Abstract
The λtI terminator is located approximately 280 bp beyond the λint gene, and it has a typical structure of an intrinsic terminator. To identify sequences required for λtI transcription termination a set of deletion mutants were generated, either from the 5' or the 3' end onto the λtI region. The termination efficiency was determined by measuring galactokinase (galK) levels by Northern blot assays and by in vitro transcription termination. The importance of the uridines and the stability of the stem structure in the termination were demonstrated. The nontranscribed DNA beyond the 3' end also affects termination. Additionally, sequences upstream have a small effect on transcription termination. The in vivo RNA termination sites at λtI were determined by S1 mapping and were located at 8 different positions. Processing of transcripts from the 3' end confirmed the importance of the hairpin stem in protection against exonuclease.
Keywords: λtI, PNPase, degradation, transcription termination
Résumé
Le terminateur λtlest localisé approximativement 280 pb au-delá du géne λint, et il posséde une structure typique d’un terminateur intrinséque. Afin d’identifier les séquences requises à la terminaison de la transcription de λtl, une série de mutants de délétion ont été générés, aux extrémités 5' ou 3', dans la région du géne λint. L’efficacité de terminaison a été déterminée en mesurant les niveaux de galactokinase (galK) par buvardage Northern et par terminaison de la transcription in vitro. L’importance de la présence de résidus uridine et de la stabilité de la structure en tige dans la terminaison ont été démontrées. L’ADN non transcrit situé au-delà de l’extrémité 3' affecte aussi la terminaison. De plus, les séquences présentes en amont ont un léger effet sur la terminaison de la transcription. Les sites de terminaison de l’ARN in vivo sur λtlont été determinés par cartographie S1 et ont été localisés à 8 positions différentes. La maturation moléculaire des transcrits de l'extrémité 3' a confirmé l'importance de la tige en épingle à cheveux dans la protection contre les exonucléases.
Introduction
There are 2 classes of transcription terminators in Escherichia coli: factor-dependent terminators, such as ρ-dependent terminators, and intrinsic terminators that are efficient in vitro in absence of factors others than the RNA polymerase (Richardson and Roberts 1993). The intrinsic terminators have a region of hyphenated dyad symmetry rich in GC preceding the termination site, followed by a stretch of thymidines (Rosenberg and Court 1979). It has been proposed that the transcription of the inverted repeat sequence results in an RNA hairpin with terminal uridine residues (Farnham and Platt 1980). Escherichia coli has 135 intrinsic terminators and 960 putative terminator sequences at the 3' end of transcription units, which represents about half of the terminator sequences reported (Lesnik et al. 2001). Further, 36.5% of the genes studied in 378 genomes analyzed in eubacteria bear a transcriptional terminator of this kind downstream of the coding region (Mitra et al. 2009).
Farnham and Platt (1980) have suggested that instability of uridines (in the RNA) and adenines (in the DNA) pairing may reduce transcription termination. Recent models consider that the interactions between nucleic acids and the RNA polymerase are very important for the process (Korzheva et al. 2000; Nudler 1999). Besides this, the pause by the RNA polymerase is proposed to be induced by the T-stretch probably enhanced by DNA downstream sequences, which provides additional time for the hairpin to form and consequently destabilize the transcription elongation Complex (Nudler and Gottesman 2002).
The λtI terminator is located approximately 280 bp beyond the λint gene, and it has a typical structure of an intrinsic terminator (Luk et al. 1982). The λtI terminator is nested within an alternative structure called sib; the RNA of which is processed by RNase III and degraded by a polynucleotide phosphorylase (PNPase) leading to the suppression of int expression by a process of retroregulation (Guarneros et al. 1982; Court et al. 1983; Montañez et al. 1986). When the int gene is transcribed from the pL promoter, the transcript does not terminate at tI because of the anti-termination activity of the N protein, allowing the transcription of sib. When the transcription begins at pI. the RNA hairpin structure of the λtI terminator is produced and the transcripts are terminated avoiding the formation of the sib structure and the nuclease degradation of transcripts (Schmeissner et al. 1984a, 1984b). The importance of λtI in maintaining the RNA stability of terminated transcripts has been demonstrated previously (Cisneros et al. 1996).
The efficiency of λtI was estimated at 95%-99% in vivo, measured by levels of galactokinase (galK) activity (Montañez et al. 1986; Cisneros et al. 1996), and at 81% by quantifying the in vivo transcripts by dot blot analysis (Bermúdez-Cruz et al. 1999). The role of NusA protein was evident by the reduction of the efficiency of λtI at 60% in a nusA1 mutant strain at nonpermissive temperatures (Bermúdez-Cruz et al. 1999).
In this work we investigated the sequences around λtI that affected termination, by making a set of deletions upstream and downstream of the λtI hairpin. Transcription termination was analyzed in vivo by measuring galK activity and galK transcripts by Northern blot as well as in vitro by transcription termination analysis. Nuclease RNA protection assay was carried out to determine exonucleolytic degradation pattern of the transcripts.
Materials and methods
Materials
The Escherichia coli strain SA1943, carrying a mutation in the galK gene (Adhya et al. 1968), was used in this work for the in vivo assays. SA1943pnp− (Cisneros et al. 1996) was also used for the in vivo nuclease RNA protection assay. Plasmids and phages used are shown in Table 1. Restriction enzymes, Bal31 exonuclease, and Klenow fragment were used as described by manufacturer specifications. Radioactive nucleotides for DNA sequencing and in vitro transcription as well as [14C]galactose were purchased from Amersham (Buckinghamshire, UK). Bacteria were grown in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 1% NaCl). Cells transformed with ampicillin-resistant plasmids were selected on MacConkey agar plates supplemented with 0.5% galactose and 50 μg/mL ampicillin (Mac-gal-amp plates).
Table 1.
Plasmids and phages used in this study.
| Plasmid or phage | Relevant characteristics | λtI ΔG (kcal/mol at 37 °C)* | Reference |
|---|---|---|---|
| pKG1800 | ampR, galK+, without terminator between Pgal and galK | — | McKenney et al. 1981 |
| pUS6 | ampR, galK+, with wild λtI terminator between Pgal and galK | −18.2 | Schmeissner et al. 1984a |
| pMC+32 | 3′ deletion +32 in λtI | −18.2 | This work |
| pMC+22 | 3′ deletion +22 in λtI | −18.2 | This work |
| pMC+3 | 3′ deletion +3 in λtI | −18.2 | This work |
| pMC−9 | 3′ deletion −9 in λtI | −15.6 | This work |
| pMC−15 | 3′ deletion −15 in λtI | −2.1 | This work |
| pMC−34 | 3′ deletion −34 in λtI | 0.0 | This work |
| pMS−106 | 5′ deletion −106 in λtI | −18.2 | This work |
| pMS−92 | 5′ deletion −92 in λtI | −18.2 | This work |
| pMS−64 | 5′ deletion −64 in λtI | −18.2 | This work |
| pMS−37 | 5′ deletion −37 in λtI | −15.0 | This work |
| pMS−32 | 5′ deletion −32 in λtI | −1.5 | This work |
| pMS−31 | 5′ deletion −31 in λtI | −0.3 | This work |
| pMS1 | Point mutation in the λtI stem | −12.0 | Montanez et al. 1986 |
| M13mp8 | Lac I′ | — | Zinder and Boeke 1982 |
Stability was calculated considering that the stretch of uridines was forming a hybrid with the DNA strand.
Methods
Generation of deletions in the 5' and 3' ends of λtI
To generate 5 deletions in λtI, plasmid pUS6 (Schmeissner et al. 1984a) was digested with HindIII and treated with Bal31 exonuclease to remove DNA from the HindIII ends. DNA ends were repaired with Klenow fragment of DNA polymerase, and HindIII DNA linkers were inserted with DNA ligase to join the ends. Amp-resistant transformants were selected, and plasmid DNA from each colony was purified. All fragments were cloned into M13mp8, manually sequenced (Sanger et al. 1977), and subcloned in pKG1800 (McKenney et al. 1981) between the Pgal promoter and the galK gene. The 3' deletions used in this work were previously generated by Court et al. (1983), and AluI-AluI fragments (approximately 230 bp) containing the region of λtI were also subcloned in the SmaI site of pKG1800. Additionally, the HindIII-EcoV fragments containing either 3' and 5' deletions were subcloned in pBlttescribe+/- (pBS series) for in vitro analysis. The prediction of the secondary structures at 37 °C was made using the RNA structure software 4.2 version (Mathews et al. 2004).
galK assay
Cells carrying plasmids were grown overnight in minimal media (M56) supplemented with fructose as the carbon source and 50 μg/mL ampicillin at 37 °C. Cells were diluted 50-fold in M56 medium containing 0.2% fucose and allowed to grow to OD 0.2–0.5 at 650 nm at 37 °C. One millilitre of culture was mixed and vortexed with 40 μL of M3 buffer (100 mmol/L EDTA, 100 mmol/L dithiothreitol, and 50 mmol/L TrisHC1 (pH 8)) and 3 drops of toluene. After toluene evaporation at 37 °C, 100 μL was mixed with a reaction solution of 4 mmol/L MgCl2, 100 mmol/L Tris-HCl (pH 8.0), 1.6 mmol/L ATP, 1 mmol/L dithiothreitol, 3.2 mmol/L NaF, and 20 mmol/L D-[14C]galactose at a specific activity of approximately 7.5 × 10−4 Bq/μmoL. After 20 min of incubation at 32 °C. 50 μL of the samples were spotted onto DE81 filters (Whatman). Filters were washed with distilled water until the blank showed no more than 1000 cycles/min. Galactokinase units are expressed as nmol of galactose phosphorylated·min−1·mL−1 of cells at an absorbance of 1.0 at 650 nm, as previously described (McKenney et al. 1981).
RNA extraction and Northern blots
Cellular RNA isolation was performed as described by Mackie (1985). RNA was extracted from bacteria grown on LB-ampicillin broth medium with fucose at 37 °C to OD 0.3–0.4 at 600 nm; 2 mL of the cultures were mixed with 500 μL of Lysis solution (1 mol/LNaCl, 50 mmol/L EDTA, and 2.5% SDS) and boiled. Shaking was performed until lysis, and after this, samples were placed in a cold water bath and treated with 2.5 mL of phenol. The mixture was vortexed twice and centrifuged at 20 200g at 4 °C. The aqueous phase was extracted with chloroform and RNA was precipitated with ethanol either overnight at −20 °C or for 15 min at −70 °C. The RNA pellet was resuspended in 400 μL of 0.3 mol/L sodium acetate (pH 7) and again ethanol precipitated. After washing the pellet with cold 70% ethanolDEPC, sample precipitates were vacuum dried and dissolved in 17 μL of H20-DEPC. Two microlitres of 10× DNase Buffer (400 mmol/L Tris-HCl (pH 7.9), 100 mmol/L NaCl, and 60 mmol/L MgCl2) and 1 μL of DNase RQ1 Promega (1000 U/mL) were added to the reactions. After 20–30 min of incubation at 37 °, 20 μL of denaturing solution (2æ MOPS, 12% formaldehyde, and 50% formamide) was added to the mixture, which was then heated to 65 °C for 15 min and frozen with dry ice.
RNA probes were prepared by in vitro transcription according to the procedure described by Promega. [32P]-labeled antisense RNA probes were made for β-lactamase, galK, and λtI transcripts. The anti-galK (αgalK), anti-tI (α-tl), and <mi\-$-lactamase (a-P-lactamase) probes were 813, 149, and 152 nt long, respectively. [32P]-labeled RNA was precipitated with ethanol, dried tinder vacuum, and dissolved in 20 μL of denaturing solution.
Denatured RNA was separated by electrophoresis in 1.8% agarose - 37% formaldehyde gels (20 μg in each lane) and transferred onto Hybond (Amersham) membranes. The blots were prehybridized at 65 °C overnight and hybridized for 6 h at 55 C or 65 C (2× SSC and 0.1% SDS) with the appropriate antisense RNA [α-32P]UTP-labeled riboprobe (1 × 106 cpm; 1 cpm = 0.0167 Bq). After hybridization, membranes were rinsed for 15 min at 68 °C (0.1 × SSC and 0.1% SDS) twice and then air dried before exposure to photographic film. The autoradiograms were scanned in an IS1000 Digital System and the β-lactamase signal was used to normalize the galK and tI expression levels.
In vitro transcription termination
The T3 polymerase has been successlully used lor analyzing point mutations as described in the Discussion. Plasmids used for in vitro transcription were constructed by inserting the HindIII-EcoRV restriction fragments derived from the plasmids containing λtI+ (pUS6) or the deletions (pMC+32, pMC+3, pMC-9, pMC-15, pMS-32, pMS-37, pMS-64, and pMS-92) into the pBS+ plasmid (Stratagene, Cedar Creek, Tex.) that contains the T3 promoter (pBS series). Transcription conditions were as the manufacturer recommended with a few modifications. Briefly, in a final volume of 10 μL, 2 μCi of [α-32P]UTP, 0.4 μg of EcoRI-linearized plasmid containing λtI derivatives, 2 U RNAsin, 15 U T3 RNA polymerase (Invitrogen, Carlsbad, Calif.) with the corresponding buffer as indicated by manufacturer, 2 mmol/L ATP/GTP/CTP. and 0.2 mmol/L UTP were mixed and incubated at 37 °C for 15 min. Template was removed by adding RNase-free DNase I (Invitrogen) and incubated at room temperature for 15 min as recommended by the manufacturer. Terminated and nonterminated transcripts were ethanol precipitated and resuspended in denaturing buffer to be resolved by electrophoresis. The termination efficiency was determined by (λtI counts per minute terminated transcript x 100)/(counts per minute terminated transcript + counts per minute nonterminated transcript).
S1 nuclease RNA protection assay
Ten micrograms of pUS6 (Schmeissner et al. 1984a) was digested with HindIII. End labeling was performed by adding 50 μCi of [32P]dATP, 80 μmol/L [dGTP, dCTP, dTTP], and 10 U of Klenow for 20 min at room temperature in a final volume of 25 μL. The 3' end labeled linearized plasmid was digested with EcoRV, and a 3' end labeled 413 bp fragment was released. DNA was ethanol precipitated and centrifuged at 20 200g. Approximately, 10 000 cycles/min of 3' end labeled 413 bp HindIII were hybridized to 20 μg of total RNA isolated in vivo. Nucleic acids were ethanol precipitated. Pellets were washed with 70% ethanol, dried and resuspended in 10 μL of 40 mmol/L PIPES (pH 6.4), 0.4 mol/L NaCl, and 1 mmol/L EDTA. Nucleic acids were denatured by heating for 5 min at 90 °C and then transferred to a 66 °C water bath for 3 h. Hybridization was stopped by adding 200 μL of S1 buffer and 100 U of S1. After 30 min at 37 °C, S1-treated hybrids were precipitated with ethanol and resolved by electrophoresis in an 8.3 mol/L urea - 6% acrylamide gel.
Results
Construction of λtI deletions
The 3' and 5' deletions of the λtI region were generated and then sequenced after subcloning in M13mp8 (see Materials and methods). Six deletions from the 3' distal end of λtI were obtained and referred as tIΔ3'+32, tIΔ3'+22, tIΔ3'+3, tIΔ3'-9, tIΔ3'-15, and tIΔ3'-34. The eliminated regions in tIΔ3'+32, tIΔ3'+22, and tIΔ3'+3 are not transcribed as part of the terminated transcript. Deletion tIΔ3'-9 eliminates the stretch of uridines, 2 adenines and 1 uridine in the transcribed RNA hairpin diminishing the hairpin stability by 2.6 kcal/mol with respect to wild-type λtI (-18.2 kcal/mol) (Table 1). Deletions tIΔ3'-15 and tIΔ3'-34 remove most or all of the stem structure, respectively. Six deletions from the 5' end of λtI were also obtained; tIΔ5'-106, tIΔ5'-92, and tIΔ5'-64 are located 106, 92, and 64 nt upstream of position + 1, respectively (Fig. 1). The tIΔ5'-37, tIΔ5'-32, and tIΔ5'-31 deletions affect the stem structure decreasing the stability of the hairpin by 3.2, 16.7, and 17.9 kcal/mol, respectively, relative to the wild type. The different λtI derivatives were subcloned between the Pgal promoter and the galK gene, as shown in Fig. 2 and Table 1, and assayed to determine the termination efficiency in in vivo conditions.
Fig. 1.
λtI deletions end points. (A) The deletions are located according to the predicted stem-loop structure in the RNA. Asterisks indicate the different sites for termination of the transcripts. (B) The base G after the last thymine of the stem-loop structure is considered arbitrarily as +1, and the deletions are numbered with this nucleotide as a reference. The arrows located above and pointing down towards the λtI terminator sequence indicate the 5' deletions, while the arrows located under and pointing up indicate the 3' deletions.
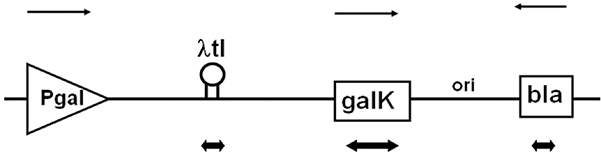
Fig. 2.
Linear map of the plasmids harboring the λtI regions. Pgal, galK, and bla represent the promoter, galactokinase gene, and (3lactamase gene, respectively. The transcription direction of Pgal, galK, and bla is indicated by the upper arrows. λtI region is shown as a stem-loop structure. Antisense riboprobes are denoted by the double direction arrows (↔)
In vivo termination efficiency
The in vivo termination efficiency of the different λtI derivatives were determined by two criteria, galK reporter assay and Northern blot analysis. The different plasmids, pMC and pMS series, containing the deletions were used to transform the galK mutant E. coli strain SA1943, and the activity of the galK enzyme was determined as described in materials and methods. Table 2 shows these results. The 3' deletions tIΔ3'+32 and tIΔ3'+22 and 5' deletions tIΔ5'-106 and tIΔ5'-92 do not modify the transcription termination efficiency, whereas the other deletions reduce the termination efficiency by different magnitudes. Although the tIΔ3'+3 deletion does not eliminate nucleotides from the transcript, it reduces the termination to 56%. Elimination of the uridines (tIΔ3'-9 deletion) decreases the termination to 23% and deletions drastically destabilizing the hairpin structure (tIΔ3'-15 and tIΔ3'-34 deletions) also reduce the termination to almost 20%, The tIΔ5'-64 does not eliminate nucleotides from the hairpin but reduces the termination to 80% and the elimination of 3 nt of the hairpin by the tIΔ5'-37 deletion reduces to 64% the efficiency. The drastic destabilization of the hairpin by the tIΔ5'-32 and tIΔ5'-31 deletions reduce the termination to only 45% and 28%, respectively.
Table 2.
In vivo termination efficiencies of the wild-type λtI and its deletions quantified by galK activity.
| Terminator or deletion | Galactokinase units* ± SE† | % termination‡ |
|---|---|---|
| Without terminator | 1642±213 | 0 |
| Wild λtI terminator | 30±3 | 98 |
| tIΔ3′+32 | 62±8 | 96 |
| tIΔ3′+22 | 53±6 | 97 |
| tIΔ3′+3 | 730±110 | 56 |
| tIΔ3′−9 | 1260±202 | 23 |
| tIΔ3′−15 | 1269±190 | 23 |
| tIΔ3′−34 | 1282±203 | 22 |
| tIΔ5′−106 | 65±9 | 96 |
| tIΔ5′−92 | 62±7 | 96 |
| tIΔ5′−64 | 329±48 | 80 |
| tIΔ5′−37 | 593±77 | 64 |
| tIΔ5′−32 | 904±140 | 45 |
| tIΔ5′−31 | 1182±170 | 28 |
Galactokinase units are expressed as nmol from cells at an absorbance of 1.0 at 650 nm. pMC and pMS series were used.
Confidence interval was calculated from 3 different measurements with α = 0.05 using Microsoft Office Excel 2007. SE, standard error.
Percent termination was calculated by subtracting galactokinase units from those of pKG1800 (without terminator), then dividing bythe units obtained for pKG1800 and multiplying by 100.
For Northern blot assays, total RNA isolated Irom the SA1943 E. coli strain, bearing the wild-type terminator or each deletion, was hybridized with different antisense riboprobes (Fig. 2). Transcripts reading through λtI into galK were detected by antisense galK riboprobe (α-galK), and terminated transcripts were detected by an antisense tl region riboprobe (α-tI). To normalize the RNA input, the constant β-lactamase RNA from the plasmid was detected after hybridization with antisense β-lactamase riboprobe (Fig. 2). Figure 3 shows typical autoradiograms of in vivo transcription products detected by the α-galK, α-tI, and α-βlactamase riboprobes. The galK transcripts are more than 2 kb long (see arrow in Fig. 3, panels A and B) and the ones stopped at λtI are approximately 800 nt (see arrow in Fig. 3, panels C and D). Quantification of the galK transcripts normalized as described in Materials and methods is shown in Table 3. These results reveal that the 3' deletions tIΔ3'+32 and tIΔ3'+22 and 5' deletions tIΔ5'-106 and tIΔ5'-92 did not affect the transcription termination efficiency (consistent with galK assay), whereas the other deletions reduced the termination by different degrees. The tIΔ3'+3 reduced the transcription to 74% and the 3' deletions tIΔ3'-9, 3'Δ-15, and 3'Δ-34 that drastically destabilize the hairpin did not show termination in comparison with the galK assay in which the termination is approximately 20%. The tIΔ5'-32 and tIΔ5'-31 deletions that also destabilize the haiipin showed termination of 44% and 21% by Northern assay. The difference in measured termination efficiency between the galK assay and the Northern is likely due to measuring enzymatic activity in the first case and actual transcript levels in the second case.
Fig. 3.
Northern blot assays. Autoradiograms were hybridized with anti-galK riboprobe (A and B), anti-tI riboprobe (C and D), and with anti-β-lactamase riboprobe (E and F, used to normalize the galK and tI transcripts). 3' (A, C, and E) and 5' deletions (B, D, and F) used are indicated above each blot. λtI+ corresponds to pUS6. M, RNA molecular weight markers.
Table 3.
In vivo and in vitro termination efficiencies of the λtI+ and its λtI deletions quantified by transcripts.
| Terminator or deletion | galK RNA level* | % in vivo termination† | % in vitro termination‡ |
|---|---|---|---|
| Without terminator | 52.0 | 0 | nd |
| Wild λtI terminator | 1.0 | 98 | 65 |
| tIΔ3′+32 | 4.0 | 92 | 38 |
| tIΔ3′+22 | 3.6 | 93 | nd |
| tIΔ3′·+3 | 13.4 | 74 | 39 |
| tIΔ3′−9 | 52.1 | 0 | 18 |
| tIΔ3′−15 | 52.3 | 0 | 18 |
| tIΔ3′−34 | 52.3 | 0 | nd |
| tIΔ5′−106 | 3.1 | 94 | nd |
| tIΔ5−92 | 4.3 | 92 | 31 |
| tIΔ5′−64 | 9.9 | 81 | 13 |
| tIΔ5′−37 | 10.6 | 80 | 14 |
| tIΔ5−32 | 29.2 | 44 | 14 |
| tIΔ5′−31 | 41.1 | 21 | nd |
Note: nd, not determined.
Measurements are the average of 3 different pairs of autoradiograms in which galK RNA is normalized to β-lactamase RNA levels. pMC and pMS series were used.
Percent in vivo termination was calculated by subtracting galK RNA levels for thevarious tI derivatives from the pKG1800 control, lacking a terminator, then dividingby the units found for pKG1800 and multiplying by 100%.
Percent in vitro termination was calculated as follows: (counts per minute of terminated transcript × 100)/(counts per minute of terminated transcript + counts per minute of nonterminated transcript); 1 cpm = 0.0167 Bq. The pBS series was used.
In vitro transcription termination
The effect or some λtI deletions was also analyzed with the T3 RNA polymerase transcription system. A typical autoradiogram of the in vitro transcription with the λtI deletions is shown in Fig. 4. The lower RNA products correspond to the terminated transcripts, whereas the upper ones are the read-through or nonterminated transcripts. The percent termination was calculated as described in Materials and methods and is shown in Table 3 for each case. The wild λtI terminator was less efficient (65%) and consequently the other values for transcription termination efficiency were lower than those from the Northern blot assays; however, the deletions had a proportional effect on the λtI transcription termination, mostly affecting those that destabilize the hairpin.
Fig. 4.
Autoradiogram of in vitro termination assays. The 5' and 3' deletions were used to perform in vitro transcription as described in the Materials and methods. “T” represents the transcripts terminated at λtI, and "NT" the read-through transcripts (nonterminated at λtI).
The in vivo termination sites at λtI
S1 mapping of λtI and derivative termination sites was performed, and it revealed multiple termination sites located along several U residues of the λtI terminator (data not shown). The 3' ends of transcripts of sibl (tI point mutant; Montañez et al. 1986) were also mapped by SI and the same termination sites were found (data not shown), which is consistent with previous data (Bermúdez-Cruz et al. 1999). Interestingly, when S1 nuclease RNA protection assay was performed on RNA isolated from isogenic pnpstrains transformed either with the wild-type or mutant terminators, though longer RNAs were found when compared with isogenic pnp+, there were transcripts with ends located at the termination sites previously described (Fig. 5, lanes 3 and 4, respectively; Bermúdez-Cruz et al. 1999). The longer transcripts are stabilized in pnp− strains, showing that pnp carries out processing of λtI transcripts, which is consistent with previous data (Cisneros et al. 1996). Further, when pnp processing is separated from transcription termination, in vivo termination efficiency performed on the tl transcript exhibited a reduction (60% in a pnp− strain vs, 80% in a wild-type strain; Fig. 5), showing that λtI is less efficient that previously thought (Schmeissner et al. 1984/?). It can also be observed that sib1 is deficient in termination compared with wild-type tI. Additionally the pup processing of the readthough transcripts from sib1 and tI is different.
Fig. 5.
In vivo SI nuclease RNA protection assay. Lane 1, in vivo RNA isolated from pMS1/SA1943 strain; lane 2, in vivo RNA isolated from pUS6(tI+)/SA1943 strain; lane 3, in vivo RNA isolated from pMS1/SA1943pnp_; lane 4, in vivo RNA isolated from pUS6(tI+)/SA1943pnp−; and lane 5, probe. Based on previous published data, termination sites occur in the 6 uridines (Fig. 1). Bracketed region indicates termination sites along uridine residues.
Discussion
The λtI terminator corresponds to the structure of a typical intrinsic terminator, with a dyad symmetry followed by a stretch of uridines (Luk et al. 1982; Guarneros et al. 1982). Although the importance of the stem and the stretch of uridines has been demonstrated for many intrinsic terminators, the specific roles assigned to these regions have changed. Originally the hairpin was proposed to elicit a pause in the polymerase and the uridines participated in the dissociation of the weak hybrid dDNA-rRNA (Rosenberg and Court 1979; Farnham and Platt 1980; Martin and Tinoco 1980; Platt 1986). In a recent model, the interactions between nucleic acids and the RNA polymerase are considered more important. The 3 nucleic acid binding sites within the RNA polymerase that stabilize the ternary elongation complex (TEC) have been characterized biochemically and structurally and are as follows; the RNA:DNA heteroduplex binding site (HBS), the single-stranded RNA binding site (RBS), and the double stranded DNA binding site (DBS) (Korzheva et al. 2000; Nudler et al. 1997, 1998). The pause in the RNA polymerase is not induced by the hairpin. Instead, the T-stretch probably enhanced by DNA downstream sequences induces this pause that provides additional time for the hairpin to form and consequently destabilize the TEC by weakening interactions in the RNA binding site and the HBS binding site; this leads to the dissociation of the RNA polymerase (Nudler et al. 1995, 1997; Komissarova and Kashlev 1997; Nudler 1999; Gusarov and Nudler 2001; Nudler and Gottesman 2002).
The role of the stem structure of the λtI terminator has been analyzed by point mutations that decrease the stability of the hairpin by half, but the efficiency of the terminator is only reduced from 99% to 81% in vivo (Cisneros et al. 1996). A simple correlation between stability of the hairpin and termination efficiency has not held for other terminators as well (Revnolds et al. 1992).
The importance of the uridines in λtI is demonstrated with the tIΔ3'-9 deletion, which abolishes the termination efficiency as determined by in vivo or in vitro transcripts of galK (Fig. 3 and Fig. 4, respectively, and Table 3) and leaves only a residual activity as determined by the enzymatic galK assay (Table 2). These results are in agreement with the thr and trp terminators in which removal of 4 uridines essentially abolishes the transcription termination activity (Lynn et al. 1988; Christie et al. 1981).
Sequences downstream in the nontranscribed DNA have been reported for some terminators to enhance the termination efficiency (Telesnitsky and Chamberlin 1989; Reynolds et al. 1992). Lee et al. (1990) demonstrated that the transcription pausing by E. coli RNA polymerase is modulated by downstream sequences. In λtI deletion tIΔ3'+3 diminished the termination efficiency to 56% by the enzymatic assay, 51% by in vitro assay, and 74% by Northern blot (Tables 2 and 3). The importance of downstream DNA sequences might be a consequence of the interaction of a 9 bp DNA region with the RNA polymerase (Nudler et al. 1996; Korzheva et al. 2000) that could increase the pause induced by the T-stretch (Gusarov and Nudler 2001). In the tIΔ3'+3 deletion, the downstream ATCAAA is replaced by the GC-rich sequence CGGGCA and the termination is reduced. In the T7 terminator, changes of nucleotides between 3 and 7 residues downstream of the RNA release site are critical in determining the strength of the terminator, and the change of TATAAG (38) by CCGAGG (3–8) reduces the termination efficiency from 65% to 3% (Telesnitsky and Chamberlin 1989). This suggests that in some terminators, the presence of thymines and adenines downstream of the transcript may reinforce transcription termination efficiency. Some terminators that have AT rich sequences 9 bp downstream of the predicted RNA stem are S10 and 5S in E. coli and λ6S and G4 in phages (Rosenberg and Court 1979); however, there are no studies on DNA downstream sequences in these terminators. With the establishment of the atomic structure of the RNA polymerase and crosslinking studies, the sequences downstream of the transcription termination site are shown as essential for termination (Santangelo and Roberts 2004), and the forward translocation of the RNA polymerase without RNA synthesis and DNA unwinding is suggested as the normal pathway for the dissociation of the RNA polymerase complex (Park and Roberts 2006). With this model, the AT rich segments in front of the termination site will facilitate the termination, which is in agreement with our findings.
The destabilization of the stem structure in the tIΔ5'-32 and tIΔ5'-31 deletions (Table 1) reduces the termination efficiency drastically from 98% (wild-type λtI terminator) to 45% and 28%, respectively (galK assays), or 44% and 21%, respectively (Northern assays) (Tables 2 and 3). The residual termination activity of these deletions may be due to the presence of the uridines combined with the weak secondary structure. As an example, a remaining termination activity (9%) has been observed in the crp terminator when the predicted RNA stem structure is eliminated but the uridines stay, and only when the uridines are also eliminated the termination is abolished (Abe and Aiba 1996).
The sequence upstream of the predicted stem structure may affect the termination efficiency, as has been demonstrated in the trp terminator, where changing 23 bp of the original sequence by the corresponding sequence of the T7 terminator reduces the termination efficiency from 68% to 46% (Reynolds et al. 1992). In the λtI tIΔ5'-64 deletion, the interaction of the sequence upstream of the predicted stem structure (24 bp) is altered since the absence of region 1 would allow that during transcription, region 2 and 3 pair before region 4 is transcribed (Fig. 6B) when compared with the wild-type tl predicted structure (-7.3 kcal/mol) where regions 1 and 2 (-11.4 kcal/mol) and regions 3 and 4 (tI structure is contained in this region, −18.2 kcal/mol) are normally paired (Fig. 1, Fig. 6A). This reduced the efficiency from 96% to 80% and 81% and 13% by the enzymatic Northern assay or in vitro assay, respectively. Using oligonucleotides complementary to the tR2 sequence, Larson et al. (2008) found that the formation of upstream weak secondary structures may compete with the formation of the termination hairpin in tR2, which support our findings.
Fig. 6.
Predicted secondary structures in the RNA of the λtI terminator region. (A) Regions 3 and 4 correspond to the stem-loop structure of the λtI terminator with a stability of −18.2 kcal/mol, while upstream regions 1 and 2 form a predicted secondary structure with a stability of −11.4 kcal/mol. The position of the tIΔ5'-64 deletion is indicated. (B) Secondary structure predicted for regions 2 and 3 in the absence of region 4 with a stability of −7.3 kcal/mol.
The in vitro transcription termination was performed with the T3 polymerase system. Although this phage monomeric polymerase terminates the transcription in a GC-rich stem-loop structure in the phage T3 (Sengupta et al. 1989), it has been used successfully in analyzing point mutations in the λtI terminator (Cisneros et al. 2000). The percent termination in λtI+ in this in vitro system is 65%, which is lower than the in vivo Northern assays (98%). This lower in vitro termination has been reported for the T3 and T7 terminators (Telesnitsky and Chamberlin 1989) and for λtI (Cisneros et al. 2000). However, the in vitro transcription termination obtained with the different deletions follows the pattern obtained with the Northern assays, with less magnitude in the effects for the 3' deletions and with more magnimde for the 5' deletions (Table 3). This strengthens our in vivo results for the effects of the different deletions of λtI in transcription termination. Lambda tI has an unusual structure in that the hairpin helix is almost 50% AT rich. Usually, mapping of some intrinsic terminators shows 1 or 2 termination sites. Interestingly, termination sites found on λtI previously as well as for sib1 (tI point mutant) map along each U residue in the poly U tail (data not shown). Once processing (pnp-background) is removed, the λtI terminator seems to be less efficient, which is consistent with the in vitro data. Also, the point mutation in sib1 is providing an RNA element that seems to destabilize RNA transcript consistent with a previous report (García et al. 1999).
Taken together, the use of the generated deletions in the analysis of in vivo and in vitro termination efficiencies supports the importance of the stretch of uridines and the hairpin as the most important determinants of the intrinsic terminator λtI and also provides evidence of the downstream DNA and upstream hairpin RNA as elements that influence the transcription termination. The knowledge of the parameters affecting the λtI terminator performance contributes to the understanding of the functioning of an intrinsic terminator as well as the cis (DNA/RNA sequences) and trans (protein factor) determinants involved in transcription termination.
Acknowledgements
We thank Adalberto Herrera and Veronica Ramírez for technical assistance.
Contributor Information
Miguel Martínez-Trujillo, Departamento de Genética y Biología Molecular, Centro de Investigación y de Estudios Avanzados del I.P.N, Apartado postal 14-740, C.P. 07360 México, D.F., México.
Alejandra Sánchez-Trujillo, Departamento de Genética y Biología Molecular, Centro de Investigación y de Estudios Avanzados del I.P.N, Apartado postal 14-740, C.P. 07360 México, D.F., México.
Víctor Ceja, Departamento de Genética y Biología Molecular, Centro de Investigación y de Estudios Avanzados del I.P.N, Apartado postal 14-740, C.P. 07360 México, D.F., México.
Federico Ávila-Moreno, Departamento de Genética y Biología Molecular, Centro de Investigación y de Estudios Avanzados del I.P.N, Apartado postal 14-740, C.P. 07360 México, D.F., México.
Rosa María Bermúdez-Cruz, Departamento de Genética y Biología Molecular, Centro de Investigación y de Estudios Avanzados del I.P.N, Apartado postal 14-740, C.P. 07360 México, D.F., México.
Donald Court, Gene Regulation and Chromosome Biology, National Cancer Institute-Frederick, Frederick, MD 21702-1201, USA.
Cecilia Montañez, Departamento de Genética y Biología Molecular, Centro de Investigación y de Estudios Avanzados del I.P.N, Apartado postal 14-740, C.P. 07360 México, D.F., México.
References
- Abe H, and Aiba H 1996. Differential contributions of two elements of rho-independent terminator to transcription termination and mRNA stabilization. Biochimie, 78(11–12): 1035–1042. 10.1016/S0300-9084(97)86727-2. [DOI] [PubMed] [Google Scholar]
- Adhya S, Cleary P, and Campbell A 1968. A deletion analysis of prophage lambda and adjacent genetic regions. Proc. Natl. Acad. Sci. U.S.A. 61(3): 956–962. 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Cruz RM, Chamberlin MJ, and Montañez C 1999. Nus A is involved in transcriptional termination on lambda tl. Biochimie, 81(7): 757–764. 10.1016/S0300-9084(99)801345. [DOI] [PubMed] [Google Scholar]
- Christie GE, Farnham PJ, and Platt T 1981. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc. Natl. Acad. Sci. U.S.A. 78(7): 4180–1184. 10.1073/pnas.78.7.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros B, Court D, Sánchez A, and Montañez C, 1996. Point mutations in a transcription terminator, λtI, that affect both transcription termination and RNA stability. Gene, 181(1–2): 127–133.. 10.1016/S0378-1119(96)00492-1. [DOI] [PubMed] [Google Scholar]
- Cisneros B, Bermúdez-Cruz RM, Sanchez A, and Montanez C 2000. Effect of λ tI point mutations on T3 RNA polymerasemediated transcription termination. Rev. Latinoam. Microbiol. 42: 95–100. [Google Scholar]
- Court D, Huang TF, and Oppenheim AB 1983. Deletion analysis of the retroregulatory site for the lambda int gene. J. Mol. Biol. 166(2): 233–240. 10.1016/S0022-2836(83)80010-2. [DOI] [PubMed] [Google Scholar]
- Farnham PJ, and Platt T 1980. A model for transcription termination suggested by studies on the tip attenuator in vitro using base analogs. Cell, 20(3): 739–748. 10.1016/0092-8674(80)90320-7. [DOI] [PubMed] [Google Scholar]
- García-Mena J, Das A, Portier C, Sánchez-Trujillo A, and Montańez C 1999. A novel mutation in the KH domain of polynucleotide phosphorylase affects autoregulation and mRNA decay in E. coli. Mol. Microbiol. 33(2): 235–248. 10.1046/j.1365-2958.1999.01451.x. [DOI] [PubMed] [Google Scholar]
- Guameros G, Montańez C, Hernández T, and Court D 1982. Posttranscriptional control of bacteriophage λ int gene expression from a site distal to the gene. Proc. Natl. Acad. Sci. U.S.A. 79(2): 238–242. 10.1073/pnas.79.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I, and Nudler E 2001. Control of intrinsic transcription termination by N and NusA: the basic mechanisms. Cell, 107(4): 437–149. 10.1016/S0092-8674(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Komissarova N, and Kashlev M 1997. Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3' end of the RNA intact and extruded. Proc. Natl. Acad. Sci. U.S.A. 94(5): 1755–1760. 10.1073/pnas.94.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzheva N, Mustaev A, Kozlov M, Malhotra A, Nikiforov V, Goldfarb A, and Darst SA 2000. A structural model of transcription elongation. Science (Washington, D.C.), 289(5479): 619–625. 10.1126/science.289.5479.619. [DOI] [PubMed] [Google Scholar]
- Larson MH, Greenleaf WJ, Landick R, Block SM, and Block SM 2008. Applied force reveals mechanistic and energetic details of transcription termination. Cell, 132(6): 971–982. 10.1016/j.cell.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DN, Phung L, Stewart J, and Landick R 1990. Transcription pausing by Escherichia coli RNA polymerase is modulated by downstream DNA sequences. J. Biol. Chem. 265(25): 15145–15153. [PubMed] [Google Scholar]
- Lesnik EA, Sampath R, Levene HV, Henderson TJ, McNeil JA, and Ecker DJ 2001. Prediction of rho-independent transcriptional terminators in Escherichia coli. Nucleic Acids Res. 29: 3583–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Dobrzański P, and Szybalski W 1982. Cloning and characterization of the termination site tI for the gene int transcript in phage λ. Gene, 17(3): 259–262. 10.1016/03781119(82)90141-X. [DOI] [PubMed] [Google Scholar]
- Lynn SP, Kasper LM, and Gardner JF 1988. Contributions of RNA secondary structure and length of the thymidine tract to transcription termination at the thr operon attenuator. J. Biol. Chem. 263(1): 472–A79. [PubMed] [Google Scholar]
- Mackie GA 1985. Stabilization of the 3' one-third of Escherichia coli ribosomal protein S20 mRNA in mutants lacking polynucleotide phosphorylase. J. Bacteriol. 171: 4112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FH, and Tinoco IJ Jr. 1980. DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 8(10): 2295–2299. 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH, Zuker M, and Turner DH 2004. RNAstructure ver. 4.2 Rensselaer Polytechnic Institute, Troy, N.Y. [Google Scholar]
- McKenney K, Shimatake H, Court D, Schmeissner U, Brady C, and Rosenberg M 1981. Analysis of nucleic acids by enzymatic methods In Gene amplifications and analysis. Edited by Chrikjian JC and Papas TS Elsevier/North-Holland, New York, pp. 383–415. [PubMed] [Google Scholar]
- Mitra A, Angamuthu K, Jayashree HV, and Nagaraja V 2009. Occurrence, divergence and evolution of intrinsic terminators across eubacteria. Genomics, 94(2): 110–116. 10.1016/j.ygeno.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Montañez C, Bueno J, Schmeissner U, Court DL, and Guarneros G 1986. Mutations of bacteriophage lambda that define independent but overlapping RNA processing and transcription termination sites. J. Mol. Biol. 191(1): 29–37. 10.1016/0022-2836(86)90420-1. [DOI] [PubMed] [Google Scholar]
- Nudler E 1999. Transcription elongation: structural basis and mechanisms. J. Mol. Biol. 288(1): 1–12. 10.1006/jmbi,1999.2641. [DOI] [PubMed] [Google Scholar]
- Nudler E, and Gottesman ME 2002. Transcription termination and anti-termination in E. coli. Genes Cells, 7(8): 755–768. 10.1046/j.1365-2443.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- Nudler E, Kashlev M, Nikiforov V, and Goldfarb A 1995. Coupling between transcription termination and RNA polymerase inchworming. Cell, 81(3): 351–357. 10.1016/00928674(95)90388-7. [DOI] [PubMed] [Google Scholar]
- Nudler E, Avetissova E, Markovtsov V, and Goldfarb A 1996. Transcription processivity: protein-DNA interactions holding together the elongation complex. Science (Washington, D.C.), 273(5272): 211–217. 10.1126/science.273.5272.211. [DOI] [PubMed] [Google Scholar]
- Nudler E, Mustaev A, Lukhtanov E, and Goldfarb A 1997. The RNA-DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell, 89(1): 33–41. 10.1016/S0092-8674(00)80180-4. [DOI] [PubMed] [Google Scholar]
- Nudler E, Gusarov I, Avetissova E, Kozlov M, and Goldfarb A 1998. Spatial organization of transcription elongation complex in Escherichia coli. Science (Washington, D.C.), 281(5375): 424–428. 10.1126/science.281.5375A24. [DOI] [PubMed] [Google Scholar]
- Park JS, and Roberts JW 2006. Role of DNA bubble rewinding in enzymatic transcription termination. Proc. Natl. Acad. Sci. U.S.A. 103(13): 4870–4875. 10.1073/pnas.0600145103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T 1986. Transcription termination and the regulation of gene expression. Annu. Rev. Biochem. 55(1): 339–372. 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Bermudez-Cruz RM, and Chamberlin MJ 1992. Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J. Mol. Biol. 224(1): 31–51. 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- Richardson JP, and Roberts JW 1993. Transcription termination. Crit. Rev. Biochem. Mol. Biol. 28(1): 1–30. 10.3109/10409239309082571. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, and Court D 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13(1): 319–353. 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nickleti S, and Coulson AR 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 74(12): 5463–5467. 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo TJ, and Roberts JW 2004. Forward translocation is the natural pathway of RNA release at an intrinsic terminator. Mol. Cell, 14(1): 117–126. 10.1016/S1097-2765(04)001546. [DOI] [PubMed] [Google Scholar]
- Schmeissner U, McKenney K, Rosenberg M, and Court D 1984a. Transcription terminator involved in the expression of the int gene of phage lambda. Gene, 28(3): 343–350. 10.1016/0378-1119(84)90152-5. [DOI] [PubMed] [Google Scholar]
- Schmeissner l. McKenney K, Rosenberg M, and Court D 1984b. Removal of a terminator structure by RNA processing regulates int gene expression. J. Mol. Biol. 176(1): 39–53. 10.1016/0022-2836(84)90381-4. [DOI] [PubMed] [Google Scholar]
- Sengupta D, Chakravarti D, and Maitra U 1989. Relative efficiency of utilization of promoter and termination sites by bacteriophage T3 RNA polymerase. J. Biol. Chem. 264(24): 14246–14255.. [PubMed] [Google Scholar]
- Telesnitsky AP, and Chamberlin MJ 1989. Terminator-distal sequences determine the in vitro efficiency of the early terminators of bacteriophages T3 and T7. Biochemistry, 28(12): 5210–5218.. 10.1021/bi00438a044. [DOI] [PubMed] [Google Scholar]
- Zinder ND, and Boeke JD 1982. The filamentous phage (Ff) as vectors for recombinant DNA. Gene, 19(1): 1–10. 10.1016/0378-1119(82)90183-4. [DOI] [PubMed] [Google Scholar]