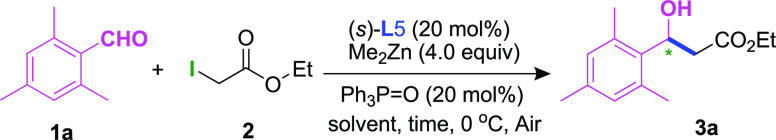Abstract
A highly Me2Zn-mediated catalytic enantioselective Reformatsky reaction of aldehydes and ketones with ethyl iodoacetate using a readily available prolinol ligand is reported. This reaction provides an efficient method for the construction of β-hydroxy esters in up to 98% yield and 95% enantiomeric excess (ee) value. A wide range of functional groups are tolerated and the practicality of this protocol is demonstrated by performing the reaction on a gram scale.
Introduction
The Reformatsky reaction was first discovered in 1887; it involved the zinc-induced synthesis of β-hydroxy esters through the addition of zinc enolate to aldehydes or ketones.1 Since its introduction, the Reformatsky reaction has emerged as one of the most widespread strategies for the formation of new carbon–carbon bonds.2 To date, various methods based on metal-mediated Reformatsky reactions of α-halo carbonyl compounds with aldehydes or ketones have been widely developed.3−12 Among the reported methods, homogeneous enantioselective Reformatsky-type reactions based on the use of Me2Zn have been successfully employed to generate chiral β-hydroxy esters.4 A breakthrough in the development of Me2Zn-mediated catalytic enantioselective Reformatsky reactions with aldehydes or ketones as electrophiles has been made by Cozzi and coauthors. Medium yields and high enantioselectivities have been obtained by the use of a chiral amino alcohol ligand for the reaction of aldehydes and chiral [MnCl(salen)] ligands for the reaction of ketones (Scheme 1a).13 Feringa and co-workers also have described an efficient catalytic enantioselective Reformatsky reaction with aldehydes and ketones using Binol derivatives as the chiral ligands (Scheme 1b).14 In 2008, a Me2Zn-mediated reaction with a Schiff base ligand for an asymmetric Reformatsky reaction of aldehydes was achieved (Scheme 1c).15 Afterward, ethyl-3-hydroxy-3-phenylpropanoates with up to 80% enantiomeric excess (ee) were produced using a chiral bisoxazolidine ligand (Scheme 1d).16
Scheme 1. Methods for Catalytic Enantioselective Reformatsky Reactions.
Although breakthrough processes on enantioselective Reformatsky reaction have been made,13−16 high yields and excellent enantioselectivity are still an imposing challenge for chemists. Our group has previously developed an efficient asymmetric Michael reaction of aldehydes to nitroalkenes by the use of chiral diphenylperhydroindolinol silyl ethers as catalysts.17 Hydroindolinol derivatives were found to facilitate the Michael reactions efficiently, providing the corresponding products in good yields and high diasteroselectivities. In addition, a catalytic highly enantioselective aza-Reformatsky reaction with cyclic imines using a chiral diary prolinol ligand was reported.18 Hence, it is highly desirable to explore a practical protocol for the Reformatsky reaction with both aldehydes and ketones in high yields and excellent enantioselectivities.
Inspired by the reported advances and based on our previous work,13−18 we envisioned that since a catalytic enantioselective aza-Reformatsky reaction with imines is feasible, highly enantioselective Reformatsky reactions with aldehydes and ketones using hydroindolinols might represent a possible alternative strategy to realize the construction of β-hydro esters. Fortunately, a highly Me2Zn-mediated enantioselective Reformatsky reaction with aldehydes and ketones that affords a variety of β-hydro ester derivatives has been developed (Scheme 1e).
Results and Discussion
We began our investigation with the reaction of low-activity 2,3,6-trimethylbenzaldehyde (1a), previously reported in the asymmetric catalytic Reformatsky reaction,4a,13a with ethyl iodoacetate (2a) in the presence of chiral indolinols, Ph3P=O (20 mol %), and mediated by Me2Zn (Scheme 2). Fortunately, our attempts with the use of perhydroindolinols (L1, L2) and indolinols (L3, L4) led to excellent yields and medium to good enantioselectivities. Based on the above-mentioned features reported for the catalytic enantioselective aza-Reformatsky reaction with imines,18 we then chose a series of chiral prolinols as ligands to study the Reformatsky reaction with aldehydes (L5–L8). To our delight, the reactions using prolinols L1 and L2 gave the products 3a in excellent yields and enantioselectivities.
Scheme 2. Ligand Screening,,
The reaction was performed using 1a (0.5 mmol), 2 (1.0 mmol), ligand (20 mol %), Me2Zn (4.0 mol), Ph3P=O (20 mol %), and Et2O (5 mL) under air, and stirred at 0 °C for 12 h.
Isolated yields.
ee values were determined by high-performance liquid chromatography (HPLC) with an OD-H column.
Further solvent screening showed that all of the solvents performed the reaction with excellent yields (Table 1, entries 1–10). Among the solvents screened, Et2O gave rise to the best enantioselectivity (93% ee) (Table 1, entry 10). Further investigation revealed that 40 mol % amount of prolinol (L5) and 8.0 equiv amount of Me2Zn exhibited the best reaction efficiency and enantioselectivity in up to 95% yield and 96% ee at 0 °C for 12 h (Table 1, entries 11–13).
Table 1. Optimization of Reaction Conditionsa.
| entry | solvent | yield / %b | ee / %c |
|---|---|---|---|
| 1 | THF | 90 | 77 |
| 2 | Toluene | 93 | 75 |
| 3 | Hexane | 91 | 63 |
| 4 | DCM | 92 | 81 |
| 5 | CH3CN | 93 | 78 |
| 6 | DCE | 92 | 80 |
| 7 | MTBE | 91 | 71 |
| 8 | Xylene | 92 | 75 |
| 9 | 1,2-dichlorobenzene | 93 | 73 |
| 10 | Et2O | 94 | 93 |
| 11 | Et2O | 94d | 93 |
| 12 | Et2O | 95e | 96 |
| 13 | Et2O | 93f | 90 |
A mixture of 1a (0.5 mmol, 1 equiv), 2 (1.0 mmol, 2 equiv), Me2Zn (2.0 mmol, 4 equiv), L5 (20 mol %), Ph3P=O (20 mol %), and solvent (5 mL) was sealed in a 25 mL Schlenk tube at 0 °C for 12 h under air.
Yields of the isolated product.
Determined by an OD-H column.
60 mol % L5 was used.
40 mol % L5 and 8.0 equiv Me2Zn were used.
Performed at −10 °C.
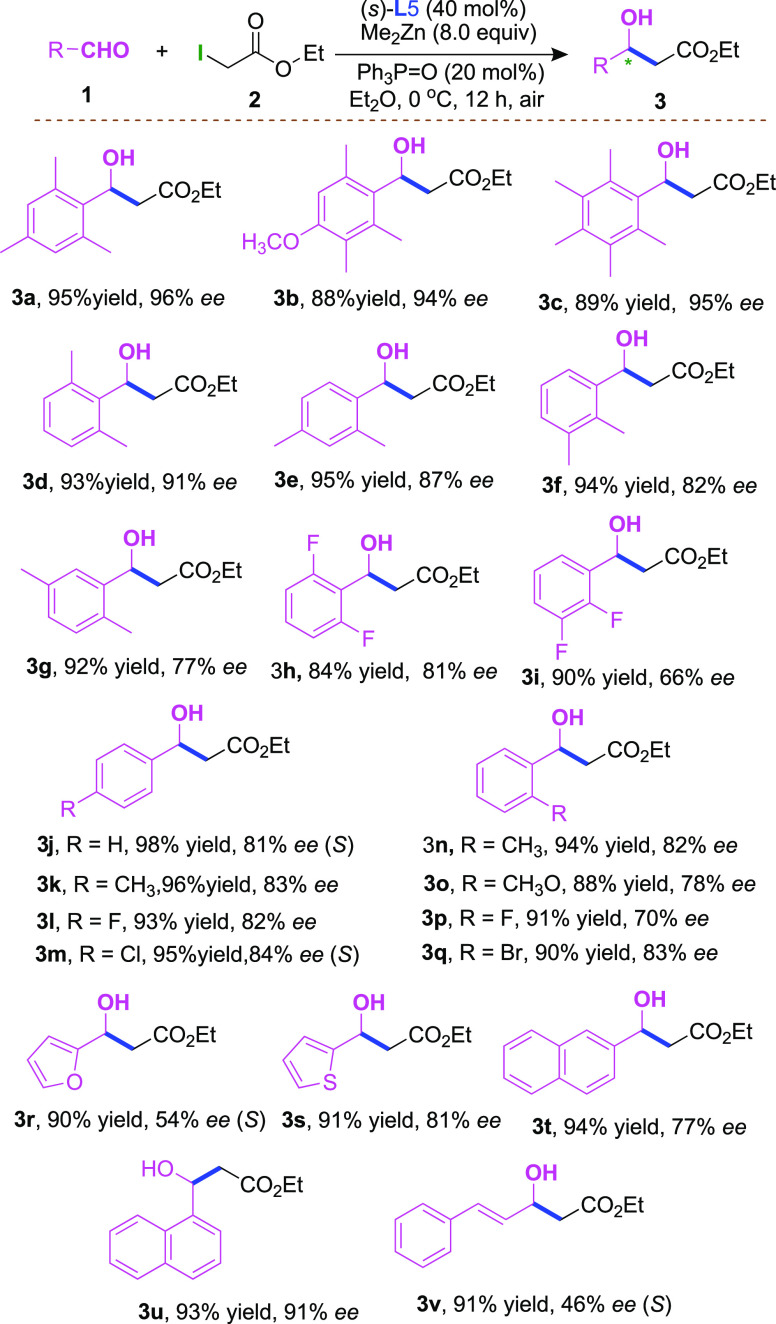
With the optimized conditions established, we next explored the reactivity of different aldehydes (Scheme 3). A series of multisubstituted aryl aldehydes with electron-donating substituents were all well tolerated, giving the obtained products 3a–3e in 88–95% yields with 93–96% ee. Additionally, a variety of dimethyl-substituted aryl aldehydes in the para-, meta-, or ortho-position of the benzene ring were also converted to the corresponding β-hydro esters 3d–3g with high yields (92–95%) and good enantioselectivities (77–91% ee). Moreover, substituents with difluoro-functional groups at the para- or meta-position of the arene proceeded smoothly to afford the Reformatsky reaction products (3h, 3i). Furthermore, substrates with electron-neutral (-H), electron-donating (−Me, CH3O−), and electron-withdrawing (−F, −Cl, −Br) functional groups at the para- or ortho-position of the phenyl ring were found to be suitable substrates, and the corresponding products were formed in 88–98% yields with 70–84% ee (3j–3q). Of particular note is that substrates with electron-rich groups at 2,6-positions of the arene contributed to the efficiency of high enantioselectivities (3a–3d). Importantly, the heteroaryl substrates were also compatible with this transformation (3r, 3s). Delightfully, the polyarene naphthalene ring substrates could react efficiently, which gave 3t and 3u in 77 and 91% ee, respectively. Notably, an alkenyl-substituted aldehyde could also be employed in this transformation to generate 3v in 91% yield with moderate enantioselectivity, which is currently underway in our laboratory.
Scheme 3. Substrate Scope of Aldehydes,,
The reaction was performed using 1 (0.5 mmol, 1 equiv), 2 (1.0 mmol, 2 equiv), ligand (40 mol %), Me2Zn (4.0 mol, 4 equiv), Ph3P=O (20 mol %), and Et2O (5 mL) under air, and was stirred at 0 °C for 12 h.
Isolated yields.
ee values were determined by HPLC with an OD-H column.
To further explore the scope of the electrophile substrate, a variety of ketones were examined (Scheme 4). To our delight, excellent yields and good enantioselectivities were obtained with a range of substituents in the para- and ortho-position of the aryl ketones (5a–5e). The introduction of electron-donating (CH3O−) or electron-withdrawing (−CF3) as well as halo-functional groups (−F, −Br) does not compromise the efficiency of the Reformatsky reaction (5b–5g). Heterocyclic ketones were also suitable for this transformation (5h, 5i). Note that the naphthalene substituent was tolerated with 93% yield and 81% ee (5j). Importantly, the cyclic aromatic ketones afforded the products 5k and 5l in 93% yield with 82% ee and 90% yield with 92% ee, respectively.
Scheme 4. Substrate Scope of Ketones,,
The reaction was performed using 4 (0.5 mmol, 1 equiv), 2 (1.0 mmol, 2 equiv), ligand (40 mol %), Me2Zn (4.0 mol, 8 equiv), Ph3P=O (20 mol %), and Et2O (5 mL) under air, and stirred at 0 °C for 12 h.
Isolated yields.
ee values were determined by HPLC with an OD-H column.
To evaluate the potential synthetic utility of this Reformatsky reaction, gram-scale reactions of 1j and 1s were carried out under the standard conditions (Scheme 5). The corresponding desired products 3j and 3s, which are important in synthesis because they serve as useful precursors to (R)-tomoxetine and (R)-duloxetine,19 were finally isolated in good to excellent yields and enantioselectivities.
Scheme 5. Gram-Scale Reaction Synthesis.
Based on our previous works17 and the reported literature by Cozzi13 and the Feringa group,14 a similar mechanism for our catalytic cycle was proposed in Scheme 6. Me radicals were first generated from Me2Zn in the presence of air, and the zinc species played a key role in the high enantioselectivities for the Reformatsky reactions.
Scheme 6. Proposed Reaction Mechanism.
Conclusions
We have presented a practical highly Me2Zn-mediated catalytic enantioselective Reformatsky reaction of aldehydes and ketones with ethyl iodoacetate using an available chiral prolinol ligand. This procedure provided an efficient method for the construction of β-hydroxy esters in good to excellent yields and enantioselectivities. The synthetic utility of this transformation was demonstrated by performing the reaction on a gram scale, which would extend the potential application of β-hydroxy esters in pharmaceutical and synthetic chemistry.
Experimental Section
General Information
Commercially available reagents were purchased and used without further purification. The starting materials of ethyl iodoacetate, aldehydes, ketones, and prolinols (L5–L8) were commercially available. Indolin ligands (L1–L4) were synthesized as previously reported.17 Thin-layer chromatography was performed on regular 250 μm silica gel plates using ultraviolet light as a visualizing agent. NMR spectra were recorded using a 400 MHz NMR spectrometer. Chemical shifts were referenced to residual CDCl3 solvent signals, which referenced at δH 7.26 ppm and δC 77.16 ppm. Gas chromatography–mass spectrometry (GC–MS) was performed using electron ionization. HPLC analyses were performed using a Chiralcel OD-H, Chiralpak AD-H, or AS-H column (0.46 cm diameter × 25 cm length). Optical rotations were recorded on a PerkinElmer polarimetry.
General Procedures for the Synthesis of Indolin Ligands (L3 and L4)
RMgBr (40 mL; 40 mmol, 4 equiv, 1 M in Et2O) was added dropwise to a solution of 1-(tert-butyl) 2-methyl (S)-indoline-1,2-dicarboxylate20 (2.77 g, 10 mmol, 1 equiv) in 20 mL of Et2O at 0 °C and the resulting mixture was stirred at 0 °C for 4 h. Then, the reaction was quenched with a saturated aqueous solution of NH4Cl and extracted with ethyl acetate. The combined organic phases were washed with brine, dried over MgSO4, and concentrated under vacuum. The residue was used in the next procedure without purification. CF3COOH (5 mL) was added to the above residue in 20 mL of dichloromethane (DCM) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 12 h. The solvent was then evaporated under vacuum after completion. A saturated aqueous Na2CO3 solution was added to neutralize the solution. The aqueous phase was extracted with CH2Cl2, washed with brine, dried over MgSO4, and concentrated under vacuum. The residue was purified on a column chromatograph to afford a pure product.
General Procedure for the Synthesis of β-Hydroxy Esters
In a 25 mL dried single-neck round-bottom flask equipped with a CaCl2 tube, 50.6 mg of L5 (0.2 mmol, 40 mol%) was added at room temperature. The flask was transferred to 0 °C and 5 mL of Et2O was added, stirring for 15 min, after which 60 μL of ethyl iodoacetate (0.5 mmol, 1.0 equiv) and 1.7 mL of Me2Zn (2.0 mmol, 4 equiv, 1.2 M solution in toluene) were added. Subsequently, benzaldehydes or ketones (0.5 mmol, 1.0 equiv) and 27.8 mg of Ph3PO (0.1 mmol, 20 mol%) were added immediately. After another 15 min, 1.7 mL of Me2Zn (2.0 mmol, 4 equiv, 1.2 M solution in toluene) and 60 μL of ethyl iodoacetate (0.5 mmol, 1.0 equiv) were added again. The outlet of the other end of the CaCl2 tube directly contacts the atmosphere so that air could enter into the reaction flask. The resulting solution was stirred for 12 h and quenched by 1 M HCl aqueous after completion and then extracted with Et2O (5 mL × 2). The combined organic phase was then dried over anhydrous MgSO4 and concentrated in vacuum. The residue was purified by silica gel flash column chromatography eluting with the mixture of ethyl acetate/petroleum ether (1/10 v/v), which eventually afforded the pure product. The enantioselectivities of the products are determined by a Chiralcel OD-H column.
Procedure for Gram-Scale Synthesis of β-Hydroxy Esters
In a 250 mL dried single-neck round-bottom flask equipped with a CaCl2 tube, 1.012 g of L5 (4.0 mmol, 40 mol %) was added at room temperature. The flask was transferred to 0 °C, and 100 mL of Et2O was added. The mixture was stirred for 15 min, after which 1.2 mL of ethyl iodoacetate(10.0 mmol, 1.0 equiv) and 34 mL of Me2Zn (40.0 mmol, 4 equiv, 1.2 M solution in toluene) were added. Subsequently, benzaldehydes (10 mmol, 1.0 equiv) and 556 mg of Ph3PO (2.0 mmol, 20 mol %) were added immediately. After another 15 min, 34 mL of Me2Zn (40.0 mmol, 4 equiv, 1.2 M solution in toluene) and 1.2 mL of ethyl iodoacetate (10.0 mmol, 1.0 equiv) were added again. The outlet of the other end of the CaCl2 tube directly contacts the atmosphere so that air could enter into the reaction flask. The resulting solution was stirred for 18 h and quenched by 1 M HCl aqueous after completion and then extracted with Et2O (100 mL × 2). The combined organic phase was then dried over anhydrous MgSO4 and concentrated in vacuum. The residue was purified by silica gel flash column chromatography eluting with the mixture of ethyl acetate/petroleum ether (1/10 v/v), which eventually afforded the pure product. The enantioselectivities of the products are determined by a Chiralcel OD-H column.
Ethyl-3-hydroxy-3-mesitylpropanoate (3a)
[α]D20 = −21.8 (c 0.15, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 6.83 (s, 2H), 5.61 (dd, J = 10.6, 2.1 Hz, 1H), 4.21 (q, J = 7.1 Hz, 2H), 3.05 (dd, J = 16.5, 10.7 Hz, 1H), 2.64 (s, 1H), 2.54 (dd, J = 16.6, 2.3 Hz, 1H), 2.43 (s, 6H), 2.25 (s, 3H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 136.9, 136.1, 134.5, 130.1, 67.5, 60.8, 40.0, 20.7, 20.6, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 208 nm, tmajor = 7.819 min, tminor = 10.562 min, 96% ee.
Ethyl-3-hydroxy-3-(4-methoxy-2,3,6-trimethylphenyl)propanoate (3b)
[α]D20 = −22.5 (c 0.28, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 6.53 (s, 1H), 5.64 (d, J = 10.4 Hz, 1H), 4.20 (q, J = 7.1 Hz, 2H), 3.80 (s, 3H), 3.09 (dd, J = 16.6, 10.6 Hz, 1H), 2.84 (s, 1H), 2.56 (dd, J = 16.6, 3.3 Hz, 1H), 2.41 (d, J = 13.6 Hz, 6H), 2.13 (s, 3H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.7, 156.3, 136.6, 133.9, 129.9, 123.9, 110.8, 67.5, 60.7, 55.4, 40.5, 21.2, 16.7, 14.1, 11.6. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 8.735 min, tminor = 12.383 min, 94% ee; HRMS (ESI, m/z): [M + Na]+ Calcd for C15H22O4Na, 289.1410; found, 289.1382.
Ethyl-3-hydroxy-3-(2,3,4,5,6-pentamethylphenyl)propanoate (3c)
[α]D20 = −11.8 (c 0.44, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 5.82 (dt, J = 10.7, 3.1 Hz, 1H), 4.25 (qd, J = 7.1, 0.8 Hz, 2H), 3.17 (dd, J = 16.6, 10.7 Hz, 1H), 2.91 (d, J = 3.1 Hz, 1H), 2.63 (dd, J = 16.6, 3.2 Hz, 1H), 2.43 (s, 6H), 2.27 (d, J = 9.9 Hz, 9H), 1.34 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 135.1, 134.4, 133.3, 131.8, 68.0, 60.7, 40.5, 17.0, 17.0, 16.6, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 6.894 min, tminor = 9.474 min, 95% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C16H24O3Na, 287.1618; found, 287.1593.
Ethyl-3-(2,6-dimethylphenyl)-3-hydroxypropanoate (3d)
[α]D20 = −13.5 (c 0.28, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.12–7.06 (m, 1H), 7.02 (d, J = 7.3 Hz, 2H), 5.66 (d, J = 10.6 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.08 (dd, J = 16.5, 10.7 Hz, 1H), 2.99 (s, 1H), 2.58 (dd, J = 16.8, 2.5 Hz, 1H), 2.48 (s, 6H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 137.4, 136.1, 129.4, 127.4, 67.6, 60.8, 39.8, 20.7, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 8.463 min, tmajor = 9.276 min, 91% ee.
Ethyl-3-(2,4-dimethylphenyl)-3-hydroxypropanoate (3e)
[α]D20 = −20.1 (c 0.53, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.8 Hz, 1H), 7.07 (d, J = 7.9 Hz, 1H), 6.99 (s, 1H), 5.38–5.30 (m, 1H), 4.22 (q, J = 7.1 Hz, 2H), 3.30 (s, 1H), 2.76–2.62 (m, 2H), 2.34 (d, J = 4.5 Hz, 6H), 1.31 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.5, 137.5, 137.0, 134.1, 131.1, 126.9, 125.1, 66.7, 60.7, 42.1, 20.9, 18.8, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 10.159 min, tmajor = 10.758 min, 87% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C13H18O3Na, 245.1148; found, 245.1133.
Ethyl-3-(2,3-dimethylphenyl)-3-hydroxypropanoate (3f)
[α]D20 = −33.5 (c 0.16, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.5 Hz, 1H), 7.19–7.10 (m, 2H), 5.44 (dd, J = 8.2, 4.5 Hz, 1H), 4.23 (q, J = 7.2 Hz, 2H), 3.29 (s, 1H), 2.75–2.65 (m, 2H), 2.32 (s, 3H), 2.26 (s, 3H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.5, 140.4, 136.8, 132.8, 129.1, 125.7, 122.9, 67.2, 60.8, 42.2, 20.5, 14.5, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 9.766 min, tmajor = 12.00 min, 82% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C13H18O3Na, 245.1148; found, 245.1133.
Ethyl-3-(2,5-dimethylphenyl)-3-hydroxypropanoate (3g)
[α]D20 = −27.5 (c 0.21, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.29 (s, 1H), 6.97 (q, J = 7.7 Hz, 2H), 5.28 (d, J = 9.1 Hz, 1H), 4.15 (q, J = 7.1 Hz, 2H), 3.40 (s, 1H), 2.68–2.56 (m, 2H), 2.28 (d, J = 11.4 Hz, 6H), 1.24 (td, J = 7.1, 1.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.4, 140.2, 135.5, 130.8, 130.1, 128.0, 125.7, 66.7, 60.6, 42.1, 20.8, 18.3, 13.9. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 9.976 min, tmajor = 10.272 min, 77% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C13H18O3Na, 245.1148; found, 245.1133.
Ethyl-3-(2,6-difluorophenyl)-3-hydroxypropanoate (3h)
[α]D20 = −6.4 (c 0.17, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.26 (td, J = 8.2, 4.3 Hz, 1H), 6.95–6.83 (m, 2H), 5.55 (dt, J = 9.4, 4.9 Hz, 1H), 4.18 (q, J = 7.2 Hz, 2H), 3.26 (d, J = 6.1 Hz, 1H), 3.15 (dd, J = 16.3, 9.5 Hz, 1H), 2.75 (dd, J = 16.4, 4.2 Hz, 1H), 1.26 (td, J = 7.2, 1.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.6, 162.2 (d, J = 8.0 Hz), 159.8 (d, J = 9.0 Hz), 129.7, 117.5, 111.86, 111.8, 111.7, 62.2, 60.9, 40.8, 40.8, 40.8, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 98:2, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 38.572min, tminor = 41.707 min, 81% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C11H12F2O3Na, 253.0647; found, 253.0633.
Ethyl-3-(2,3-difluorophenyl)-3-hydroxypropanoate (3i)
[α]D20 = −26.3 (c 0.29, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.32–7.27 (m, 1H), 7.12–7.05 (m, 2H), 5.42 (dd, J = 8.9, 3.4 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.74 (s, 1H), 2.75 (qd, J = 16.5, 6.3 Hz, 2H), 1.26 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.1, 150.2 (dd, J = 13.0 Hz, J = 247.0 Hz), 147.3 (dd, J = 13.0 Hz, J = 246.0 Hz), 131.9 (d, J = 10.0 Hz), 124.2 (q, J = 5.0 Hz, J = 7.0 Hz), 121.8 (t, J = 3.0 Hz), 116.2 (d, J = 17.0 Hz), 64.2, 61.0, 41.7, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 98:2, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 17.499 min, tminor = 20.280 min, 66% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C11H12F2O3Na, 253.0647; found, 253.0633.
(S)-Ethyl-3-hydroxy-3-phenylpropanoate (3j)14a
[α]D20 = −25.5 (c 0.22, CH2Cl2). 1H NMR (400 MHz,) δ 7.38–7.32 (m), 7.30–7.25 (m), 5.16–5.08 (m), 4.17 (q, J = 7.1 Hz), 3.36 (d, J = 3.4 Hz), 2.78–2.66 (m), 1.25 (t, J = 7.1 Hz).13C NMR (100 MHz, CDCl3) δ 172.3, 142.5, 128.5, 127.7, 125.6, 70.3, 60.8, 43.3, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 11.207 min, tminor = 12.868 min, 81% ee.
Ethyl-3-hydroxy-3-(p-tolyl)propanoate (3k)
[α]D20 = −30.1 (c 0.16, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 7.9 Hz, 2H), 7.15 (d, J = 7.9 Hz, 2H), 5.08 (dd, J = 9.0, 3.5 Hz, 1H), 4.16 (q, J = 7.1 Hz, 2H), 3.30 (s, 1H), 2.70 (qd, J = 16.3, 6.5 Hz, 2H), 2.33 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.4, 139.6, 137.4, 129.1, 125.6, 70.1, 60.8, 43.3, 21.0, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 98:2, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 25.515 min, tmajor = 27.005 min, 83% ee.
Ethyl-3-(4-fluorophenyl)-3-hydroxypropanoate (3l)
[α]D20 = −21.5 (c 0.14, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.38–7.31 (m, 2H), 7.07–6.99 (m, 2H), 5.14–5.06 (m, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.39 (d, J = 2.6 Hz, 1H), 2.76–2.64 (m, 2H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.3, 162.3 (d, J = 244 Hz), 138.3, 127.4, 115.3, 69.7, 60.9, 43.3, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 99:1, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 57.433 min, tmajor = 59.097 min, 82% ee.
(S)-Ethyl-3-(4-chlorophenyl)-3-hydroxypropanoate (3m).14a
[α]D20 = −37.9 (c 0.11, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.33–7.27 (m, 4H), 5.08 (dd, J = 8.2, 4.5 Hz, 1H), 4.16 (q, J = 7.1 Hz, 2H), 3.57 (s, 1H), 2.73–2.62 (m, 2H), 1.25 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.1, 141.0, 133.3, 128.6, 127.0, 69.5, 60.9, 43.2, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 16.849 min, tminor = 18.165 min, 84% ee.
Ethyl-3-hydroxy-3-(o-tolyl)propanoate (3n)
[α]D20 = −44.6 (c 0.52, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 7.6 Hz, 1H), 7.19 (ddd, J = 30.6, 15.8, 7.7 Hz, 3H), 5.35 (dt, J = 6.4, 2.9 Hz, 1H), 4.20 (q, J = 7.2 Hz, 2H), 3.23 (d, J = 2.8 Hz, 1H), 2.73–2.62 (m, 2H), 2.35 (s, 3H), 1.28 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 140.4, 134.2, 130.4, 127.5, 126.4, 125.2, 66.9, 60.9, 42.1, 19.0, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 16.773 min, tmajor = 21.200 min, 82% ee.
Ethyl-3-hydroxy-3-(2-methoxyphenyl)propanoate (3o)
[α]D20 = −42.7 (c 0.46, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.43 (dd, J = 7.5, 1.1 Hz, 1H), 7.28–7.24 (m, 1H), 6.98 (t, J = 7.4 Hz, 1H), 6.87 (d, J = 8.2 Hz, 1H), 5.36 (dd, J = 9.2, 3.6 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.85 (s, 3H), 2.82 (dd, J = 16.1, 3.6 Hz, 1H), 2.70 (dd, J = 16.1, 9.2 Hz, 1H), 1.26 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 156.0, 130.5, 128.6, 126.6, 120.8, 110.3, 66.6, 60.7, 55.3, 41.7, 14.2. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 98:2, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 23.861 min, tminor = 27.973 min, 78% ee.
Ethyl-3-(2-fluorophenyl)-3-hydroxypropanoate (3p)
[α]D20 = −22.7 (c 0.42, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.53 (t, J = 7.5 Hz, 1H), 7.30–7.22 (m, 1H), 7.15 (t, J = 7.5 Hz, 1H), 7.06–6.97 (m, 1H), 5.45–5.36 (m, 1H), 4.18 (q, J = 7.2 Hz, 2H), 3.64 (d, J = 4.0 Hz, 1H), 2.82–2.67 (m, 2H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.31, 159.3 (d, J = 245 Hz), 129.4, 129.0, 127.1, 124.3, 115.1, 64.5, 60.8, 41.8, 41.8, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 15.333 min, tmajor = 18.309 min, 70% ee.
Ethyl-3-(2-bromophenyl)-3-hydroxypropanoate (3q)
[α]D20 = −45.9 (c 0.33, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.62 (dd, J = 7.8, 1.6 Hz, 1H), 7.51 (dd, J = 7.9, 1.1 Hz, 1H), 7.37–7.31 (m, 1H), 7.14 (td, J = 7.7, 1.7 Hz, 1H), 5.43 (dt, J = 9.6, 2.5 Hz, 1H), 4.23–4.17 (m, 2H), 3.66 (d, J = 3.4 Hz, 1H), 2.85 (dd, J = 8.4, 2.7 Hz, 1H), 2.55 (dd, J = 16.6, 9.8 Hz, 1H), 1.27 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.4, 141.4, 132.6, 129.0, 127.8, 127.3, 121.3, 69.2, 61.0, 41.4, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OJ-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 12.830 min, tminor = 16.016 min, 83% ee.
(S)-Ethyl-3-(furan-2-yl)-3-hydroxypropanoate (3r)14a
[α]D20 = −14.0 (c 0.14, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.38 (dd, J = 1.8, 0.8 Hz, 1H), 6.34 (dd, J = 3.2, 1.8 Hz, 1H), 6.28 (d, J = 3.3 Hz, 1H), 5.14 (dt, J = 8.6, 4.4 Hz, 1H), 4.20 (q, J = 7.1 Hz, 2H), 3.23 (d, J = 5.0 Hz, 1H), 2.94–2.81 (m, 2H), 1.28 (d, J = 3.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.9, 154.7, 142.2, 110.2, 106.3, 64.2, 61.0, 39.7, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 90:10, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 8.648 min, tminor = 25.430 min, 54% ee.
Ethyl-3-hydroxy-3-(thiophen-2-yl)propanoate (3s)
[α]D20 = −9.7 (c 0.15, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.24 (dd, J = 4.7, 1.6 Hz, 1H), 6.96 (dd, J = 3.9, 2.7 Hz, 2H), 5.35 (d, J = 5.2 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.64 (s, 1H), 2.83 (dd, J = 14.1, 9.9 Hz, 2H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.8, 146.3, 126.6, 124.7, 123.5, 66.4, 60.9, 43.1, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 90:10, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 8.363 min, tminor = 17.743 min, 81% ee.
Ethyl-3-hydroxy-3-(naphthalen-2-yl)propanoate (3t)
[α]D20 = −35.1 (c 0.13, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 5.2, 2.8 Hz, 4H), 7.54–7.46 (m, 3H), 5.32 (dd, J = 8.6, 3.7 Hz, 1H), 4.20 (q, J = 7.1 Hz, 2H), 3.67 (s, 1H), 2.84 (qd, J = 16.2, 6.5 Hz, 2H), 1.30–1.25 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 172.3, 139.9, 133.1, 132.9, 128.2, 127.9, 127.6, 126.1, 125.8, 124.4, 123.7, 70.3, 60.8, 43.3, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 16.891 min, tmajor = 19.909 min, 77% ee.
Ethyl-3-hydroxy-3-(naphthalen-1-yl)propanoate (3u)
[α]D20 = −114.2 (c 0.09, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 8.10–8.06 (m, 1H), 7.89 (dd, J = 7.4, 2.1 Hz, 1H), 7.81 (d, J = 8.2 Hz, 1H), 7.73 (d, J = 7.1 Hz, 1H), 7.57–7.47 (m, 3H), 5.94 (dt, J = 9.1, 2.9 Hz, 1H), 4.24 (q, J = 7.2 Hz, 2H), 3.67 (d, J = 3.4 Hz, 1H), 2.95–2.82 (m, 2H), 1.30 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.5, 138.0, 133.6, 129.8, 128.8, 128.1, 126.1, 125.5, 125.4, 122.8, 122.7, 67.2, 60.8, 42.7, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 23.334 min, tminor = 29.751 min, 91% ee.
(S)-Ethyl-(E)-3-hydroxy-5-phenylpent-4-enoate (3v)14a
[α]D20 = −2.6 (c 0.38, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 2H), 7.33–7.28 (m, 2H), 7.26–7.21 (m, 1H), 6.65 (dd, J = 15.9, 0.9 Hz, 1H), 6.22 (dd, J = 15.9, 6.1 Hz, 1H), 4.72 (s, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.24 (d, J = 3.8 Hz, 1H), 2.69–2.57 (m, 2H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.2, 136.3, 130.6, 129.9, 128.5, 127.7, 126.5, 68.8, 60.8, 41.5, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 22.528 min, tmajor = 34.451 min, 46% ee.
(R)-Ethyl-3-hydroxy-3-phenylbutanoate (5a)13b
[α]D20 = +12.0 (c 0.03, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.47–7.43 (m, 2H), 7.36–7.31 (m, 2H), 7.26–7.21 (m, 1H), 4.41 (s, 1H), 4.05 (q, J = 7.1 Hz, 2H), 2.98 (d, J = 15.9 Hz, 1H), 2.79 (d, J = 15.9 Hz, 1H), 1.54 (s, 3H), 1.13 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.7, 146.8, 128.2, 126.8, 124.4, 77.3, 60.7, 46.4, 30.6, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 6.465 min, tmajor = 7.081 min, 87% ee.
(S)-Ethyl-3-(4-bromophenyl)-3-hydroxybutanoate (5b)13b
[α]D20 = +19.3 (c 0.16, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.6 Hz, 2H), 7.32 (d, J = 8.6 Hz, 2H), 4.44 (s, 1H), 4.06 (tt, J = 7.2, 3.7 Hz, 2H), 2.92 (d, J = 16.0 Hz, 1H), 2.76 (d, J = 16.0 Hz, 1H), 1.50 (s, 3H), 1.14 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.5, 146.0, 131.2, 126.4, 120.8, 72.5, 60.8, 46.1, 30.5, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 6.430 min, tmajor = 7.317 min, 83% ee.
Ethyl-3-hydroxy-3-(4-(trifluoromethyl)phenyl)butanoate (5c)
[α]D20 = +16.9 (c 0.21, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.63–7.56 (m, 4H), 4.54 (s, 1H), 4.07 (q, J = 7.1 Hz, 2H), 2.98 (d, J = 16.1 Hz, 1H), 2.82 (d, J = 16.1 Hz, 1H), 1.54 (s, 3H), 1.14 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.5, 150.9, 129.1 (q, J = 32.0 Hz, J = 64.0 Hz), 124.1, 125.2 (q, J = 4.0 Hz, J = 7.0 Hz), 125.0, 72.6, 60.9, 46.0, 30.5, 13.8. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 5.450 min, tmajor = 6.362 min, 73% ee.
Ethyl-3-(2-bromophenyl)-3-hydroxybutanoate (5d)
[α]D20 = +45.9 (c 0.33, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.9 Hz, 1H), 7.07 (d, J = 7.9 Hz, 1H), 6.99 (s, 1H), 5.34 (dt, J = 9.2, 3.0 Hz, 1H), 4.25–4.19 (m, 2H), 3.21 (d, J = 3.1 Hz, 1H), 2.75–2.65 (m, 2H), 2.33 (d, J = 5.9 Hz, 6H), 1.29 (d, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 137.5, 137.1, 134.2, 131.2, 127.0, 125.2, 66.8, 60.8, 42.1, 20.9, 18.9, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 9.957 min, tmajor = 10.649 min, 85% ee.
Ethyl-3-hydroxy-3-(2-methoxyphenyl)butanoate (5e)
[α]D20 = +7.3 (c 0.30, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.58 (dd, J = 7.7, 1.7 Hz, 1H), 7.23 (ddd, J = 8.1, 7.5, 1.7 Hz, 1H), 6.96 (td, J = 7.6, 1.1 Hz, 1H), 6.88 (dd, J = 8.2, 0.9 Hz, 1H), 4.58 (s, 1H), 3.98 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.28 (d, J = 15.0 Hz, 1H), 2.85 (d, J = 15.0 Hz, 1H), 1.63 (s, 3H), 1.06 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.7, 155.8, 133.7, 128.4, 126.7, 120.7, 111.0, 72.5, 60.3, 55.2, 45.0, 27.4, 13.9. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 98:2, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 22.861 min, tminor = 27.973 min, 80% ee.
Ethyl-3-(3-bromo-4-fluorophenyl)-3-hydroxybutanoate (5f)
[α]D20 = +18.2 (c 0.07, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 6.6, 2.3 Hz, 1H), 7.33 (ddd, J = 8.6, 4.5, 2.3 Hz, 1H), 7.05 (t, J = 8.5 Hz, 1H), 4.49 (s, 1H), 4.07 (q, J = 7.1 Hz, 2H), 2.90 (d, J = 16.0 Hz, 1H), 2.75 (d, J = 16.0 Hz, 1H), 1.50 (s, 3H), 1.15 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.39, 157.9 (d, J = 245), 144.4, 130.0, 125.2, 116.0, 108.8, 72.1, 60.9, 46.1, 30.6, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel AS-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 9.386 min, tmajor = 10.395 min, 83% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C12H14BrFO3Na, 327.0003; found, 327.0067.
Ethyl-3-(3,4-difluorophenyl)-3-hydroxybutanoate (5g)
[α]D20 = +19.6 (c 0.21, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.36–7.28 (m, 1H), 7.17–7.06 (m, 2H), 4.52 (s, 1H), 4.09 (q, J = 7.1 Hz, 2H), 2.92 (d, J = 16.0 Hz, 1H), 2.78 (d, J = 16.0 Hz, 1H), 1.51 (s, 3H), 1.17 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.40, 150.8 (dd, J = 12.0 Hz, J = 88.0 Hz), 148.3 (dd, J = 13.0 Hz, J = 90.0 Hz), 144.2, 120.4 (q, J = 4.0 Hz, J = 6.0 Hz), 116.8 (d, J = 17.0 Hz), 114.1(d, J = 19.0 Hz), 72.1, 60.9, 46.0, 30.5, 13.9. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 7.700 min, tmajor = 8.981 min, 78% ee. HRMS (ESI, m/z): [M + H]+ Calcd for C12H15F2O3, 245.0984; found, 245.1351.
Ethyl-3-hydroxy-3-(thiophen-2-yl)butanoate (5h)
[α]D20 = +15.5 (c 0.26, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.18 (d, J = 5.0 Hz, 1H), 6.94–6.91 (m, 1H), 6.89 (d, J = 3.4 Hz, 1H), 4.76 (s, 1H), 4.12 (d, J = 7.1 Hz, 2H), 2.98 (d, J = 16.0 Hz, 1H), 2.81 (d, J = 16.0 Hz, 1H), 1.63 (s, 3H), 1.19 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, Acetone) δ 172.3, 152.2, 126.6, 123.9, 121.9, 71.8, 60.9, 46.8, 31.3, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 11.162 min, tminor = 12.474 min, 85% ee.
Ethyl-2-(4-hydroxychroman-4-yl)acetate (5i)
[α]D20 = +36.3 (c 0.16, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 7.8, 1.6 Hz, 1H), 7.18 (ddd, J = 8.7, 7.3, 1.6 Hz, 1H), 6.93 (td, J = 7.9, 1.2 Hz, 1H), 6.83 (dd, J = 8.2, 1.1 Hz, 1H), 4.28 (ddd, J = 11.8, 7.3, 4.6 Hz, 1H), 4.21 (ddd, J = 14.2, 5.9, 2.4 Hz, 3H), 4.12 (s, 1H), 3.06 (d, J = 15.7 Hz, 1H), 2.72 (d, J = 15.7 Hz, 1H), 2.23–2.16 (m, 2H), 1.28 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.1, 154.0, 129.2, 126.6, 126.0, 120.6, 117.0, 66.7, 62.8, 60.9, 45.0, 35.3, 14.0. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 12.703 min, tmajor = 13.893 min, 92% ee.
Ethyl-3-hydroxy-3-(naphthalen-2-yl)butanoate (5j)
[α]D20 = +16.9 (c 0.21, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H), 7.89–7.83 (m, 3H), 7.59 (dd, J = 8.6, 1.8 Hz, 1H), 7.53–7.46 (m, 2H), 4.65 (s, 1H), 4.07 (qd, J = 7.1, 3.6 Hz, 2H), 3.13 (d, J = 15.9 Hz, 1H), 2.92 (d, J = 15.9 Hz, 1H), 1.66 (s, 3H), 1.13 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.7, 144.1, 133.1, 132.3, 128.1, 127.9, 127.4, 126.0, 125.7, 123.1, 123.0, 72.9, 60.7, 46.2, 30.6, 13.9. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tmajor = 11.128 min, tminor = 12.328 min, 81% ee.
Ethyl-2-(5-fluoro-1-hydroxy-2,3-dihydro-1H-inden-1-yl)acetate (5k)
[α]D20 = −4.9 (c 0.53, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.27 (dd, J = 9.1, 5.2 Hz, 1H), 6.94–6.85 (m, 2H), 4.24–4.15 (m, 3H), 3.06–2.96 (m, 1H), 2.88–2.76 (m, 2H), 2.68 (d, J = 15.9 Hz, 1H), 2.29 (t, J = 7.0 Hz, 2H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.6, 163.2 (d, J = 243 Hz), 145.1, 141.6, 124.1, 113.8, 111.7, 80.3, 60.9, 43.9, 40.5, 29.2, 29.2, 14.1. The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 8.334 min, tmajor = 15.408 min, 82% ee.
Ethyl-2-(1-hydroxy-2-methyl-1,2,3,4-tetrahydronaphthalen-1-yl)acetate (5l)
[α]D20 = −7.2 (c 0.24, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ 7.64 (dd, J = 7.7, 1.4 Hz, 1H), 7.25–7.17 (m, 2H), 7.10–7.06 (m, 1H), 4.20–4.14 (m, 2H), 3.94 (s, 1H), 2.97–2.88 (m, 2H), 2.80–2.72 (m, 2H), 2.31–2.22 (m, 1H), 2.07–1.98 (m, 1H), 1.81–1.74 (m, 1H), 1.25 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 6.9 Hz, 3H). The enantiomeric excess was determined by HPLC using the Daicel Chiralcel OD-H column, hexane/i-PrOH 95:5, flow rate 1.0 mL/min, UV detection at 220 nm, tminor = 5.590 min, tmajor = 7.735 min, 92% ee. HRMS (ESI, m/z): [M + Na]+ Calcd for C15H20NaO3Na, 271.1305; found, 271.1287.
Acknowledgments
The authors thank the National Natural Science Foundation of China (21961002, 21962004, and 21562004), the Jiangxi provincial department of science and technology (20192BAB203004), Fundamental Research Funds for Gannan Medical University (QD201810), and the COVID-19 emergency project of Gannan Medical University (YJ202027) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02400.
Schematic diagram for the synthesis of ligands (L1–L4); HPLC spectra; 1H and 13C NMR spectra of all compounds prepared (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Reformatsky S. Neue Synthese zweiatomiger einbasischer Säuren aus den Ketonen. Ber. Dtsch. Chem. Ges. 1887, 20, 1210–1211. 10.1002/cber.188702001268. [DOI] [Google Scholar]; b Ocampo R.; Dolbier W. R. Jr. The Reformatsky reaction in organic synthesis. Recent advances. Tetrahedron 2004, 60, 9325–9374. 10.1016/j.tet.2004.07.018. [DOI] [Google Scholar]; c Babu S. A.; Yasuda M.; Shibata I.; Baba A. In- or In(I)-employed tailoring of the stereogenic centers in the Reformatsky-type reactions of simple ketones, α-alkoxy ketones, and β-keto esters. J. Org. Chem. 2005, 70, 10408–10419. 10.1021/jo051659w. [DOI] [PubMed] [Google Scholar]
- a Fürstner A. Recent advancements in the Reformatsky reaction. Synthesis 1989, 1989, 571–590. 10.1055/s-1989-27326. [DOI] [Google Scholar]; b Fürstner A.Organozinc Reagents; Knochel P.; Jones P., Eds.; Oxford University Press: New York, 1999; pp 287–305. [Google Scholar]; c Marshall J. A. Rhodium-catalyzed Reformatskii reaction. Chemtracts 2000, 13, 705–707. [Google Scholar]; d Podlech J.; Maier T. C. Indium in organic synthesis. Synthesis 2003, 0633–0655. 10.1055/s-2003-38064. [DOI] [Google Scholar]; e Orsini F.; Sello G. Transition metals-mediated Reformatsky reactions. Curr. Org. Synth. 2004, 1, 111–135. 10.2174/1570179043485385. [DOI] [Google Scholar]; f Nakamura E.Organometallics in Synthesis: A Manual; Schlosser M., Ed.; Wiley: New York, 2002; pp 579–664. [Google Scholar]
- a Huck L.; Berton M.; de la Hoz A.; Díaz-Ortiz A.; Alca′zar J. Reformatsky and Blaise reactions in flow as a tool for drug discovery. One pot diversity oriented synthesis of valuable intermediates and heterocycles. Green Chem. 2017, 19, 1420–1424. 10.1039/C6GC02619B. [DOI] [Google Scholar]; b Kloetzing R. J.; Thaler T.; Knochel P. An improved asymmetric Reformatsky reaction mediated by (−)-N, N-dimethylaminoisoborneol. Org. Lett. 2006, 8, 1125–1128. 10.1021/ol0531381. [DOI] [PubMed] [Google Scholar]; c Greszler S. N.; Malinowski J. T.; Johnson J. S. Remote stereoinduction in the acylation of fully substituted enolates: tandem Reformatsky/quaternary Claisen condensations of silyl glyoxylates and β-lactones. J. Am. Chem. Soc. 2010, 132, 17393–17395. 10.1021/ja108848d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Fernández-Ibáñez M. Á.; Maciá B.; Alonso D. A.; Pastor I. M. Recent Advances in the Catalytic Enantioselective Reformatsky Reaction. Eur. J. Org. Chem. 2013, 2013, 7028–7034. 10.1002/ejoc.201300571. [DOI] [Google Scholar]; e Pellissier H. Recent developments in the asymmetric Reformatsky-type reaction. Beilstein J. Org. Chem. 2018, 14, 325–344. 10.3762/bjoc.14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fornalczyk M.; Singh K.; Stuart A. M. Synthesis of α-fluoro-β-hydroxy esters by an enantioselective Reformatsky-type reaction. Chem. Commun. 2012, 48, 3500–3502. 10.1039/c2cc17985g. [DOI] [PubMed] [Google Scholar]; b Cozzi P. G. A Catalytic, Me2Z-mediated, enantioselective Reformatsky reaction with ketones. Angew. Chem., Int. Ed. 2006, 45, 2951–2954. 10.1002/anie.200504239. [DOI] [PubMed] [Google Scholar]; c Kanai K.; Wakabayashi H.; Honda T. Rhodium-catalyzed Reformatsky-type reaction. Org. Lett. 2000, 2, 2549–2551. 10.1021/ol006268c. [DOI] [PubMed] [Google Scholar]
- a Chattopadhyay A.; Dubey A. K. A simple and efficient procedure of low valent iron-or copper-mediated reformatsky reaction of aldehydes. J. Org. Chem. 2007, 72, 9357–9359. 10.1021/jo0710984. [DOI] [PubMed] [Google Scholar]; b Moriwake T. The Reformatsky reaction. I. Condensation of ketones and t-butyl bromoacetate by magnesium. J. Org. Chem. 1966, 31, 983–985. 10.1021/jo01341a524. [DOI] [Google Scholar]
- a Hojo M.; Harada H.; Ito H.; Hosomi A. Manganese ate complexes as new reducing agents: Perfectly regiocontrolled generation and reactions of the manganese enolates with electrophiles. J. Am. Chem. Soc. 1997, 119, 5459–5460. 10.1021/ja964054d. [DOI] [Google Scholar]; b Suh Y. S.; Rieke R. D. Synthesis of β-hydroxy esters using highly active manganese. Tetrahedron Lett. 2004, 45, 1807–1809. 10.1016/j.tetlet.2003.11.045. [DOI] [Google Scholar]; c Durandetti M.; Pe′richon J. Iron-catalysed reformatsky-type reactions. Synthesis 2006, 2006, 1542–1548. 10.1055/s-2006-926432. [DOI] [Google Scholar]
- a Orsini F.; Lucci E. M. Reformatsky reactions with SmI2 in catalytic amount. Tetrahedron Lett. 2005, 46, 1909–1911. 10.1016/j.tetlet.2005.01.079. [DOI] [Google Scholar]; b Segade Y.; Montaos M. A.; Rodríguez J.; Jimenez C. A short stereoselective synthesis of prepiscibactin using a SmI2-mediated Reformatsky reaction and Zn2+-induced asymmetric thiazolidine formation. Org. Lett. 2014, 16, 5820–5823. 10.1021/ol502958u. [DOI] [PubMed] [Google Scholar]; c Nelson C. G. Jr.; Burke T. R. Samarium iodide-mediated reformatsky reactions for the stereoselective preparation of β-hydroxy-γ-amino acids: synthesis of isostatine and dolaisoleucine. J. Org. Chem. 2012, 77, 733–738. 10.1021/jo202091r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessjohann L.; Gabriel T. Chromium (II)-mediated Reformatsky reactions of carboxylic esters with aldehydes. J. Org. Chem. 1997, 62, 3772–3774. 10.1021/jo961910v. [DOI] [Google Scholar]
- Burkhardt E.; Rieke R. D. The direct preparation of organocadmium compounds from highly reactive cadmium metal powders. J. Org. Chem. 1985, 50, 416–417. 10.1021/jo00203a036. [DOI] [Google Scholar]
- Kagoshima H.; Hashimoto Y.; Oguro D.; Saigo K. An activated Germanium metal-promoted, highly diastereoselective Reformatsky reaction. J. Org. Chem. 1998, 63, 691–697. 10.1021/jo971672j. [DOI] [PubMed] [Google Scholar]
- Imamoto T.; Kusumoto T.; Tawarayama Y.; Sugiura Y.; Mita T.; Hatanaka Y.; Yokoyama M. Carbon-carbon bond-forming reactions using cerium metal or organocerium (III) reagents. J. Org. Chem. 1984, 49, 3904–3912. 10.1021/jo00195a006. [DOI] [Google Scholar]
- a Chao L.; Rieke R. D. Activated metals. IX. New reformatsky reagent involving activated indium for the preparation of beta-hydroxy esters. J. Org. Chem. 1975, 40, 2253–2255. 10.1021/jo00903a031. [DOI] [Google Scholar]; b Babu S. A.; Yasuda M.; Shibata I.; Baba A. In- or In (I)-employed diastereoselective Reformatsky-type reactions with ketones: 1H NMR investigations on the active species. Org. Lett. 2004, 6, 4475–4478. 10.1021/ol0482846. [DOI] [PubMed] [Google Scholar]; c Ishihara J.; Tsuru H.; Hatakeyama S. Total synthesis of (−)-dihydrosporothriolide utilizing an Indium-mediated Reformatsky–Claisen rearrangement. J. Org. Chem. 2014, 79, 5908–5913. 10.1021/jo5008948. [DOI] [PubMed] [Google Scholar]; d Poisson T.; Belhomme M.-C.; Pannecoucke X. Indium-promoted Reformatsky reaction: a straightforward access to β-amino and β-hydroxy α, α-difluoro carbonyl compounds. J. Org. Chem. 2012, 77, 9277–9285. 10.1021/jo301873y. [DOI] [PubMed] [Google Scholar]; e Liu X.-Y.; Li X.-R.; Zhang C.; Chu X.-Q.; Rao W.; Loh T.-P.; Shen Z.-L. Iron (0)-mediated Reformatsky reaction for the synthesis of β-hydroxyl carbonyl compounds. Org. Lett. 2019, 21, 5873–5878. 10.1021/acs.orglett.9b01999. [DOI] [PubMed] [Google Scholar]
- a Cozzi P. G.; Benfatti F.; Capdevila M. G.; Mignogna A. Me2Zn mediated, tert-butylhydroperoxide promoted, catalytic enantioselective Reformatsky reaction with aldehydes. Chem. Commun. 2008, 3317–3318. 10.1039/b805197f. [DOI] [PubMed] [Google Scholar]; b Cozzi P. G.; Mignogna A.; Vicennati P. Dimethylzinc-mediated, oxidatively promoted Reformatsky reaction of ethyl iodoacetate with aldehydes and ketones. Adv. Synth. Catal. 2008, 350, 975–978. 10.1002/adsc.200700572. [DOI] [Google Scholar]; c Benfatti F.; Cozzi P. G. Copper-promoted enantioselective Reformatsky-type reaction with ketones. Tetrahedron: Asymmetry 2010, 21, 1503–1506. 10.1016/j.tetasy.2010.04.058. [DOI] [Google Scholar]; d Cozzi P. G. A catalytic, Me2Zn-mediated, enantioselective Reformatsky reaction with ketones. Angew. Chem., Int. Ed. 2006, 45, 2951–2954. 10.1002/anie.200504239. [DOI] [PubMed] [Google Scholar]; e Cozzi P. G.; Mignogna A.; Zoli L. Practical chloromanganese–salen-catalyzed enantioselective Reformatsky reaction with ketones. Synthesis 2007, 2007, 2746–2750. 10.1055/s-2007-983780. [DOI] [Google Scholar]
- a Fernández-Ibáñez M. Á.; Maciá B.; Minnaard A. J.; Feringa B. L. Catalytic enantioselective Reformatsky reaction with aldehydes. Angew. Chem., Int. Ed. 2008, 47, 1317–1319. 10.1002/anie.200704841. [DOI] [PubMed] [Google Scholar]; b Fernández-Ibáñez M. Á.; Maciá B.; Minnaard A. J.; Feringa B. L. Catalytic enantioselective Reformatsky reaction with ketones. Chem. Commun. 2008, 2571–2573. 10.1039/b801749b. [DOI] [PubMed] [Google Scholar]; c Fernández-Ibáñez M. Á.; Maciá B.; Minnaard A. J.; Feringa B. L. Catalytic enantioselective Reformatsky reaction with ortho-substituted diarylketones. Org. Lett. 2008, 10, 4041–4044. 10.1021/ol801574m. [DOI] [PubMed] [Google Scholar]
- Wolf C.; Moskowitz M. Bisoxazolidine-catalyzed enantioselective Reformatsky reaction. J. Org. Chem. 2011, 76, 6372–6376. 10.1021/jo200774e. [DOI] [PubMed] [Google Scholar]
- Tanaka T.; Hayashi M. Catalytic enantioselective Reformatsky reaction of alkyl iodoacetate with aldehydes catalyzed by chiral Schiff base. Chem. Lett. 2008, 37, 1298–1299. 10.1246/cl.2008.1298. [DOI] [Google Scholar]
- a Luo R.-S.; Weng J.; Ai H.-B.; Lu G.; Chan A. S. C. Highly efficient asymmetric Michael reaction of aldehydes to nitroalkenes with diphenylperhydroindolinol silyl ethers as organocatalysts. Adv. Synth. Catal. 2009, 351, 2449–2459. 10.1002/adsc.200900355. [DOI] [Google Scholar]; b Lin N.; Chen M.-M.; Luo R.-S.; Deng Y.-Q.; Lu G. Indolinylmethanol catalyzed enantioselective Reformatsky reaction with ketones. Tetrahedron: Asymmetry 2010, 21, 2816–2824. 10.1016/j.tetasy.2010.11.004. [DOI] [Google Scholar]
- a De Munck L.; Vila C.; Muñoz M. C.; Pedro J. R. Catalytic enantioselective aza-Reformatsky reaction with cyclic imines. Chem. - Eur. J. 2016, 22, 17590–17594. 10.1002/chem.201604606. [DOI] [PubMed] [Google Scholar]; b De Munck L.; Sukowski V.; Vila C.; Muñoz M. C.; Pedro J. R. Catalytic enantioselective aza-Reformatsky reaction with seven-membered cyclic imines dibenzo[b,f][1,4]oxazepines. Org. Chem. Front. 2017, 4, 1624–1628. 10.1039/C7QO00329C. [DOI] [Google Scholar]
- Kumar A.; Ner D. H.; Dike S. Y. A new chemoenzymatic enantioselective synthesis of R-(−)-tomoxetine, (R)- and (S)-fluoxetine. Tetrahedron Lett. 1991, 32, 1901–1904. 10.1016/S0040-4039(00)85993-6. [DOI] [Google Scholar]
- 1-(tert-Butyl)-2-methyl-(S)-indoline-1,2-dicarboxylate was synthesized as literatureGross K. M. B.; Jun Y. M.; Beak P. Asymmetric deprotonations: lithiation of N-(tert-butoxycarbonyl)indoline with sec-butyllithium/(−)-Sparteine. J. Org. Chem. 1997, 62, 7679–7689. 10.1021/jo9708856. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.