To the Editor:
Approximately 15 million people, including 8% of children, in the United States have food allergy and are at risk of having life-threatening anaphylactic reactions.1 There is an unmet need for the prevention of such reactions. Tyrosine kinases, including Bruton’s tyrosine kinase (BTK), have been shown to be critical for allergen reactivity by transducing FcεRI crosslinking signals into cellular activation and mediator release from mast cells and basophils.2 Pharmacyclics, Inc, together with Janssen Pharmaceuticals, recently received Food and Drug Administration approval of ibrutinib (Imbruvica, PCI-32765) as a selective, irreversible BTK inhibitor for the treatment of mantle cell lymphoma, chronic lymphocytic leukemia (CLL), and Waldenstrom’s macroglobulinemia. Studies have demonstrated broad and durable activity, with excellent overall tolerability. Regarding tolerability, ibrutinib treatment has been shown to cause a transient increase in blood lymphocytes at the same time as a reduction in lymph node size is typically noted. Neutropenia has also been noted as a side effect in clinical trials with a frequency ranging from 15% to 22% of patients.3–5 Regarding effects on allergic cell reactivity, Advanti et al6 reported inhibition of IgE-dependent basophil activation in subjects receiving ibrutinib as determined ex vivo using the basophil activation test (BAT). In vitro studies done by MacGlashan et al7 showed that ibrutinib, tested at clinically relevant concentrations, completely blocked FcεRI-dependent basophil release of histamine and leukotrienes, secretion of IL-4, and the BAT. Furthermore, multiple studies have shown defects in FcεRI-dependent mast cell degranulation and cytokine production in patients with X-linked agammaglobulinemia, a disease caused by BTK deficiency, or in BTK null mice.2,8 These data suggest that BTK signaling plays a critical role in basophil and mast cell IgE-dependent activation, and therefore, its safe and effective pharmacologic inhibition could result in profound antiallergic effects.
We hypothesized that treatment with ibrutinib would attenuate allergen reactivity by interfering with IgE-dependent basophil and mast cell activation. To evaluate the effect of BTK inhibition on allergen reactivity in humans, we initiated a pilot study in patients who were prescribed ibrutinib as standard of care for treatment of underlying lymphoid malignancy. We performed skin testing and the BAT immediately before and during prescribed ibrutinib treatment. Using a Northwestern University institutional review board (IRB)-approved protocol, we screened a total of 28 out of 35 patients before the start of commercial ibrutinib treatment for treatment of mantle cell lymphoma, CLL, or Waldenstrom’s macroglobulinemia over a period of approximately 18 months for the presence of aeroallergen sensitivity by brief questionnaire (see this article’s Online Repository at www.jacionline.org), BAT, and aeroallergen skin testing using the Multi-Test II device (Lincoln Diagnostics, Inc, Decatur, Ill) using 8 skin test materials (saline, histamine, cat allergen, dust mite mix, ragweed mix, mold mix, tree mix, and grass mix from ALK-Abello, Round Rock, Tex). We enrolled 2 allergic patients, both of whom were initiating treatment with ibrutinib 420 mg daily for CLL. Subject 1 was on daily intranasal fluticasone and remained on the same dosing regimen throughout the entire study. Subject 2 was not taking any antiallergy medication. Per the IRB-approved protocol, subjects underwent repeat skin testing to the same 6 aeroallergen panel and BAT after 7 days and again after 30 days or more of ibrutinib treatment. At each time point, serum was collected and total and specific IgE levels were measured by Immunocap.
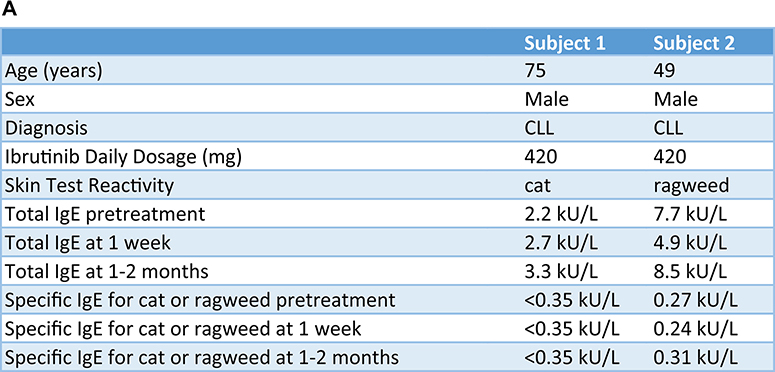
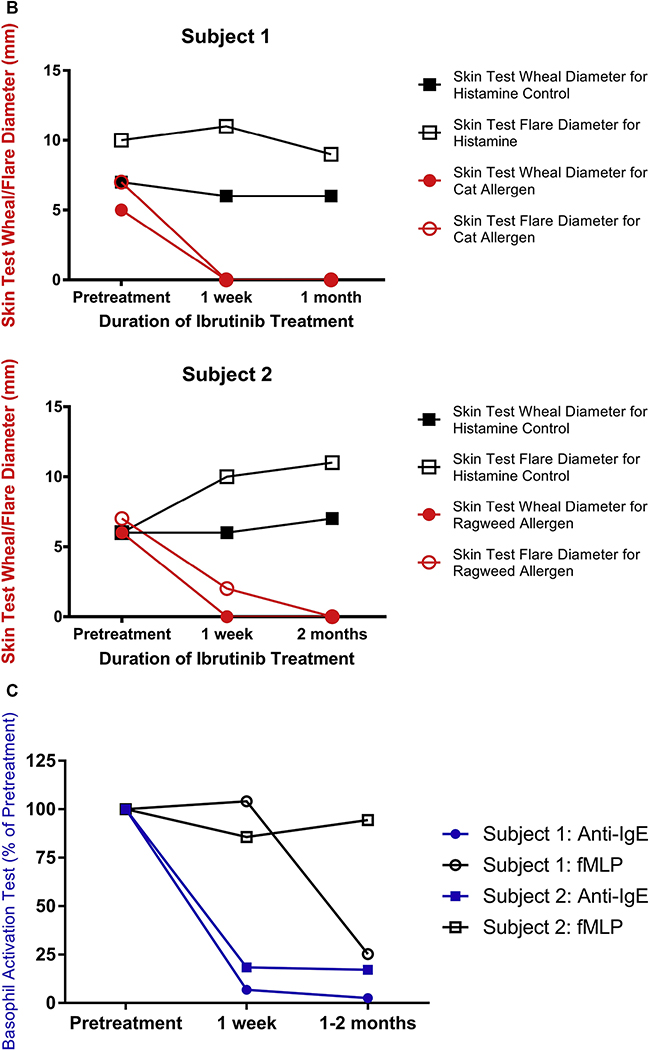
As shown in Fig 1, the enrolled subjects initially had a positive skin test result to either cat allergen (subject 1, Fig 1, B) or ragweed (subject 2, Fig 1, B) in addition to a positive BAT result (Fig 1, C). Each of the 2 subjects was skin test positive to only a single aeroallergen, and neither became skin test positive to a new aeroallergen (among the 6 allergens tested) during the study. One week after starting ibrutinib, there was an almost complete loss of skin test reactivity as assessed by wheal and flare size, and basophil activation as measured by the BAT. In contrast, but as expected, skin test responses to histamine, and BAT responses to a non–IgE-dependent stimulus formyl-methionyl-leucyl-phenylalanine (fMLP), were unaffected (Fig 1, B and C). These reduced IgE-dependent responses were sustained at 1 to 2 months of ibrutinib treatment (Fig 1, B and C), although 1 of the 2 subjects showed a decreased fMLP BAT response at 1 to 2 months. Loss of skin test reactivity and BAT responses was not due to changes in IgE levels because they were not altered during the study (Fig 1, A), although these allergen-specific and total levels were below 0.35 kU/L. Whether these low levels were due, at least in part, to previous treatments received for their CLL is unknown.
FIG 1.
Ibrutinib inhibits basophil and mast cell activation in 2 subjects prescribed ibrutinib for CLL treatment. A, Demographic characteristics, BTK inhibitor clinical indication, ibrutinib dose, and IgE levels are listed for each subject. B, Skin test responses to cat allergen (subject 1) and ragweed (subject 2) before and during ibrutinib treatment (420 mg daily) in 2 patients being treated for CLL. C, Basophil activation responses to anti-IgE (72% and 89% positive, respectively, before starting ibrutinib) or fMLP (12% and 16% positive, respectively, before starting ibrutinib) are shown for these same 2 subjects.
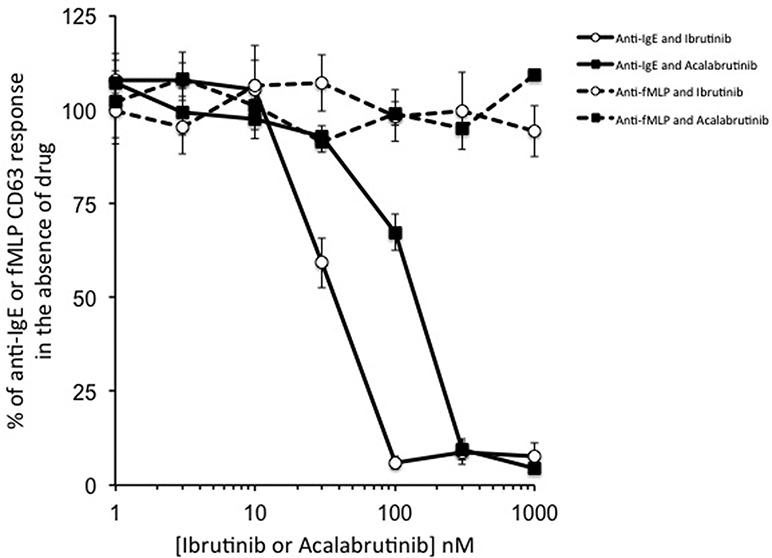
To evaluate whether other BTK inhibitors could also inhibit basophil activation, we performed in vitro BAT studies to compare effects of acalabrutinib with those of ibrutinib. As shown in Fig 2, when added in vitro to whole blood of normal donors (using a separate IRB-approved protocol), these drugs, as expected, had no effect on fMLP-induced BAT because signaling via fMLP does not depend on tyrosine kinases. In contrast, both ibrutinib and acalabrutinib completely blocked BAT responses, with an IC50 of approximately 40 nM for ibrutinib and 105 nM for acalabrutinib. These IC50s were higher than those previously reported,7,9 probably because unlike previous studies, our experiments were performed with impure cells in whole blood in the presence of albumin, red blood cells, and other substances capable of binding to drug and thus lowering effective drug concentrations. Regardless, both ibrutinib and acalabrutinib inhibit IgE-dependent, but not IgE-independent, basophil activation at concentrations that are readily achieved with current clinical dosing regimens.
FIG 2.
Ibrutinib and acalabrutinib inhibit basophil activation in vitro as measured by the BAT in whole blood samples obtained from healthy volunteers. Whole blood samples were obtained from healthy volunteers and preincubated with the indicated concentrations of the drug for 30 minutes; then, BAT was performed with the addition of anti-IgE or fMLP. Levels of anti-IgE and fMLP BAT from which the values were normalized averaged 62% ± 9% and 26% ± 1% positive. Values represent mean ± SEM (N = 3–4).
These results suggest that through inhibition of IgE-dependent basophil and mast cell activation, BTK antagonists may be able to block allergic reactions including food allergy. Although its ability to inhibit BAT in vitro and ex vivo, as well as passive cutaneous anaphylaxis in mice, has been reported,8 to our knowledge, this is the first report of a pharmacological agent having true mast cell–stabilizing activity capable of markedly inhibiting mast cell activation as measured by skin test reactivity. There are currently no treatments to prevent anaphylaxis, so agents such as BTK inhibitors have the potential to fill this void. It remains to be determined whether ibrutinib treatment will be similarly efficacious in suppressing skin test responses and BAT in polyallergic subjects, and in subjects with higher levels of allergen-specific IgE. However, a separate IRB-approved protocol is now in place that will allow us to expand our preliminary results to allergic subjects without lymphoproliferative disorders, to confirm the inhibitory mechanisms involved, to explore the duration of such inhibitory effects, and to determine whether this strategy could be used in the management and treatment of allergic diseases including food allergy.
Acknowledgments
We thank Drs Shikha Jain, Jason Kaplan, Regina Stein, and Jane Winter for referring their patients for screening and possible participation in our study.
Disclosure of potential conflict of interest: J. A. Regan has received a grant from the 2016 Dixon Translational Innovation Award through the Northwestern University Clinical and Translational Sciences Institute and Northwestern Memorial Foundation, is employed by Northwest Asthma and Allergy Center, and has received travel support from the American Academy of Allergy, Asthma & Immunology and the American College of Asthma, Allergy, and Immunology. M. C. Dispenza has received a grant from the 2016 Dixon Translational Innovation Award through the Northwestern University Clinical and Translational Sciences Institute and Northwestern Memorial Foundation and the National Institutes of Health (grant nos. P01HL107151, AI072265, and T32AI083216). S. Ma has consultant arrangements with Novartis, AbbVie, Pharmacyclics, and Gilead; has received grants from Novartis, Pharmacyclics, Gilead, AbbVie, Xeme, and Acerta; and has received payment for lectures from Genentech, Pharmacyclics, and Gilead. L. I. Gordon has received a grant from the 2016 Dixon Translational Innovation Award through the Northwestern University Clinical and Translational Sciences Institute and Northwestern Memorial Foundation. A. M. Petrich is employed by AbbVie. B. S. Bochner has received a grant from the 2016 Dixon Translational Innovation Award through the Northwestern University Clinical and Translational Sciences Institute and Northwestern Memorial Foundation and the National Institutes of Health; is on the scientific advisory board for Allakos Inc, has consultant arrangements with TEVA; has several patents related to Siglec-8 that belong to Johns Hopkins, some of which have been licensed to Allakos; is entitled to receive a share of royalties regarding milestones and the sale of Siglec-8-related products from Allakos; and has received stock/stock options from Allakos. Y. Cao declares no relevant conflicts of interest.
Footnotes
This work was funded in part by a 2016 Dixon Translational Innovation Award through the Northwestern University Clinical and Translational Sciences Institute and Northwestern Memorial Foundation.
REFERENCES
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:9–17. [DOI] [PubMed] [Google Scholar]
- 2.Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, et al. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med 1998;187:1235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting Btk with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting Btk with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med 2015;372:1430–40. [DOI] [PubMed] [Google Scholar]
- 6.Advanti RB, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton’s tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacGlashan D Jr, Honigberg LA, Smith A, Buggy J, Schroeder JT. Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton’s tyrosine kinase, Btk, inhibitor. Int Immunopharmacol 2011;11:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, et al. The Bruton’s tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther 2011;13:R115, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]