Synopsis:
Chronic lung disease is a frequent complication of primary antibody deficiency (PAD) associated with significant morbidity and mortality. The manifestations of lung disease in PAD are numerous and may or may not be related to infection depending upon the circumstance. Accordingly, thoughtful application of diagnostic approaches is imperative to accurately identify the form of lung disease present. While much of the treatment approach employed is adapted from immunocompetent populations, recent genomic and translational medicine advances have led to treatments that are more specific than ever for PAD patients. As chronic lung disease has continued to affect PAD patients despite immunoglobulin replacement therapy and antibiotics, it is the hope that continued advancements in our understanding of pulmonary pathology will ultimately lead to effective methods that alleviate impact upon quality of life and survival for those with immune deficiency.
Keywords: primary antibody deficiency, common variable immunodeficiency, lung disease, asthma, bronchiectasis, interstitial lung disease, GLILD
Introduction
Primary antibody deficiency (PAD) is the most commonly diagnosed inborn error of immunity, consisting of numerous conditions in which impairment of immunoglobulin production is the dominant phenotype.1 PAD consists of a spectrum of clinical phenotypes from the relatively mild, like selective IgA deficiency (IgAD), to those with severe antibody loss, as in X-linked agammaglobulinemia (XLA).2 The most prevalent symptomatic form of PAD is common variable immunodeficiency (CVID), which is defined by profoundly low IgG and IgA and/or IgM as well as failure to mount antibodies against vaccination.3 Since CVID is the most frequently encountered, most studies of PAD and lung disease have focused upon this diagnosis.4 Recognition of PAD is often delayed by many years, which may increase the risk of chronic lung disease.5
Evaluation of PAD is most frequently preceded by a history of recurrent acute respiratory infections, often of bacterial etiology.6, 7 Frequent and/or severe respiratory infections can cause structural lung damage that may promote chronic pulmonary disease.8 Moreover, delay in PAD diagnosis is associated with fixed pulmonary obstruction, chronic atelectasis, pulmonary fibrosis, and bronchiectasis.6, 9, 10 Earlier recognition of PAD potentially can lead to interventions that reduce the frequency of respiratory infections, hospital admissions, and improve survival.11–13 It is widely accepted that the infection susceptibility in PAD stems from loss of the important contribution of antibodies in immunity against bacteria.14 Supporting this concept, encapsulated bacteria, for which immunity is thought to be particularly reliant upon antibodies, are the most frequently cultured pathogens from sputum of PAD patients.15, 16 Efficacy of antibiotic prophylaxis for reduction of annual respiratory exacerbations in PAD patients was recently demonstrated in a large placebo-controlled trial, resulting in encouragingly low levels of antibiotic resistance.17 In addition to bacteria, viruses are also frequent instigators of respiratory disease in PAD and should not be overlooked.18–20
Despite improved recognition of PAD and management of acute respiratory infections in these patients, chronic lung disease still occurs frequently and pulmonary function declines over time.21 In a single center study of 473 CVID patients, functional or structural lung impairment was reported in 28.5% of subjects, with significantly reduced survival compared to CVID patients without chronic lung disease.22 Lower respiratory tract infections with encapsulated bacteria may be of particular importance to the development of chronic lung disease as H. influenzae and S. pneumoniae have been shown to lead to pleurisy, empyema, and bronchospasm in CVID.23 It appears that early intervention with prophylactic antibiotics and/or immunoglobulin replacement therapy (IRT) can reduce respiratory infections and decrease morbidity and mortality from pulmonary disease in PAD.11, 12, 24–27 However, chronic lung disease progresses in many PAD patients despite widespread usage of antibiotic prophylaxis and IRT.28–30 This may be explained, at least in part, by immune dysregulation occurring independent of infection.31 Alternatively, there may be important host defense mechanisms that remain deficient despite IRT, such as mucosal IgM and IgA responses. Infection- and non-infection-driven pathways of chronic lung disease alike must be better understood in order to improve care of PAD.
One particularly interesting observation which demonstrates the complexity of lung disease susceptibility in PAD patients is the fact that respiratory infections and chronic lung disease occurs more frequently in those with CVID compared to XLA, despite XLA resulting in more profound antibody deficiency.32, 33 Comparison of CVID and XLA suggests that differences in genetic etiology, concurrent T cell defects, propensity for immune dysregulation, and/or differences in diagnostic delay may explain why these two forms of severe PAD have differing prevalence of lung disease.33 For example, PAD with concurrent disruption of T cell function, such as hyper IgM syndrome due to defects of CD40:CD40L interaction, can lead to a broader range of respiratory pathogens, including Histoplasma and Pneumocystis.34 Though increased susceptibility to respiratory infections and chronic lung disease is shared amongst the diverse forms of PAD, immunological differences shape individual variations in pulmonary manifestations.
Key points regarding respiratory infections in PAD are highlighted in Box 1. As with many chronic diseases, thoughtful clinical surveillance and intervention is vital for management of lung disease in PAD. Unfortunately, there is significant variance among physicians regarding follow-up and monitoring of respiratory disease in PAD.35 Appropriate usage of chest imaging and pulmonary function testing (PFT) forms the basis of the diagnostic work-up of a PAD patient with suspected chronic lung disease, with tissue biopsy employed when indicated. Therapeutic response, or lack thereof, often provides an additional modality of clinical evaluation. Emerging diagnostic tools and precision medical therapies are dramatically altering the landscape of lung disease management in PAD. Specific diagnostic and therapeutic concerns applicable to common forms of chronic lung disease affecting PAD patients will now be explored further.
Box 1: Key points regarding respiratory infection in PAD patients.
Encapsulated bacteria are the most common pathogens isolated from the respiratory tract of PAD patients, but viruses are also frequent and may be underappreciated in importance.
Prophylactic antibiotics can be used to reduce the frequency of respiratory infections in PAD patients without a significant increase in antibiotic resistance.
Chronic lung disease develops in some PAD patients despite improved recognition of immune deficiency and usage of IRT.
Chronic lung disease may develop because of immune dysregulation independent of infection or deficiencies in host defense that are not alleviated by IRT and/or antibiotic prophylaxis.
Asthma and COPD
Asthma and COPD are common forms of chronic lung disease in the general population that also frequently affect those with PAD. These are obstructive forms of lung disease resulting from airway constriction owing, at least in part, to chronic inflammation. Depending on the cohort studied, obstructive lung disease, or at least bronchial hyperresponsiveness, has been reported in 15 – 50% of those with CVID and at least 5% of XLA patients.36–38 Leveraging the United States Immunodeficiency Network we found further evidence that asthma is more common in CVID than in XLA, as 31.2% of CVID patients in the national registry had a diagnosis of asthma compared to 10.3% of those with XLA.33 In the same study, we found asthma to be the most common chronic pulmonary complication in CVID, but there was no association with age of symptom onset or CVID diagnosis. Like CVID, those with IgAD have an increased likelihood of asthma.39, 40 Even though IgE may contribute to asthma pathology, severe antibody deficiency does not seem to be protective as airway bronchoconstriction in response to allergens can occur in CVID when IgE-mediated allergy testing is negative.40, 41 It should be noted that while obstructive lung function is often used to diagnose asthma, one study found only 9/29 CVID patients with obstructive lung disease to have asthma.41 Thus, alternative etiologies of obstructive lung disease must be considered in PAD as many cases may be misdiagnosed as asthma.
Little is known regarding the relationship of PAD with COPD. PAD appears to result in more frequent COPD exacerbations and low levels of IgA have been proposed to underlie COPD exacerbation and/or progression.42–44 Population studies of PAD have not differentiated rates of COPD from that of asthma definitively, but when both forms of obstructive lung disease are considered together they were found in 19% of CVID patients in one study.45 It is unclear whether risks of smoking are heightened in PAD patients, but it is a habit that is certainly advisable to halt as in any patient. Thus, while the data is limited, evidence suggests that, like asthma, COPD prevalence and exacerbation rate are increased in PAD.
It is reasonable to postulate that PAD predisposes to respiratory infections that can promote asthma or COPD. Yet, the precise mechanism by which PAD promotes asthma or COPD remains to be conclusively defined. Numerous studies have demonstrated benefit of IRT for asthma or COPD in various types of PAD.13, 46–48 Other than the provision of IRT, management of asthma and COPD in PAD patients follows standardized diagnostic and treatment guidelines reviewed in detail elsewhere.49, 50 However, the efficacy of specific treatments have not been tested in PAD and it is possible that some agents, most notably immunomodulatory biologics, may have differing efficacy compared to the general population. It should be noted that some PAD patients may have immune defects contributing to obstructive lung disease that are not fully ameliorated by IRT. Indeed, we found asthma to be associated with lower levels of IgA and IgM in CVID, and these antibody isotypes are not significantly provided by IRT therapy.33 Considerations regarding asthma and COPD in PAD patients are highlighted in Box 2. Further efforts are needed to better understand asthma and COPD within the unique concerns of PAD.
Box 2: Considerations regarding asthma and COPD in PAD patients.
Risk of developing obstructive lung disease appears to be increased by PAD.
As many cases of pulmonary obstruction in PAD may be misdiagnosed as asthma, it is important to confirm the diagnosis and/or rule-out alternatives.
Other than the provision of IRT when indicated, management of asthma and COPD in PAD patients follows the standard diagnostic and treatment guidelines.
No studies have been conducted to determine whether changes in conventional management of asthma or COPD is warranted in PAD, or whether specific biologic therapies have more or less utility for patients with immune deficiency.
Bronchiectasis
PAD patients are particularly at risk of severe lung infections, with 60% or more of CVID and XLA patients reporting a history of pneumonia prior to diagnosis of immune deficiency.11, 12, 23, 32, 51 Bronchiectasis can be a complication of recurrent pneumonia and results from irreversible dilation of the airways due to repeated episodes of inflammatory damage.23, 52 PAD is likely to increase the frequency of pulmonary infections that drive airway inflammation and lead to the fixed changes that define bronchiectasis.53 Chronic rhinosinusitis was found in nearly half of the 900 bronchiectasis patients and significantly associated with antibody deficiency in these subjects from a recent report.54 Further highlighting the cumulative impact of pulmonary infections, bronchiectasis was associated with history of pneumonia, older age, and diagnostic delay in CVID as well as reduced levels of CD4+ T cells and IgM.29, 55–57
Patients with less severe forms of PAD, such as IgG subclass deficiency, selective IgA deficiency, and specific antibody deficiency, may also have an increased risk of bronchiectasis.58–62 Lower levels of IgA and/or IgM are associated with bronchiectasis, highlighting the fact that isotypes other than the IgG replaced by IRT could be important in limiting this chronic lung complication.63 Indeed, local production of IgA and IgM is thought to contribute significantly to mucosal immunity.64 Specific genetic defects are also key, as exemplified by the high rate of bronchiectasis occurring in those with gain-of-function mutations of PI3KD.65 Excess mucus production and impaired mucociliary clearance that results from bronchiectasis alters the microbial composition of the airways in a manner that promotes a viscous cycle of pulmonary exacerbations.66 Antibody deficiency itself also clearly alters the constituency and reduces the diversity of mucosal microbiota.67, 68 Another key factor is time, as bronchiectasis is more common in older CVID and XLA patients.29, 55 Bronchiectasis emerges and progresses in PAD patients for numerous reasons that must be better targeted therapeutically to improve patient care (Box 3).
Box 3: Key factors that may increase risk of bronchiectasis in PAD.
History of pneumonia, older age, and diagnostic delay.
Lower levels of CD4+ T cells, IgA, or IgM.
Specific genetic defects of the immune system, such as gain-of-function mutations PI3KDI.
Alterations in microbial composition of the airways.
Clinical presentation of bronchiectasis typically includes chronic cough with purulent sputum, occasional hemoptysis, and dyspnea. Airflow obstruction is often evident on PFT and diagnosis is typically made by computed tomography (CT) as chest radiographs can be inadequate (Figure 1).69–71 As further evidence that imaging is key for diagnosis, a recent European study found that cough was more strongly associated with bronchiectasis on CT than spirometry findings in PAD patients.57 Observations that can precede bronchiectasis, such as early bronchial wall thickening, may also be identified by chest CT.72 The clinical impact of bronchiectasis may be more severe in PAD as these patients appear to have heighted inflammation in the lungs and circulation compared with those without immune deficiency but similar degree of bronchiectasis evident on CT scan.73 Colonization of the airways with non-tuberculous mycobacteria (NTM) or Pseudomonas aeruginosa can further worsen disease course in patients with bronchiectasis.74 Sputum culture to rule out such colonization is often helpful in selection of antibiotics and is of particular importance to limit NTM antimicrobial resistance associated with macrolide single therapy.75 The key points for the diagnostic algorithm of bronchiectasis is highlighted in Box 4.
Figure 1.
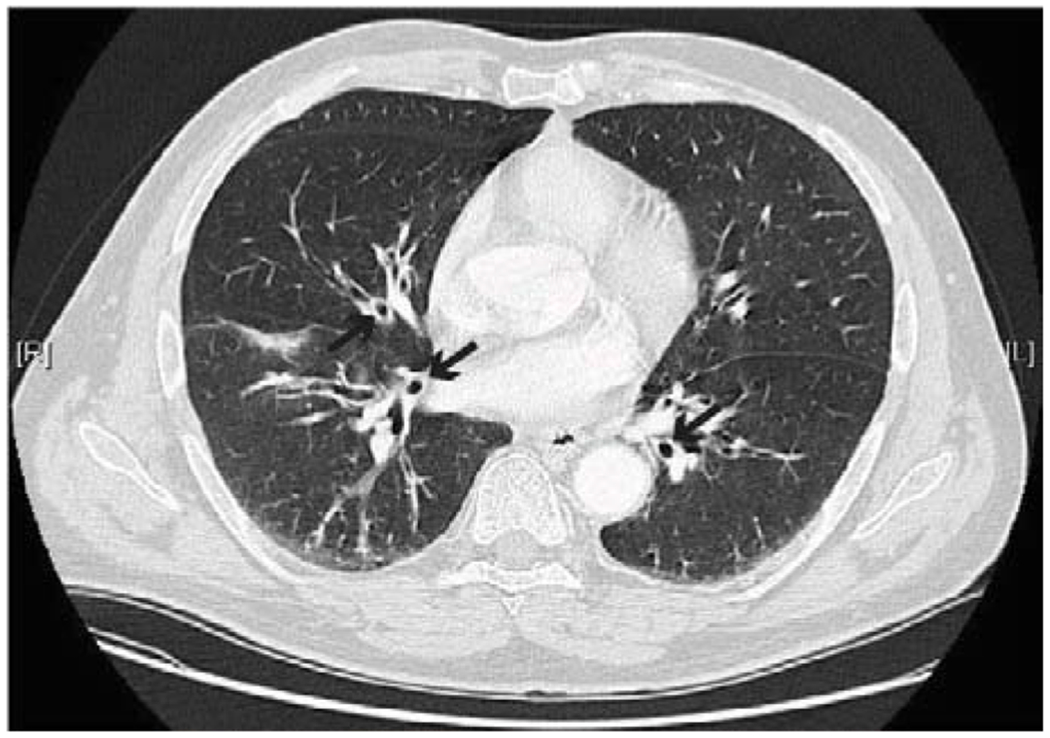
Bronchial wall thickening (marked by black arrows) consistent with bronchiectasis in a PAD patient.
Box 4: Diagnosis of bronchiectasis in PAD.
Clinical presentation includes chronic cough, often with purulent sputum, and dyspnea.
Airflow obstruction is often evident on PFT.
CT is usually needed for diagnosis because chest radiographs are frequently inadequate.
CT can identify precursors of bronchiectasis such as early bronchial wall thickening.
Sputum culture can be helpful for selection of antibiotics and identification of colonization with NTM or Pseudomonas.
Upon recognition of the immune deficiency, bronchiectasis course in PAD patients parallels those developing this pulmonary complication from other causes.76 Treatment options for bronchiectasis in PAD patients include IgG replacement with a high goal trough (such as 1000 mg/dL), inhaled corticosteroids and long-acting β-agonists, extended course macrolide therapy, and pulmonary rehabilitation (Figure 2).77–80 As previously mentioned, prior to initiating extended course macrolide therapy, sputum culture is recommended to limit NTM resistance. Macrolide therapy has been studied from 8 weeks to 24 months in those with non-cystic fibrosis bronchiectasis, but not specifically PAD. The macrolides studied include azithromycin (typically 250 or 500 mg three days a week), clarithromycin, and erythromycin.80–82 Inhaled antibiotics and mucolytics are other potential therapeutic options for PAD-associated bronchiectasis that have been less frequently used.83, 84 Unfortunately, studies evaluating the efficacy of individual therapies for bronchiectasis in PAD are lacking.
Figure 2.
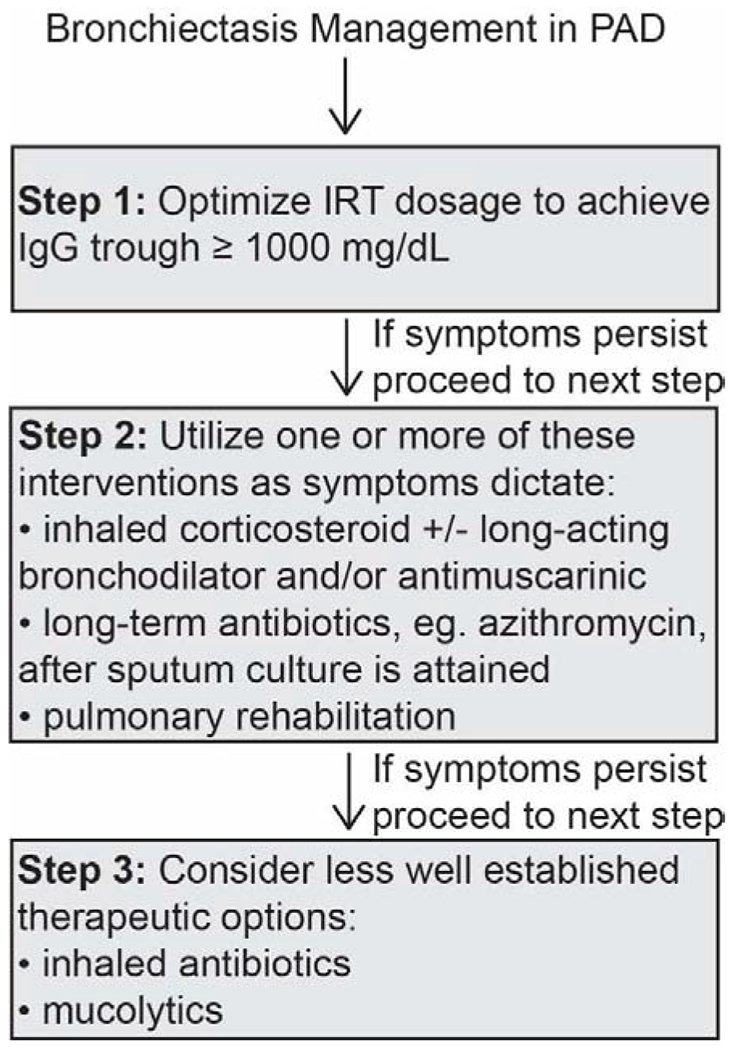
Proposed algorithm for bronchiectasis management in PAD.
IRT = immunoglobulin replacement therapy.
Interstitial Lung Disease
The exact prevalence of ILD in PAD is unclear and varies by immune deficiency. Based upon best estimates, ILD occurs in approximately 10-20% of CVID patients.85 This prevalence may be underestimated as CT findings consistent with ILD (ground glass opacity and/or numerous pulmonary nodules) was noted in 64% of CVID patients with respiratory symptoms at a tertiary referral center.55 Interpretation of these CT findings is complicated by the fact that PFT is not always diminished and they may not be indicative of clinically significant ILD.86, 87 ILD appears far less common in other forms of PAD. ILD was absent or exquisitely rare in numerous large studies of X-linked and autosomal recessive hyper IgM syndrome.88–90 ILD is also very rare in those with congential agammaglobulinemia, particularly XLA.32,91–95 We leveraged the expansive USIDNET registry to more closely examine why ILD is more common in CVID than XLA despite both being severe forms of PAD. We found that concurrent autoimmunity and deficiency of T cells were associated with ILD in CVID, immunological characteristics that occur far less frequently in XLA.33 Reduction of CD8+ T cells, CD4+ regulatory T cells, and isotype-switched memory B cells as well as elevated serum IgM and CD38+IgM+CD27-transitional B cells have also been associated with ILD presence or progression in CVID.96–100 Similarly, ILD has been reported in IgAD patients with autoimmunity and more severe immune defect, such as concurrent IgG subclass deficiency.4, 59, 101, 102 Thus, alterations in T cell function, generalized immune dysregulation, greater infection susceptibility, and/or the presence of pathogenic B cells may be fundamental to the development of ILD in PAD (Box 5). Given the heterogeneity of ILD seen in CVID, not all patients should be expected to manifest all these immunological alterations.
Box 5: Immunological characteristics associated with PAD patients with ILD.
Greater immune defect as manifested by more profound loss of immunoglobulins, isotype-switched memory B cells, and T cells.
Deficient or impaired regulatory T cells.
Elevation of serum IgM or IgM+CD38+CD27-transitional B cells.
Presence of systemic immune dysregulation as evidenced by history of autoimmunity, lymphadenopathy, and/or splenomegaly.
ILD can be challenging to differentiate from other forms of chronic lung disease based upon clinical history alone, as the most common presenting symptoms, chronic cough and dyspnea, are nonspecific. Physical exam can be useful, with crackles more indicative of ILD and wheezing more likely to be associated with obstructive disease, like asthma or COPD. However, physical exam findings can straddle multiple potential diagnoses, as inspiratory ronchi can be heard in certain types of ILD as well as some asthmatics. PFT can be useful to differentiate obstructive forms of chronic lung disease (asthma, bronchiectasis, COPD) from restrictive lung disease (ILD). Yet, obstructive forms of lung disease, such as bronchiectasis, can occur together with ILD. Thus, high resolution CT is frequently vital for the evaluation of chronic lung disease in PAD, with ILD often manifesting as large pulmonary nodules (Figure 3). Lung biopsy can be helpful to confirm a diagnosis of ILD, rule-out lymphoma or other malignancy, and perhaps even help guide therapy.103 The diagnosis of ILD and differentiation from other forms of pulmonary disease requires employing various modalities of clinical evaluation.
Figure 3.
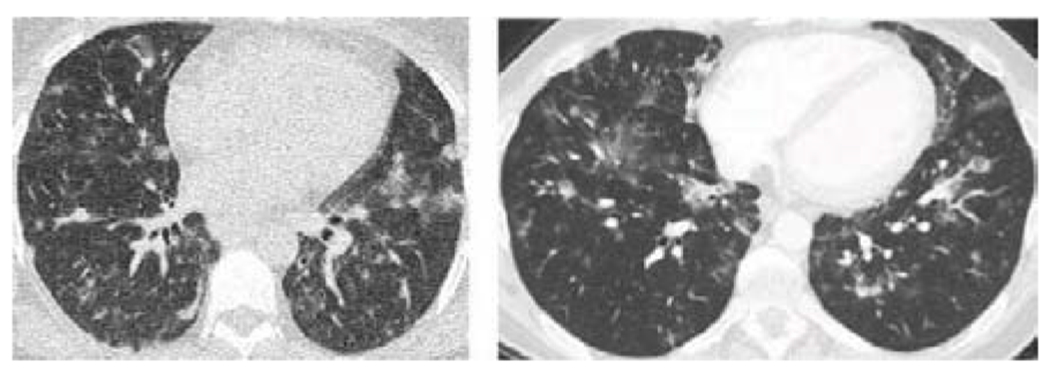
CT chest imaging from two distinct CVID patients demonstrating large nodules typical of PAD-associated ILD.
ILD pathology in PAD patients is typically consistent with one or more forms of benign pulmonary lymphoproliferation, usually follicular bronchiolitis, lymphocytic interstitial pneumonia (LIP), or nodular lymphoid hyperplasia.55, 97, 104 In follicular bronchiolitis, there is benign hyperplasia of lymphoid follicles adjacent to airways (Figure 4A). LIP is considered a progression of this pulmonary lymphoid hyperplasia into the lung interstitium with expansion of alveoli septa (Figure 4B). Nodular lymphoid hyperplasia in the lungs is characterized by lymphoid follicles that are more cleanly demarcated than in LIP and is speculated to be a precursor of mucosal-associated lymphoid tissue (MALT) lymphoma.104 Of note, CVID patients are occasionally misdiagnosed with MALT lymphoma when this diagnosis is not adequately differentiated from the benign lymphoproliferative lung pathology that is most common.105 Granulomatous inflammation and organizing pneumonia may also be found in lungs of PAD patients together with one of the lymphoproliferative pathologies, and the term granulomatous lymphocytic interstitial lung disease (GLILD) is frequently used to described the ILD associated with PAD.106–108 Granulomas and organizing pneumonia are features of LIP, so some may prefer this terminology over GLILD.109, 110 As the multifaceted pathology of GLILD is not always appreciated, it is imperative recognize the spectrum of ILD pathologies found in PAD (Table 1).
Figure 4.
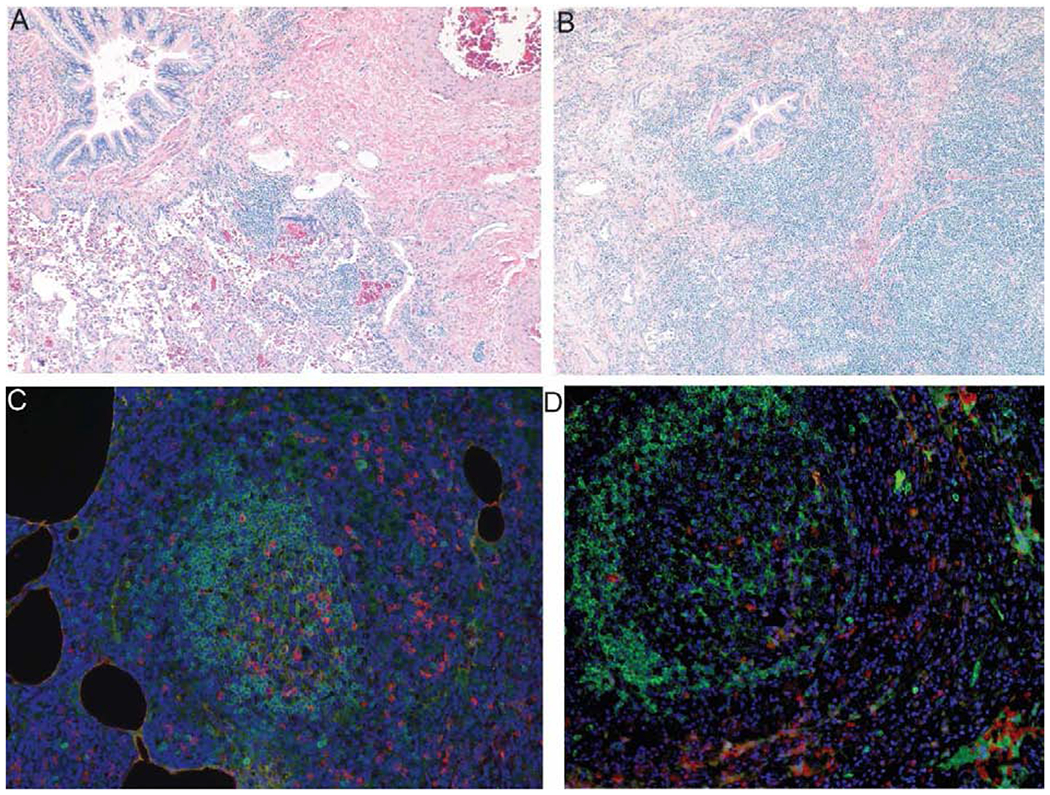
Immunopathology of CVID ILD. A. Hematoxylin and eosin (H&E) stain demonstrating peribronchial lymphocytic inflammation of follicular bronchiolitis in a CVID patient. B. H&E stain showing expansion of peribronchial inflammation into lymphocytic interstitial pneumonia (LIP) in a CVID patient. C. Immunofluorescence demonstrating IgD+ B cells (green) in ectopic pulmonary follicles with adjacent IgMbright B cells (red) with diamidinophenylindole (DAPI) (blue) nuclear stain. D. IgD+ B cells (green) and B cell activating factor (BAFF) in CVID ILD with DAPI (blue) nuclear stain.
Table 1.
Types of interstitial lung disease pathology seen in primary antibody deficiency
| Pathology | Characteristics |
|---|---|
| Follicular bronchiolitis | Benign lymphoid hyperplasia bordering the airways |
| Lymphocytic interstitial pneumonia (LIP) | Pulmonary lymphoid hyperplasia involving lung interstitium with expansion of alveoli septa. Considered progression of follicular bronchiolitis. Granulomas may be present. |
| Nodular lymphoid hyperplasia | Well-demarcated lymphoid follicles considered a precursor to mucosal-associated lymphoid tissue (MALT) lymphoma |
| Non-necrotizing granulomatous inflammation | Inflammation containing circumscribed macrophages lacking a central area of necrosis |
| Organizing pneumonia | Production of granulation tissue within the alveolar space in response to lung injury. Formerly called bronchiolitis obliterans with organizing pneumonia or BOOP. |
| Granulomatous-lymphocytic interstitial lung disease (GLILD) | Broadly encompassing term typically implying the presence of granulomatous inflammation together with pulmonary lymphoid hyperplasia and sometimes also organizing pneumonia |
Perceived similarities with sarcoidosis can present another challenge when evaluating ILD in PAD. In both conditions, granulomatous inflammation can occur inside as well as outside the lungs and pulmonary imaging can look similar at first glance. Sarcoidosis is typically distinguished radiologically by smaller pulmonary nodules that have an apical or perilymphatic distribution, compared to the larger nodules with generalized lung distribution in PAD.111 When available, biopsies can differentiate the conditions, as PAD ILD typically demonstrates benign lymphoproliferation (follicular bronchiolitis, LIP, etc) that is generally absent in sarcoidosis. Clinical and laboratory features of PAD patients, such as history of autoimmune cytopenia, nodular regenerative hyperplasia of the liver, recurrent infections, and splenomegaly as well as antibody deficiency and diminished isotype-switched memory B cells should raise suspicion of PAD more so than sarcoidosis. Though not in all PAD ILD cases, bronchiectasis is far more suggestive of immune deficiency than sarcoidosis when it is found. Newer tests allowing for rapid identification of patients with severe PAD, like measurement of serum B cell maturation antigen, may also prove to be useful.112 Thus, numerous differences between PAD-associated ILD and sarcoidosis can be exploited to differentiate these chronic lung diseases (Table 2).
Table 2.
Differentiating PAD-associated ILD from sarcoidosis
| Clinical Feature | PAD-associated ILD | Sarcoidosis |
|---|---|---|
| Low immunoglobulins | + | - |
| Low isotype-switched memory B cells | + | - |
| Recurrent infections | + | - |
| Autoimmune cytopenia, nodular regenerative hyperplasia of the liver, and/or splenomegaly | + | - |
| Follicular bronchiolitis | + | - |
| Lymphocytic interstitial pneumonia | + | - |
| Radiology Feature | ||
| Large nodules (>1 cm) | + | - |
| Small nodules (< 1 cm) | - | + |
| Nodules localized to apical lung zones and perilymphatic regions | - | + |
| Nodules with generalized distribution within lungs | + | - |
| Bronchiectasis | + | - |
The pathological basis of ILD in PAD remains largely unknown. Initial perceptions held that infection drove ILD development. Associations have been made with human herpes virus 8 virus and Epstein-Barr virus, which have been shown to underlie lymphoproliferation in other atients.113–117 These viral etiologies have not been identified in the vast majority of PAD ILD ases, though improved diagnostic approaches, such as next generation sequencing, may be needed.118 Bronchiectasis, a frequent complication of PAD, can develop into follicular bronchiolitis, a common form of ILD seen in PAD.119, 120 While we found a significant association between bronchiectasis and ILD in CVID patients from USIDNET, there was no association of these complications in our single center study.33, 55 This discrepancy may be explained by input error in USIDNET, as the registry did not require CT or biopsy confirmation of the pulmonary diagnosis as was required in the single center study. Alternatively, tertiary referral sites like the single center study may receive a higher proportion of patients for which the ILD results from systemic immune regulation, due to the multisystem problems that often lead to their referral, whereas at other sites chronic infection may be a key driver of the forms of ILD encountered. As there is heterogeneity among the ILD occurring in PAD, etiologies could vary.121
There is numerous lines of evidence that indicate infection is not required for the development of ILD in PAD. Particularly in CVID patients, ILD frequently occurs in conjunction with lymphoid hyperplasia in other tissues, such as lymph nodes, spleen, and the mucosal lymphoid tissue of the gastrointestinal tract.55, 96, 97, 122, 123 Thus, the pulmonary lymphoid hyperplasia that characterizes the ILD seen in PAD may reflect systemic immune dysregulation inherent to the patient.31, 124 Along these lines, autoimmunity is more common in CVID patients with ILD, mirroring the association observed in other immunological diseases like rheumatoid arthritis and Sjogren’s syndrome.73, 100, 125, 126 The importance of intrinsic immune dysregulation is also exemplified by CVID-like disorders with monogenic etiologies. Haploinsufficiency of cytotoxic T lymphocyte-associated protein 4 (CTLA-4) can result in an immune dysregulation syndrome in which PAD is a prominent feature and ILD occurs frequently.127–129 Autosomal recessive defects of lipopolysaccharide-responsive beige-like anchor protein (LRBA) impair trafficking of CTLA-4 and result in a similar immune dysregulation syndrome in which ILD occurs frequently.130–132 Autosomal dominant gain-of-function mutations of PI3KD or STAT3 also result in immune dysregulation disorders characterized by antibody deficiency and ILD along with autoimmunity and generalized lymphoproliferation.133–136 These strong links with intrinsic immune dysregulation provide evidence that infection is not required for ILD in PAD.
A predominant feature of the pulmonary lymphoid hyperplasia seen in CVID ILD are ectopic B cell follicles that predominantly express IgD with agent IgM-expressing cells that express markers of plasmablasts and are more numerous in patients with PFT decline (Figure 4C).98 In addition to showing evidence of active proliferation, these B cell follicles express markers of germinal centers, a curious finding considering the extensive B cell maturation defect in many of the affected forms of PAD, like CVID, and the apparent absence of antibody isotype switching.137 As both protective and autoreactive immune responses can be generated within ectopic follicles, these lymphoid sites have important roles in both immune protection and pathology.138 The clinical significance of pathogenic B cells in CVID ILD has been recognized for years and rituximab has been used for treatment as part of either single or combination therapy by numerous groups.137,139–142 We recently reported the largest study of rituximab for CVID ILD, demonstrating clear efficacy of the intervention over supportive care.98 ILD recurred after B cell depletion in about 1/3rd of our subjects in association with elevated B cell activating factor (BAFF) (Figure 4D), which we demonstrated in the same study to help oppose apoptosis of B cells and serve as a potential driver of lymphoid hyperplasia in the lungs of CVID patients. Research efforts are ongoing to enhance our understanding of this potential key mechanism of disease pathogenesis.
The question of when and who to treat for ILD can be challenging. It is clear that pulmonary nodules can wax and wane in PAD patients and PFT can remain stable for extended periods.87, 98 It is imperative that IgG replacement therapy is optimized in patients with ILD because there may be a subset of patients for which ILD is stabilized by this intervention and CVID patients with troughs of 1000 mg/dL or greater are less like to have pulmonary function decline.87, 143 A reasonable option for the next intervention in PAD with mild to moderate ILD symptoms is inhaled corticosteroids, with or without long-acting beta agonists, and/or prophylactic macrolides as these therapies have been shown efficacious in follicular bronchiolitis.144–146 Systemic corticosteroid treatment may also be efficacious, with the caveats that long-term usage of these agents is frequently associated with adverse effects that outweigh their utility and many cases end up refractory to this intervention.147
In addition to the previously mentioned rituximab, a variety of immunomodulators have been employed to manage PAD-associated ILD. These include azathioprine, cyclosporine, cyclophosphamide, hydroxychorloquine, methotrexate, and mycophenolate mofetil, individually or used together with rituximab.139, 148 CVID patients with prominent granulomatous inflammation may be responsive to tumor necrosis factor (TNF) antagonists.149, 150 Rituximab offers the advantage of not adding new additional forms of immune suppression in patients who already have PAD and are on IRT, as well as having lower risk of adverse effects like infection and malignancy as other immunomodulatory agents in these patients.151, 152 In those in which ILD recurs after rituximab, we have found that addition of azathioprine or mycophenolate mofetil is effective in inducing longer remission after B cell depletion.98 Given the evidence that BAFF promotes ILD recurrence, BAFF depletive therapies such as belimumab may also be useful to prevent recurrence. Patients without prominent pulmonary B cell follicles may not benefit as much from B cell targeted therapy and those with significant fibrosis may not see significant improvement from any type of immunomodulation.
Precision therapy is available for CVID-like diseases with monogenic etiologies. Abatacept can rectify the protein deficiencies of CTLA-4 due to mutations of CTLA4 or LRBA.153 In another example of treatment tailored to the genetic defect, those with gain-of-function mutation of PI3KD are responsive to targeted inhibition of phosphoinositide 3-kinase δ with leniolisib.154 A less precise, but effective, alternative for these forms of PAD with lymphoproliferation is rapamycin, as they each have hyperactivation of the mTOR pathway.127, 136 Rapamycin has shown efficacy in other PAD patients with GLILD and may have its greatest potential benefit in those with elevated ratios of CD45RO+ effector to CD45RA+ naive T cells and/or reduced levels of regulatory T cells.99 Targeted inhibition of gain-of-function mutations in STAT3 with jakinibs also may provide benefit for ILD in these patients.155 In addition to pathological characteristics, genetic findings can profoundly shape therapeutic options and potential efficacy. Given the monumental risks involved and generally poor results, lung transplant is reserved as a last resort.156 Bone marrow transplant may improve complications of immune dysregulation in CVID, but the exorbitant mortality rate (upwards of 50%) makes this option undesirable until outcomes are improved.157 Approach to the treatment of ILD in PAD is presented in Figure 5.
Figure 5.

Proposed algorithm for ILD management in PAD.
mTOR = mammalian target of rapamycin.
Summary
The evaluation of a PAD patient with suspected chronic lung disease involves several fundamental diagnostic tools (Box 6). Imaging and PFT form the basis of any diagnostic work-up and together can help differentiate various forms of lung disease occurring in PAD. Even in those without respiratory symptoms, a “baseline” high resolution chest CT and PFT seem prudent at the time of PAD diagnosis to look for evidence of chronic lung disease that may be present prior to onset of symptoms. These baseline findings can be useful to determine any changes in association with clinical symptoms at a later date. It is usually unnecessary to conduct repeated chest imaging in asymptomatic and uncomplicated patients that are adherent with therapy. ILD is usually present at PAD diagnosis and does not develop insidiously in those without evidence of lung disease or other complications.31, 55, 158 Bronchiectasis may develop over the course of years with minimal symptoms, but the utility of repeated imaging to look for asymptomatic progression is unclear.
Box 6: Fundamental diagnostic tools for evaluation of chronic lung disease in PAD.
“Baseline” PFT at PAD diagnosis and then as dictated by symptoms or physician discretion.
“Baseline” CT chest at PAD diagnosis and then as dictated by symptoms and/or findings.
If biopsy is pursued, video-assisted thoracoscopic biopsy is preferred over endobronchial.
Sputum microbiology is helpful to rule out NTM and Pseudomonas colonization.
In ILD patients, abrupt rises in serum IgM or circulating B cells post-rituximab could be harbingers of disease progression or recurrence.
Monitoring of pulmonary nodules as per typical guidelines may be controversial in PAD patients given the increased risk of malignancy in some patients, such as those with CVID. However, in our experience many of these nodules are benign and many wax and wane over time, indicating that aggressively low thresholds to biopsy (and repeated biopsies) may not be necessary. Magnetic resonance imaging offers an option for repeated chest surveillance without radiation exposure and may be a useful for clinicians desiring more frequent monitoring.159, 160 Biopsies are useful to determine etiology of expanding nodules or potentially guide therapy on the basis of the predominant pathology in those with clinical worsening. Video-assisted thoracoscopic surgical biopsy is preferable over endobronchial biopsy to increase tissue yield so that the pulmonary pathology can be more accurately discerned.
PFT is another useful tool for monitoring chronic lung disease. We typically advise PFT every 6–12 months in PAD patients with moderate to severe chronic lung disease, with more frequent testing as symptoms dictate. Notably, diffusion capacity of the lung for carbon monoxide (DLCO) decline may precede changes in other PFT parameters in PAD patients with ILD, thus evaluations that exclude this measurement may be suboptimal.87 In PAD patients with ILD there are other, less validated, tools that can be used to monitor disease progression. We found serum IgM to elevate with PFT decline in a subset of CVID patients with ILD consistent with pulmonary lymphoid hyperplasia.87, 98 Rapid replenishment and/or elevation of circulating B cells may be a harbinger of ILD recurrence in PAD patients after rituximab therapy. However, given the heterogeneity of ILD pathology in PAD patients, some with minimal presence of B cells, a unifying biomarker for monitoring disease progression in all patients remains elusive. Efforts remain ongoing to enhance our understanding and improve management of the spectrum of chronic lung diseases affecting PAD patients.
Key Points:
Chronic lung disease is a frequent complication of primary antibody deficiency that can manifest in numerous distinct forms.
Asthma and chronic obstructive pulmonary disease are common forms of lung disease with unique considerations relating to pathogenesis and treatment when they occur in PAD.
Bronchiectasis development and progression are linked with delayed diagnosis and inadequate treatment of PAD, but can still worsen despite prophylactic measures to limit infection.
Interstitial lung disease is associated with high morbidity and mortality in PAD and results from pulmonary lymphoproliferative pathology that is responsive to immunomodulatory therapy.
Acknowledgments
P. J. M. is supported by NIH grant AI137183, a faculty development award from the American Association of Allergy, Asthma and Immunology Foundation, Boston University, and investigator-initiated grants from Horizon and Takeda.
Abbreviations:
- BAFF
B cell activating factor
- COPD
chronic obstructive pulmonary disease
- CVID
common variable immunodeficiency
- CO
diffusion capacity of the lung for carbon monoxide
- IgAD
selective IgA deficiency
- ILD
interstitial lung disease
- IRT
immunoglobulin replacement therapy
- LIP
lymphocytic interstitial pneumonia
- NTM
non-tuberculous mycobacteria
- PAD
primary antibody deficiency
- PFT
pulmonary function testing
- XLA
X-linked agammaglobulinemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith T, Cunningham-Rundles C. Primary B-cell immunodeficiencies. Hum Immunol 2019;80:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol 2013;13:519–33. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol 2012;129:1425–6.e3. [DOI] [PubMed] [Google Scholar]
- 4.Schussler E, Beasley MB, Maglione PJ. Lung Disease in Primary Antibody Deficiencies. J Allergy Clin Immunol Pract 2016;4:1039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slade CA, Bosco JJ, Binh Giang T, Kruse E, Stirling RG, Cameron PU, et al. Delayed Diagnosis and Complications of Predominantly Antibody Deficiencies in a Cohort of Australian Adults. Front Immunol 2018;9:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood P Primary antibody deficiencies: recognition, clinical diagnosis and referral of patients. Clin Med (Lond) 2009;9:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 2015;136:1186-205.e1-78. [DOI] [PubMed] [Google Scholar]
- 8.Gregersen S, Aalokken TM, Mynarek G, Fevang B, Holm AM, Ueland T, et al. Development of pulmonary abnormalities in patients with common variable immunodeficiency: associations with clinical and immunologic factors. Ann Allergy Asthma Immunol 2010;104:503–10. [DOI] [PubMed] [Google Scholar]
- 9.Sweinberg SK, Wodell RA, Grodofsky MP, Greene JM, Conley ME. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinemia. J Allergy Clin Immunol 1991;88:96–104. [DOI] [PubMed] [Google Scholar]
- 10.Obregon RG, Lynch DA, Kaske T, Newell JD Jr., Kirkpatrick CH. Radiologic findings of adult primary immunodeficiency disorders. Contribution of CT. Chest 1994; 106:490–5. [DOI] [PubMed] [Google Scholar]
- 11.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol 2002;109:1001–4. [DOI] [PubMed] [Google Scholar]
- 12.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol 2010;137:21–30. [DOI] [PubMed] [Google Scholar]
- 13.van Kessel DA, Hoffman TW, van Velzen-Blad H, Zanen P, Grutters JC, Rijkers GT. Long-term Clinical Outcome of Antibody Replacement Therapy in Humoral Immunodeficient Adults With Respiratory Tract Infections. EBioMedicine 2017;18:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gernez Y, Baker MG, Maglione PJ. Humoral immunodeficiencies: conferred risk of infections and benefits of immunoglobulin replacement therapy. Transfusion 2018;58 Suppl 3:3056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederman HM, Winkelstein JA. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 1985;64:145–56. [PubMed] [Google Scholar]
- 16.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med 1993;86:31–42. [PubMed] [Google Scholar]
- 17.Milito C, Pulvirenti F, Cinetto F, Lougaris V, Soresina A, Pecoraro A, et al. Double-blind, placebo-controlled, randomized trial on low-dose azithromycin prophylaxis in patients with primary antibody deficiencies. J Allergy Clin Immunol 2019;144:584–93.e7. [DOI] [PubMed] [Google Scholar]
- 18.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol 2010;126:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones TPW, Buckland M, Breuer J, Lowe DM. Viral infection in primary antibody deficiency syndromes. Rev Med Virol 2019;29:e2049. [DOI] [PubMed] [Google Scholar]
- 20.Peltola V, Waris M, Kainulainen L, Kero J, Ruuskanen O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin Microbiol Infect 2013;19:E322–7. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Stirling RG, Paul E, Hore-Lacy F, Thompson BR, Douglass JA. Longitudinal decline in lung function in patients with primary immunoglobulin deficiencies. J Allergy Clin Immunol 2011;127:1414–7. [DOI] [PubMed] [Google Scholar]
- 22.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012;119:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Notarangelo LD, Plebani A, Mazzolari E, Soresina A, Bondioni MP. Genetic causes of bronchiectasis: primary immune deficiencies and the lung. Respiration 2007;74:264–75. [DOI] [PubMed] [Google Scholar]
- 24.Buckley RH. Pulmonary complications of primary immunodeficiencies. Paediatr Respir Rev 2004;5 Suppl A:S225–33. [DOI] [PubMed] [Google Scholar]
- 25.Eijkhout HW, van Der Meer JW, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med 2001;135:165–74. [DOI] [PubMed] [Google Scholar]
- 26.Stiehm ER, Chin TW, Haas A, Peerless AG. Infectious complications of the primary immunodeficiencies. Clin Immunol Immunopathol 1986;40:69–86. [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA, Rosen FS. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med 1966;275:826–31. [DOI] [PubMed] [Google Scholar]
- 28.Stubbs A, Bangs C, Shillitoe B, Edgar JD, Burns SO, Thomas M, et al. Bronchiectasis and deteriorating lung function in agammaglobulinaemia despite immunoglobulin replacement therapy. Clin Exp Immunol 2018;191:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol 2011;31:315–22. [DOI] [PubMed] [Google Scholar]
- 30.Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F, et al. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol 2002;104:221–30. [DOI] [PubMed] [Google Scholar]
- 31.Maglione PJ. Autoimmune and Lymphoproliferative Complications of Common Variable Immunodeficiency. Curr Allergy Asthma Rep 2016;16:19. [DOI] [PubMed] [Google Scholar]
- 32.Aghamohammadi A, Allahverdi A, Abolhassani H, Moazzami K, Alizadeh H, Gharagozlou M, et al. Comparison of pulmonary diseases in common variable immunodeficiency and X-linked agammaglobulinaemia. Respirology 2010;15:289–95. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger T, Fuleihan R, Cunningham-Rundles C, Maglione PJ. Factors Beyond Lack of Antibody Govern Pulmonary Complications in Primary Antibody Deficiency. J Clin Immunol 2019;39:440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durandy A, Peron S, Fischer A. Hyper-IgM syndromes. Curr Opin Rheumatol 2006;18:369–76. [DOI] [PubMed] [Google Scholar]
- 35.Jolles S, Sanchez-Ramon S, Quinti I, Soler-Palacin P, Agostini C, Florkin B, et al. Screening protocols to monitor respiratory status in primary immunodeficiency disease: findings from a European survey and subclinical infection working group. Clin Exp Immunol 2017;190:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reisi M, Azizi G, Kiaee F, Masiha F, Shirzadi R, Momen T, et al. Evaluation of pulmonary complications in patients with primary immunodeficiency disorders. Eur Ann Allergy Clin Immunol 2017;49:122–8. [PubMed] [Google Scholar]
- 37.Milota T, Bloomfield M, Parackova Z, Sediva A, Bartunkova J, Horvath R. Bronchial Asthma and Bronchial Hyperresponsiveness and Their Characteristics in Patients with Common Variable Immunodeficiency. Int Arch Allergy Immunol 2019;178:192–200. [DOI] [PubMed] [Google Scholar]
- 38.Farmer JR, Ong MS, Barmettler S, Yonker LM, Fuleihan R, Sullivan KE, et al. Common Variable Immunodeficiency Non-Infectious Disease Endotypes Redefined Using Unbiased Network Clustering in Large Electronic Datasets. Front Immunol 2017;8:1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozcan C, Metin A, Erkocoglu M, Kocabas CN. Bronchial hyperreactivity in children with antibody deficiencies. Allergol Immunopathol (Madr) 2015;43:57–61. [DOI] [PubMed] [Google Scholar]
- 40.Urm SH, Yun HD, Fenta YA, Yoo KH, Abraham RS, Hagan J, et al. Asthma and risk of selective IgA deficiency or common variable immunodeficiency: a population-based case-control study. Mayo Clin Proc 2013;88:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agondi RC, Barros MT, Rizzo LV, Kalil J, Giavina-Bianchi P. Allergic asthma in patients with common variable immunodeficiency. Allergy 2010;65:510–5. [DOI] [PubMed] [Google Scholar]
- 42.McCullagh BN, Comellas AP, Ballas ZK, Newell JD Jr., Zimmerman MB, Azar AE. Antibody deficiency in patients with frequent exacerbations of Chronic Obstructive Pulmonary Disease (COPD). PLoS One 2017;12:e0172437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, et al. Secretory IgA Deficiency in Individual Small Airways Is Associated with Persistent Inflammation and Remodeling. Am J Respir Crit Care Med 2017;195:1010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Putcha N, Paul GG, Azar A, Wise RA, O’Neal WK, Dransfield MT, et al. Lower serum IgA is associated with COPD exacerbation risk in SPIROMICS. PLoS One 2018;13:e0194924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maarschalk-Ellerbroek LJ, de Jong PA, van Montfrans JM, Lammers JW, Bloem AC, Hoepelman AI, et al. CT screening for pulmonary pathology in common variable immunodeficiency disorders and the correlation with clinical and immunological parameters. J Clin Immunol 2014;34:642–54. [DOI] [PubMed] [Google Scholar]
- 46.Roifman CM, Gelfand EW. Replacement therapy with high dose intravenous gamma-globulin improves chronic sinopulmonary disease in patients with hypogammaglobulinemia. Pediatr Infect Dis J 1988;7:S92–6. [PubMed] [Google Scholar]
- 47.Tiotiu A, Salvator H, Jaussaud R, Jankowski R, Couderc LJ, Catherinot E, et al. Efficacy of immunoglobulin replacement therapy and azithromycin in severe asthma with antibody deficiency. Allergol Int 2019; [DOI] [PubMed] [Google Scholar]
- 48.Kim JH, Ye YM, Ban GY, Shin YS, Lee HY, Nam YH, et al. Effects of Immunoglobulin Replacement on Asthma Exacerbation in Adult Asthmatics with IgG Subclass Deficiency. Allergy Asthma Immunol Res 2017;9:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley CM, Sciurba FC. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. Jama 2019;321:786–97. [DOI] [PubMed] [Google Scholar]
- 50.Wu TD, Brigham EP, McCormack MC. Asthma in the Primary Care Setting. Med Clin North Am 2019;103:435–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. [DOI] [PubMed] [Google Scholar]
- 52.Barker AF. Bronchiectasis. N Engl J Med 2002;346:1383–93. [DOI] [PubMed] [Google Scholar]
- 53.Cole PJ. Inflammation: a two-edged sword--the model of bronchiectasis. Eur J Respir Dis Suppl 1986;147:6–15. [PubMed] [Google Scholar]
- 54.Somani SN, Kwah JH, Yeh C, Conley DB, Grammer LC 3rd, Kern RC, et al. Prevalence and characterization of chronic rhinosinusitis in patients with non-cystic fibrosis bronchiectasis at a tertiary care center in the United States. Int Forum Allergy Rhinol 2019;9:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maglione PJ, Overbey JR, Radigan L, Bagiella E, Cunningham-Rundles C. Pulmonary radiologic findings in common variable immunodeficiency: clinical and immunological correlations. Ann Allergy Asthma Immunol 2014;113:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gathmann B, Mahlaoui N, Gerard L, Oksenhendler E, Warnatz K, Schulze I, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014;134:116–26. [DOI] [PubMed] [Google Scholar]
- 57.Schutz K, Alecsandru D, Grimbacher B, Haddock J, Bruining A, Driessen G, et al. Imaging of Bronchial Pathology in Antibody Deficiency: Data from the European Chest CT Group. J Clin Immunol 2019;39:45–54. [DOI] [PubMed] [Google Scholar]
- 58.Aghamohammadi A, Cheraghi T, Gharagozlou M, Movahedi M, Rezaei N, Yeganeh M, et al. IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol 2009;29:130–6. [DOI] [PubMed] [Google Scholar]
- 59.Bjorkander J, Bake B, Oxelius VA, Hanson LA. Impaired lung function in patients with IgA deficiency and low levels of IgG2 or IgG3. N Engl J Med 1985;313:720–4. [DOI] [PubMed] [Google Scholar]
- 60.Stanley PJ, Corbo G, Cole PJ. Serum IgG subclasses in chronic and recurrent respiratory infections. Clin Exp Immunol 1984;58:703–8. [PMC free article] [PubMed] [Google Scholar]
- 61.De Gracia J, Rodrigo MJ, Morell F, Vendrell M, Miravitlles M, Cruz MJ, et al. IgG subclass deficiencies associated with bronchiectasis. Am J Respir Crit Care Med 1996;153:650–5. [DOI] [PubMed] [Google Scholar]
- 62.van Kessel DA, van Velzen-Blad H, van den Bosch JM, Rijkers GT. Impaired pneumococcal antibody response in bronchiectasis of unknown aetiology. Eur Respir J 2005;25:482–9. [DOI] [PubMed] [Google Scholar]
- 63.Hodkinson JP, Bangs C, Wartenberg-Demand A, Bauhofer A, Langohr P, Buckland MS, et al. Low IgA and IgM Is Associated with a Higher Prevalence of Bronchiectasis in Primary Antibody Deficiency. J Clin Immunol 2017;37:329–31. [DOI] [PubMed] [Google Scholar]
- 64.Langereis JD, van der Flier M, de Jonge MI. Limited Innovations After More Than 65 Years of Immunoglobulin Replacement Therapy: Potential of IgA- and IgM-Enriched Formulations to Prevent Bacterial Respiratory Tract Infections. Front Immunol 2018;9:1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science 2013;342:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira AC, Kokron CM, Romagnolo BM, Yagi CS, Saldiva PH, Lorenzi Filho G, et al. Analysis of the sputum and inflammatory alterations of the airways in patients with common variable immunodeficiency and bronchiectasis. Clinics (Sao Paulo) 2009;64:1155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jorgensen SF, Troseid M, Kummen M, Anmarkrud JA, Michelsen AE, Osnes LT, et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol 2016;9:1455–65. [DOI] [PubMed] [Google Scholar]
- 68.Fadlallah J, El Kafsi H, Sterlin D, Juste C, Parizot C, Dorgham K, et al. Microbial ecology perturbation in human IgA deficiency. Sci Transl Med 2018;10: [DOI] [PubMed] [Google Scholar]
- 69.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Gallagher M, Holmes PW. Outcome in adult bronchiectasis. Copd 2005;2:27–34. [DOI] [PubMed] [Google Scholar]
- 70.Twiss J, Stewart AW, Byrnes CA. Longitudinal pulmonary function of childhood bronchiectasis and comparison with cystic fibrosis. Thorax 2006;61:414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, Roman-Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007;132:1565–72. [DOI] [PubMed] [Google Scholar]
- 72.Hampson FA, Chandra A, Screaton NJ, Condliffe A, Kumararatne DS, Exley AR, et al. Respiratory disease in common variable immunodeficiency and other primary immunodeficiency disorders. Clin Radiol 2012;67:587–95. [DOI] [PubMed] [Google Scholar]
- 73.Hurst JR, Workman S, Garcha DS, Seneviratne SL, Haddock JA, Grimbacher B. Activity, severity and impact of respiratory disease in primary antibody deficiency syndromes. J Clin Immunol 2014;34:68–75. [DOI] [PubMed] [Google Scholar]
- 74.Griffith DE, Aksamit TR. Bronchiectasis and nontuberculous mycobacterial disease. Clin Chest Med 2012;33:283–95. [DOI] [PubMed] [Google Scholar]
- 75.Kipourou M, Manika K, Papavasileiou A, Pitsiou G, Lada M, Ntinapogias E, et al. Immunomodulatory effect of macrolides: At what cost? Respir Med Case Rep 2016;17:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goussault H, Salvator H, Catherinot E, Chabi ML, Tcherakian C, Chabrol A, et al. Primary immunodeficiency-related bronchiectasis in adults: comparison with bronchiectasis of other etiologies in a French reference center. Respir Res 2019;20:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J 2015;45:1446–62. [DOI] [PubMed] [Google Scholar]
- 78.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol 2005;94:S1–63. [DOI] [PubMed] [Google Scholar]
- 79.Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2015;Cd010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. Jama 2013;309:1251–9. [DOI] [PubMed] [Google Scholar]
- 81.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:660–7. [DOI] [PubMed] [Google Scholar]
- 82.Fan LC, Lu HW, Wei P, Ji XB, Liang S, Xu JF. Effects of long-term use of macrolides in patients with non-cystic fibrosis bronchiectasis: a meta-analysis of randomized controlled trials. BMC Infect Dis 2015;15:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tay GT, Reid DW, Bell SC. Inhaled antibiotics in Cystic Fibrosis (CF) and non-CF bronchiectasis. Semin Respir Crit Care Med 2015;36:267–86. [DOI] [PubMed] [Google Scholar]
- 84.Mall MA, Danahay H, Boucher RC. Emerging Concepts and Therapies for Mucoobstructive Lung Disease. Ann Am Thorac Soc 2018;15:S216–s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verma N, Grimbacher B, Hurst JR. Lung disease in primary antibody deficiency. Lancet Respir Med 2015;3:651–60. [DOI] [PubMed] [Google Scholar]
- 86.Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol 1999;104:1031–6. [DOI] [PubMed] [Google Scholar]
- 87.Maglione PJ, Overbey JR, Cunningham-Rundles C. Progression of Common Variable Immunodeficiency Interstitial Lung Disease Accompanies Distinct Pulmonary and Laboratory Findings. J Allergy Clin Immunol Pract 2015;3:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–84. [DOI] [PubMed] [Google Scholar]
- 89.Quartier P, Bustamante J, Sanal O, Plebani A, Debre M, Deville A, et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to Activation-Induced Cytidine Deaminase deficiency. Clin Immunol 2004;110:22–9. [DOI] [PubMed] [Google Scholar]
- 90.Cabral-Marques O, Klaver S, Schimke LF, Ascendino EH, Khan TA, Pereira PV, et al. First report of the Hyper-IgM syndrome Registry of the Latin American Society for Immunodeficiencies: novel mutations, unique infections, and outcomes. J Clin Immunol 2014;34:146–56. [DOI] [PubMed] [Google Scholar]
- 91.Costa-Carvalho BT, Wandalsen GF, Pulici G, Aranda CS, Sole D. Pulmonary complications in patients with antibody deficiency. Allergol Immunopathol (Madr) 2011;39:128–32. [DOI] [PubMed] [Google Scholar]
- 92.Toth B, Volokha A, Mihas A, Pac M, Bernatowska E, Kondratenko I, et al. Genetic and demographic features of X-linked agammaglobulinemia in Eastern and Central Europe: a cohort study. Mol Immunol 2009;46:2140–6. [DOI] [PubMed] [Google Scholar]
- 93.Lee PP, Chen TX, Jiang LP, Chan KW, Yang W, Lee BW, et al. Clinical characteristics and genotype-phenotype correlation in 62 patients with X-linked agammaglobulinemia. J Clin Immunol 2010;30:121–31. [DOI] [PubMed] [Google Scholar]
- 94.Aadam Z, Kechout N, Barakat A, Chan KW, Ben-Ali M, Ben-Mustapha I, et al. X-Linked Agammagobulinemia in a Large Series of North African Patients: Frequency, Clinical Features and Novel BTK Mutations. J Clin Immunol 2016;36:187–94. [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Garcia E, Staines-Boone AT, Vargas-Hernandez A, Gonzalez-Serrano ME, Carrillo-Tapia E, Mogica-Martinez D, et al. Clinical and mutational features of X-linked agammaglobulinemia in Mexico. Clin Immunol 2016;165:38–44. [DOI] [PubMed] [Google Scholar]
- 96.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 2008;112:277–86. [DOI] [PubMed] [Google Scholar]
- 97.Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol 2004;114:415–21. [DOI] [PubMed] [Google Scholar]
- 98.Maglione PJ, Gyimesi G, Cols M, Radigan L, Ko HM, Weinberger T, et al. BAFF-driven B cell hyperplasia underlies lung disease in common variable immunodeficiency. JCI Insight 2019;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deya-Martinez A, Esteve-Sole A, Velez-Tirado N, Celis V, Costa J, Cols M, et al. Sirolimus as an alternative treatment in patients with granulomatous-lymphocytic lung disease and humoral immunodeficiency with impaired regulatory T cells. Pediatr Allergy Immunol 2018;29:425–32. [DOI] [PubMed] [Google Scholar]
- 100.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 2008;111:77–85. [DOI] [PubMed] [Google Scholar]
- 101.Roca B, Ferran G, Simon E, Cortes V. Lymphoid hyperplasia of the lung and Evans’ syndrome in IgA deficiency. Am J Med 1999;106:121–2. [DOI] [PubMed] [Google Scholar]
- 102.Ozkan H, Atlihan F, Genel F, Targan S, Gunvar T. IgA and/or IgG subclass deficiency in children with recurrent respiratory infections and its relationship with chronic pulmonary damage. J Investig Allergol Clin Immunol 2005;15:69–74. [PubMed] [Google Scholar]
- 103.Reichenberger F, Wyser C, Gonon M, Cathomas G, Tamm M. Pulmonary mucosa-associated lymphoid tissue lymphoma in a patient with common variable immunodeficiency syndrome. Respiration 2001;68:109–12. [DOI] [PubMed] [Google Scholar]
- 104.Carrillo J, Restrepo CS, Rosado de Christenson M, Ojeda Leon P, Lucia Rivera A, Koss MN. Lymphoproliferative lung disorders: a radiologic-pathologic overview. Part I: Reactive disorders. Semin Ultrasound CT MR 2013;34:525–34. [DOI] [PubMed] [Google Scholar]
- 105.da Silva SP, Resnick E, Lucas M, Lortan J, Patel S, Cunningham-Rundles C, et al. Lymphoid proliferations of indeterminate malignant potential arising in adults with common variable immunodeficiency disorders: unusual case studies and immunohistological review in the light of possible causative events. J Clin Immunol 2011;31:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol 2009;133:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roberton BJ, Hansell DM. Organizing pneumonia: a kaleidoscope of concepts and morphologies. Eur Radiol 2011;21:2244–54. [DOI] [PubMed] [Google Scholar]
- 108.Rao N, Mackinnon AC, Routes JM. Granulomatous and lymphocytic interstitial lung disease: a spectrum of pulmonary histopathologic lesions in common variable immunodeficiency--histologic and immunohistochemical analyses of 16 cases. Hum Pathol 2015;46:1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian X, Yi ES, Ryu JH. Lymphocytic interstitial pneumonia and other benign lymphoid disorders. Semin Respir Crit Care Med 2012;33:450–61. [DOI] [PubMed] [Google Scholar]
- 110.Guinee DG Jr. Update on nonneoplastic pulmonary lymphoproliferative disorders and related entities. Arch Pathol Lab Med 2010;134:691–701. [DOI] [PubMed] [Google Scholar]
- 111.Verbsky JW, Routes JM. Sarcoidosis and common variable immunodeficiency: similarities and differences. Semin Respir Crit Care Med 2014;35:330–5. [DOI] [PubMed] [Google Scholar]
- 112.Maglione PJ, Ko HM, Tokuyama M, Gyimesi G, Soof C, Li M, et al. Serum B-Cell Maturation Antigen (BCMA) Levels Differentiate Primary Antibody Deficiencies. J Allergy Clin Immunol Pract 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med 2005;202:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bailey MD, Mitchell GL, Dhaliwal DK, Boxer Wachler BS, Zadnik K. Patient satisfaction and visual symptoms after laser in situ keratomileusis. Ophthalmology 2003;110:1371–8. [DOI] [PubMed] [Google Scholar]
- 115.Barbera JA, Hayashi S, Hegele RG, Hogg JC. Detection of Epstein-Barr virus in lymphocytic interstitial pneumonia by in situ hybridization. Am Rev Respir Dis 1992;145:940–6. [DOI] [PubMed] [Google Scholar]
- 116.San-Juan R, Comoli P, Caillard S, Moulin B, Hirsch HH, Meylan P. Epstein-Barr virus-related post-transplant lymphoproliferative disorder in solid organ transplant recipients. Clin Microbiol Infect 2014;20 Suppl 7:109–18. [DOI] [PubMed] [Google Scholar]
- 117.Unger S, Seidl M, Schmitt-Graeff A, Bohm J, Schrenk K, Wehr C, et al. Ill-defined germinal centers and severely reduced plasma cells are histological hallmarks of lymphadenopathy in patients with common variable immunodeficiency. J Clin Immunol 2014;34:615–26. [DOI] [PubMed] [Google Scholar]
- 118.Young BA, Hanson KE, Gomez CA. Molecular Diagnostic Advances in Transplant Infectious Diseases. Curr Infect Dis Rep 2019;21:52. [DOI] [PubMed] [Google Scholar]
- 119.Ryu JH. Classification and approach to bronchiolar diseases. Curr Opin Pulm Med 2006;12:145–51. [DOI] [PubMed] [Google Scholar]
- 120.Wakamatsu K, Nagata N, Taguchi K, Takakura K, Harada C, Kumazoe H, et al. A Case of Follicular Bronchiolitis as the Histological Counterpart to Nodular Opacities in Bronchiectatic Mycobacterium avium Complex Disease. Case Rep Pulmonol 2012;2012:214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel S, Anzilotti C, Lucas M, Moore N, Chapel H. Interstitial lung disease in patients with common variable immunodeficiency disorders: several different pathologies? Clin Exp Immunol 2019;198:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Torigian DA, LaRosa DF, Levinson AI, Litzky LA, Miller WT Jr. Granulomatous-lymphocytic interstitial lung disease associated with common variable immunodeficiency: CT findings. J Thorac Imaging 2008;23:162–9. [DOI] [PubMed] [Google Scholar]
- 123.Bondioni MP, Soresina A, Lougaris V, Gatta D, Plebani A, Maroldi R. Common variable immunodeficiency: computed tomography evaluation of bronchopulmonary changes including nodular lesions in 40 patients. Correlation with clinical and immunological data. J Comput Assist Tomogr 2010;34:395–401. [DOI] [PubMed] [Google Scholar]
- 124.Allenspach E, Torgerson TR. Autoimmunity and Primary Immunodeficiency Disorders. J Clin Immunol 2016;36 Suppl 1:57–67. [DOI] [PubMed] [Google Scholar]
- 125.Bendstrup E, Moller J, Kronborg-White S, Prior TS, Hyldgaard C. Interstitial Lung Disease in Rheumatoid Arthritis Remains a Challenge for Clinicians. J Clin Med 2019;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gupta S, Ferrada MA, Hasni SA. Pulmonary Manifestations of Primary Sjogren’s Syndrome: Underlying Immunological Mechanisms, Clinical Presentation, and Management. Front Immunol 2019;10:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014;345:1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 2014;20:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwab C, Gabrysch A, Olbrich P, Patino V, Warnatz K, Wolff D, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol 2018;142:1932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gamez-Diaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol 2016;137:223–30. [DOI] [PubMed] [Google Scholar]
- 131.Habibi S, Zaki-Dizaji M, Rafiemanesh H, Lo B, Jamee M, Gamez-Diaz L, et al. Clinical, Immunologic, and Molecular Spectrum of Patients with LPS-Responsive Beige-Like Anchor Protein Deficiency: A Systematic Review. J Allergy Clin Immunol Pract 2019;7:2379–86.e5. [DOI] [PubMed] [Google Scholar]
- 132.Cagdas D, Halacli SO, Tan C, Lo B, Cetinkaya PG, Esenboga S, et al. A Spectrum of Clinical Findings from ALPS to CVID: Several Novel LRBA Defects. J Clin Immunol 2019;39:726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 2015;125:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet 2014;46:812–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haapaniemi EM, Kaustio M, Rajala HL, van Adrichem AJ, Kainulainen L, Glumoff V, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood 2015;125:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol 2014;15:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maglione PJ, Ko HM, Beasley MB, Strauchen JA, Cunningham-Rundles C. Tertiary lymphoid neogenesis is a component of pulmonary lymphoid hyperplasia in patients with common variable immunodeficiency. J Allergy Clin Immunol 2014;133:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology 2016;147:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boursiquot JN, Gerard L, Malphettes M, Fieschi C, Galicier L, Boutboul D, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol 2013;33:84–95. [DOI] [PubMed] [Google Scholar]
- 140.Chase NM, Verbsky JW, Hintermeyer MK, Waukau JK, Tomita-Mitchell A, Casper JT, et al. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID). J Clin Immunol 2013;33:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jolles S, Carne E, Brouns M, El-Shanawany T, Williams P, Marshall C, et al. FDG PET-CT imaging of therapeutic response in granulomatous lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID). Clin Exp Immunol 2017;187:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zdziarski P, Gamian A. Lymphoid Interstitial Pneumonia in Common Variable Immune Deficiency - Case Report With Disease Monitoring in Various Therapeutic Options: Pleiotropic Effects of Rituximab Regimens. Front Pharmacol 2018;9:1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arish N, Eldor R, Fellig Y, Bogot N, Laxer U, Izhar U, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006;61:1096–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hayakawa H, Sato A, Imokawa S, Toyoshima M, Chida K, Iwata M. Bronchiolar disease in rheumatoid arthritis. Am J Respir Crit Care Med 1996; 154:1531–6. [DOI] [PubMed] [Google Scholar]
- 145.Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med 2003;168:1277–92. [DOI] [PubMed] [Google Scholar]
- 146.Aerni MR, Vassallo R, Myers JL, Lindell RM, Ryu JH. Follicular bronchiolitis in surgical lung biopsies: clinical implications in 12 patients. Respir Med 2008;102:307–12. [DOI] [PubMed] [Google Scholar]
- 147.Kohler PF, Cook RD, Brown WR, Manguso RL. Common variable hypogammaglobulinemia with T-cell nodular lymphoid interstitial pneumonitis and B-cell nodular lymphoid hyperplasia: different lymphocyte populations with a similar response to prednisone therapy. J Allergy Clin Immunol 1982;70:299–305. [DOI] [PubMed] [Google Scholar]
- 148.Davies CW, Juniper MC, Gray W, Gleeson FV, Chapel HM, Davies RJ. Lymphoid interstitial pneumonitis associated with common variable hypogammaglobulinaemia treated with cyclosporin A. Thorax 2000;55:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol 2005;95:293–300. [DOI] [PubMed] [Google Scholar]
- 150.Franxman TJ, Howe LE, Baker JR Jr. Infliximab for treatment of granulomatous disease in patients with common variable immunodeficiency. J Clin Immunol 2014;34:820–7. [DOI] [PubMed] [Google Scholar]
- 151.Chien SH, Liu CJ, Hong YC, Teng CJ, Hu YW, Shen CC, et al. Use of azathioprine for graft-vs-host disease is the major risk for development of secondary malignancies after haematopoietic stem cell transplantation: a nationwide population-based study. Br J Cancer 2015;112:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gobert D, Bussel JB, Cunningham-Rundles C, Galicier L, Dechartres A, Berezne A, et al. Efficacy and safety of rituximab in common variable immunodeficiency-associated immune cytopenias: a retrospective multicentre study on 33 patients. Br J Haematol 2011;155:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 2015;349:436–40. [DOI] [PubMed] [Google Scholar]
- 154.Rao VK, Webster S, Dalm V, Sediva A, van Hagen PM, Holland S, et al. Effective “activated PI3Kdelta syndrome”-targeted therapy with the PI3Kdelta inhibitor leniolisib. Blood 2017;130:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol 2018;142:1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Burton CM, Milman N, Andersen CB, Marquart H, Iversen M. Common variable immune deficiency and lung transplantation. Scand J Infect Dis 2007;39:362–7. [DOI] [PubMed] [Google Scholar]
- 157.Wehr C, Gennery AR, Lindemans C, Schulz A, Hoenig M, Marks R, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol 2015;135:988–97.e6. [DOI] [PubMed] [Google Scholar]
- 158.Prasse A, Kayser G, Warnatz K. Common variable immunodeficiency-associated granulomatous and interstitial lung disease. Curr Opin Pulm Med 2013;19:503–9. [DOI] [PubMed] [Google Scholar]
- 159.Milito C, Pulvirenti F, Serra G, Valente M, Pesce AM, Granata G, et al. Lung magnetic resonance imaging with diffusion weighted imaging provides regional structural as well as functional information without radiation exposure in primary antibody deficiencies. J Clin Immunol 2015;35:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arslan S, Poyraz N, Ucar R, Yesildag M, Yesildag A, Caliskaner AZ. Magnetic Resonance Imaging May Be a Valuable Radiation-Free Technique for Lung Pathologies in Patients with Primary Immunodeficiency. J Clin Immunol 2016;36:66–72. [DOI] [PubMed] [Google Scholar]


