Abstract
Background:
The aim of this study was to determine the frequency and characteristics of bone and joint complications, specifically bone fragility, joint replacement surgery, and arthropathy, in hereditary hemochromatosis (HH) and related factors.
Methods:
This study was a cross-sectional observational study of 93 patients with HH. Radiographs of the hands, wrists, knees, and ankles were scored for joint space narrowing, erosions and cysts, osteophytes, and chondrocalcinosis. Prevalent (vertebral and non-vertebral) fragility fractures were recorded and bone mineral density (BMD) was systematically evaluated by dual energy X-ray absorptiometry. Bone fragility was defined as (i) a T-score ⩽ −2.5 at any site with or without a prevalent fragility fracture, or (ii) a T-score between −1.0 and −2.5 at any site and a prevalent fragility fracture.
Results:
The mean age of the patients was 60.0 (11.2) years, and 58.0% of them were men. The frequency of radiographic MCP2–3 arthropathy was 37.6% (95% CI 0.28–0.48). Radiographic MCP2–3 arthropathy was independently associated with older age [OR 1.17 (1.09–1.26) per year, p < 0.0001], male sex [OR 3.89 (1.17–12.97), p = 0.027] and C282Y+/+ genotype [OR 4.78 (1.46–15.68), p = 0.010]. The frequency of joint replacement surgery was 12.9% (95% CI 0.07–0.21). The frequency of bone fragility was 20.4% (95% CI 0.13–0.30). Bone fragility was independently associated with hepatic cirrhosis [OR 8.20 (1.74–38.68), p = 0.008].
Discussion:
Radiographic MCP2–3 arthropathy was found to occur in 37.6% of patients with HH. The association observed between this form of arthropathy and C282Y homozygosity, male sex, and older age suggests that demographic characteristics and genetic background are likely to be major determinants of this joint disorder and play a more important role than severity of iron overload. Bone fragility was observed in a fifth of the patients with HH, independently of genetic background and severity of iron overload, and was strongly associated with hepatic cirrhosis.
Conclusion:
Future investigations should focus on pathogenesis and early identification of patients at risk of developing bone and joint complications secondary to HH.
Keywords: arthropathy, bone mineral density, hemochromatosis, hepatic cirrhosis, iron overload, osteoporosis
Introduction
Hereditary hemochromatosis (HH) is an autosomal recessive disorder. It is characterized by an increase in the absorption of dietary iron and rapid iron release from macrophages leading to an abnormal accumulation of iron in several organs, particularly the liver, heart, joints, and bones.1,2 In patients of northern European descent, the prevalence of the disorder is about 1 in 300 and the most common genotype among HH patients is C282Y homozygosity in the HFE gene, which is found in about 80–90% of HH cases.2 Bone and joint complications, arthropathy, joint replacement surgery, and osteoporosis have been consistently described in C282Y homozygous patients.3–6
Between 70% and 80% of individuals with HH report symptoms of arthropathy involving the metacarpophalangeal (MCP), ankle, knee, hip, or proximal interphalangeal joints by the time they reach the fifth or sixth decade of life.7,8 HH patients also exhibit a higher risk of large-joint involvement, which may later require joint replacement surgery.9,10 Indeed, up to 16% of HH patients undergo joint replacement surgery.9
Radiographically, arthropathy in HH mimics osteoarthritis and is variably accompanied by chondrocalcinosis.3,4 There is a predilection for the MCP joints and the proportion of HH patients with radiographically diagnosed arthropathy has been reported in the range 24–81%. Most of these frequency estimates are derived from retrospective case series, which tend to involve more severe cases of HH. However, the investigations conducted by Carroll et al.11 are especially salient: Unselected cases of definite or probable HH were clinically evaluated and, in those cases with demonstrable iron overload, the frequency of MCP2–5 joint arthropathy was determined by radiological assessment. Arthropathy was observed in 10 of 41 patients (24%), all of whom were homozygous for the C282Y mutation in the HFE gene. Moreover, iron load was found to be a major determinant of arthropathy in patients with HH.11 Likewise, three other studies have reported a strong association between arthropathy and iron load in patients with HH.4,12,13
Several animal studies provide evidence of bone impairment in HH. However, only a few studies to date have sought to determine whether this is associated with an increase in fracture risk in humans.14,15 In a cross-sectional survey, significantly more HH patients were diagnosed with osteoporosis than age- and gender-matched controls.12 Moreover, iron overload was associated with wrist or vertebral fractures.12 Based on bone mineral density (BMD) measurements determined by dual energy X-ray absorptiometry (DXA), the prevalence of osteoporosis and osteopenia in these patients ranges from 25% to 34% and from 74% to 79% respectively,5,6 and the risk of vertebral fractures has been reported at up to 20%.14 To date, no data have been reported on the risk of non-vertebral fractures in HH. The mechanisms leading to bone loss in hemochromatosis are not well understood. However, osteoporosis might also be associated with severity of iron overload, independently of cirrhosis and hypogonadism, which may be aggravating factors.2,5
Previous investigations aimed at determining the frequency of bone and joint complications (arthropathy, joint replacement surgery, and osteoporosis) in patients with HH have been conducted on small patient populations and may not accurately represent the full scope of the problem. Data on bone and joint complications in patients with HH are scarce and the frequency and characteristics of these complications, as well as related factors, need to be better evaluated. Thus, we investigated the frequency and determinants of radiographic arthropathy, joint replacement surgery, and bone fragility in a large series of unselected HH patients with different degrees of iron overload and different genetic backgrounds.
Patients and methods
Study design
In this cross-sectional study, 93 HH patients attending the Department of Rheumatology outpatient clinic at Lille University Hospital, Lille, France were included between April 2016 and April 2018. The study protocol was approved by the local Institutional Review Board (number DEC2015-130). The study procedures complied with the Helsinki Declaration of 1975, as revised in 2000. Since this is a non-interventional cross-sectional study, it did not require ethical committee approval in accordance with the French laws and regulations. Moreover, all participants provided a verbal informed consent prior to enrolment in the study.
Study population
The main inclusion criteria for patients with HH were: volunteers age ⩾18 years in whom HH had been diagnosed by hepatologists at Lille University Hospital; serological signs of iron overload (increased transferrin saturation up to 45% and initial serum ferritin level ⩾300 ng/ml for men and postmenopausal women, and ⩾200 ng/ml for premenopausal women) at the time of the diagnosis of HH in the presence of C282Y homozygosity, H63D homozygosity, compound C282Y/H63D heterozygosity or C282Y/wt genotype for mutations of the HFE gene; and ability to understand the study’s objectives and procedures. The main exclusion criteria were: weight >160 kg, lack of understanding of French, severe cognitive disorders, guardianship, and lack of social welfare access.
Eligible subjects were identified using data from the patient database in the Department of Hepatology at Lille University Hospital. All potential study subjects (n = 159), even those with no musculoskeletal complaints, were sent a letter inviting them to participate in the study. A total of 97 patients volunteered and were screened, and 93 of them satisfied the inclusion criteria. All of these patients (n = 93) were included in the study.
Study protocol
Information was obtained by means of a structured interview, a physical examination, DXA and X-ray examinations, and a review of medical records.
Patient disease assessment
Demographic and clinical characteristics were recorded by two physicians (CDN and JP) with experience in managing patients with HH, and a complete musculoskeletal examination was performed. Information on HFE genotype and initial serum ferritin values at the time of diagnosis was retrieved. The disease manifestations of HH, including hepatic cirrhosis, diabetes mellitus, hypogonadism, and cardiomyopathy, were assessed. Disease duration was defined as duration since diagnosis of HH. Body mass index (BMI) was determined at inclusion and calculated as weight divided by height squared (kg/m²). Smoking status (non/past/current smoker) and excessive alcohol consumption (3 or more units of alcohol daily) were recorded. The number and type of joint replacement surgeries were recorded.
Risk factors for osteoporosis were collected and included low BMI (<18.5 kg/m²), current smoking, excessive alcohol consumption, history of rheumatoid arthritis, use of oral corticosteroids (exposed to ⩾5 mg/day of prednisolone for ⩾3 months), history of fragility fracture after the age of 40, secondary osteoporosis, and family history of osteoporosis (hip fracture in mother or father). Data on prior use of menopausal hormone therapy and anti-osteoporosis treatment were also collected.
Radiographic assessment
Standard radiographs of hand, wrist, knee, and ankle joints were obtained for the assessment of characteristic radiographic changes. A validated dichotomous radiographic scoring system assessing the presence of four radiographic features (joint space narrowing, erosions and cysts, osteophytes, and chondrocalcinosis) was used for the evaluation of all radiographs.16 In this scoring system, one point is given for the presence of each of the four features. The presence of osteophytes and erosive/cyst changes is scored separately at the proximal and distal portions of the assessed joint, thus yielding a total of six points for a joint if all features are present.16 All radiographs were assessed by a first reader with experience in the evaluation of radiographs (CDN). An aggregate score incorporating the presence of radiographic changes in the second and third MCP joints was used for the main analysis (MCP2/MCP3 score; maximum 24 points). Patients were diagnosed as having specific radiographic MCP2–3 arthropathy when the sum of their scores from all four MCP2 and MCP3 joints was ⩾2. Arthropathy at other sites was also investigated. The second to fifth MCP joints, as well as the wrist (radiocarpal joint) and ankle (talocrural) joints, were scored using the same dichotomous scoring system. Knee joints were assessed for the presence of chondrocalcinosis only. Hand (MCP2–5 joints, wrist joints; maximum 60 points) and total radiographic scores (MCP2–5 joints, wrist and ankle joints; maximum 72 points), calculated by summing up the respective points, were used for further analyses.16
All radiographs were also read by a second reader who was an experienced musculoskeletal radiologist (VM) who did not have access to any of the clinical data and who was blinded to the assigned diagnostic categories. Scores were not validated between the two readers and arthropathy evaluations made by the first reader (CDN) are presented.
In line with French guidelines on the management of postmenopausal osteoporosis,17 conventional anterior–posterior and lateral radiographs of the thoracic spine and posterior–anterior and lateral radiographs of the lumbar spine were performed both in men and women when indicated. Radiographs are indicated in postmenopausal women with spinal pain or any of the following criteria: loss of height ⩾4 cm compared with historical height (at 20 years of age); loss of height ⩾2 cm as established prospectively during follow-up; previous vertebral fracture; chronic comorbidities; and treatments associated with a high risk of vertebral fracture (glucocorticoids and aromatase inhibitors). The French guidelines were used for all of the patients. All radiographs were blindly assessed for the presence and severity (grade) of vertebral fractures by two independent observers, that is, an experienced musculoskeletal radiologist (VM) and an experienced bone and mineral disorders specialist (JP). Fractures were assessed using Genant’s semiquantitative method of vertebral fracture assessment.
BMD measurement by DXA
BMD was measured at the lumbar spine (L1–L4) and at the non-dominant hip by DXA (HOLOGIC Discovery A S/N 81360). The machine was calibrated daily and quality-assurance tests were carried out daily and weekly. World Health Organization (WHO) criteria were used to define osteoporosis and osteopenia based on BMD (T-score ⩽ −2.5 and T-score between −1.0 and −2.5 respectively).
Definition of “bone fragility”
In this study, “bone fragility” was defined in terms of BMD T-score and presence/absence of prevalent (vertebral or non-vertebral) fragility fracture. Specifically, “bone fragility” was defined as (i) a T-score ⩽ −2.5 at any site with or without a prevalent fragility fracture, or (ii) a T-score between −1.0 and −2.5 at any site and a prevalent fragility fracture.
Statistical analysis
Categorical variables are expressed as numbers (percentage). Continuous variables are expressed as mean [standard deviation (SD)]. Normality of distributions was assessed using histograms and the Shapiro–Wilk test. Radiographic MCP2–3 arthropathy, joint replacement surgery, and bone fragility rates were estimated by calculating 95% binomial confidence intervals (95% CIs). Inter-rater reliability for radiographic arthropathy evaluations was assessed by calculating intraclass correlation coefficients (ICCs) with their 95% CIs.
Bivariate analyses were performed to assess the associations between clinical and biochemical features (age, gender, BMI, disease duration, HFE gene status (C282Y+/+ genotype versus others), severe iron overload (serum ferritin ⩾1000 ng/ml at diagnosis), diabetes mellitus and hepatic cirrhosis), and the presence of radiographic MCP2–3 arthropathy. Student’s t tests were used for continuous variables and chi-squared tests (or Fisher’s exact tests when expected cell frequency was <5) for binary variables. All of the clinical and biochemical features found to be associated with radiographic MCP2–3 arthropathy at p < 0.10 in the bivariate analyses were then analyzed using a stepwise forward selection multivariate logistic regression model. Before developing the multivariable model, we examined the log-linearity assumption for continuous features using restricted cubic spline functions. Odds ratios (ORs) were calculated as effect size. The same approach was used to assess associations between clinical and biochemical features and presence of “bone fragility” and joint replacement surgery.
Statistical testing was conducted at the two-tailed α-level of 0.05. Data were analyzed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
General characteristics
Demographic and clinical data for the 93 subjects included in the study (58% men) are shown in Table 1. Mean ± SD age of the patients at the time of inclusion was 60.0 ± 11.2 years. Mean ± SD disease duration was 8.5 ± 5.9 years. At the time of inclusion, all 93 patients had started phlebotomy therapy. A total of 47 patients (50%) were homozygous for the C282Y mutation of the HFE gene, and 28/85 (33%) exhibited signs of severe iron overload (serum ferritin levels ⩾1000 ng/ml) at the time of diagnosis of HH. As expected, severe iron overload was frequently found in the presence of C282Y homozygosity (p = 0.003) but not the case in the presence of H63D homozygosity (Appendix 1).
Table 1.
Characteristics of 93 patients with hereditary hemochromatosis.
| Characteristics | n = 93 |
|---|---|
| Age, years | 60.0 (11.2) |
| Male sex ⩾50 years | 43 (46.2) |
| Male sex <50 years | 11 (11.8) |
| Postmenopausal women | 22 (23.7) |
| Premenopausal women | 17 (18.3) |
| Body height, cm | 169.1 (8.4) |
| Body weight, kg | 78.2 (16.8) |
| Body mass index (BMI), kg/m² | 27.4 (5.4) |
| BMI<18.5 kg/m² | 2 (2.1) |
| 18.5⩽BMI<25 kg/m² | 25 (26.9) |
| 25⩽BMI<30 kg/m² | 41 (44.1) |
| BMI⩾30 kg/m² | 25 (26.9) |
| Smoking status | |
| Non-smoker | 59 (63.5) |
| Past smoker | 24 (25.8) |
| Current smoker | 10 (10.7) |
|
Excessive alcohol consumption
(⩾3 units of alcohol daily) |
12 (12.9) |
| Disease duration, years | 8.5 (5.9) |
| HFE gene status | |
| C282Y/C282Y | 47 (50.6) |
| C282Y/H63D | 27 (29.0) |
| H63D/H63D | 12 (12.9) |
| C282Y/wt | 7 (7.5) |
|
Iron overload1
(Serum ferritin level ⩾1000 ng/ml) |
28 (32.9) |
| Organ involvement | |
| Diabetes mellitus | 10 (10.7) |
| Hepatic cirrhosis | 8 (8.6) |
| Hypogonadism | 3 (3.2) |
| Cardiomyopathy | 1 (1.1) |
| Cause of referral | |
| Clinical manifestations | 40 (43.0) |
| Altered biochemical values | 28 (30.1) |
| Family screening | 25 (26.9) |
Values are expressed as mean (standard deviation) or numbers (percentage).
Eight missing values.
MCP2–3 arthropathy: frequency and related factors
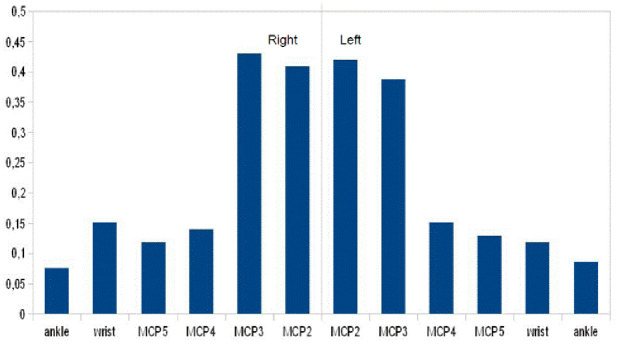
A complete musculoskeletal examination was performed. None of the subjects exhibited evidence of other inflammatory rheumatic diseases as defined by clinical, laboratory, and radiological criteria. Eleven patients (11.8%) exhibited limited range of motion in either the second or third MCP joint, with flexion restricted to less than 70°. Limited range of motion in these joints, as determined by physical examination, was significantly associated with presence of radiographic MCP2–3 arthropathy (p < 0.0001), which was more common in C282 homozygotes (n = 9) than in H63D homozygotes (n = 1) or C282Y/H63D heterozygotes (n = 1). Clinical evidence of arthropathy, per joint and per genotype, is shown in Appendix 2. The severity of articular changes for each joint is shown in Figure 1. The second and third MCP joints were more severely affected than the fourth and fifth MCP joints. Radiographic examinations revealed signs of chondrocalcinosis in 23 patients (24.7%). The mean (SD) (min–max) aggregate MCP2-3 score, the hand score and the total score assessed by the first reader (CDN) were respectively 2.2 (4.3) (0–14), 3.1 (6.4) (0–31), 3.3 (6.6) (0–32) Inter-rater reliability for arthropathy evaluations was good, with an ICC of 0.89 (95% CI 0.82–0.93) for the MCP2–3 score, 0.82 (95% CI 0.72–0.88) for the hand score and 0.79 (95% CI 0.69–0.86) for the total score.
Figure 1.
Severity of articular changes as assessed by a 4-score scale. The left and right second through fifth metacarpophalangeal (MCP2 - MCP5), wrist, and ankle joints of the patients were scored. Values are the mean.
The prevalence of radiographic MCP2–3 arthropathy (score ⩾2) was 37.6% (95% CI 0.28–0.48). The clinical and biochemical features associated with the presence of specific radiographic MCP2–3 joint arthropathy are shown in Table 2. Patients with radiographic MCP2–3 arthropathy were older (p < 0.001), had higher BMI (p = 0.032) and were more likely to be men (p = 0.014). They were also more likely to have diabetes (p = 0.037), hepatic cirrhosis (p = 0.004) and ferritin ⩾1000 ng/ml at diagnosis (p = 0.015) than patients without radiographic MCP2–3 arthropathy. We then performed multivariate analyses to determine independent predictors of radiographic MCP2–3 joint arthropathy (Table 3). Radiographic MCP2–3 arthropathy was independently associated with older age [OR 1.17 (1.09–1.26) per year, p < 0.0001], male gender [OR 3.89 (1.17–12.97), p = 0.027], and C282Y+/+ genotype [OR 4.78 (1.46–15.68), p = 0.010].
Table 2.
Clinical and biochemical features of 93 patients with HH subdivided according to presence or not of radiographic MCP2–3 arthropathy.
| Radiographic arthropathy of MCP2–3 | p-value | ||
|---|---|---|---|
| Present (n = 35) |
Absent (n = 58) |
||
| Age, years | 67.1 (5.3) | 55.7 (11.6) | <0.001 |
| Gender, women | 9 (25.7) | 30 (51.7) | 0.014 |
| Body Mass Index, kg/m² | 28.9 (5.8) | 26.5 (5.0) | 0.032 |
| Disease duration, years | 9.0 (7.0) | 8.2 (4.9) | 0.54 |
| HFE gene status, C282Y/C282Y | 22 (62.9) | 25 (43.1) | 0.065 |
| Ferritin ⩾1000 ng/ml at diagnosis1 | 16 (48.5) | 12 (23.1) | 0.015 |
| Diabetes | 7 (20.0) | 3 (5.2) | 0.037 |
| Hepatic cirrhosis | 7 (20.0) | 1 (1.7) | 0.004 |
Values are expressed as mean (standard deviation) or numbers (percentage).
Eight missing values (two patients with radiographic arthropathy of MCP2–3).
Table 3.
Multivariate logistic regression analysis of determinants of radiographic MCP2–3 arthropathy in 93 patients with hereditary hemochromatosis.
| Radiographic MCP2–3 arthropathy | OR | 95% CI | p-value |
|---|---|---|---|
| Age at assessment (per 1-year increase) | 1.17 | 1.09–1.26 | <0.0001 |
| Gender, men (versus women) | 3.89 | 1.17–12.97 | 0.027 |
| HFE gene status (C282Y/C282Y versus others) | 4.78 | 1.46–15.68 | 0.010 |
Odds ratios (ORs) were calculated using a stepwise forward selection logistic regression model to which the following candidate variables were added: age, gender, body mass index, HFE gene status, ferritin ⩾1000 ng/ml at diagnosis, diabetes, and hepatic cirrhosis.
Joint replacement surgery: frequency and related factors
Twelve patients (12.9%) (95% CI 0.07–0.21) underwent total joint replacement surgery. The mean (SD) age of these patients (9 men and 3 women) at the time of evaluation was 68.1 (5.9) years. Overall, a total of 21 joints were replaced. Four patients had one joint replaced, seven had two joints replaced, and one underwent three joint replacement surgeries. The most common sites for replacement were the hip joints (n = 13 joints). Knee joints (n = 8) were replaced less frequently.
Patients who underwent joint replacement surgery were older (p < 0.001), had higher BMI (p = 0.008) and were more likely to be homozygous for the C282Y mutation of the HFE gene (p = 0.002). They were also more likely to have diabetes (p = 0.003) than those who did not undergo surgery. The clinical and biochemical features associated with joint replacement surgery are shown in Appendix 3. Independent predictors of joint replacement surgery were determined by multivariate analysis. Joint replacement surgery was independently associated with older age [OR 1.14 (1.02–1.27) per year, p = 0.02], BMI [OR 1.19 (1.01–1.40) per kg/m², p = 0.03], and C282Y+/+ genotype [OR 36.27 (3.03–434.42), p = 0.010].
“Bone fragility”: frequency and related factors
In 93 consecutive patients with HH, BMD was systematically evaluated by DXA scans of the lumbar spine (LS; n = 92, lumbar osteosynthesis in one patient) and femoral neck (FN; n = 88, bilateral hip replacement in 5 patients). Mean LS and FN Z-scores were 0.8 (1.6) and 0.1 (1.0) respectively. The corresponding T-scores were 0.0 (1.8) and −1.0 (1.0) respectively. LS and/or FN osteoporosis was detected in 9 patients (9.7%), and osteopenia in a further 54 patients (58.1%). LS osteoporosis was detected in 8 patients (8.6%), FN osteoporosis in 4 patients (4.3%), LS osteopenia in 17 patients (18.3%) and FN osteopenia in 44 patient (47.3%).
At evaluation, one patient had premature menopause (below the age of 45), 11 had a family history of first-degree hip fracture (11.8%), 4 had prolonged exposure to corticosteroids, 10 were current smokers, and 12 were excessive consumers of alcohol. Regarding history of fragility fracture after the age of 40, some patients had several fractures, and 21 fractures were found in 16 patients. There was one vertebral fracture, seven distal forearm or wrist fractures, seven leg or ankle fractures, two pelvis fractures, one proximal humerus fracture, and three other fractures (femur, rib and elbow).
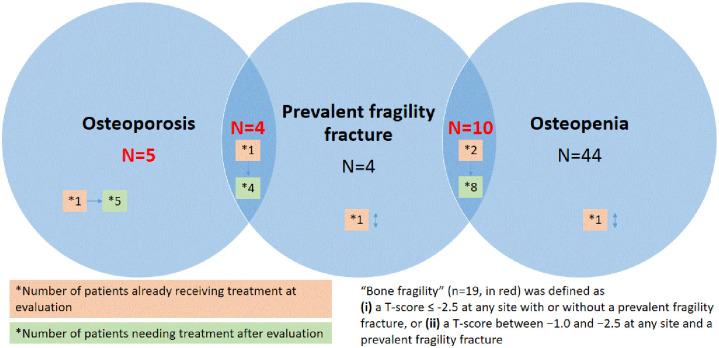
In line with French guidelines, 29 patients (34.9%) underwent a morphological assessment of the spine. An unknown vertebral fracture was only diagnosed in three patients, with no discrepancies between the two readers (JP and VM). Among those patients, two had no history of fragility fracture. In all, therefore, there were 18 patients with at least 1 prevalent (vertebral or non-vertebral) fragility fracture. Among the patients with BMD-determined osteoporosis (n = 9), four had at least one prevalent fragility fracture. Among those with osteopenia (n = 54), 10 had at least 1 prevalent fragility fracture. Four patients with fragility fractures had normal BMDs. At evaluation, 6 patients were already undergoing anti-osteoporosis treatment and, after evaluation, 17 patients needed anti-osteoporosis treatment (Figure 2).
Figure 2.
Patients with “bone fragility” according to bone mineral density and prevalent fragility fractures.
“Bone fragility” was defined as (i) a T-score ⩽ −2.5 at any site with or without a prevalent fragility fracture, or (ii) a T-score between −1.0 and −2.5 at any site and a prevalent fragility fracture. The frequency of “bone fragility” was 20.4% (95% CI 0.13–0.30). The characteristics of the 19 patients with bone fragility are shown in Appendix 4. The clinical and biochemical features associated with “bone fragility” are shown in Table 4. Patients with “bone fragility” were more likely to have hepatic cirrhosis (26.3% versus 4.1%, p = 0.008) and tended to have longer disease durations (p = 0.06) and severe iron overload (p = 0.08). Multivariate analyses were then performed to determine independent predictors of “bone fragility.” “Bone fragility” was strongly and independently associated with hepatic cirrhosis [OR 8.20 (1.74–38.68), p = 0.008] (Appendix 5). The same results were found regardless of whether we used the BMD criteria for osteoporosis (n = 9) or the presence of (vertebral and non-vertebral) fragility fractures at evaluation (n = 18) as the indicator of “bone fragility” (Appendixes 6 and 7).
Table 4.
Clinical features of 93 patients with HH subdivided according to presence or not of bone fragility.
| Bone fragility | p-value | ||
|---|---|---|---|
| Present (n = 19) |
Absent (74) |
||
| Age, years | 63.5 (8.5) | 59.1 (11.6) | 0.12 |
| Sex, women | 7 (36.8) | 32 (43.2) | 0.61 |
| Body mass index, kg/m² | 27.3 (7.1) | 27.4 (4.9) | 0.95 |
| Disease duration, years | 10.7 (6.7) | 7.9 (5.4) | 0.06 |
| HFE gene status, C282Y/C282Y | 8 (42.1) | 39 (52.7) | 0.41 |
| Ferritin ⩾1000 ng/ml at diagnosis1 | 9 (50.0) | 19 (28.4) | 0.08 |
| Parent fractured hip | 2 (10.5) | 9 (12.2) | 1.00 |
| Excessive alcohol consumption | 1 (5.3) | 11 (14.9) | 0.45 |
| Current smoker | 1 (5.3) | 10 (13.5) | 0.45 |
| Diabetes mellitus | 2 (10.5) | 8 (10.8) | 1.00 |
| Hepatic cirrhosis | 5 (26.3) | 3 (4.1) | 0.008 |
Values are expressed as mean (standard deviation) or numbers (percentage).
Eight missing values (one patient with osteoporosis).
Discussion
This cross-sectional study included 93 patients with HH enrolled at the Department of Rheumatology outpatient clinic at Lille University Hospital over a period of 2 years. The frequency of radiographic MCP2-3 arthropathy (37.6%) was higher than expected and independently associated with older age, male sex, and C282Y+/+ genotype rather than severity of iron overload. The frequency of joint replacement surgery was 12.9% and independently associated with older age, BMI and C282Y+/+ genotype. “Bone fragility” was observed in a fifth of the HH patients, independently of genetic background and severity of iron overload, and was strongly associated with hepatic cirrhosis.
Our results confirm data in the literature on the demographic (age and gender) and anthropometric characteristics (BMI) of patients with HH.3–6 In contrast with previous findings, C282Y homozygosity was lower than expected.9,11 The frequencies of C282Y and H63D mutations of the HFE gene vary between different populations. In our patients, the frequency of C282Y was unexpectedly low, and the frequency of H63D unexpectedly high. Similar results were previously found in a study on the distribution of HFE mutations in French Basque Country patients with HH.18 The C282Y mutation was underrepresented (53.3% in autochthonous Basques and 59.2% in the whole sample) and H63D was highly represented (5.6% in autochthonous Basques and 13.3% in the whole sample). Regarding organ involvement, the prevalence of hypogonadism and hepatic cirrhosis was quite low.9
One of the main finding in our study is that radiographic arthropathy of MCP2 and MCP3 joints occurs in a higher proportion of patients with HH than expected. In our study, and in those by others, radiographic arthropathy was strongly associated with age and C282Y homozygosity.5,11 C282Y homozygosity and compound C282Y/H63D heterozygosity are the most commonly acknowledged genotypes in the pathogenesis of HH. In our study, two patients with clinical evidence of arthropathy were H63D homozygotes (Appendix 2). This result corroborates the findings of Alizadeh et al.,19 who reported a higher prevalence of arthralgia and arthropathy in patients with the H63D +/+ genotype. We also found an association with male gender, which has only been reported in one previous study.20 In the Melbourne Collaborative Cohort Study, the presence of abnormal MCP2 and MCP3 joints at physical examination was more common in male (but not female) C282Y homozygotes than in control subjects, regardless of serum ferritin levels.20 Sandhu et al. suggest that factors other than iron overload might be contributing to clinical arthropathy in HFE HH.21 This view is supported by evidence that phlebotomy therapy does not always relieve joint symptoms in patients with HH. This is in accordance with our findings where severe iron overload (serum ferritin concentration >1000 mg/l) at the time of diagnosis was not found to be an important predictor of radiographic MCP2–3 arthropathy in HH. Nevertheless, other investigators have reported a strong relationship between iron load and radiographic arthropathy in HH in general, and MCP arthropathy in the hand in particular.4,11–13
Two small studies have suggested that the risk of joint replacement surgery might be higher in patients with HH.9,12 In a large nationwide, population-based study, Elmberg et al. reported that patients with HH were at higher risk of undergoing joint replacement surgery.10 This finding was also found in a retrospective cohort study in which HH was found to be associated with increased odds of replacement arthroplasty, particularly in the elderly.22 We found that a high proportion of patients (~13%) at inclusion had undergone total joint replacement surgery, and this is quite similar to the findings reported by Sahinbegovic et al.9 When we sought to determine risk factors for joint replacement surgery in the general population, we found that age and BMI, which are established risk factors for primary osteoarthritis, also determined the risk of joint replacement surgery in patients with HH. In addition, the presence of the C282Y+/+ genotype was strongly associated with joint replacement surgery, suggesting that genetic background is a major determinant. In a prospective cohort of healthy, middle-aged Australians over an 8-year period, C282Y homozygosity was associated with a higher risk of both single and bilateral total hip replacement due to osteoarthritis.23 Our findings that radiographic MCP2–3 arthropathy and joint replacement surgery in HH seem to be related to HFE genotype rather than severe iron overload is interesting.
A higher prevalence of osteoporosis based on BMD criteria (T-score ⩽ –2.5 at any site) has been previously reported and found to correlate with severity of iron overload, independently of cirrhosis and hypogonadism.5,6,24 Indeed, osteoporosis was detected in 25–34% of the patients, and osteopenia in 41–79% of them.5,6,24 In contrast with previous findings, we observed a lower prevalence of osteoporosis (~10%) based on a T-score ⩽ −2.5 at any site, but we acknowledge that a small proportion of our patients (~6%) were already undergoing anti-osteoporosis treatment, which may have biased our BMD results in comparison with other studies where no patients were being treated.5,6,24
In a study involving a small cohort of men with HH, the prevalence of radiologically confirmed vertebral fractures was reported at up to 20%.14 However, the prevalence of vertebral and non-vertebral fractures was not reported in other studies assessing bone status in patients with HH.5,6,24 A novel aspect of this study is that it provides data on the prevalence of vertebral and non-vertebral fragility fractures in a large cohort of patients with HH. Furthermore, the prevalence of vertebral and non-vertebral fractures was quite high (~20%) and mainly accounted for by non-vertebral fractures rather than vertebral fractures.
Since osteoporosis is not only defined in terms of a low T-score based on DXA measurements of BMD, we opted to use a definition of “bone fragility” based on T-score and prevalence of (vertebral and non-vertebral) fragility fractures. Using this definition, we found a strong and independent association between “bone fragility” and hepatic cirrhosis, but none between “bone fragility” and C282Y homozygosity or severe iron overload at diagnosis.
The mechanisms leading to bone impairment in HH are not well understood. However, it has been assumed that the iron overload itself leads to this condition. Indeed, excess iron is reported to inhibit osteoblastic cell proliferation, differentiation and primary mineralization in vitro,25 possibly inducing low bone formation.26 In animal studies with iron-overloaded mice, an increase in reactive oxygen species and dose-dependent tissue iron content has been observed, leading to impaired bone microarchitecture and an increase in the non-mineralized matrix.27 However, in various mouse models of genetic HFE-hemochromatosis, Wagner et al. demonstrated that iron overload alone is not sufficient to induce bone loss.28 Our results are consistent with this finding. The impact of cirrhosis on bone is difficult to evaluate on account of the presence of numerous cofactors for osteoporosis, such as hypogonadism and excessive alcohol consumption.29–31 However, the prevalence of hypogonadism was low in our study, and excessive alcohol consumption was not associated with “bone fragility.”
Using high-resolution peripheral quantitative computed tomography (HRpQCT), bone microarchitecture was found to be impaired in patients with HH. Cortical volumetric BMD and cortical thickness were lower compared with age- and gender-adjusted reference values from the literature, whereas trabecular microstructure and volumetric BMD were preserved.32
Study strengths and weaknesses
We acknowledge that there are several limitations to this study. The prevalence of fractures and radiographic arthropathy may be biased since all of our patient data were from an outpatient clinic specializing in bone and joint diseases. As such, patients with HH but without bone and joint complaints may not have come to our clinic. As the study was hospital-based (rather than population-based) its findings cannot be extrapolated to other populations. Furthermore, findings on patients in tertiary care hospitals cannot be extrapolated to other patients. We did not systematically evaluate morphological vertebral fractures in all patients using conventional spine X-ray or vertebral fracture assessment by DXA. Another limitation is that no biological evaluations (ferritin, markers of bone turnover, 25-hydroxyvitamin D, etc.) were systematically performed for this study at inclusion. It may have been useful to evaluate sex steroid hormones such as testosterone and estradiol in order to determine the prevalence of hypogonadism. Another potential limitation in interpreting the results of this study is that the patients with HH were not compared with an age-matched control group. Lastly, given the size of our study sample, we cannot exclude the risk of over-fitting in multivariate analyses, or a lack of statistical power in detecting associations.
Conclusion
All things considered, we believe that our study makes an important contribution to the knowledge of bone and joint involvement in HH. In a population of 93 patients with well-defined genetic HFE-related hemochromatosis, radiographic MCP2–3 arthropathy was found to occur in ~38% of patients. Moreover, the association observed between radiographic MCP2–3 arthropathy and C282Y homozygosity, male sex, and older age suggests that demographic characteristics and genetic background, rather than severity of iron overload, are likely to be major determinants of this form of joint impairment. The frequency of joint replacement surgery was 12.9% and independently associated with older age, BMI and C282Y+/+ genotype. Furthermore, “bone fragility” was observed in a fifth of patients, independently of genetic background and severity of iron overload, and was strongly associated with hepatic cirrhosis. Future investigations should focus on pathogenesis and early identification of patients at risk for developing bone and joint complications secondary to HH. Prospective studies should investigate possible treatment options to slow the progression of bone and joint complications in HH.
Appendix
Appendix 1.
Iron overload at time of diagnosis among HFE HH.
| Iron overload | p-value | ||
|---|---|---|---|
| Serum ferritin level<1000 ng/ml (n = 57) | Serum ferritin level⩾1000
ng/ml (n = 28) |
||
| - C282Y/C282Y1 | 21 (36.8) | 20 (71.4) | 0.003 |
| - C282Y/H63D | 23 (40.3) | 4 (14.3) | 0.015 |
| - H63D/H63D2 | 8 (14.0) | 3 (10.7) | 1.00 |
| - C282Y/wt2 | 5 (8.8) | 1 (3.6) | 0.66 |
Values are expressed as numbers (percentage).
Six missing values.
One missing value.
Appendix 2.
Criteria for qualifying “clinical evidence of arthropathy.”
| Joint | Criteria | n (%) | HFE gene status |
|---|---|---|---|
| MCP2–3# | Passive flexion <70° | 11 (11.8) | - C282Y/C282Y, n = 9 - C282Y/H63D, n = 1 - H63D/H63D, n = 1 - C282Y/wt, n = 0 |
| Radiocarpal# | Passive extension <50° or passive flexion <40° | 8 (8.6) | - C282Y/C282Y, n = 5 - C282Y/H63D, n = 1 - H63D/H63D, n = 1 - C282Y/wt, n = 1 |
| Elbow | Passive extension deficit of at least 10° or
passive flexion <120° |
1 (1.1) | - C282Y/C282Y, n = 0 - C282Y/H63D, n = 1 - H63D/H63D, n = 0 - C282Y/wt, n = 0 |
| Hip | Passive flexion <100° or passive internal rotation <20° | 6 (6.5) | - C282Y/C282Y, n = 5 - C282Y/H63D, n = 0 - H63D/H63D, n = 0 - C282Y/wt, n = 1 |
| Ankle# | Passive plantar flexion <20° | 1 (1.1) | - C282Y/C282Y, n = 1 - C282Y/H63D, n = 0 - H63D/H63D, n = 0 - C282Y/wt, n = 0 |
| Any joint | Any limited range of motion | 19 (20.4) | - C282Y/C282Y, n = 13 - C282Y/H63D, n = 2 - H63D/H63D, n = 2 - C282Y/wt, n = 2 |
All patients had radiographs.
Appendix 3.
Clinical and biochemical features of 93 patients with HH subdivided according to presence or not of joint replacement surgery.
| Joint replacement surgery | p-value | ||
|---|---|---|---|
| Present (n = 12) |
Absent (n = 81) |
||
| Age, years | 68.1 (5.9) | 58.8 (11.3) | <0.001 |
| Gender, women | 3 (25.0) | 36 (44.4) | 0.20 |
| Body mass index, kg/m² | 33.1 (7.0) | 26.6 (4.6) | 0.008 |
| Disease duration, years | 10.3 (7.9) | 8.2 (5.4) | 0.24 |
| HFE gene status, C282Y/C282Y | 11 (91.7) | 36 (44.4) | 0.002 |
| Ferritin ⩾1000 ng/ml at diagnosis1 | 7 (58.3) | 21 (28.8) | 0.054 |
| Diabetes | 5 (41.7) | 5 (6.2) | 0.003 |
| Hepatic cirrhosis | 3 (25.0) | 5 (6.2) | 0.064 |
Values are expressed as mean (standard deviation) or numbers (percentage).
Eight missing values.
Appendix 4.
Characteristics of patients with “bone fragility.”.
| Sex | Age | Type of fracture | LS T-score | FN T-score | TH T-score | Osteoporosis treatment needed after evaluation | |
|---|---|---|---|---|---|---|---|
| 1 | M | 73 | Ribs and ankle | –2.5 | Bilateral hip replacement | Yes, oral BisP | |
| 2 | F | 57 | No | LS osteosynthesis | −3.4 | −3.2 | Yes, ZOL |
| 3 | M | 65 | Wrist and femur | −2.9 | −2.3 | −2.0 | Yes, ZOL |
| 4 | F | 70 | Wrist | 1.8 | −1.0 | −0.1 | No, already treated by oral BisP and DNB for 9 years |
| 5 | F | 72 | Shoulder and ankle Vertebral fracture (D6) |
−1.6 | −1.3 | −0.6 | Yes, ZOL. Already treated by oral BisP for 7 years |
| 6 | M | 70 | Wrist | −2.7 | −3.4 | −2.9 | Yes, already treated by ZOL for 3 years. |
| 7 | M | 72 | Leg | 1 | −1.9 | −1.3 | Yes, oral BisP |
| 8 | M | 56 | Wrist | −0.6 | −1.7 | −0.8 | Yes, ZOL |
| 9 | M | 55 | No | −4.0 | −2.3 | −2.3 | Yes, already treated by oral BisP for 10 years |
| 10 | F | 66 | Hip and bilateral leg | −0.3 | −2.4 | −2.3 | Yes, ZOL |
| 11 | M | 79 | Left wrist and right forearm | 5.3 | −1.1 | −0.9 | Yes, ZOL |
| 12 | F | 54 | Sacrum | −1.0 | −1.9 | −1.1 | Yes, HRT |
| 13 | M | 57 | No | −2.6 | −2.8 | −1.7 | Yes, ZOL |
| 14 | F | 57 | Vertebral fracture (D12) | −0.6 | −2.3 | −2.0 | Yes, ZOL |
| 15 | F | 56 | No | 0.0 | −2.5 | −0.9 | Yes, already treated by oral BisP for 10 years |
| 16 | M | 69 | Vertebral fractures (D6-D7*) | −3.6 | −2.9 | −2.2 | Yes, patient loss of follow-up |
| 17 | F | 69 | Vertebral fracture (D12*) | −1.9 | −1.6 | −1.0 | Yes, oral BisP |
| 18 | M | 59 | Pelvis | −1.7 | Bilateral hip replacement | No, already treated by oral BisP and ZOL for 7 years | |
| 19 | M | 60 | No | −2.6 | −0.9 | −0.1 | Yes, ZOL |
BisP, bisphosphonate; DNB, denosumab; LS, lumbar spine; ZOL, zoledronic acid.
New vertebral fractures discovered at evaluation.
Appendix 5.
Multivariate logistic regression analysis of determinants of “bone fragility” in 93 patients with hereditary hemochromatosis.
| “Bone fragility” | OR | 95% CI | p-value |
|---|---|---|---|
| Cirrhosis | 8.20 | 1.74–38.68 | 0.008 |
Odds ratios (ORs) were calculated using a stepwise forward selection logistic regression model to which the following candidate variables were applied: age, disease duration, ferritin ⩾1000 ng/ml at diagnosis and cirrhosis.
Appendix 6.
Clinical and biochemical features of 93 patients with HH subdivided according to presence or not of bone mineral density (BMD) osteoporosis at any site.
| BMD osteoporosis | p-value | ||
|---|---|---|---|
| Present (n = 9) |
Absent (n = 84) |
||
| Age, years | 61.2 (8.9) | 59.8 (11.4) | 0.73 |
| Gender, women | 1 (11.1) | 38 (45.2) | 0.074 |
| Body mass index, kg/m² | 26.2 (8.8) | 27.5 (5.0) | 0.67 |
| Disease duration, years | 12.4 (8.2) | 8.1 (5.4) | 0.030 |
| HFE gene status, C282Y/C282Y | 6 (66.7) | 41 (48.8) | 0.49 |
| Ferritin ⩾1000 ng/ml at diagnosis1 | 5 (62.5) | 23 (29.9) | 0.11 |
| Diabetes | 2 (22.2) | 8 (9.5) | 0.25 |
| Hepatic cirrhosis | 3 (33.3) | 5 (6.0) | 0.028 |
| Parent fractured hip | 1 (11.1) | 10 (11.9) | 1.00 |
| Excessive alcohol consumption | 0 (0) | 12 (14.3) | 0.60 |
| Current smoker | 1 (11.1) | 10 (11.9) | 1.00 |
Values are expressed as mean (standard deviation) or numbers (percentage).
Eight missing values.
Appendix 7.
Clinical and biochemical features of 93 patients with HH subdivided according to presence or not of fragility fracture.
| fragility fracture | p-value | ||
|---|---|---|---|
| Present (n = 18) |
Absent (n = 75) |
||
| Age, years | 64.2 (8.5) | 59.0 (11.6) | 0.076 |
| Gender, women | 9 (50.0) | 30 (40.0) | 0.44 |
| Body mass index, kg/m² | 28.4 (7.3) | 27.2 (4.9) | 0.51 |
| Disease duration, years | 10.2 (7.4) | 8.1 (5.3) | 0.17 |
| HFE gene status, C282Y/C282Y | 11 (61.1) | 36 (48.0) | 0.32 |
| Ferritin ⩾1000 ng/ml at diagnosis1 | 8 (47.1) | 20 (29.4) | 0.17 |
| Diabetes | 2 (11.1) | 8 (10.7) | 1.00 |
| Hepatic cirrhosis | 5 (27.8) | 3 (4.0) | 0.006 |
| Parent fractured hip | 3 (16.7) | 8 (10.7) | 0.44 |
| Excessive alcohol consumption | 2 (11.1) | 10 (13.3) | 1.00 |
| Current smoker | 3 (16.7) | 8 (10.7) | 0.44 |
Values are expressed as mean (standard deviation) or numbers (percentage).
Eight missing values.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Julien Paccou  https://orcid.org/0000-0001-6599-8623
https://orcid.org/0000-0001-6599-8623
Contributor Information
Chi-Duc Nguyen, Service de rhumatologie, CHU Lille, Lille, France.
Vincent Morel, Service de radiologie ostéoarticulaire, CHU Lille, Lille, France.
Adeline Pierache, EA 2694 – Santé Publique: épidémiologie et qualité des soins, Université de Lille, CHU Lille, F-Lille, France.
Georges Lion, Service de médecine nucléaire, CHU Lille, Lille, France.
Bernard Cortet, Service de rhumatologie, CHU Lille, Lille, France; MABLaB ULR 4490 faculté de chirurgie dentaire, Université de Lille, Université Littoral Côte d’Opale, place de Verdun, Lille, France.
René-Marc Flipo, Service de rhumatologie, CHU Lille, Lille, France.
Valérie Canva-Delcambre, Service d’hépatologie, CHU Lille, Lille, France.
Julien Paccou, Service de rhumatologie, Hôpital Roger Salengro, Rue Emile Laine, CHRU, 59037 Lille cedex, France; MABLaB ULR 4490 faculté de chirurgie dentaire, Université de Lille, Université Littoral Côte d’Opale, place de Verdun, Lille, 59000, France.
References
- 1. Pietrangelo A, Deugnier Y, Dooley J, et al. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010; 53: 3–22. [DOI] [PubMed] [Google Scholar]
- 2. Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet 2016; 388: 706–716. [DOI] [PubMed] [Google Scholar]
- 3. Carroll GJ, Breidahl WH, Olynyk JK. Characteristics of the arthropathy described in hereditary hemochromatosis. Arthritis Care Res (Hoboken) 2012; 64: 9–14. [DOI] [PubMed] [Google Scholar]
- 4. Sahinbegovic E, Dallos T, Aigner E, et al. Musculoskeletal disease burden of hereditary hemochromatosis. Arthritis Rheum 2010; 62: 3792–3798. [DOI] [PubMed] [Google Scholar]
- 5. Valenti L, Varenna M, Fracanzani AL, et al. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 2009; 20: 549–555. [DOI] [PubMed] [Google Scholar]
- 6. Guggenbuhl P, Deugnier Y, Boisdet JF, et al. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 2005; 16: 1809–1814. [DOI] [PubMed] [Google Scholar]
- 7. Richardson A, Prideaux A, Kiely P. Haemochromatosis: unexplained metacarpophalangeal or ankle arthropathy should prompt diagnostic tests: findings from two UK observational cohort studies. Scand J Rheumatol 2017; 46: 69–74. [DOI] [PubMed] [Google Scholar]
- 8. Husar-Memmer E, Stadlmayr A, Datz C, et al. HFE-Related hemochromatosis: an update for the rheumatologist. Curr Rheumatol Rep 2014; 16: 393. [DOI] [PubMed] [Google Scholar]
- 9. Sahinbegovic E, Dallos T, Aigner E, et al. Hereditary hemochromatosis as a risk factor for joint replacement surgery. Am J Med 2010; 123: 659–662. [DOI] [PubMed] [Google Scholar]
- 10. Elmberg M, Hultcrantz R, Simard JF, et al. Increased risk of arthropathies and joint replacement surgery in patients with genetic hemochromatosis: a study of 3,531 patients and their 11,794 first-degree relatives. Arthritis Care Res (Hoboken) 2013; 65: 678–685. [DOI] [PubMed] [Google Scholar]
- 11. Carroll GJ, Breidahl WH, Bulsara MK, et al. Hereditary hemochromatosis is characterized by a clinically definable arthropathy that correlates with iron load. Arthritis Rheum 2011; 63: 286–294. [DOI] [PubMed] [Google Scholar]
- 12. Richette P, Ottaviani S, Vicaut E, et al. Musculoskeletal complications of hereditary hemochromatosis: a case-control study. J Rheumatol 2010; 37: 2145–2150. [DOI] [PubMed] [Google Scholar]
- 13. Valenti L, Fracanzani AL, Rossi V, et al. The hand arthropathy of hereditary hemochromatosis is strongly associated with iron overload. J Rheumatol 2008; 35: 153–158. [PubMed] [Google Scholar]
- 14. Diamond T, Stiel D, Posen S. Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or other factors? Ann Intern Med 1989; 110: 430–436. [DOI] [PubMed] [Google Scholar]
- 15. Pilling LC, Tamosauskaite J, Jones G, et al. Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ 2019; 364: k5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dallos T, Sahinbegovic E, Aigner E, et al. Validation of a radiographic scoring system for haemochromatosis arthropathy. Ann Rheum Dis 2010; 69: 2145–2151. [DOI] [PubMed] [Google Scholar]
- 17. Briot K, Roux C, Thomas T, et al. 2018 update of French recommendations on the management of postmenopausal osteoporosis. Joint Bone Spine 2018; 85: 519–530. [DOI] [PubMed] [Google Scholar]
- 18. Bauduer F, Scribans C, Degioanni A, et al. Distribution of the C282Y and H63D polymorphisms in hereditary hemochromatosis patients from the French Basque country. Ann Hematol 2005; 84: 99–102. [DOI] [PubMed] [Google Scholar]
- 19. Alizadeh BZ, Njajou OT, Hazes JM, et al. The H63D variant in the HFE gene predisposes to arthralgia, chondrocalcinosis and osteoarthritis. Ann Rheum Dis 2007; 66: 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med 2008; 358: 221–230. [DOI] [PubMed] [Google Scholar]
- 21. Sandhu K, Flintoff K, Chatfield MD, et al. Phenotypic analysis of hemochromatosis subtypes reveals variations in severity of iron overload and clinical disease. Blood 2018; 132: 101–110. [DOI] [PubMed] [Google Scholar]
- 22. Kröner PT, Mareth KF, Wijarnpreecha K, et al. Hereditary hemochromatosis is associated with increased use of joint replacement surgery: results of a nationwide analysis. Semin Arthritis Rheum. Epub ahead of print 11 November 2019. DOI: 10.1016/j.semarthrit.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Gurrin LC, Wluka AE, et al. HFE C282Y homozygosity is associated with an increased risk of total hip replacement for osteoarthritis. Semin Arthritis Rheum 2012; 41: 872–878. [DOI] [PubMed] [Google Scholar]
- 24. Sinigaglia L, Fargion S, Fracanzani AL, et al. Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 1997; 24: 1809–1813. [PubMed] [Google Scholar]
- 25. Yamasaki K, Hagiwara H. Excess iron inhibits osteoblast metabolism. Toxicol Lett 2009; 191: 211–215. [DOI] [PubMed] [Google Scholar]
- 26. Doyard M, Chappard D, Leroyer P, et al. Decreased bone formation explains osteoporosis in a genetic mouse model of hemochromatosiss. PLoS One 2016; 11: e0148292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsay J, Yang Z, Ross FP, et al. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 2010; 116: 2582–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner A, Alan B, Yilmaz D, et al. Despite genetic iron overload, Hfe-hemochromatosis mice do not show bone loss. JBMR Plus 2019; 3: e10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krol CG, Dekkers OM, Kroon HM, et al. No association between BMD and prevalent vertebral fractures in liver transplant recipients at time of screening before transplantation. J Clin Endocrinol Metab 2014; 99: 3677–3685. [DOI] [PubMed] [Google Scholar]
- 30. Wibaux C, Legroux-Gerot I, Dharancy S, et al. Assessing bone status in patients awaiting liver transplantation. J Bone Spine 2011; 78: 387–391. [DOI] [PubMed] [Google Scholar]
- 31. Paccou J, Edwards MH, Ward K, et al. Relationships between bone geometry, volumetric bone mineral density and bone microarchitecture of the distal radius and tibia with alcohol consumption. Bone 2015; 78: 122–129. [DOI] [PubMed] [Google Scholar]
- 32. Jandl NM, Rolvien T, Schmidt T, et al. Impaired bone microarchitecture in patients with hereditary hemochromatosis and skeletal complications. Calcif Tissue Int 2020; 106: 465–475. [DOI] [PubMed] [Google Scholar]