Abstract
Introduction
Neurofilament light (NFL) in cerebrospinal fluid (CSF) is elevated in neurodegenerative disease patients, and may track disease progression and treatment. Macaque monkeys are emerging as important translational models of neurodegeneration, and NFL may be a useful biomarker.
Methods
To determine the influence of a previous lumbar puncture (LP) on NFL, we collected CSF at multiple time points in macaque monkeys via LP or cisterna magna puncture. NFL, amyloid beta (Aβ40, Aβ42), and tau (tTau, pTau) in CSF were measured by standard enzyme‐linked immunosorbent assay and multiplex.
Results
NFL was significantly elevated at 14 to 23 days after an LP (median increase: 162%). Aβ and tau biomarkers remained stable. NFL peaked and decayed over 1 to 2 months after LP. NFL was not elevated after cisterna magna puncture.
Discussion
Results suggest damage of the cauda equina during LP may increase NFL. Caution should be taken in interpreting NFL concentration in studies in which repeat LPs are performed.
Keywords: amyloid beta, biomarkers, cerebrospinal fluid, cisterna magna puncture, lumbar puncture, neurofilament light, non‐human primate models, tau
1. INTRODUCTION
Neurofilaments form part of the neuronal cytoskeleton, are particularly prevalent in high‐caliber myelinated axons, and are released into the cerebrospinal fluid (CSF) when neurons are damaged or degenerating. 1 , 2 Neurofilament light (NFL) in CSF and blood has been proposed as a putative biomarker relevant to a wide variety of pathological conditions that involve breakdown of white matter, 3 including Alzheimer's disease (AD), 4 , 5 , 6 , 7 , 8 , 9 mild cognitive impairment risk, 10 frontal‐temporal dementia, 11 , 12 , 13 Parkinson's disease, 14 , 15 , 16 Huntington's disease, 17 amyotrophic lateral sclerosis, 18 and multiple sclerosis (MS). 19 , 20 , 21 NFL concentrations have also been compared across various disorders. 8 , 18 NFL is touted as a sensitive index of disease severity 12 and is being used to track the effects of therapeutics in clinical trials, especially in MS (clinicaltrials.gov).
Non‐human primates (NHPs) are increasingly being used as models of neurodegenerative diseases, 22 and there is a need to track CSF biomarkers in these models. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Similar to human patient trials, serial sampling of CSF in NHP models can be used to track disease progression and treatment effects, inferred by CSF biomarkers such as NFL, amyloid beta (Aβ), and tau. Understanding factors influencing biomarker concentration levels, such as the method of sampling, is important to biomarker interpretation. Studying the effect of repeated sampling via lumbar puncture (LP) in a controlled manner is difficult to do in humans; however, NHPs afford an opportunity to do so as they have similar anatomy and LPs are performed in a similar way. We tracked the CSF biomarkers NFL, Aβ40, Aβ42, and total and phosphorylated tau (t‐tau, p‐tau) after repeated LPs in rhesus and cynomolgus macaque monkeys. When a subsequent LP was performed, NFL was significantly elevated while other biomarkers remained stable. NFL concentrations required more than a month to return to baseline. This increase in NFL was not reliably observed when CSF was repeatedly sampled from the cisterna magna, suggesting the mechanism of the increase after LP may be due to local damage of cauda equina axons, rather than anesthesia or removal of CSF.
2. METHODS
All procedures were approved by the Queen's University Animal Care Committee and in compliance with the Canadian Council on Animal Care (Munoz, 2011‐039‐Or). Animals were maintained at the Centre for Neuroscience Studies at Queen's University (Kingston, ON, Canada) under the supervision of a lab animal technician and the Institute veterinarian.
2.1. Subjects
In total, 35 animals (19 rhesus macaques, 4‐15 years, 5.2‐16.9 kg, five females; 16 cynomolgus macaques, 3‐6 years, 3.0‐9.2 kg, two females) were used. For Figure 1, CSF was collected repeatedly via LP from a subset of 23 animals who were completely naïve to experimental central nervous system (CNS) procedures, including LP: 11 cynomolgus (4‐6 years, 5.0‐9.2 kg, one female) and 12 rhesus macaques (4‐10 years, 5.2‐16.9 kg, five females). After baseline CSF collection via LP, a second LP was performed 14 to 23 days later. One male cynomolgus was removed from t‐tau analysis as a sample was below detection limits; another male rhesus was removed from all biomarker analysis because his baseline NFL (>2000 pg/mL) was four standard deviations (SD) above the mean and flagged by Grubb's test as an outlier (P < .01).
FIGURE 1.
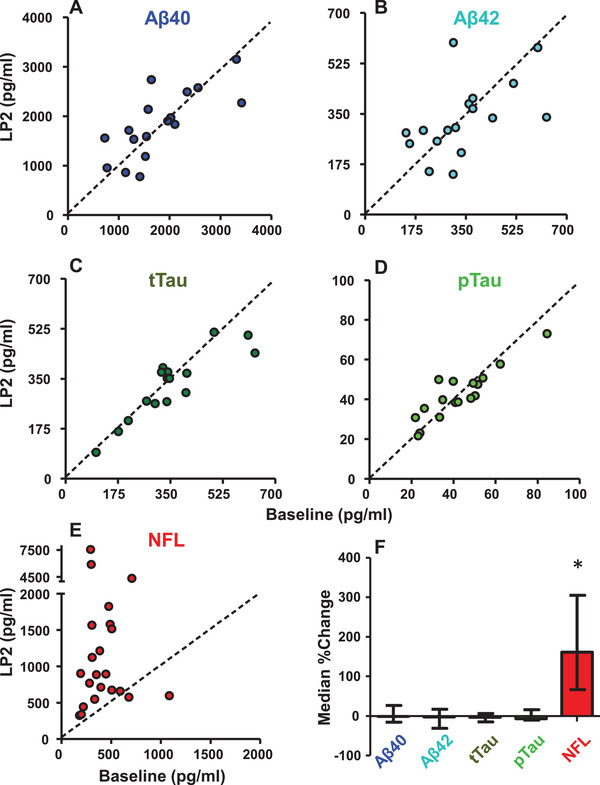
The effect of previous lumbar puncture (LP) on cerebrospinal fluid biomarkers. The value of each biomarker obtained from the baseline LP is plotted against the value obtained from an LP performed 14 to 23 days later. A, Amyloid beta (Aβ)40 (n = 16); B, Aβ42 (n = 16); C, t‐tau (n = 15); D, p‐tau (n = 16); and E, neurofilament light (NFL;n = 22, 11 rhesus and 11 cynos). F, The percent change in biomarkers from LP1 to LP2 (%change = [LP2‐LP1]/LP1*100) is plotted along with their median and interquartile range, revealing that NFL was significantly elevated by LP (P < .0002, Wilcoxon rank sum test, two‐tailed), but Aβ and tau biomarkers were not (all ps>0.17, paired t tests, two‐tailed). Most animals were only anesthetized with ketamine for both LPs (16/22), the rest also received briefly masked isoflurane for LP1 and three of those were under full isoflurane anesthetic via intubation for LP2.
HIGHLIGHTS
Lumbar puncture (LP) in monkeys increased neurofilament light (NFL), but not amyloid beta or tau, at 2 to 3 weeks.
Return of NFL concentrations to baseline after LP requires at least 1 to 2 months.
Axonal damage during LP may be the mechanism of NFL release.
Caution should be taken in interpreting NFL in invasive animal models.
Caution should be taken in interpreting NFL in patients with history of LP.
RESEARCH IN CONTEXT
Systematic review: A review of recent literature reveals neurofilament light (NFL) in cerebrospinal fluid as a promising biomarker of axonal degeneration in many neurodegenerative disorders, including Alzheimer's disease. We examined whether a previous lumbar puncture (LP) influenced NFL levels on subsequent LPs using macaque monkeys.
Interpretation: LPs in macaque monkeys elevate NFL, but not amyloid beta and tau, when sampled after 2 to 3 weeks. This elevation lasts at least 1 to 2 months depending on initial elevation. Repeat cisterna magna punctures did not elevate NFL, suggesting local cauda equina damage from LPs as a putative mechanism.
Future directions: Elevated NFL after LP is a potential confound for repeated LPs in preclinical primate models, and future research should be conducted to determine whether this confound translates to humans.
For Figure 2A, in three additional rhesus (13‐15 years, 11.2‐15.5 kg) and four additional cynomolgus (5‐6 years, 6.4‐7.2 kg), CSF was collected after various delays (30, 51, 58, 94, 118, 201, 365 days) longer than that for the animals from Figure 1 (14‐23 days), with one rhesus having a 3rd LP 365 days after the second. For Figure 2B, in a subset of three rhesus (4‐6 years, 5.2‐6.5 kg) and eight cynomolgus (5‐6 years, 6.1‐8.9 kg) macaques from Figure 1E, a third LP was performed 69 days later to determine whether biomarkers had returned to baseline levels. For Figure 2C, a single male rhesus macaque (11 years, 16.9 kg) was studied. For Figure 3A, six rhesus (6‐11 years, 7.6‐15.5 kg) and four cynomolgus macaques (3‐6 years, 3.0‐7.6 kg, one female) had two cisterna magna samples taken 15 days apart. Of these 10 animals, 7 had been given an LP in the previous year (typically 5 or more months prior to the baseline cisterna puncture) and the rest were experimentally naïve.
FIGURE 2.
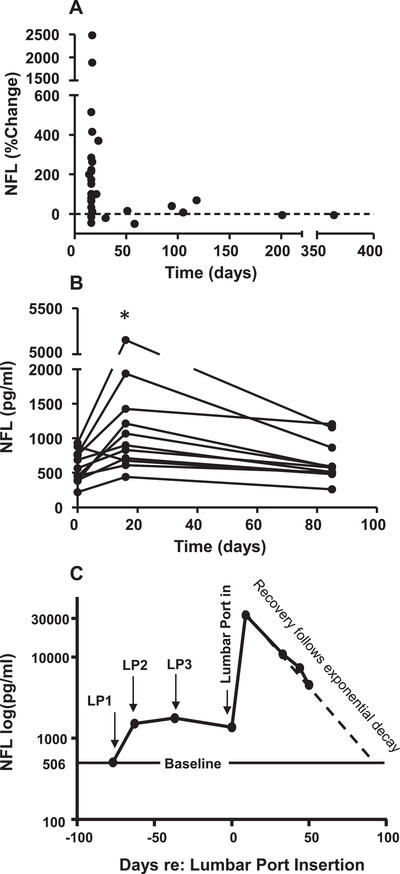
Timecourse of neurofilament light (NFL) elevation and decline after repeated lumbar punctures (LPs). A, The percent change in NFL from Figure 1E is plotted along with data from seven additional animals who had second LPs at longer delays, demonstrating that increases in NFL due to LP are not evident after 1 to 2 months delay. Values at 58, 118, 201, and 365 days of separation were obtained from animals under full isoflurane anesthetic via intubation, in addition to ketamine. B, Values of NFL are plotted from a subset of NHPs from Figure 1E (n = 11) who had a second LP after 16 days, and a third LP after another 69 days (85 days after original baseline). NFL concentrations were significantly elevated after 16 days (P < .03), and had decreased to near baseline levels after an additional 69 days. Most animals (8/11) had all LPs conducted under ketamine only. The others (3/11) received ketamine and masked isoflurane, with one under full anesthesia for LP2. C, In another animal, three LPs were performed (0, 14, and 26 days) and NFL was elevated after both the first and second LPs. Then, a lumbar port was implanted (requiring a fourth LP) to track the decline in NFL over time. NFL increased dramatically after lumbar port insertion, and then declined over 50 days after an exponential decay function (y = 50371e‐0.046x, R2 = 0.99) which predicted a return to baseline at 100 days. In addition to ketamine, this animal received brief masked isoflurane for LP1 and was under full isoflurane anesthetic via intubation for LP2 and lumbar port insertion. Samples after port insertion were taken with the animal awake in the home cage.
FIGURE 3.
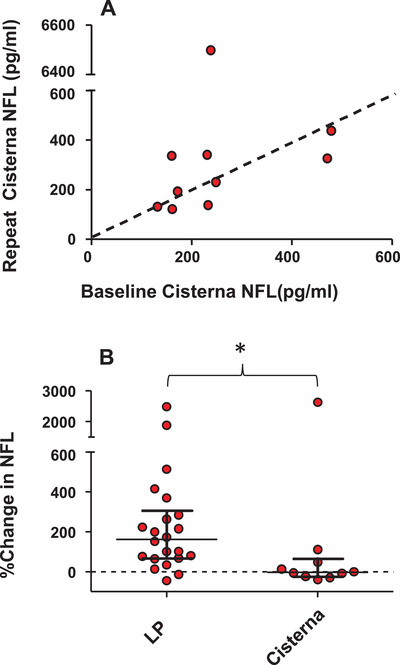
The effect of previous cisterna magna puncture on neurofilament light (NFL) concentration. A, The value of NFL obtained from the baseline cisterna magna puncture is plotted against the value obtained from a cisterna magna puncture performed 15 days later (n = 10, six rhesus and four cynomolgus). There is no significant change in NFL measured from repeat cisterna magna samples (P = .92, Wilcoxon rank sum test, two‐tailed). All animals were anesthetized using only ketamine. B, The median percent change in NFL for repeated lumbar punctures (14‐23 days apart) from Figure 1 is plotted next to the median percent change in NFL for repeated cisterna punctures (15 days apart). The change observed for repeated LPs is significantly greater than that observed for repeated cisterna punctures (P = .01, Mann‐Whitney U, two‐tailed).
2.2. CSF sampling and storage
A trained veterinarian or veterinary technician performed LPs and cisterna magna punctures with animals receiving the dissociative anesthetic ketamine (5‐15 mg/kg, intramuscular). When required to reduce movement, six animals were masked briefly (2‐5 minutes) with 2% isoflurane—an inhaled anesthetic. If additional procedures were planned (eg, magnetic resonance imaging [MRI]), full anesthesia was induced as described previously 30 (n = 10/98). Specifically, anesthesia was induced with ketamine (10 mg/kg, intramuscular) and diazepam (5 mg/kg, intramuscular). Glycopyrrolate (0.013 mg/kg, intramuscular) was given and the animal was intubated. Anesthesia was maintained using isoflurane (1%‐3%) and oxygen (2%).
To prepare for CSF sampling, the lumbar or cisterna area was shaved and cleaned using chlorhexidine, alcohol, and betadine. Depending on animal size, a 20 or 22 g Quincke spinal needle (BD™) was inserted into the intrathecal space between L4/5, or occasionally between L3/4 or L5/6 (n = 5/70). CSF was collected into a sterile 1.5 mL polypropylene tube (Thermo Fisher) and immediately kept on ice. For cisterna samples, a 23 g needle was typically used and CSF was drawn into a 1 mL (BD Luer‐Lok) polypropylene syringe. Any samples that were not clear (ie, tinted with a drop of blood contamination caused by needle insertion through tissue; n = 13/98) were centrifuged (1800 × g, 10 minutes, 4°C). Using data from the first LP or cisterna samples taken, we found that mean NFL concentration in blood contaminated samples (n = 6, 354 pg/mL) did not differ appreciably from clear samples (n = 26, 370 pg/mL, SD = 204). Samples with substantial blood contamination, suggestive of blood contamination in CSF flow—a very rare occurrence—were not taken and the sampling attempt was abandoned. Samples were typically aliquoted within 30 minutes and stored in 0.6 mL tubes at −80°C.
2.3. Lumbar port surgery and sampling
In one rhesus macaque, an indwelling lumbar port and catheter was implanted following a previously published protocol. 31 The animal was sedated with ketamine (10 mg/kg, intramuscular) and diazepam (5 mg/kg, intramuscular). Glycopyrrolate (0.013 mg/kg, intramuscular) was given and the animal was intubated. Anesthesia was maintained using isoflurane (1%‐3%) and oxygen (2%). The lumbar area was shaved and cleaned using chlorhexidine, alcohol, and betadine. An incision was made over the vertebral spaces between L4 and L5. A Tuohy epidural needle (17 g, CMD) was used as an introducing needle, and a 3‐french hydromer‐coated catheter (Access Technologies, Skokie, Illinois) was inserted through the introducing needle ≈13 cm into the intrathecal space. The catheter had x‐ray opaque markings and placement of the catheter was verified using x‐ray. The catheter was attached to a MIN LoVol port (15 μl volume, Access Technologies, Skokie, Illinois), which was then sutured to the muscle. Upon verification of port and catheter patency by drawing CSF through the port, the skin was sutured and the animal was recovered. Subsequent port sampling occurred cage‐side, with the awake animal trained to present the port. A non‐coring Huber needle (22‐25 g, Access Technologies, Skokie, Illinois) was inserted through shaved and cleaned skin into the reservoir and CSF was extracted with a 1cc polypropylene syringe and aliquoted as described above. The lumbar port was an important refinement because it allowed us to repeatedly sample CSF (for 50 days) without anesthesia, and without repeated puncturing of the lumbar area. The dead volume of CSF in the port was <20 μl and typically a 300 μl sample was obtained. While the dead volume was not discarded, it represented <7% of the sample volume so was unlikely to substantially influence the NFL concentration. Further, because the procedure was consistent for all samples, any error was constant.
2.4. CSF biomarker analysis
CSF samples were thawed in a biological safety cabinet just before analysis. To measure NFL concentration, a commercial sandwich enzyme‐linked immunosorbent assay (ELISA; NF‐light ELISA kit, Uman Diagnostics, Umeå, Sweden) was performed according to the manufacturer's instructions. Concentrations of Aβ40, Aβ42, p‐tau (pThr181), and t‐tau were determined using a MILLIPLEX Human Amyloid Beta Tau Magnetic BeadPanel (HNABTMAG‐68K, EMD Millipore, Billerica, MA, USA) completed on a Biorad Luminex platform according to the manufacturer's instructions. Within plate and interplate coefficients of variation were <5% and <15% for NFL; and <10% and <15% for the multiplex, respectively. Repeat samples collected from a given animal were run together on the same plate, except for two in Figure 2A (long delays).
2.5. Data/statistical analysis
Statistical analyses were performed in GraphPad Prism 5.01. A paired t test (two‐tailed) was used to test if biomarker values differed on the second LP or cisterna puncture. A P value of <.05 was considered significant. If the samples failed the Kolmogorov‐Smirnov test for normality of data, which only occurred for the second sample of NFL values for both LP and cisterna punctures, a Wilcoxon signed‐rank test for paired samples was conducted. For comparison of the percent change in NFL values obtained by LP and cisterna magna the Mann‐Whitney U test for independent samples was conducted as the samples were not normally distributed.
Anonymized data will be shared by request from any qualified investigator.
3. RESULTS
3.1. Lumbar puncture elevated CSF concentration of NFL, but not Aβ or tau biomarkers
We conducted a controlled study in which CSF was collected via LP in 23 experimentally naïve rhesus (n = 12) and cynomolgus (n = 11) macaques of similar age to obtain a baseline CSF sample, and then a second sample was collected via LP 14 to 23 days later. No other procedures were performed during this time interval. No animals were observed to have any sensory or motor effects after LP. Thus, any differences between the biomarker values in the CSF were attributable to the original LP sampling event. NFL was measured from samples at these two time points in 23 animals (one excluded as an outlier, see Methods); Aβ40, Aβ42, t‐tau, and p‐tau were measured in 16 of these animals. The means, standard deviations, medians, and interquartile ranges of these are provided in Table 1 and the individual datapoints are provided in scatter plots in Figure 1A‐E. A previous LP resulted in a mean increase in NFL of 348% (t[21] = 2.88, P = .009, paired t test, two‐tailed) but no change in Aβ40 (P = .75), Aβ42 (P = .75), t‐tau (P = .17), and p‐tau (P = .95). Because the second sample of NFL values was not normally distributed, we repeated the analysis using non‐parametric statistics to avoid bias from some extremely high values. The median percentage increase in NFL was 162% (Figure 1F) and a Wilcoxon rank sum test revealed this increase to be a highly significant (P < .0002, two‐tailed). That Aβ did not change during this repeat sampling timescale is consistent with a previous report using human trial data. 32 However, the timescale of any increase or decline of Aβ and tau may have occurred during the first days after LP.33 To test if there was any difference between cynomolgus and rhesus macaques in these effects, we compared the percent change in biomarker values after LP, and observed no species difference for any of the biomarkers (independent samples t test, two‐tailed, all p's>0.08). For NFL we confirmed the lack of species difference with a non‐parametric test (Mann‐Whitney for independent samples, P = .55).
TABLE 1.
Summary statistics for CSF biomarkers obtained from two LPs taken 14 to 23 days apart
| LP1 mean (SD)pg/mL | LP2 mean (SD)pg/mL | LP1 median (IQR)pg/mL | LP2 median (IQR)pg/mL | Mean % change(SD) | Median % change(IQR) | |
|---|---|---|---|---|---|---|
| Aβ40 | 1796.54 (772.52) | 1837.89 (678.49) | 1580.37 (977.15) | 1833.81 (1023.85) | 9.59 (39.05) | 0.72 (47.03) |
| Aβ42 | 341.59 (140.41) | 331.42 (127.28) | 314.21 (171.78) | 301.7 (143.49) | 6.18 (44.88) | −1.49 (55) |
| t‐tau | 350.63 (140.48) | 326.77 (114.66) | 338.49 (127.36) | 351.43 (120.25) | −4.9 (14.22) | −4.6 (19.74) |
| p‐tau | 42.32 (16.12) | 42.21 (12.68) | 40.87 (21.36) | 40.51 (16.36) | 3.74 (21.20) | −6.02 (26.69) |
| NFL | 419.27 (210.36) | 1586.95 (1878.25) | 369 (216.75) | 888.5 (979) | 347.74 (617.28) | 162.19 (238.88) |
Abbreviations: Aβ, amyloid beta; CSF, cerebrospinal fluid; IQR, interquartile range; LP, lumbar puncture; NFL, neurofilament light; SD, standard deviation.
For most animals (n = 16/22) CSF was collected exclusively under ketamine—a dissociative anesthetic—for both LPs. The six other animals were additionally masked with isoflurane for a few minutes to reduce movement, and three of those were under full anesthetic procedures (see section 2.2) for the second LP, because additional procedures were performed after the LP. We repeated our analyses using only those animals exclusively anesthetized with ketamine. The effect of the previous LP on NFL concentration remained strong, with a mean percentage increase of 421% (t[15] = 2.7, P = .017, paired t test, two‐tailed). The median percentage increase remained at 162% (P = .0006, Wilcoxon rank sum, two‐tailed). Those animals receiving ketamine and masked isoflurane on the first LP showed a percentage increase in NFL of 153%. Thus, exposure to isoflurane did not contribute to the increased NFL concentration from the second LP.
3.2. How long did NFL remain elevated?
Figure 2A illustrates how the percentage change in NFL values persisted after LP, including seven additional animals with delays longer than 30 days between LPs. For several of these animals CSF was sampled under full anesthetic procedures (see figure caption). For delays longer than 30 days there were only small, inconsistent changes in NFL values (Figure 2A). This suggested that the increase in NFL we observed at 2 to 3 weeks was followed by a decline over time back to baseline levels. To test this in a controlled fashion, in a subset of the animals (n = 11) who had all received second LPs at 16 days (from Figure 1), a third LP was performed after 69 additional days to determine whether enough time had passed for NFL to return to baseline. The samples of NFL values from the first, second (16 days later), and the third LP (69 days later) were normally distributed with mean (+SD) values of 591.5 (229), 1361.6 (1327), and 657.7 (295) pg/mL, respectively. NFL concentration was elevated at 16 days, and had declined to baseline levels for some but not all animals after an additional 69 days (Figure 2B). A one‐way repeated‐measures analysis of variance was performed revealing a significant main effect (F[2,20] = 4.34, P = .027). Paired comparisons with Tukey honestly significant difference (HSD) test revealed that there was a significant increase from baseline to day 16 (P < .01), a marginally significant decrease between day 16 and 69 (P < .06), and no difference between baseline and 69 days after the second LP (P = .97).
In an additional animal, we further examined NFL in CSF obtained from repeated LPs (14, 26, and 37 days separations), and confirmed NFL remained elevated relative to baseline with repeated sampling (Figure 2C, left). We then inserted a lumbar port to allow for chronic sampling without further LPs or anesthetic events. Insertion of the port resulted in a dramatic elevation in NFL (>2000%). This is consistent with a report that NFL was highly elevated after the insertion of lumbar ports in cynomolgus macaques, 24 and only declined by about 100 days after port insertion. We tracked the subsequent decline in NFL by taking repeated port samples in the absence of anesthesia for 50 days (Figure 2C, right). Consistently, NFL declined after an exponential decay function that predicted a return to baseline by 100 days.
3.3. Is damage of cauda equina axons the mechanism of NFL increase?
Possible mechanisms of the NFL increase we observed after LP could include damage to the NFL‐rich axons 1 of the cauda equina, some consequence of the anesthesia 34 required for LP sampling in monkeys, or of the removal of a bolus of CSF from circulation. One way to elucidate this is to examine whether a previous cisterna magna sample affects NFL values obtained from a subsequent cisterna magna sample. Sampling from the cisterna magna is not impeded by nerve roots in the way that an LP may be. In a group of animals (n = 10), we obtained a CSF sample via puncture of the cisterna magna using the same protocols required for most LP sampling (ie, ketamine anesthesia), thereby controlling for any effects due to anesthetic procedure or the removal of a volume of CSF. In Figure 3A, NFL values measured from the first cisterna magna sample are plotted against the value obtained from a cisterna magna sample performed 15 days later. In contrast to repeated LP sampling, a previous cisterna sample (mean = 252, SD = 124) did not result in a consistent increase in NFL on the second cisterna puncture (mean = 875, SD = 1979; t[9] = 0.99, P = .35, two‐tailed). Because the data were not normally distributed, they were also analyzed non‐parametrically. The medians for the two cisterna samples were 232 and 278 pg/mL, and the median percentage change was −3.94%. A Wilcoxon rank sum test revealed no significant difference (P = .92, two‐tailed). Comparing the effect of repeat LP (median % change = 162%) with repeat cisterna magna puncture (median % change = −3.94%) using a Mann‐Whitney U test for independent samples revealed that the increase with repeated LPs was significantly greater than that for repeated cisterna magna punctures (P = .01, two‐tailed, see Figure 3B). In summary, there was no consistent change in NFL with repeat cisterna magna puncture suggesting that the increase in NFL with repeat LP sampling was more likely due to local damage in the cauda equina, rather than ketamine anesthesia or removal of CSF. Note that while increases in NFL have been associated with general anesthesia in human patients undergoing surgical procedures, 34 most of the patients in that study also received spinal anesthesia (ie, a LP). Indeed, when general anesthesia was induced with sevoflurane in the absence of any surgical procedure, plasma NFL actually decreased over 5 hours. 35
4. DISCUSSION
When measured 2 to 3 weeks after an initial LP, NFL was highly elevated and Aβ and tau biomarkers did not change. While we observed a dramatic increase in NFL at time of measurement, the peak increase likely occurred earlier than that, and our subsequent experiments revealed that it may take 1 to 2 months or more for NFL to return to baseline, depending on the degree of elevation. Our final experiment shed insight on the mechanism underlying the increase in NFL after LP. We observed no increase in NFL when CSF was obtained by cisterna magna puncture, which is not impeded by nerve roots as is LP. This suggests that the increase in NFL after LP was not caused by ketamine or removal of a bolus of CSF (held constant in both experiments), but more likely by nerve root damage in the cauda equina.
4.1. Implications for non‐human primate models
These results reveal a major limitation in the use of NFL as a biomarker in preclinical animal models of neurological disease, in which invasive CNS procedures are routinely performed. In our experience, any invasive CNS procedure, such as insertion of catheters into the lateral ventricle, 30 , 36 insertion of electrodes into brain tissue, 37 insertion of lumbar ports, and even simple atraumatic LPs can increase NFL concentrations. When using NHP models, caution should be taken in interpreting NFL when repeated LPs are performed to track disease progression or treatment effects. Increases in NFL due to LP could artificially inflate disease progression or might mask reductions in NFL due to treatment. Indeed, in a recent study tracking NFL levels during induction of an NHP model of AD, 23 NFL concentrations reported were extremely elevated at baseline by almost an order of magnitude compared to our baseline levels, suggesting the cause was due to invasive procedures prior to first sample. Our results also confirm those reported by Barten et al. 24 that insertion of a lumbar port results in an extreme elevation in NFL. Consistent with that study, we found that the return to baseline NFL levels can take up to 100 days, reflecting the long half‐life of neurofilament in the brain.
For studies using NFL as a biomarker, cisterna magna puncture might be a better choice over LP to obtain CSF to avoid this confound. Alternatively, moving to blood based measures of NFL is advisable. Replicating our results in blood using SIMOA is a critical future direction. However, given that NFL concentrations in blood and CSF are highly correlated, 38 even blood samples would be affected by a previous LP or invasive neurological procedures.
4.2. Potential implications for human studies
There are two published studies in which NFL was analyzed after repeat LPs in humans; however, they are difficult to interpret. In one, there was no difference in NFL values taken about 2 weeks apart, but patients were undergoing electroconvulsive therapy; 39 in the other, the data were not presented in a way that the reader can determine the order of sampling. 40 A recently published re‐analysis of this later study demonstrated that repeat sampling (3 days apart) resulted in elevated Aβ and tau, but not NFL. 33 This suggests: (1) LPs in NHP models may cause more damage than LPs in humans, suggesting a problem with translating the use of NFL between NHP models and the human studies; (2) the failure to observe an NFL increase was biased by the small sample size in that human study; (3) the NFL increase takes longer to manifest than the 3 days between samples taken in that study.
We strongly recommend that the influence of LP on NFL levels receive further study in humans, especially in older individuals, because, should our results translate, this potential confound could have serious implications for human trials in which NFL is used as a biomarker and repeat CSF sampling via LP is conducted. Further, patient groups are more likely than controls to have had a previous LP for diagnostic workup prior to enrollment in studies, which could inflate patient‐control differences in NFL.
CONFLICTS OF INTEREST
The authors have no financial disclosures to declare.
ACKNOWLEDGMENTS
This work was funded by a Canadian Institute for Health Research (CIHR) Foundation Grant [#MOP‐FDN‐148418] to DPM; Brain Canada Project Grant to DPM, RL, DJC; CIHR Fellowship to RGW; and a Canada Research Chair Program to DPM.
Boehnke SE, Robertson EL, Armitage‐Brown B, et al. The effect of lumbar puncture on the neurodegeneration biomarker neurofilament light in macaque monkeys. Alzheimer's Dement. 2020;12:e12069 10.1002/dad2.12069
Susan E. Boehnke and Emma L. Robertson: These authors contributed equally to the manuscript.
Contributor Information
Susan E. Boehnke, Email: susan.boehnke@queensu.ca.
Douglas P. Munoz, Email: doug.munoz@queensu.ca.
REFERENCES
- 1. Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb Perspect Biol. 2017;9:a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27‐65. [DOI] [PubMed] [Google Scholar]
- 3. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577‐589. [DOI] [PubMed] [Google Scholar]
- 4. Rosén C, Hansson O, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer's disease – current concepts. Mol Neurodegener. 2013;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattsson N, Insel PS, Palmqvist S, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO Mol Med. 2016;8:1184‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattsson N, Andreasson U, Zetterberg H, et al. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta‐analysis. Lancet Neurol. 2016;15:673‐684. [DOI] [PubMed] [Google Scholar]
- 8. Olsson B, Portelius E, Cullen NC, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019;76:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weston PSJ, Poole T, Ryan NS, et al. Serum neurofilament light in familial Alzheimer disease. Neurology. 2017;89:2167‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kern S, Syrjanen JA, Blennow K, et al. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol. 2019;76:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landqvist Waldö M, Frizell Santillo A, Passant U, et al. Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol. 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skillbäck T, Farahmand B, Bartlett JW, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945‐1953. [DOI] [PubMed] [Google Scholar]
- 13. Rohrer JD, Woollacott IOC, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansson O, Janelidze S, Hall S, et al. Blood‐based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88:930‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical parkinsonian disorders. Parkinsonism Relat Disord. 2010;16:142‐145. [DOI] [PubMed] [Google Scholar]
- 16. Lin Y‐S, Lee W‐J, Wang S‐J, Fuh J‐L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep. 2018;8:17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrne LM, Rodrigues FB, Blennow K, et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington's disease: a retrospective cohort analysis. Lancet Neurol. 2017;16:601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosengren LE, Karlsson J‐E, Karlsson J‐O, Persson LI, Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 2002;67:2013‐2018. [DOI] [PubMed] [Google Scholar]
- 19. Burman J, Zetterberg H, Fransson M, Loskog AS, Raininko R, Fagius J. Assessing tissue damage in multiple sclerosis: a biomarker approach. Acta Neurol Scand. 2014;130:81‐89. [DOI] [PubMed] [Google Scholar]
- 20. Novakova L, Axelsson M, Khademi M, et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler J. 2017;23:62‐71. [DOI] [PubMed] [Google Scholar]
- 21. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89:2230‐2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Dam D, De Deyn PP. Non human primate models for Alzheimer's disease‐related research and drug discovery. Expert Opin Drug Discov. 2017;12:187‐200. [DOI] [PubMed] [Google Scholar]
- 23. Beckman D, Ott S, Donis‐Cox K, et al. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc Natl Acad Sci U S A. 2019;116:26239‐26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barten DM, Cadelina GW, Weed MR. Dosing, collection, and quality control issues in cerebrospinal fluid research using animal models. Handb Clin Neurol. 2018;146:47‐64. [DOI] [PubMed] [Google Scholar]
- 25. Darusman HS, Sajuthi D, Kalliokoski O, et al. Correlations between serum levels of beta amyloid, cerebrospinal levels of tau and phospho tau, and delayed response tasks in young and aged cynomolgus monkeys (Macaca fascicularis). J Med Primatol. 2013;42:137‐146. [DOI] [PubMed] [Google Scholar]
- 26. Darusman H, Pandelaki J, Mulyadi R, et al. Poor memory performance in aged cynomolgus monkeys with hippocampal atrophy, depletion of amyloid Beta 1‐42 and accumulation of tau proteins in cerebrospinal fluid. In Vivo (Brooklyn). 2014;28:173‐184. [PubMed] [Google Scholar]
- 27. Yue F, Lu C, Ai Y, Chan P, Zhang Z. Age‐associated changes of cerebrospinal fluid amyloid‐β and tau in cynomolgus monkeys. Neurobiol Aging. 2014;35:1656‐1659. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Q, Lu J, Yao Z, et al. Upregulation of Aβ42 in the Brain and Bodily Fluids of Rhesus Monkeys with Aging. J Mol Neurosci. 2017;61:79‐87. [DOI] [PubMed] [Google Scholar]
- 29. Latimer CS, Shively CA, Keene CD, et al. A nonhuman primate model of early Alzheimer's disease pathologic change: implications for disease pathogenesis. Alzheimer's Dement. 2019;15:93‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forny‐Germano L, Lyra e Silva NM, Batista AF, et al. Alzheimer's disease‐like pathology induced by amyloid‐β oligomers in nonhuman primates. J Neurosci. 2014;34:13629‐13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacAllister RP, Lester McCully CM, Bacher J, et al. Minimally invasive lumbar port system for the collection of cerebrospinal fluid from rhesus macaques (Macaca mulatta). Comp Med. 2016;66:349‐352. [PMC free article] [PubMed] [Google Scholar]
- 32. Lucey BP, Gonzales C, Das U, et al. An integrated multi‐study analysis of intra‐subject variability in cerebrospinal fluid amyloid‐β concentrations collected by lumbar puncture and indwelling lumbar catheter. Alzheimer's Res Ther. 2015;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olsson M, Ärlig J, Hedner J, Blennow K, Zetterberg H. Repeated lumbar punctures within 3 days may affect CSF biomarker levels. Fluids Barriers CNS. 2019;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K. Association of Changes in Plasma Neurofilament Light and Tau Levels With Anesthesia and Surgery: Results From the CAPACITY and ARCADIAN Studies. JAMA Neurol. 2018;75:542‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deiner S, Baxter MG, Mincer JS, et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery [published online ahead of print, 2020 Jun 11]. Br J Anaesth. 2020;S0007‐0912(20)30339‐1. 10.1016/j.bja.2020.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Batista AF, Forny‐Germano L, Clarke JR, et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non‐human primate model of Alzheimer's disease. J Pathol. 2018;245:85‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Constantinescu R, Holmberg B, Rosengren L, Corneliusson O, Johnels B, Zetterberg H. Light subunit of neurofilament triplet protein in the cerebrospinal fluid after subthalamic nucleus stimulation for Parkinson's disease. Acta Neurol Scand. 2011;124:206‐210. [DOI] [PubMed] [Google Scholar]
- 38. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in hiv infection: a cross‐sectional study. EBioMedicine. 2016;3:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zachrisson OCG, Balldin J, Ekman R, et al. No evident neuronal damage after electroconvulsive therapy. Psychiatry Res. 2000;96:157‐165. [DOI] [PubMed] [Google Scholar]
- 40. Olsson M, Ärlig J, Hedner J, Blennow K, Zetterberg H. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer's disease. Sleep. 2018;41:1‐8. [DOI] [PubMed] [Google Scholar]


