the first 2 weeks, recipients are started on antibiotics to avoid opportunistic infection. Our standard regimen includes cefazolin, Levaquin, and ganciclovir. The animal’s WBC typically falls below 1000 cells/μL for the first week. While the animal is leukopenic, platelet counts are a good surrogate for WBC concerning infection. Clinically, we have observed that platelets fall in the setting of bacteremia, despite a very low WBC, in a potentially normal appearing animal. Like humans, immunosuppressed baboons may display no signs of sickness while immunosuppressed, despite active infection. For this reason, any clinically concerning signs (decreased appetite, activity, etc.) should prompt blood cultures and empiric widening of antibiotic coverage. Typically, coverage is increased to vancomycin and cefazolin is stopped. If there is concern for gram-negative infection, piperacillin tazobactam can be started. In these situations, it is best to consult an infectious disease expert.
Antibody/complement-mediated cytotoxicity assay: Rabbit complement is kept on ice to avoid deactivation. Appropriate dilution should be determined by using control naive porcine serum. To avoid background staining, 7-AAD should be stopped within 60 min.
References
- 1.Moses RD, Pierson RN 3rd, Winn HJ, Auchincloss H Jr (1990) Xenogeneic proliferation and lympholdne production are dependent on CD4 + helper T cells and self antigen-presenting cells in the mouse. J Exp Med 172(2):567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deschamps JY, Roux FA, Sai P, Gouin E (2005) History of xenotransplantation. Xenotransplantation 12(2):91–109. 10.1111/j.1399-3089.2004.00199.x [DOI] [PubMed] [Google Scholar]
- 3.Murray AG, Khodadoust MM, Pober JS, Bothwell AL (1994) Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity 1 (1):57–63 [DOI] [PubMed] [Google Scholar]
- 4.Yamada K, Sachs DH, DerSimonian H (1995) Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol 155(11):5249–5256 [PubMed] [Google Scholar]
- 5.Cozzi E, White DJ (1995) The generation of transgenic pigs as potential organ donors for humans. Nat Med 1(9):964–966 [DOI] [PubMed] [Google Scholar]
- 6.Mollnes TE, Fiane AE (2003) Perspectives on complement in xenotransplantation. Mol Immunol 40(2–4):135–143 [DOI] [PubMed] [Google Scholar]
- 7.Adams DH, Kadner A, Chen KH, Farivar RS (2001) Human membrane cofactor protein (MCP, CD 46) protects transgenic pig hearts from hyperacute rejection in primates. Xenotransplantation 8(1):36–40 [DOI] [PubMed] [Google Scholar]
- 8.Buhler L, Yamada K, Kitamura H et al. (2001) Pig kidney transplantation in baboons: anti-Gal (alpha)1–3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation 72(11):1743–1752 [DOI] [PubMed] [Google Scholar]
- 9.Kolber-Simonds D, Lai L, Watt SR et al. (2004) Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA 101 (19):7335–7340. 10.1073/pnas.0307819101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada K, Yazawa K, Shimizu A et al. (2005) Marked prolongation of porcine renal xenograft survival in baboons through the use of alphal,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11(1):32–34. 10.1038/nm1172 [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Qian H, Starzl T et al. (2005) Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med 11 (12):1295–1298. 10.1038/nm1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benda B, Karlsson-Parra A, Ridderstad A, Korsgren O (1996) Xenograft rejection of porcine islet-like cell clusters in immunoglobulinor Fc-receptor gamma-deficient mice. Transplantation 62(9):1207–1211 [DOI] [PubMed] [Google Scholar]
- 13.Sandberg JO, Benda B, Lycke N, Korsgren O (1997) Xenograft rejection of porcine islet-like cell clusters in normal, interferon-gamma, and interferon-gamma receptor deficient mice. Transplantation 63(10):1446–1452 [DOI] [PubMed] [Google Scholar]
- 14.Shin JS, Kim JM, Kim JS et al. (2015) Longterm control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant 15 (11):2837–2850. 10.1111/ajt.13345 [DOI] [PubMed] [Google Scholar]
- 15.Shin JS, Min BH, Kim JM et al. (2016) Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation 23(4):300–309. 10.1111/xen.12246 [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M (1996) Skin graft tolerance across a discordant xenogeneic barrier. Nat Med 2(11):1211–1216 [DOI] [PubMed] [Google Scholar]
- 17.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M (1999) Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol 162(6):3402–3407 [PubMed] [Google Scholar]
- 18.Rodriguez-Barbosa JI, Zhao Y, Barth R et al. (2001) Enhanced CD4 reconstitution by grafting neonatal porcine tissue in alternative locations is associated with donor-specific tolerance and suppression of preexisting xenoreactive T cells. Transplantation 72(7):1223–1231 [DOI] [PubMed] [Google Scholar]
- 19.Haller GW, Esnaola N, Yamada K et al. (1999) Thymic transplantation across an MHC class I barrier in swine. J Immunol 163 (7):3785–3792 [PubMed] [Google Scholar]
- 20.Yamada K, Shimizu A, Ierino F et al. (1999) Thymic transplantation in miniature swine. I. Development and function of the “thymokidney”. Transplantation 68(11)4684–1692 [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, Shimizu A, Utsugi R et al. (2000) Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol 164(6):3079–3086 [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Vagefi PA, Utsugi R et al. (2003) Thymic transplantation in miniature swine: III. Induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex-mismatched barriers. Transplantation 76(3):530–536. 10.1097/01.Tp.0000080608.42480.E8 [DOI] [PubMed] [Google Scholar]
- 23.LaMattina JC, Kumagai N, Barth RN et al. (2002) Vascularized thymic lobe transplantation in miniature swine: I. Vascularized thymic lobe allografts support thymopoiesis. Transplantation 73(5):826–831 [DOI] [PubMed] [Google Scholar]
- 24.Kamano C, Vagefi PA, Kumagai N et al. (2004) Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci USA 101 (11):3827–3832. 10.1073/pnas.0306666101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nobori S, Shimizu A, Okumi M et al. (2006) Thymic rejuvenation and the induction of tolerance by adult thymic grafts. Proc Natl Acad Sci USA 103(50):19081–19086. 10.1073/pnas.0605159103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postrach J, Bauer A, Schmoeckel M, Reichart B, Brenner P (2012) Heart xenotransplantation in primate models. Methods Mol Biol 885:155–168. 10.1007/978-1-61779-845-0_10 [DOI] [PubMed] [Google Scholar]
- 27.Tanabe T, Watanabe H, Shah JA et al. (2017) Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 17 (7):1778–1790. 10.1111/ajt.14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivard CJ, Tanabe T, Lanaspa MA et al. (2018) Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xenorenal transplantation–an experimental study. Transpl Int 31(10):1164–1177. 10.1111/tri.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tena AA, Sachs DH, Mallard C et al. (2017) Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 101(2):316–321. 10.1097/TP.0000000000001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada K, Gianello PR, Ierino FL et al. (1997) Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med 186(4):497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]