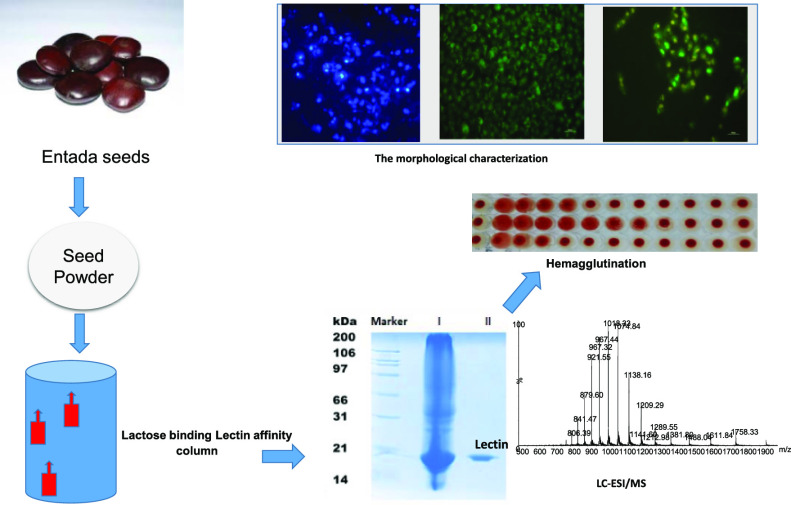
Abstract
A novel Entadin lectin was isolated, purified, and characterized from the seeds of Entada rheedii by ammonium sulfate precipitation, followed by lactose affinity chromatography. On sodium dodecyl sulfate polyacrylamide gel electrophoresis, the purified Entadin lectin appeared as a single band (monomeric in nature) with a molecular mass of approximately 20 kDa in both reducing and nonreducing conditions. Mass spectroscopic analysis confirms the molecular weight of Entadin lectin as 19,333 Da. Entadin lectin showed a highest titer value in agglutination against human blood group B red blood cells, and its hemagglutination activity was inhibited by lactose, cellobiose, and galactose. Periodic acid Schiff staining confirmed the glycoprotein nature of Entadin lectin with an approximately 5% carbohydrate content. This lectin is highly stable even after incubation at a wide range of temperatures (30–60 °C) and pHs (6–10). The antiproliferative effect of Entadin lectin against lung cancer cells A549 and cervical cancer cells HeLa showed IC50 values of 28 and 32 μg/mL, respectively, and no antiproliferative activity against normal cells was observed. Cell morphological studies revealed that Entadin lectin induced apoptosis in both A549 and HeLa cancer cells, which was confirmed by acridine orange/ethidium bromide and Hoechst (33258) nuclear counterstaining.
1. Introduction
Diverse carbohydrate structures present on the surface of the cells help them to interact with the outer environment.1 The interaction of cell surface glycans with various glycoproteins plays a crucial role in cell signaling, cell growth, metastasis, host–pathogen interaction, cell adhesion, and cell–cell communication.2,3 Lectins are glycoproteins that recognize cell surface glycan moieties specifically and reversibly that help to understand the carbohydrate structure and its physiological dynamics inside the cells.4 Lectins are glycoproteins of nonimmune origin distributed ubiquitously in nature (bacteria, fungi, plants, and animals) and found in various structural and functional forms.5−10 Compared to animal and other lectins, plant lectins have been studied more extensively because of their enormous diversity and easy accessibility. Although the physiological functions of lectins in plants remain unclear, there are various reports which revealed that in plants, lectins are involved in insecticidal activities, antiparasitic activities, antifungal activities, antibacterial activities, immunological response, cell wall propagation, seed germination, hormone regulation, and various cellular activities.11−14 The ability of lectins to recognize glycan moieties is used by biotechnologists for a wide range of applications such as purification and analysis of glycoconjugates, visualization of multisynaptic neural pathways, bacterial mapping, and agglutination of cells.15−18 A number of studies demonstrated that lectins can distinguish malignant or tumor cells from normal cells.19,20 This unique property of lectins received a lot of attention from cancer biologists.20 Lectins are also known to induce apoptosis in cells, which makes them an excellent molecule against cancer treatment.21−23
In recent years, lectins from legume family have been studied more extensively because of their potential role in antitumor activity.6 Legume lectins from Phaseolus coccineus, Bauhinia ungulata, and Lotus corniculatus(24−26) showed substantial antitumor activities against various cancer cell lines. Therefore, it is important to purify and characterize new lectins from leguminous families to understand the mechanism of lectins against tumor cells. Entada rheedii plant belongs to the Leguminosae family, which is widely distributed from Africa to Australia. In Asian regions, the plant extract (roots, leaves, and seeds) of this plant was also used as an anti-inflammatory agent to treat liver dysfunction.27 In the present study, we have isolated and characterized a novel lactose binding lectin from E. rheedii seeds. Further, the biological activity of the purified lectin was elucidated, and its application in cancer biology was studied using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and cell morphological studies.
2. Results
2.1. Purification of E. rheedii Lectin
The soluble crude protein extract of Entada seeds showed significant hemagglutination activity against the human blood group B, and all experiments in this paper were done by using human blood group B erythrocyte samples. Entadin lectin was purified using the solid ammonium sulfate precipitation method, followed by single-step lactose affinity column chromatography. A fraction of 20–40% ammonium sulfate precipitation, which showed hemagglutination activity, was subjected to lactose affinity column chromatography. The unbound fraction (peak-I) was collected, whereas the bound protein (peak-II) was eluted with 400 mM lactose in an equilibrium buffer. Hemagglutination activity was observed only for peak-II. The yield and specific activity of Entadin lectin for each step have been mentioned in Table 1. The yield of Entadin lectin was approximately 21.68%.
Table 1. Percentage (%) Recovery of Lectin at Different Purification Stepsa.
| procedure/step | fraction volume (mL) | protein (mg/mL) | total protein (mg) | recovery (%) | hemagglutination unit (HU) | total hemagglutination activity | specific hemagglutination activity | fold purification |
|---|---|---|---|---|---|---|---|---|
| crude | 23 | 25.62 | 589.26 | 100.00 | 1 | 23 | 0.039 | 1 |
| (NH4)2SO4 precipitation | 13 | 15.76 | 204.88 | 034.76 | 2 | 26 | 0.126 | 3.230 |
| lactose affinity column | 15 | 08.52 | 127.80 | 021.68 | 7 | 105 | 0.466 | 11.94 |
Total hemagglutination activity = HU × volume (mL)/specific hemagglutination activity = HU/protein (mg/mL).
2.2. Molecular Mass Determination of Entadin Lectin
The obtained peak-II fraction was studied for protein purity using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis and molecular mass identification using liquid chromatography–mass spectrometry (LC–MS). As can be seen in Figure 1, lane III (Coomassie blue stain gel) and lane IV (silver stain gel) display that indeed the targeted lectin was obtained as a single band with approximately 20 kDa molecular weight both in the presence and in the absence of β-mercaptoethanol. Furthermore, electrospray ionization mass spectrometry (ESI-MS) of the same sample confirmed the intact molecular mass (M) of this purified lectin to be 19,333 Da (Maverage) (Figure 2), which indicated that Entadin lectin may exist in monomeric form in nature.
Figure 1.
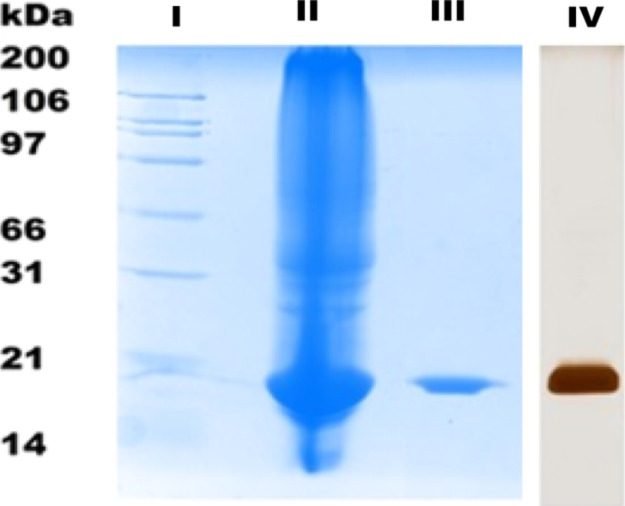
Analysis of purified Entadin lectin using a lactose affinity column: (LI) molecular weight marker, (LII) crude extract of E. rheedii seeds, (LIII) purified Entadin lectin shows 20 kDa on SDS-PAGE, and (LIV) silver staining of lactose column-purified Entadin lectin.
Figure 2.
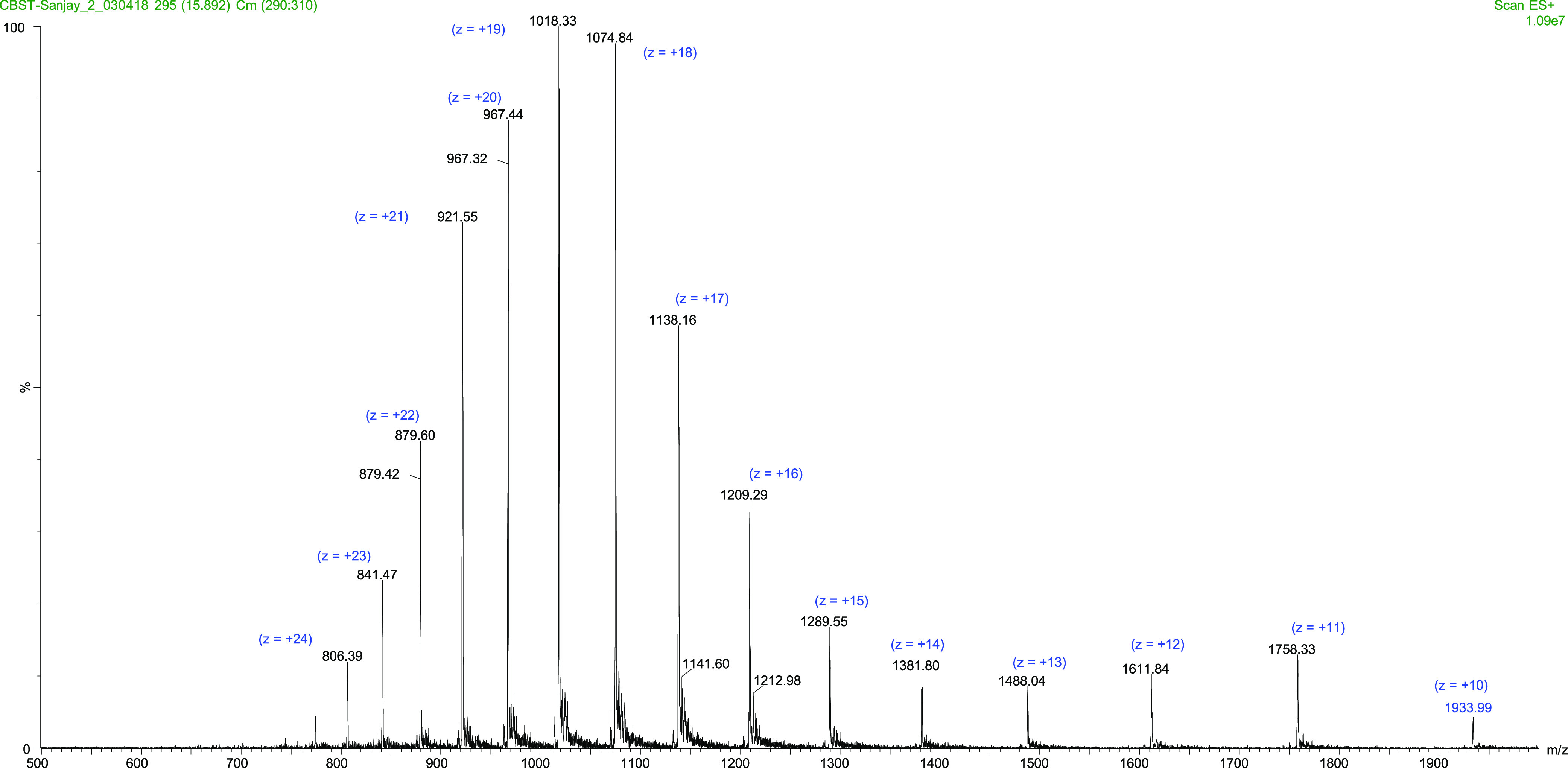
Mass spectra analysis of intact Entadin lectin through LC–ESI-MS.
2.3. Hemagglutination Activity and Hemagglutination Inhibition Assay
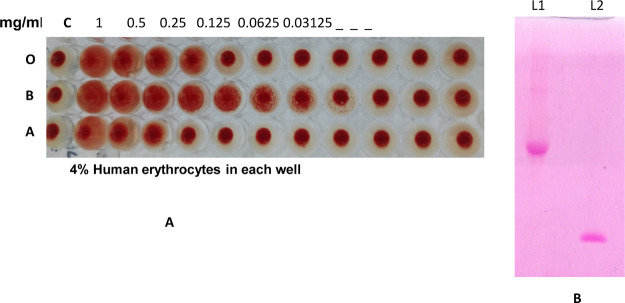
The purified Entadin lectin showed significant hemagglutination activity against B human blood group erythrocytes with a minimum agglutination concentration of 7.81 μg/mL (Figure 3A).
Figure 3.
(A) Hemagglutination activity of purified Entadin lectin using human O, B, and A blood cells. (B) PAS staining: L1—ovalbumin, L2—Entadin lectin.
The carbohydrate specificity of Entadin lectin was examined by the hemagglutination inhibition assay. The hemagglutination activity was inhibited by two disaccharides, lactose (0.625 mM) and cellobiose (5 mM), and a monosaccharide, d-galactose (5 mM) (Table 2). A similar sugar inhibition was also observed in other lectins isolated from Leguminosae plant species.28
Table 2. Minimum Inhibitory Concentration (MIC) Values of Different Sugars.
| sugars | MIC (mM) |
|---|---|
| mannose | no inhibition |
| glucose | no inhibition |
| fructose | no inhibition |
| arabinose | no inhibition |
| d-galactose | 5.00 |
| lactose | 0.625 |
| sucrose | no inhibition |
| cellobiose | 5.00 |
| ovalbumin | no inhibition |
2.4. Protein Content, Carbohydrate Content, and Glycosylation of Entadin Lectin
The total protein content in each step from crude to chromatographic fractions was determined as described by Bradford and is shown in the protein purification table (Table 1). The total carbohydrate content of Entadin lectin was calculated using anthrone and phenol–sulfuric acid methods. It was found to be 4.8% with anthrone assay and 5% with phenol–sulfuric acid. The carbohydrate content of Entadin lectin is close to that of Phaseolus lunatus lectin29 isolated from lima beans. The high concentration of carbohydrates may help in the stability of Entadin lectin,30 which needs to be tested. The purified Entadin lectin was stained with a periodic acid Schiff (PAS) reagent, which showed a pink band on the gel, indicating the presence of carbohydrate in the molecule and its glycosylic nature (Figure 3B).
2.5. pH and Temperature Stability of Entadin Lectin
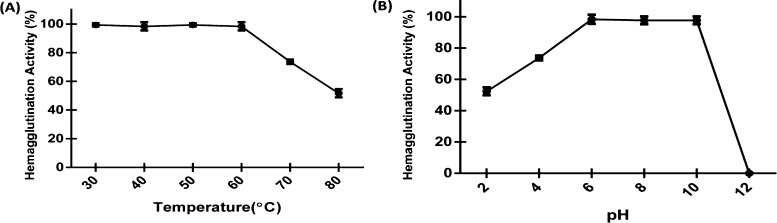
First, we tested the functional stability of purified Entadin lectin at various pHs ranging from 2 to 12. The hemagglutination activity was retained and showed stability up to pH ranging from 6 to 10. However, there was a gradual decrease in activity when the pH was below 6. The lectin retained activity up to 75% at pH 4 and up to 50% at pH 2, whereas above pH 10, the hemagglutination activity was completely lost (Figure 4B). In the next set of experiments, the temperature stability of Entadin lectin was tested over a wide range of temperatures from 30 to 80 °C. The purified Entadin lectin maintained its stability and activity up to the temperature ranging from 30 to 60 °C. In the case of temperatures above 60 °C, it retained its activity up to 75% at 70 °C and 50% at 80 °C (Figure 4A).
Figure 4.
Biophysical properties of Entadin lectin: (A) temperature stability at different temperatures and (B) pH stability at different pHs.
2.6. Metal Ion Effects on Entadin Lectin
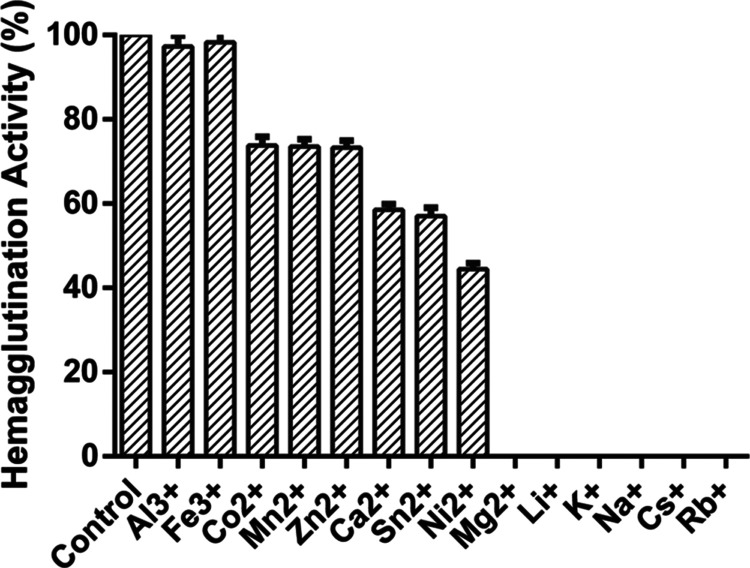
The hemagglutination assay (HA) activity of purified lectin was 100% retained after sequential dialysis against ethylenediaminetetraacetic acid (EDTA), followed by NaCl. The effect of various metal ions on purified lectin dialysis against EDTA showed that the HA activity was not affected by trivalent metal ions such as Al3+ and Fe3+. In the case of divalent ions, the presence of Mn2+, Co2+, and Zn2+ resulted in 30% activity loss; Ca2+ and Sn2+ yielded 40% activity; Ni2+ retained 60% activity, whereas Mg2+ completely inhibited the hemagglutination activity. In the case of monovalent metal ions such as Li+, K+, Na+, and Cs+, the hemagglutination activity was completely lost (Figure 5).
Figure 5.
Effect of monovalent, divalent, and trivalent metal ions on the hemagglutination activity of Entadin lectin.
2.7. Structural Aspect of Entadin Lectin
These results showed that the protein loses a considerable fraction of secondary structure during thermal denaturation. The protein maintained its secondary structure up to 60 °C with a slight change in its ellipticity (Figure 6). The major change in the mean residue ellipticity of the protein was found to be at 70, 75, and 80 °C, which confirms the existence of three intermediate states between the folding and unfolding state.
Figure 6.
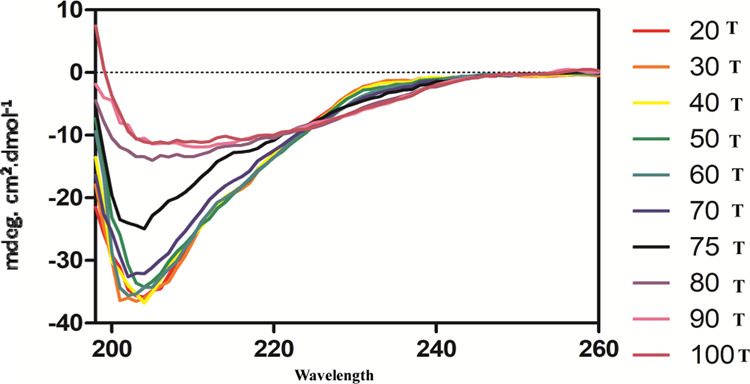
Spectroscopic studies of Entadin lectin at various temperatures.
2.8. In Vitro Cytotoxicity and Morphological Analysis of Entadin Lectin on A549, HeLa, and African Green Monkey Normal Kidney Cells (Vero)
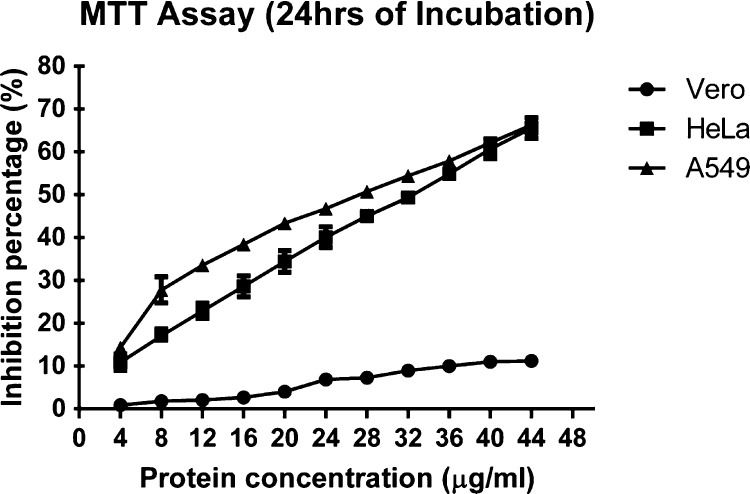
The dose-dependent MTT assay for Entadin lectin-treated cancer cells (A549 and HeLa) showed a significant reduction in cell viability, whereas in the case of normal cells, there was no significant change observed in cell viability. Entadin lectin at a concentration of 4–44 μg/mL showed a significant reduction in cell viability at 24 h with inhibitory concentrations (IC50) of 28 μg/mL for A549 cells and 32 μg/mL for HeLa cells (Figure 7). The cell killing efficiency of Entadin lectin by the MTT assay was also monitored for 48 h to observe the condition of the cells (Figure S1).
Figure 7.
Dose-dependent cytotoxic effect (MTT) of Entadin lectin on HeLa, A549, and Vero cells.
2.9. Acridine Orange/EtBr Staining for Cell Viability and Hoechst Nuclear Counterstaining Assay for Apoptosis
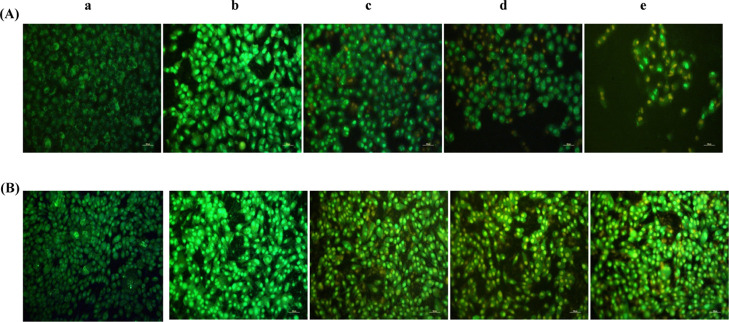
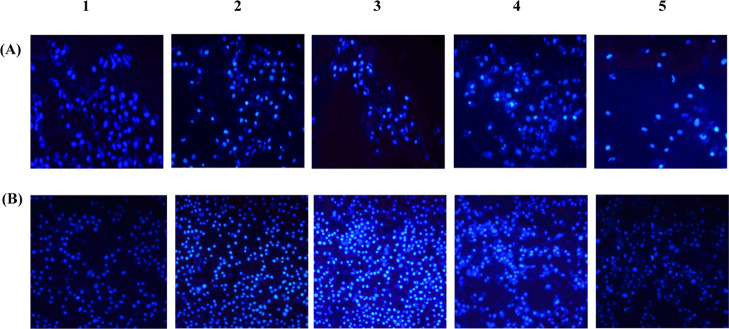
Morphological visualization of cells with fluorescent compounds such as acridine orange (AO)/EtBr provides reasonable information about cell viability. The fluorescence microscopic study of Entadin lectin treated on A549 and HeLa cells showed the formation of apoptotic bodies; after treatment with Entadin lectin, A549 and HeLa cells showed orange color because of AO staining and necrotic cells showed red color because of EtBr staining, whereas the control group showed a consistent green color (Figure 8). The nuclear fragmentation of both A549 and HeLa cells was observed using Hoechst dye stain, also used in chromatin condensation. The untreated cells showed normal nuclei, whereas the Entadin lectin-treated A549 and HeLa cells showed apoptotic bodies, which are significant to conclude that the purified lectin induces apoptosis inside the cells (Figure 9).
Figure 8.
Morphological characterization of cells treated with Entadin lectin further stained with AO/EtBr (A) human lung cancer cell line (A-549) and (B) human cervical cancer cell line (HeLa) with varying concentrations: (a) control, (b) 0.5× IC50 μg/mL, (c) 1× IC50 μg/mL, (d) 2× IC50 μg/mL, and (e) cells treated with doxorubicin considered as a positive control.
Figure 9.
(A and B) Morphological observation of treated human lung cancer cell line (A-549) and human cervical cancer cell line (HeLa) with different lectin concentrations: (1) control, (2) 0.5× IC50 μg/mL, (3) 1× IC50 μg/mL, (4) 2× IC50 μg/mL, and (5) positive control treated with doxorubicin further stained with Hoechst stain.
3. Discussion
Legume lectins are one of the most widely studied plant lectins because of their potential role in antitumor activity. Several lectins from this family were used against various cancer cell lines. E. rheedii plant is predominantly present in the southern part of India. Various parts of this plant are used for traditional medicinal purposes. In the present work, lectin from E. rheedii seeds was purified by ammonium sulfate precipitation, followed by lactose affinity chromatography. With the use of less chromatographic steps for purification, the purity and yield of Entadin lectin were high compared to conventional chromatographic methods, which were also used for the purification of Entadin lectin (data not included). Lectin agglutination activity was checked by using human blood group B, O, and A erythrocytes. The human blood group B erythrocytes exhibited the highest titer value in an agglutination assay. The lectin activity depends on various metal ions, primarily on divalent and trivalent metal ions, and categorized as metal-dependent and metal-independent lectins. Purified lectin dialyzed against EDTA showed 100% agglutination activity, indicating that Entadin lectin can be categorized as a metal-independent lectin.31 The HA activity of purified lectin dialyzed against EDTA was insensitive or unaffected in the presence of trivalent ions (Al3+ and Fe3+). In the case of divalent ions, except in the presence of Mg2+, the lectin HA activity was restored from 25 to 75%. However, in the case of monovalent ions, the Entadin lectin activity was not retained. It showed the metal ion selectivity of Entadin lectin.32 The sugar specificity of lectin was checked by using various disaccharide and monosaccharide molecules. A crude sample of Entadin lectin hemagglutination activity was strongly inhibited by lactose compared to galactose, which guided us to design a lactose affinity column for the purification of Entadin lectin. Interaction studies using Fourier transform infrared (FTIR) also showed (Figure S2) binding of Entadin lectin with lactose. The stability of Entadin lectin was assessed in various ranges of pHs and temperatures. The purified lectin maintained its HA activity up to 70 °C, whereas at 80 °C, it maintained its HA activity up to 50%. The high thermal stability of lectin, in general, is due to the rigid tertiary structure as well as high glycosylation of protein. It is very common in the case of plant lectins to possess a highly thermally stable structure. The circular dichroism (CD) data clearly indicated that up to 60 °C, the secondary structure of Entadin lectin has been preserved, due to which 100% hemagglutination activity has been achieved between 60 and 70 °C during the thermal stability experiment. With the increase of temperature up to 80 °C, circular dichroism revealed that the secondary structure of the protein was destructed or in the intermediate stage. Structural conformation change may be the possible reason for the hemagglutination activity to decrease up to 50% during the thermal stability experiment. The anticancer or antiproliferative activity of lectins has been shown using various cancer cell lines. Lectins in general bind to cancer cells or their receptors to induce either apoptosis or cytotoxic activity to reduce cancer cell growth. Entadin lectin showed antiproliferative activity with IC50 values of 28 μg/mL for A549 and 32 μg/mL for HeLa cells. A similar IC50 activity has been observed in the case of various lectins isolated from leguminous families (Table 3). The cell viability assay was done by AO/ethidium bromide (EtBr) dual staining methods and visualized by a fluorescence microscope for Entadin lectin-treated A-549 and HeLa cells. Apoptotic cells containing apoptotic bodies were observed in orange color and necrotic or dead cells were shown in red color, whereas living cells were shown in green color. The nuclear fragmentation of apoptotic cells increased when A549 and HeLa cells were treated with higher concentration of Entadin lectin, which was observed using Hoechst stain 33258. We for the first time report a highly stable lactose binding lectin with significant antiproliferative activity against various cell lines from E. rheedii seeds.
Table 3. MIC Values of Various Lectins.
| year | plant name | sugar specificity | cell line | incubation time (h) | IC50 | references | |
|---|---|---|---|---|---|---|---|
| 1 | 2013 | pod of Lotus corniculatus lectin | d-galactose binding lectin | human leukemic cell line (adherent type) (THP-1), lung cancer cell line (HOP62), and colon cancer cell line (HCT116) | 48 | THP-1, HOP62, and HCT116 with IC50 of 39, 50, and 60 μg/mL, respectively. | (26) |
| 2 | 2013 | Bauhinia ungulata L. seeds | d-galactose (2.1 mM), lactose (0.96 mM), and N-acetyl-d-galactosamine (0.63 mM) | human colon adenocarcinoma (HT-29 cell line) | 24 | 160 μg/mL | (33,34) |
| 3 | 2014 | Cicer arietinum seeds | 20 mM of N-acetyl-d-galactosamine | human oral carcinoma cells (KB) | 48 | 37.5 μg/mL (IC50) | (35) |
| 4 | 2014 | soybean/seeds | lactose specific | HeLa (cervical cancer), Hep2 (oral cancer), U373 MG (glioblastoma), HepG2 (liver cancer), MDA MB 231 (breast cancer), and HaCaT (human keratinocyte cell line) | 72 | HeLa, Hep2, HepG2, MDA MB 231, and U373 MG were 60.13 ± 3.5, 20.8 ± 1.2, 40.6 ± 6.1, 40.1 ± 7.7, and 51.0 ± 7.8 μg/mL, respectively | (36) |
| 5 | 2016 | Phaseolus lunatus billb/seeds | d-galactose, d-fructose, d-glucose, and mannitol | HepG2, HeLa, and K562 | 48 | 13.7 μM (K562), not reached to IC50 (HeLa and HepG2) | (37) |
| 6 | 2016 | lima bean variety (Phaseolus lunatus L. var. cascavel) | only N-acetyl-d-galactosamine | A375 melanoma cells | 48 and 72 | 100, 50, and 25 μg/mL at 48 h (73, 41, and 7%) at 72 h (83, 53, and 0%) | (38) |
| 7 | 2017 | Calliandra surinamensis leaf | the monosaccharides glucose, mannose, methyl-d glucopyranoside, N-acetylglucosamine, and galactose or by the disaccharide maltose | K562 (chronic myelogenous leukemia) and T47D (breast cancer) | 72 | IC50 observed with the concentrations of 67.04 ± 5.78 and 58.75 ± 2.5 μg/mL | (6) |
| 8 | 2017 | Cratylia mollis seed | NIL | human prostate adenocarcinoma (PC-3) | 24 | the native as well as recombinant Cratylia mollis seed lectin; IC50 were 29.91 and 39.69 μg/mL, respectively | (39) |
| 9 | 2018 | soybean/seeds | NIL | B16F1 melanoma cells | 24 | ∼30% cell growth inhibition of B16 melanoma cells at a concentration of 35 μg/mL | (40) |
| 10 | 2019 | E. rheedii | d-galactose (5 mM), cellobiose (5), and lactose (0.625 mM) | lung cancer cells A549 and human cervical cancer cells HeLa | 24 | A549 and HeLa cells with IC50 values 389 and 345 μg/mL, respectively | NA |
4. Conclusions
This is the first report of a highly stable lactose binding lectin from E. rheedii plant with significant antiproliferative activity against lung cancer cells A549 and cervical cancer cells HeLa with IC50 values of 28 and 32 μg/mL, respectively.
5. Methods and Materials
5.1. Extraction of Protein
Ganape kayi (E. rheedii) seeds were collected from the Shimoga Malnad region of the Western Ghats of Karnataka, India, during the summer season (April to May) and stored in 4 °C until used for study. The outer strong coats of the seeds were peeled off and the seeds were processed into a white powder. The white powder sample was suspended in 50 mM Tris buffer at pH 7.2 and kept overnight for homogenization at 4 °C. The homogenated sample was centrifuged at 8000 rpm (Eppendorf Centrifuge 5810 R) at 4 °C for 30 min. After centrifugation, the supernatant was collected and further processed for ammonium sulfate precipitation ((NH4)2SO4) at different concentrations of saturation (0–20, 20–40, 40–60, and 60–80%) at 4 °C. The sample after each step of ammonium sulfate precipitation was centrifuged at 8000 rpm at 4 °C for 20 min, and the protein in the form of pellet was collected. The pellet was dialyzed against a continuous flow of the same buffer (50 mM Tris buffer) at pH 7.2 using a dialysis membrane (HIMEDIA-Dialysis Membrane-60). Further, the sample which displayed hemagglutination activity (40% ammonium sulfate precipitate) was used for the purification of lectin. Sugar specificity (40% ammonium sulfate precipitate) was also checked to prepare the affinity column for the purification of lectin.
5.2. Preparation of a Lactose Affinity Column
Purification of lectins from different sources using lactose sugar specificity has been reported in the literature.41,42 A lactose affinity column has been developed, where Sepharose 4B (8 g) was washed with Milli-Q water and stored at 40 °C in absolute alcohol for 8 h, followed by alcohol decanting, and the resin was washed twice with Milli-Q water. Subsequently, Sepharose 4B was incubated in a buffer containing 5% epichlorohydrin and 0.4 M NaOH at 40 °C for 2 h with continuous shaking. Sepharose 4B suspension was transferred to a glass filter funnel and washed with 500 mL of Milli-Q water. Epoxy-activated Sepharose 4B was incubated in ammonium solution (12 mL) at 40 °C for 90 min, followed by transferring the suspension to a glass filter funnel and washed with 500 mL of Milli-Q water. Amino Sepharose 4B was then suspended in a buffer containing 0.2 M K2HPO4 (dipotassium phosphate), 208 mg of lactose, and 102 mg of NaCNBH3 (sodium cyanoborohydride) solution. The suspension was incubated at 25 °C for 10 days with occasional shaking. After this, the suspension was incubated in acetic anhydride (2 mL) to block free amino groups present on the suspension at 25 °C for 1 h. Lactamyl Sepharose 4B was transferred to a glass filter funnel and washed subsequently with 500 mL of Milli-Q water, followed by 0.1 M NaOH and again with 500 mL of Milli-Q water.
5.3. Purification and Quantification of Lectin
A dialyzed 40% ammonium sulfate precipitate sample of E. rheedii was loaded in a pre-equilibrated lactose affinity column with 50 mM Tris buffer at pH 7.2. Bound protein was eluted using 400 mM lactose. The eluted fraction was dialyzed against 50 mM Tris buffer at pH 7.2 to remove lactose, and the dialyzed solution was concentrated using the Centricon membrane (Millipore Merck 3 kDa). Total protein content from crude extract to purified fractions of lectin was determined by the Bradford assay43 using bovine serum albumin as a standard, and absorbance was measured at 595 nm.
5.4. SDS-PAGE and Mass Spectrometry
The molecular weight and purity of purified Entadin lectin were determined by SDS-PAGE (12.5%) in the presence or absence of β-mercaptoethanol. Standard broad-range protein markers (5 μL; Bio-Rad—β-galactosidase 116, phosphorylase b97, serum albumin 66, ovalbumin 45, carbonic anhydrase 31, trypsin inhibitor 21, lysozyme 14, and aprotinin 6), 10 μL of purified protein, and a crude sample of E. rheedii were loaded on SDS-PAGE. The gel was stained with Coomassie brilliant blue G-250 stain. Furthermore, the purity of Entadin lectin was determined by silver staining. To determine the mass, the purified Entadin lectin was injected into the Acquity UPLC coupled to Quattro premier XE (Waters), which is a triple quadrupole mass spectrometer. LC was carried out in the ACQUITY UPLC BEH C18 column using Milli-Q water containing 0.1% formic acid as solvent A and acetonitrile containing 0.1% formic acid as solvent B. A linear gradient was followed by keeping 5–80% of solvent B for 28 min with a flow rate of 0.1 mL/min. MS was performed in the positive ion mode by keeping the mass range of m/z 500–2000. Other parameters of MS to record the sample are capillary voltage ∼3 kV, cone voltage 25 V, source temperature 100 °C, and desolvation temperature 200 °C. Moreover, here hydrogen is used for the cone gas flow and desolvation gas flow with 75 and 600 L/h, respectively. The recorded data were processed by mass lynx.
5.5. Hemagglutination Activity and Hemagglutination Inhibition Assay
The HA was conducted by using human erythrocytes (A, B, and O) in a 96-well microtiter U-plate. For erythrocytes, fresh blood from healthy volunteers was collected following the approval from the Institutional Ethical Committee for studies on human samples (IECH) of VIT (Ref. no. VIT/IECH/08/May 2019). Purified Entadin lectin (100 μL, 1 mg/mL) was added to the first well of the 96-well microtiter U-plate, from which 50 μL was serially diluted into the consecutive well with phosphate-buffered saline (PBS) at pH 7.2 containing 4% human red blood cell suspension. After incubation at 37 °C for 30 min, the hemagglutination activity was observed visually and compared with the negative control, erythrocytes, incubated with PBS at pH 7.2. Hemagglutination unit (HU) was defined as the reciprocal of the highest dilution of Entadin lectin that showed visible agglutination under assay conditions. However, specific hemagglutination was calculated as the ratio between HU and protein concentration (mg/mL). All the hemagglutination experiments were done in triplicates. The carbohydrate binding specificity of Entadin lectin was studied by minimal sugar concentration adequate to inhibit hemagglutination. Monosaccharides (glucose, fructose, arabinose, mannose, and galactose), disaccharides (cellobiose, lactose, and sucrose), and glycoprotein (ovalbumin) with an initial concentration of 50 mM were used for the study. Sugar solutions of 50 μL were placed in a well and serially diluted with PBS (pH 7.2), followed by the addition of 50 μL (1 mg/mL Entadin lectin) solution into each well and incubated for 30 min at 37 °C. After incubation, 4% human erythrocytes were added to each well and again incubated at 37 °C for 30 min. Hemagglutination activity was visually observed.
5.6. Total Carbohydrate Content and Observation of Glycosylation by PAS Staining
The total carbohydrate content of purified lectin was estimated by anthrone and phenol–sulfuric acid methods. Entadin lectin (1 mg/mL) was hydrolyzed using 250 μL of 2.5 N HCl for 3 h in a boiling water bath; excess of HCl was neutralized by the addition of sodium bicarbonate and was made up to 1 mL with Milli-Q water. The sample was further centrifuged at 8000 rpm at 4 °C for 5 min, and 50 μL of supernatant was used for total carbohydrate estimation. Glucose (1 mg/mL) was used for standard curve. In the case of anthrone, absorbance was measured at 630 nm, whereas in the case of phenol–sulfuric acid method, absorbance was measured at 490 nm. Detection of glycosylation of purified lectin was determined by PAS staining.44 The purified Entadin lectin was loaded on 12.5% SDS-PAGE along with ovalbumin as a standard glycoprotein in a separate well. After running SDS-PAGE, gel bands were fixed by adding 12% of trichloroacetic acid for 30 min in room temperature under shaking condition. The gel was washed for 10 min with Milli-Q water. The washed gel was immersed in 1% periodic acid solution prepared in 3% acetic acid and incubated for 1 h. After the incubation period, the gel was washed with Milli-Q water with a 10 min interval for 100 min. The gel was then placed in fuchsin sulfate (Schiff’s reagent) for 1 h in the dark. The gel was washed with freshly prepared 0.5% sodium metabisulfite for 30 min. The gel was then immersed in 7% acetic acid solution and dried to develop glycosylate bands.
5.7. Effects of pH and Temperature on the Stability of Lectin
The pH stability of Entadin lectin was investigated by incubating 1 mg/mL of purified protein at different pHs ranging from 2 to 12; all the buffers used were at a concentration of 50 mM (glycine HCl pH 2, sodium acetate buffer pH 4, phosphate buffer 6, Tris-HCl buffer pH 8, and glycine NaOH buffer pH 10–12). The purified lectin was incubated separately in each tube at 25 °C for 24 h. The temperature stability of Entadin lectin was investigated by incubating 1 mg/mL concentration of purified lectin at various temperatures ranging from 30 to 80 °C with an interval of 10 °C for 1 h. After incubation, HA was carried out by adding 4% human blood group B erythrocytes, and plates were incubated at 37 °C for 30 min.
5.8. Effects of Metal Ions on Lectin Activity
To determine the metal ion activity, 1 mg/mL of purified lectin was dialyzed against 50 mM Tris buffer at pH 7.2 containing 100 mM EDTA and 150 mM NaCl at 4 °C for 24 h. After dialysis, the purified lectin was added on serially diluted metal ions, where the initial concentration of metal ions was 50 mM (Al3+, Fe3+, Mn2+, Co2+, Zn2+, Ca2+, Sn2+, Ni2+, Mg2+, Li+, K+, Na+, Cs+, and Rb+), and incubated at 37 °C for 30 min. After incubation, HA was carried out by adding 4% human blood group B erythrocytes, and plates were incubated at 37 °C for 30 min.
5.9. CD Spectroscopy
Far-UV CD spectra of the protein were recorded at room temperature on a Jasco J-810 spectropolarimeter, Biosciences and Bioengineering Department at IIT Bombay, India. The protein sample was prepared in 300 μL of 30 μM concentration in 20 mM Tris-HCl, pH 7.4, and 20 mM NaCl buffer. The spectra of protein samples were acquired over a wavelength of 198–260 nm in a 0.1 cm path length quartz cell (Starna, Hainault, London). Subsequently, temperature-dependent far-UV CD spectra of the protein were obtained at temperatures ranging from 20 to 100 °C at a time interval of 5 °C. The signals were averaged by taking three independent readings. The raw data were subtracted from the buffer by using the spectra manager software.
5.10. Cell Culture
The lung cancer cells A549, human cervical cancer cells HeLa, and African green monkey normal kidney cells Vero were purchased from NCCS, Pune (India). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 1% glutamine (200 mmol/L), 1% (v/v) penicillin (10,000 IU/mL), and 100 mg/L streptomycin in a humidified 5% CO2 atmosphere at 37 °C before use.
5.11. In Vitro Cell Viability Assay by MTT
In vitro cell growth inhibition by Entadin lectin on human cancer cell lines A549, HeLa, and African green monkey normal kidney cells (Vero) were observed by using the MTT assay. The monolayer adherent cells in the logarithmic growth phase were dissociated by using 1× trypsin (HIMEDIA). Cells were counted by using a hemocytometer; further 3 × 104 cells/well (A549) and 2 × 104 cells/well (HeLa) were seeded into the 96-well plates with DMEM and incubated for 24 h. After incubation, DMEM was decanted from the plate and washed with PBS. Adhered cells were given Entadin lectin dissolved in fresh DMEM as dose-dependent 4–44 μg/mL for 24 h along with the control that does not contain lectin. The cells were incubated with 50 μL of 0.5 mg/mL concentration of MTT for 4 h in the dark, and then, cells were incubated with MTT and were dissolved in 100 μL of dimethyl sulfoxide. Absorbance was taken at 570 nm under an Elisa plate reader (PerkinElmer). All the experiments were done in triplicate. The formula used to calculate cell viability is as follows
5.12. Cell Morphological Study by AO/EtBr Dual Staining
A549 and HeLa cells were seeded into a 24-well culture plate and incubated in the presence of lectin concentrations with the IC50 value, half of the IC50 value, and double of the IC50 value for 24 h along with control that does not contain lectin and an anticancerous drug, doxorubicin, as a positive control. After incubation, the medium was decanted, and cells were washed with PBS. The cells were then fixed with 4% paraformaldehyde for 15 min and washed twice with PBS, followed by staining with 50 μM AO/EtBr dye solution for 10 min at room temperature in the dark and washed twice with PBS to remove excess stain. Cellular morphology was observed under an inverted fluorescence microscope.
5.13. Hoechst Nuclear Counterstaining
Entadin lectin-induced apoptosis has been observed using Hoechst nuclear counterstaining 33258. A549 and HeLa cells were treated with the lectin according to the above methods for 24 h. The medium with lectin was decanted and washed twice with PBS. After washing, the cells were stained with 100 μL of 5 μg/mL Hoechst 33258 for 15 min at room temperature in the dark. Excess stain was removed by washing with PBS, and nuclear morphology was observed in 63× oil immersion objective of the fluorescence microscope (Nikon Eclipse Ti).
Acknowledgments
We acknowledge the financial assistance from the Department of Science & Technology (DST), Ministry of Science and Technology. S.N. thanks the Indian Council of Medical Research (ICMR) for the senior research fellowship (SRF). S.K. thanks SERB for financial assistance under the Fast Track Scheme (SB/FT/LS-190/2012). We also thank Dr. Kali Kishore Reddy Tetala for his critical comments on the work.
Glossary
Abbreviations
- AO/EtBr
acridine orange/ethidium bromide
- HA
Hemagglutination
- PAS
periodic acid Schiff
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00577.
Cell killing efficiency of Entadin lectin by MTT monitored for 48 h and lactose and Entadin lectin interaction studies using the FTIR technique (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sharon N.; Lis H. The structural basis for carbohydrate recognition by lectins. Adv. Exp. Med. Biol. 2001, 491, 1–16. 10.1007/978-1-4615-1267-7_1. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-Y.; Takahashi M.; Gu J.-G.; Miyoshi E.; Matsumoto A.; Kitazume S.; Taniguchi N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Figueiroa E.; Albuquerque da Cunha C. R.; Albuquerque P. B. S.; de Paula R. A.; Aranda-Souza M. A.; Alves M. S.; Zagmignan A.; Carneiro-da-Cunha M. G.; Nascimento da Silva L. C.; Dos Santos Correia M. T. Lectin-Carbohydrate Interactions: Implications for the Development of New Anticancer Agents. Curr. Med. Chem. 2017, 24, 3667–3680. 10.2174/0929867324666170523110400. [DOI] [PubMed] [Google Scholar]
- Lis H.; Sharon N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chem. Rev. 1998, 98, 637–674. 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- Cavada B. S.; Silva M. T. L.; Osterne V. J. S.; Pinto-Junior V. R.; Nascimento A. P. M.; Wolin I. A. V.; Heinrich I. A.; Nobre C. A. S.; Moreira C. G.; Lossio C. F.; Rocha C. R. C.; Martins J. L.; Nascimento K. S.; Leal R. B. Canavalia bonariensis lectin: Molecular bases of glycoconjugates interaction and antiglioma potential. Int. J. Biol. Macromol. 2018, 106, 369–378. 10.1016/j.ijbiomac.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Procópio T. F.; de Siqueira Patriota L. L.; de Moura M. C.; da Silva P. M.; de Oliveira A. P. S.; do Nascimento Carvalho L. V.; de Albuquerque Lima T.; Soares T.; da Silva T. D.; Breitenbach Barroso Coelho L. C.; da Rocha Pitta M. G.; de Melo Rego M. J.; Bressan Queiroz de Figueiredo R. C.; Guedes Paiva P. M.; Napoleao T. H. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 2017, 98, 419–429. 10.1016/j.ijbiomac.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Ryder S. D.; Smith J. A.; Rhodes J. M. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J. Natl. Cancer Inst. 1992, 84, 1410–1416. 10.1093/jnci/84.18.1410. [DOI] [PubMed] [Google Scholar]
- Naik S.; Rawat R. S.; Khandai S.; Kumar M.; Jena S. S.; Vijayalakshmi M. A.; Kumar S. Biochemical characterisation of lectin from Indian hyacinth plant bulbs with potential inhibitory action against human cancer cells. Int. J. Biol. Macromol. 2017, 105, 1349–1356. 10.1016/j.ijbiomac.2017.07.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y.; Kawagishi H. Fungal lectins: a growing family. Methods Mol. Biol. 2014, 1200, 15–38. 10.1007/978-1-4939-1292-6_2. [DOI] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987, 217, 145–157. 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- da Silva J. D. F.; da Silva S. P.; da Silva P. M.; Vieira A. M.; de Araújo L. C. C.; de Albuquerque Lima T.; de Oliveira A. P. S.; do Nascimento Carvalho L. V.; da Rocha Pitta M. G.; de Melo Rêgo M. J. B.; Pinheiro I. O.; Zingali R. B.; do Socorro de Mendonça Cavalcanti M.; Napoleão T. H.; Paiva P. M. G. Portulaca elatior root contains a trehalose-binding lectin with antibacterial and antifungal activities. Int. J. Biol. Macromol. 2019, 126, 291–297. 10.1016/j.ijbiomac.2018.12.188. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. C. Mannan-binding lectin and its role in innate immunity. Transfus. Med. 2002, 12, 335–352. 10.1046/j.1365-3148.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- Lam S. K.; Ng T. B. Lectins: production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. 10.1007/s00253-010-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordache F.; Ionita M.; Mitrea L.; Fafaneata C.; Pop A. Antimicrobial and antiparasitic activity of lectins. Curr. Pharmaceut. Biotechnol. 2015, 16, 152–161. 10.2174/138920101602150112151907. [DOI] [PubMed] [Google Scholar]
- Cummings R., Lectins as Tools for Glycoconjugate Purification and Characterization. Glycosciences: Status and Perspectives; Wiley, 2008; pp 191–199. [Google Scholar]
- Yoshihara Y.; Mizuno T.; Nakahira M.; Kawasaki M.; Watanabe Y.; Kagamiyama H.; Jishage K.-i.; Ueda O.; Suzuki H.; Tabuchi K.; Sawamoto K.; Okano H.; Noda T.; Mori K. A Genetic Approach to Visualization of Multisynaptic Neural Pathways Using Plant Lectin Transgene. Neuron 1999, 22, 33–41. 10.1016/s0896-6273(00)80676-5. [DOI] [PubMed] [Google Scholar]
- Magnusson G.; Hultgren S. J.; Kihlberg J. [10] Specificity mapping of bacterial lectins by inhibition of hemagglutination using deoxy and deoxyfluoro analogs of receptor-active saccharides. Methods Enzymol. 1995, 253, 105–114. 10.1016/s0076-6879(95)53012-6. [DOI] [PubMed] [Google Scholar]
- Rutishauser U.; Sachs L. Cell-to-cell binding induced by different lectins. J. Cell. Biol. 1975, 65, 247–257. 10.1083/jcb.65.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R.; Raz A. Lectins in cancer cells. Ann. N.Y. Acad. Sci. 1988, 551, 385–398. ; discussion 396–398 10.1111/j.1749-6632.1988.tb22372.x. [DOI] [PubMed] [Google Scholar]
- Ryva B.; Zhang K.; Asthana A.; Wong D.; Vicioso Y.; Parameswaran R. Wheat Germ Agglutinin as a Potential Therapeutic Agent for Leukemia. Front. Oncol. 2019, 9, 100. 10.3389/fonc.2019.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastman B.; Wang K.; Han J.; Zhu Z.-y.; Huang X.; Wang G.-Q.; Rabinowich H.; Gorelik E. A novel apoptotic pathway as defined by lectin cellular initiation. Biochem. Biophys. Res. Commun. 2004, 316, 263–271. 10.1016/j.bbrc.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Liu B.; Zhang Z.-T.; Zhou T.-T.; Bian H.-J.; Min M.-W.; Liu Y.-H.; Chen J.; Bao J.-K. A mannose-binding lectin from Sophora flavescens induces apoptosis in HeLa cells. Phytomedicine 2008, 15, 867–875. 10.1016/j.phymed.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Xiao X.; He H.; Ding X.; Yang Q.; Liu X.; Liu S.; Rang J.; Wang T.; Zuo M.; Xia L. Purification and cloning of lectin that induce cell apoptosis from Allium chinense. Phytomedicine 2015, 22, 238–244. 10.1016/j.phymed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Silva H. C.; Pinto L. d. S.; Teixeira E. H.; Nascimento K. S.; Cavada B. S.; Silva A. L. C. BUL: A novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process Biochem. 2014, 49, 203–209. 10.1016/j.procbio.2013.10.020. [DOI] [Google Scholar]
- Pan W. L.; Ng T. B. A dimeric Phaseolus coccineus lectin with anti-oxidative, anti-proliferative and cytokine-inducing activities. Int. J. Biol. Macromol. 2015, 81, 960–966. 10.1016/j.ijbiomac.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Rafiq S.; Majeed R.; Qazi A. K.; Ganai B. A.; Wani I.; Rakhshanda S.; Qurishi Y.; Sharma P. R.; Hamid A.; Masood A.; Hamid R. Isolation and antiproliferative activity of Lotus corniculatus lectin towards human tumour cell lines. Phytomedicine 2013, 21, 30–38. 10.1016/j.phymed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Nzowa L. K.; Barboni L.; Teponno R. B.; Ricciutelli M.; Lupidi G.; Quassinti L.; Bramucci M.; Tapondjou L. A. Rheediinosides A and B, two antiproliferative and antioxidant triterpene saponins from Entada rheedii. Phytochemistry 2010, 71, 254–261. 10.1016/j.phytochem.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Yadav P.; Shahane G.; Ramasamy S.; Sengupta D.; Gaikwad S. Structural–functional insights and studies on saccharide binding of Sophora japonica seed lectin. Int. J. Biol. Macromol. 2016, 91, 75–84. 10.1016/j.ijbiomac.2016.05.047. [DOI] [PubMed] [Google Scholar]
- Gould N. R.; Scheinberg S. L. Isolation and partial characterization of two anti-A hemagglutinins from P. lunatus. Arch. Biochem. Biophys. 1970, 137, 1–11. 10.1016/0003-9861(70)90405-4. [DOI] [PubMed] [Google Scholar]
- Solá R. J.; Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva H. C.; Bari A. U.; Pereira-Jenior F. N.; Simoes R. C.; Barroso-Neto I. L.; Nobre C. B.; Pereira M. G.; Nascimento K. S.; Rocha B. A.; Delatorre P.; Nagano C. S.; Assreuy A. M.; Cavada B. S. Purification and partial characterization of a new pro-inflammatory lectin from Bauhinia bauhinioides Mart (Caesalpinoideae) seeds. Protein Pept. Lett. 2011, 18, 396–402. 10.2174/092986611794653987. [DOI] [PubMed] [Google Scholar]
- Borrebaeck C. A. K.; Loennerdal B.; Etzler M. E. Metal ion content of dolichos biflorus lectin and effect of divalent cations on lectin activity. Biochemistry 1981, 20, 4119–4122. 10.1021/bi00517a026. [DOI] [PubMed] [Google Scholar]
- de Sousa L. M.; de Carvalho J. L.; da Silva H. C.; Lemos T. L. G.; Arriaga A. M. C.; Braz-Filho R.; Militão G. C. G.; Silva T. D. S.; Ribeiro P. R. V.; Santiago G. M. P. New Cytotoxic Bibenzyl and Other Constituents fromBauhinia ungulataL. (Fabaceae). Chem. Biodiversity 2016, 13, 1630–1635. 10.1002/cbdv.201600058. [DOI] [PubMed] [Google Scholar]
- Silva H.; da Silva Pinto L.; Teixeira E.; do Nascimento K. S.; Cavada B.; Silva A. L. C. BUL: A novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process Biochem. 2013, 49, 203. 10.1016/j.procbio.2013.10.020. [DOI] [Google Scholar]
- Sunkara S.; Vadivel A.; Bhatnagar-Mathur P.; Sharma K., Isolation, cloning and characterization of 5’ upstream region of galactose binding seed lectin gene of chickpea (Cicer arietinum L.). J. SAT Agric. Res 2014, 12. [Google Scholar]
- Panda P. K.; Mukhopadhyay S.; Behera B.; Bhol C. S.; Dey S.; Das D. N.; Sinha N.; Bissoyi A.; Pramanik K.; Maiti T. K.; Bhutia S. K. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. 10.1016/j.lfs.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Wu J.; Wang J.; Wang S.; Rao P. Lunatin, a novel lectin with antifungal and antiproliferative bioactivities from Phaseolus lunatus billb. Int. J. Biol. Macromol. 2016, 89, 717–724. 10.1016/j.ijbiomac.2016.04.092. [DOI] [PubMed] [Google Scholar]
- e Lacerda R. R.; do Nascimento E. S.; de Lacerda J. T.; Pinto L. D.; Rizzi C.; Bezerra M. M.; Pinto I. R.; Filho S. M.; Pinto V. P.; Filho G. C.; Gadelha C. A.; Gadelha T. S. Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int. J. Biol. Macromol. 2017, 95, 1072–1081. 10.1016/j.ijbiomac.2016.10.097. [DOI] [PubMed] [Google Scholar]
- de Oliveira Figueirôa E.; Aranda-Souza M. Â.; Varejão N.; Rossato F. A.; Costa R. A. P.; Figueira T. R.; da Silva L. C. N.; Castilho R. F.; Vercesi A. E.; Dos Santos Correia M. T. pCramoll and rCramoll lectins induce cell death in human prostate adenocarcinoma (PC-3) cells by impairment of mitochondrial homeostasis. Toxicol. In Vitro 2017, 43, 40–46. 10.1016/j.tiv.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Roy U. K.; Lavignac N.; Rahman A. M.; Nielsen B. V. Purification of lectin and Kunitz trypsin inhibitor from soya seeds. J. Chromatogr. Sci 2018, 56, 436–442. 10.1093/chromsci/bmy018. [DOI] [PubMed] [Google Scholar]
- Almanza M.; Vega N.; Pérez G. Isolating and characterising a lectin from Galactia lindenii seeds that recognises blood group H determinants. Arch. Biochem. Biophys. 2004, 429, 180–190. 10.1016/j.abb.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Kim J.-J.; Hwang Y.-H.; Kang K.-Y.; Kim I.; Kim J.-B.; Park J.-H.; Yoo Y.-C.; Yee S.-T. Enhanced dendritic cell maturation by the B-chain of Korean mistletoe lectin (KML-B), a novel TLR4 agonist. Int. Immunopharmacol. 2014, 21, 309–319. 10.1016/j.intimp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M.; Zell T. E.; Morrison J. H.; Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal. Biochem. 1969, 30, 148–152. 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.