Abstract
Purpose/Objectives
We aimed to assess the predictive value of a lung cancer gene panel for the development of brain metastases.
Materials/Methods
Between 2011 and 2015, 102 patients with lung cancer were prospectively enrolled in a clinical trial in which a diagnostic fine-needle aspirate was obtained. Gene expression was conducted on all samples that rendered a diagnosis of non-small cell lung cancer (NSCLC). Subsequent retrospective analysis of brain metastases-related outcomes was performed by reviewing patient electronic medical records. A competing risk multivariable regression was performed to estimate the adjusted hazard ratio for the development of brain metastases and non-brain metastases from NSCLC.
Results
A total of 49 of 102 patients had died by the last follow-up. Median time of follow-up was 13 months (range 0.23– 67 months). A total of 17 patients developed brain metastases. Median survival time after diagnosis of brain metastases was 3.58 months (95% confidence interval (CI) 2.17, not available). A total of 30 patients developed metastases without any evidence of brain metastases until the time of death or last follow-up. Competing risk analysis identified three genes that were downregulated differentially in the patients with brain metastases versus non-brain metastatic disease: CD37 (0.017), cystatin A (0.022), and IL-23A (0.027). Other factors associated with brain metastases include: stage T (P ⩽ 8.3e−6) and stage N (P= 6.8e−4).
Conclusions
We have identified three genes, CD37, cystatin A, and IL-23A, for which downregulation of gene expression was associated with a greater propensity for developing brain metastases. Validation of these biomarkers could have implications on surveillance patterns in patients with brain metastases from NSCLC.
Keywords: Lung cancer, brain metastases, non-small cell lung cancer, gene expression, survival
Introduction
Nearly 100,000 patients with lung cancer are diagnosed with brain metastases (BM) in the USA each year.1 Even with improving therapies, outcomes are often poor with a median survival of 1 year or less.2 When symptomatic BM are identified, patients often experience a worsening of quality of life, a shorter survival time, and an increased financial burden.3 Conversely, early detection of asymptomatic BM may allow for the preservation of quality of life and neurological function. Furthermore, if patients at increased risk for the development of BM could be identified, brain-image-guided surveillance could be performed to intervene prior to metastases becoming symptomatic. Current data suggest that this approach may decrease the number of patients that die of BM.4
While patients with locally advanced and metastatic non-small cell lung cancer (NSCLC) are generally staged with brain magnetic resonance imaging (MRI) at the time of diagnosis, there is no clear consensus on whether to obtain further brain imaging should no metastases be discovered at the initial staging. Patients with early stage disease at diagnosis generally do not require neuroimaging because of the low rate of brain metastases at the time of diagnosis. However, up to 40% of patients with lung cancer will ultimately develop brain metastases.5 Given the number of patients with NSCLC, it would be costly to perform image-guided surveillance on all lung cancer survivors with local or locally advanced treated disease. As such, there is great interest in the identification of biomarkers that predict a high risk for BM. Recent studies of BM from primary lung cancer suggest that there are brain metastasis-specific mutations.6 The existence of such mutations suggests that the identification of a biomarker may be feasible.
Our institution sought to identify genetic and/or molecular biomarkers for BM by correlating gene expression from patients with NSCLC who subsequently developed BM.
Methods
Patient Population
Institutional Review Board approval was obtained for this trial that was undertaken between 2011 and 2015. A total of 102 patients with suspected radiographic lung cancer were prospectively enrolled in a clinical trial in which a diagnostic fine-needle aspirate (FNA) was obtained. All patients underwent either computed tomography-guided trans-thoracic needle biopsy or endobronchial ultrasoundguided trans-bronchial needle sampling. A single dedicated final needle sample was placed entirely into RNAlater®. Key inclusion criteria included being older than 18 years and the ability to understand, and the willingness to sign, a written informed consent document. Exclusion criteria included patients whose FNA biopsy was unable to provide subtype classification by pathology or was non-diagnostic.
Patient characteristics including age, gender, primary histology, and cancer staging were abstracted from the electronic medical record. Medical records were also used to determine patient outcomes, including time to metastasis, time to brain metastasis, and brain metastasis number and diameter, plus time to last follow-up or death. Patient characteristics are summarized in Table 1.
Table 1.
Patient and Pathologic Characteristics.
| Patient and Pathologic Characteristics, No. (%) | All Patients 102 (100.0%) | Brain Metastasis 17 (16.7%) |
|---|---|---|
| Age (Mean) | 65.7 | 62.2 |
| Gender | ||
| Male | 60 (58.8%) | 11 (64.7%) |
| Female | 42 (41.2%) | 6 (35.3%) |
| Race | ||
| Non-Hispanic White | 89 (87.3%) | 3 (17.6%) |
| African American | 12 (11.8%) | 14 (82.4%) |
| Other | 1 (1.0%) | 0 (0.0%) |
| Pathology | ||
| Adenocarcinoma | 55 (53.9%) | 10 (58.4%) |
| Squamous cell Carcinoma | 29 (28.4%) | 5 (29.4%) |
| Non-small cell (not further specified) | 17 (16.7%) | 2 (11.8%) |
| Other | 1 (1.0%) | 0 (0.0%) |
| T-Stage (TNM at diagnosis) | ||
| Tis | 1 (1.0%) | 0 (0.0%) |
| I | 9 (8.9%) | 0 (0.0%) |
| Ia | 10 (9.9%) | 0 (0.0%) |
| Ib | 13 (12.9%) | 0 (0.0%) |
| II | 15 (14.9%) | 2 (11.8%) |
| IIa | 10 (9.9%) | 1 (5.9%) |
| IIb | 3 (3.0%) | 0 (0.0%) |
| III | 18 (17.8%) | 3 (17.6%) |
| IV | 22 (21.8%) | 11 (64.7%) |
| N-Stage | ||
| Nx | 1 (1.0%) | 0 (0.0%) |
| N0 | 42 (41.6%) | 1 (5.9%) |
| N1 | 8 (7.9%) | 1 (5.9%) |
| N2 | 28 (27.7%) | 5 (29.4%) |
| N3 | 22 (21.8%) | 10 (58.8%) |
| M-Stage | ||
| M0 | 60 (59.4%) | 4 (23.5%) |
| M1 | 4 (4.0%) | 1 (5.9%) |
| M1a | 3 (3.0%) | 1 (5.9%) |
| M1b | 29 (28.7%) | 11 (64.7%) |
| Mx | 5 (5.0%) | 0 (0.0%) |
TNM: tumor node metastasis.
TNM staging at time of diagnosis.
Genomic Data Acquisition
Genomic analysis was performed on the RNA from a diagnostic FNA of the tumor as previously described.7 All samples were reviewed by a faculty cytopathologist for significant tumor cellularity in the diagnostic sample prior to proceeding with the extraction of RNA. Tumor tissues samples collected as the study samples were stored in RNAlater® at 4°C until pathologic confirmation was achieved. Once confirmed that the biopsy was diagnostic, the samples were batched for RNA extraction and individual samples were homogenized according to the Qiagen QIAshredder protocol. Total RNA (approximately 1–10 ug per sample) was isolated from each tumor sample according to the Qiagen RNeasy Microarray Tissue Mini Kit protocol. Once an adequate quantity was confirmed using an Eppendorf BioPhotometer, the quality of the RNA was assessed using an Agilent 2100 Bioanalyzer. RNA suitable for evaluation were selected using the following criteria: (a) RNA integrity number (RIN) >8.0; and (b) absorbance ratio (A260/A280) between 1.8 and 2.1.7 RNA from FNA were processed for gene expression analysis. A gene panel was used to generate transcriptome data using the NanoString platform (see Table 2). The selection of the genes was based on their ability to be histotype-specific, immune, and survival correlates. For each sample, original expression of all genes was log2 transformed, normalized against the mean of the four housekeeping genes, and z-transformed. Cross-sample normalization was avoided to simplify clinical implementation. Next, nCounter data analysis was conducted using biometric research program array tools. The gene expression data will be submitted to the Gene Expression Omnibus for open public access.
Table 2.
Gene Panel.
| CD37 molecule |
| Cystatin A (stefin A) |
| Interleukin 23, alpha subunit p19 |
| Protein tyrosine phosphatase, receptor type, C |
| Plakophilin 1 (ectodermal dysplasia/skin fragility syndrome) |
| Major histocompatibility complex, class II, DP alpha 1 |
| Major histocompatibility complex, class II, DP beta 1 |
| Hydroxysteroid (17-beta) dehydrogenase 6 homolog (mouse) |
| Major histocompatibility complex, class II, DR alpha |
| Post-GPI attachment to proteins 1 |
| Keratin 14 |
| Creatine kinase, mitochondrial 1B |
| Lactate dehydrogenase A |
| CD9 molecule |
| Profilin 2 |
| Interleukin 8 |
| Keratin 6B |
| Transcribed locus |
| Glutathione peroxidase 2 (gastrointestinal) |
| Glycoprotein (transmembrane) nmb |
| Surfactant protein B |
| Colony stimulating factor 1 receptor |
| Kruppel-like factor 5 (intestinal) |
| Lectin, galactoside-binding, soluble, 4 |
| Cytochrome P450, family 1, subfamily A, polypeptide 2 |
| Vascular endothelial growth factor A |
| FXYD domain containing ion transport regulator 3 |
| Paraneoplastic antigen MA2 |
| Aldo-keto reductase family 1, member B10 (aldose reductase) |
| Folate receptor l (adult) |
| Phosphofructokinase, platelet |
| |MAPK1|Mitogen-activated protein kinase 1 |
| |C8orf4|Chromosome 8 open reading frame 4 |
| |UBE2S|Ubiquitin-conjugating enzyme E2S |
Brain Metastasis Surveillance and Endpoints
Patients underwent staging MRI following the diagnosis of NSCLC. Based on provider practice standard care, patients with stage II or greater underwent staging MRI of the brain. If patients had a negative initial MRI of the brain, re-imaging of the brain was done either at the time of the development of metastatic disease (incidental finding) or when indicated for neurologic symptoms.
Statistics
A competing risk analysis was performed for the effects of the prognostic factors, including pathological stages and histology (adenocarcinoma vs. squamous cell carcinoma) on the risk of developing brain metastasis versus other types of metastasis. Cumulative incidence functions of both metastasis types in each subgroup were estimated for every prognostic factor. Gray’s test was used to assess the statistical significance of these prognostic factors in competing risks.
The genes that were differentially expressed at mRNA level in tumor tissues that subsequently developed brain metastasis were sought using empirical Bayes linear model8 using R (version: 3.3.1) package “limma” (version: 3.30.13). Top genes that showed both biological significance (absolute log fold changes of expression larger than 1) and statistical significance (P ⩽ 0.050) were selected as potential differentially expressed genes.
Results
Clinical Outcomes
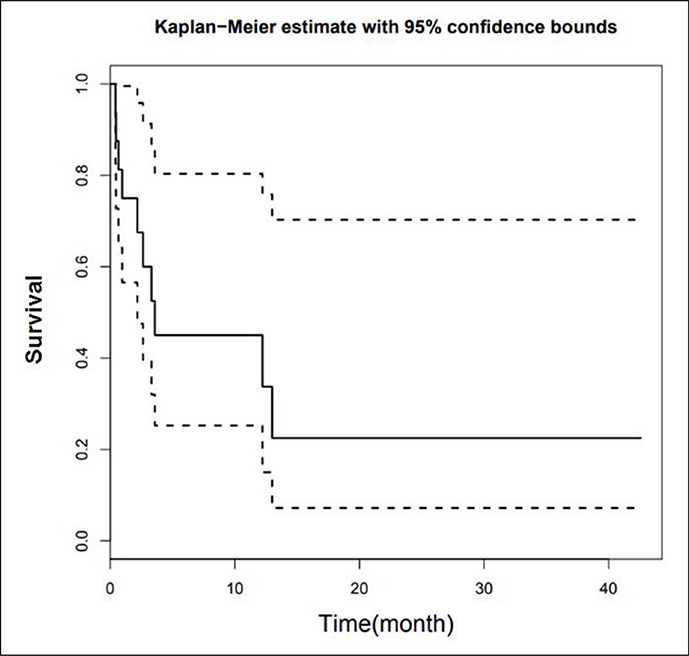
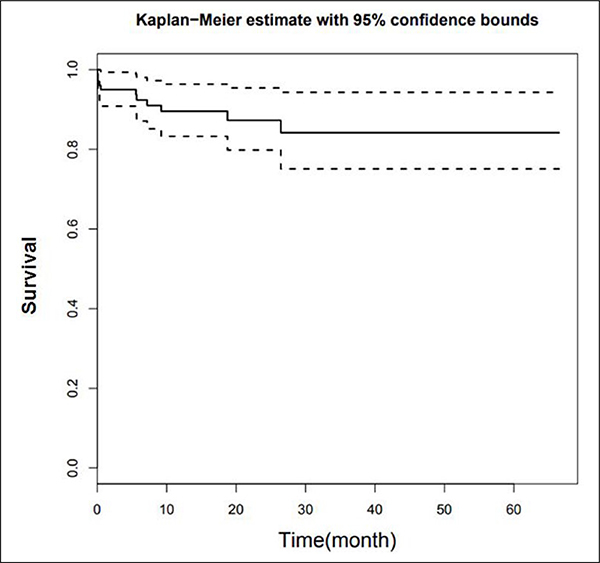
A total of 49 of 102 patients had died by the last follow-up. The median time of follow-up was 13.0 months (range 0–67 months). A total of 17 patients developed BM. The risk of developing BM was lower than 50% (1–0.84 = 0.16), so no median survival time is available (Figure 1). Median survival time after diagnosis of BM was 3.58 months (95% confidence interval (CI) 2.17, not available) (Figure 2). A total of 30 patients developed metastases outside the brain without any evidence of BM until the time of death or last follow-up.
Figure 1.
Kaplan Meier curve of survival time for patients developing BM. BM: brain metastasis.
Figure 2.
Kaplan Meier curve of time to development of BM. BM: brain metastasis.
Factors Associated with Brain Metastasis Development
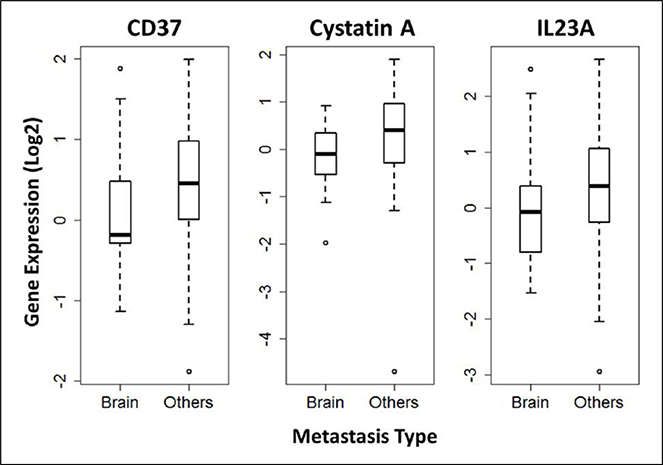
Competing risk analysis revealed the cumulative incidence of BM to be associated with higher stage T (P ⩽ 8.3e−6) and higher stage N (P = 6.8e−4). The effect of stage M (P = 0.42) or adenocarcinoma versus squamous histology (P = 0.25) were not statistically significant. Competing risk analysis identified three genes that were downregulated differentially in the primary tumor tissues of patients who later developed BM versus non-brain metastatic disease: CD37 (0.017), cystatin A (CSTA) (0.022), and IL-23A (0.027). The expression levels of these three genes were also significantly down-regulated in the primary tumor tissues of patients who later developed BM versus non-brain metastatic disease, with log fold changes of −1.3, −1.4, and −1.3, respectively (Figure 3). There was no significant expression difference of these three genes in adenocarcinoma versus squamous histology.
Figure 3.
Box plots of the expression levels in BM cases comparing with other metastasis types for genes CD37, Cystatin A, and IL23A. BM: brain metastasis.
Discussion
This study represents a retrospective analysis of a prospective study that sought to evaluate potential biomarkers related to histology, immune function, and survival.
Our study identified three downregulated genes that were associated with BM: CSTA (0.022), IL-23A (0.027), and CD37 (0.017). Each of these genes has a plausible mechanism for furthering the metastatic phenotype.
Cystatin A (CSTA), also called Stefin A, functions as a cysteine protease inhibitor and has been shown to regulate tumor growth, invasion, and metastasis.9 Specifically, CSTA mRNA is detected in benign but not malignant lesions, suggesting that the loss of CSTA promotes tumorigenesis or tumor progression.10 It has been suggested that CSTA is an inhibitor of cysteine protease cathepsin B (CatB), and the decreased expression of CSTA causes increased CatB, which is associated with tumor malignancy in brain tumors.11 Studies have also indicated that CSTA suppressed ultraviolet-induced apoptosis in keratinocytes by the inhibition of caspase 3.12 A recent study in 2018 by Shiba et al.13 showed that the mRNA expression of CSTA was significantly decreased in esophageal squamous cell carcinoma with a P<0.001; however, some portions of the tumor tissues showed high CSTA expression levels, and these areas showed the most advanced tumor invasion. The authors felt that although a significant portion of cells in the tumor were attempting to downregulate CSTA, the cells unable to do so had advanced tumor progression. Therefore, this suggested that low mRNA expression levels in tumor tissue samples may not indicate expression levels in cells that have the greatest invasion potential. Further studies with immunostaining of tumor samples will be needed to further elucidate the role of CSTA in metastases.
We found a significant downregulation of IL-23 to be associated with BM; however, it is possible that similar to the results found by Shiba et al.,13 mRNA expression could be significantly decreased overall but still have areas within the tumor showing increased expression. Again, this suggests that further studies investigating the immunostaining of tumor samples may be beneficial.
Another possibility is that IL-23, like IL-12, is an antitumor agent and inhibits metastases. One study demonstrating this used mouse models with IL-23-induced tumor cells and showed that IL-23 was effective at inhibiting the growth of tumors and lung metastases by the mediation of CD8+ T cells.14 A study by Li et al.15 similarly found that high levels of IL-23 can inhibit lung cancer cell growth. They found that low concentrations of IL-23 promoted the proliferation of IL-23 receptor-positive A549 and SPCA-1 lung cancer cells by binding to IL-23r, and that IL-23 regulated the growth of human lung cancer cells through its effects on STAT3 expression and phosphorylation in a concentration-dependent way.
Further, it has been demonstrated that IL-23 upregulates T Helper 17 cells16 and these cells have been shown to promote antitumor immunity.17 The downregulation of IL-23 could lead to the loss of this antitumor immunity.
CD37 is a transmembrane protein with known functions in the regulation of cell growth and motility. It is known as a pro-apoptotic signal, and the downregulation of CD37 has been shown to activate the IL-6 signaling pathway and to drive tumorigenesis.18 CD37 is also an important immune marker for T-cells, B-cells, macrophages/monocytes, and granulocytes. The low expression of CD37 may indicate the poor filtration of immune cells and suppressed immunity in the tumor tissue.
These genes will require validation with a larger sample size. The goal is to identify a biomarker to predict the development of future BM in patients with NSCLC. These biomarkers could allow for increased surveillance in atrisk populations; this targeted surveillance could result in the earlier detection of BM. The benefits of earlier detection of BM may include: (a) a tendency for patients to have improved functional performance status at the time of diagnosis; (b) fewer and smaller metastases; and (c) quality-of-life-sparing treatment options.19–23 The early detection of BM allows for improved functional status of patients at the time of diagnosis as the tumor has had less time to cause neurological impairment. This would allow more patients to be surgical candidates, and surgical resection has been shown to improve local control, quality of life, and survival.24,25 Early detection of BM also minimizes the time allowed for the progression of metastatic disease and allows for treatment while BM are fewer and smaller. The smaller and fewer BM are when treated the better the patient performance outcomes, local control, quality of life, and survival.19,21–23
In addition to early detection, biomarkers could increase cost effectiveness. If biomarkers for BM could be proven to be sufficiently sensitive, a negative screening would eliminate the need for brain imaging for initial staging, and decrease the need for surveillance imaging. Considering the cost of an MRI of the brain is approximately US$3,000 and the cost of adding RNA analysis to FNA and would cost about $200, the savings would be substantial. Doing RNA analysis for biomarkers would be relatively easy as tissue from FNA has often already been taken for pathological diagnosis.
Several prior studies have attempted to identify a biomarker for BM, but none have been successfully validated for NSCLC. A prospective study at Memorial Sloan Kettering Cancer Center investigated six serum proteomic makers— NSE, CYFRA 21–1, Pro-GRP, SCC-Ag, TIMP1, and HE4— in volunteers with newly diagnosed stage IV NSCLC.26 None of these proteomic markers were significantly associated with the presence of BM on logistic regression analysis. Another study by Lee et al.27 demonstrated the correlation of pre-treatment carcinoembryonic antigen to BM in NSCLC; however, no independent validation accounting for timing and survival has been conducted. A study from the Virginia Piper Cancer Center investigated microRNAs as a potential biomarker for the development of BM in NSCLC and compared microRNA microarray profiling in patients with BM and those without BM.28 This study identified miR-328 as a biomarker that was able to classify patients with BM in a small cohort (seven patients with BM, and six patients without BM). In the validation cohort (n=15), miR-328 was able to correctly classify 12 of 15 patients. Given the small numbers in this study, these findings are hypothesis-generating and likely need to be validated by a larger independent study before they can be clinically adopted.
This series has several limitations. This study is limited in power by the patient population size. As such, a larger population size will be necessary to validate the findings. As a single-institution study, this series was subject to patient-selection bias. The patient tissue sample genetic evaluation was limited to using predetermined genes and thus narrowed the analysis. Patient follow-up was limited by not having a standardized schedule for brain imaging, thus patients could have developed asymptomatic BM not discovered with imaging. Despite the limitations of this study, we build on the current literature by identifying three genes (CD37, CSTA, and IL-23A) that are differentially expressed in patients with NSCLC who develop BM compared with patients who develop non-brain metastatic disease. We theorize that the identification of NSCLC genes associated with increased brain metastasis potential could inform future protocols for image-guided surveillance and thus have the potential to identify BM before patients are symptomatic. Future retrospective and prospective studies with larger sample sizes are needed to further clarify these results.
Conclusions
We have identified three genes CD37, CSTA, and IL-23A for which the downregulation of gene expression was associated with a greater propensity for developing BM. Validation of these biomarkers could have implications on surveillance patterns in patients with BM from NSCLC.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Haughton ME, Chan MD, Watabe K, et al. Treatment of brain metastases of lung cancer in the era of precision medicine. Front Biosci 2016; 8: 219–232. [DOI] [PubMed] [Google Scholar]
- 2.Bowden G, Kano H, Caparosa E, et al. Gamma knife radiosurgery for the management of cerebral metastases from nonsmall cell lung cancer. J Neurosurg 2015; 122: 766–772. [DOI] [PubMed] [Google Scholar]
- 3.Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 2016; 45: 139–162. [DOI] [PubMed] [Google Scholar]
- 4.Lester SC, Taksler GB, Kuremsky JG, et al. Clinical and economic outcomes of patients with brain metastases based on symptoms: an argument for routine brain screening of those treated with upfront radiosurgery. Cancer 2014; 120: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali A, Goffin JR, Arnold A, et al. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol 2013; 20: e300–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aljohani HM, Aittaleb M, Furgason JM, et al. Genetic mutations associated with lung cancer metastasis to the brain. Mutagenesis 2018; 33: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash B, Miller L, Bellinger C, et al. RNA yield of FNA specimens obtained through bronchoscopic transbronchial needle aspiration (TBNA) vs trans- thoracic fine needle aspiration (TTFNA). B63 Molecular insights into lung cancer II. Am J Respir Crit Care Med 2014; 189: A3483. [Google Scholar]
- 8.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivenbark AG and Coleman WB. Epigenetic regulation of cystatins in cancer. Front Biosci 2009; 14: 453–462. [DOI] [PubMed] [Google Scholar]
- 10.Levičar N, Strojnik T, Kos J, et al. Lysosomal enzymes, cathepsins in brain tumour invasion. J Neurooncol 2002; 58: 21–32. [DOI] [PubMed] [Google Scholar]
- 11.Strojnik T, Zajc I, Bervar A, et al. Cathepsin B and its inhibitor stefin A in brain tumors. Pflugers Arch 2000; 439: r122–r123. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Komatsu N, Ibe M, et al. Cystatin A suppresses ultraviolet B-induced apoptosis of keratinocytes. J Dermatol Sci 2007; 46: 179–187. [DOI] [PubMed] [Google Scholar]
- 13.Shiba D, Terayama M, Yamada K, et al. Clinicopathological significance of cystatin A expression in progression of esophageal squamous cell carcinoma. Medicine 2018; 97: e0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo C-H, Lee S-C, Wu P-Y, et al. Antitumor and antimetastatic activity of IL-23. J Immunol 2003; 171: 600–607. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhang L, Zhang J, et al. Interleukin 23 regulates proliferation of lung cancer cells in a concentration-dependent way in association with the interleukin-23 receptor. Carcinogenesis 2012; 34: 658–666. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Franks HA, Porte J, et al. Novel approach for interleukin-23 up-regulation in human dendritic cells and the impact on T helper type 17 generation. Immunology 2011; 134: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W and Restifo NP. Erratum: TH17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 2011; 11: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Winde CM, Veenbergen S, Young KH, et al. Tetraspanin CD37 protects against the development of B cell lymphoma. J Clin Invest 2016; 126: 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363: 1665–1672. [DOI] [PubMed] [Google Scholar]
- 20.Gavrilovic IT and Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005; 75: 5–14. [DOI] [PubMed] [Google Scholar]
- 21.Gondi V and Mehta MP. Novel insights into the management of brain metastases. Curr Opin Neurol 2010; 23: 556–562. [DOI] [PubMed] [Google Scholar]
- 22.Butowski N Medical management of brain metastases. Neurosurg Clin N Am 2011; 22: 27–36, v–vi. [DOI] [PubMed] [Google Scholar]
- 23.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 24.Rades D, Kieckebusch S, Haatanen T, et al. Surgical resection followed by whole brain radiotherapy versus whole brain radiotherapy alone for single brain metastasis. Int J Radiat Oncol Biol Phys 2008; 70: 1319–1324. [DOI] [PubMed] [Google Scholar]
- 25.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990; 322: 494–500. [DOI] [PubMed] [Google Scholar]
- 26.Li BT, Lou E, Hsu M, et al. Serum biomarkers associated with clinical outcomes fail to predict brain metastases in patients with stage IV non-small cell lung cancers. PLoS One 2016; 11: e0146063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D-S, Kim Y-S, Jung S-L, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumour Biol 2012; 33: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 28.Arora S, Ranade AR, Tran NL, et al. MicroRNA-328 is associated with (non-small) cell lung cancer (NSCLC) brain metastasis and mediates NSCLC migration. Int J Cancer 2011; 129: 2621–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]