Abstract
Background:
Inhibitors targeting programmed cell death 1 (PD-1) and programmed death-ligand 1 (PD-L1) have unprecedented effects in cancer treatment. However, the objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) of PD-1/PD-L1 blockade monotherapy have not been systematically evaluated.
Methods:
We searched Embase, PubMed, and Cochrane database from inception to July 2019 for prospective clinical trials on single-agent PD-1/PD-L1 antibodies (avelumab, atezolizumab, durvalumab, cemiplimab, pembrolizumab, and nivolumab) with information regarding ORR, PFS, and OS.
Results:
Totally, 28,304 patients from 160 perspective trials were included. Overall, 4747 responses occurred in 22,165 patients treated with PD-1/PD-L1 monotherapy [ORR, 20.21%; 95% confidence interval (CI), 18.34–22.15%]. Compared with conventional therapy, PD-1/PD-L1 blockade immunotherapy was associated with more tumor responses (odds ratio, 1.98; 95% CI, 1.52–2.57) and better OS [hazard ratio (HR), 0.75; 95% CI, 0.67–0.83]. The ORRs varied significantly across cancer types and PD-L1 expression status. Line of treatment, clinical phase and drug target also impacted the response rates in some tumors. A total of 2313 of 9494 PD-L1 positive patients (ORR, 24.39%; 95% CI, 22.29–26.54%) and 456 of 4215 PD-L1 negative patients (ORR, 10.34%; 95% CI, 8.67–12.14%) achieved responses. For PD-L1 negative patients, the ORR (odds ratio, 0.92; 95% CI, 0.70–1.20) and PFS (HR, 1.15; 95% CI, 0.87–1.51) associated with immunotherapy and conventional treatment were similar. However, PD-1/PD-L1 blockade monotherapy decreased the risk of death in both PD-L1 positive (HR, 0.66; 95% CI, 0.60–0.72) and PD-L1 negative (HR, 0.86; 95% CI, 0.74–0.99) patients compared with conventional therapy.
Conclusion:
The efficacies associated with PD-1/PD-L1 monotherapy vary significantly across cancer types and PD-L1 expression. This comprehensive summary of clinical benefit from immunotherapy in cancer patients provides an important guide for clinicians.
Keywords: cancer, immunotherapy, PD-1, PD-L1, tumor response
Background
Immune checkpoint blockade therapy targeting programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) has revolutionized cancer treatment in the past decade.1 Currently, three PD-1 monoclonal antibodies (cemiplimab, nivolumab, and pembrolizumab) and three PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab) are approved by the United States (US) Food and Drug Administration (FDA) for dozens of indications. These PD-1/PD-L1 inhibitors statistically improved the overall survival (OS) of cancer patients and have emerged as the standard therapy for multiple malignancies.1,2 It should be acknowledged that, in many clinical trials, because it usually takes a long time to obtain OS information, the efficacies of immunotherapy were represented by the independently confirmed objective response rates (ORRs) evaluated based on the standard guidelines such as Response Evaluation Criteria in Solid Tumor (RECIST).
Although immunotherapy has unprecedented effects on cancer treatment, only a fraction of patients can benefit from it.1,2 Currently, a major challenge for immunotherapy is to find ideal biomarkers that can identify patients who are susceptible to treatment and avoid serious toxicities and unnecessary costs for non-responders. Previous studies have revealed that PD-L1 immunohistochemistry (IHC), microsatellite instability (MSI), tumor mutational burden (TMB), T-cell receptor clonality, T-cell infiltration levels, gene expression signatures and peripheral blood biomarkers were associated with clinical response in immunotherapy.3 Among them, PD-L1 expression was treated as biologically plausible biomarker for the prediction of treatment response to immunotherapy. Currently, companion PD-L1 expression diagnostic assays were granted by FDA for use in patients with lung cancer, urothelial cancer, renal cell cancer, and melanoma.4 In fact, only two biomarkers, namely PD-L1 and MSI, were approved for PD-1/PD-L1 based immunotherapy.1,3 However, it is unclear whether PD-L1 expression status is robustly predictive of clinical benefit across diverse human cancers, or outside of these specific clinical trial populations.
Understanding of these issues may have important public health and clinical implications given the significant increase in the application of immunotherapy is expected in the future. Here, with accumulated evidence, we conducted a comprehensive meta-analysis to assess the clinical efficacies of PD-1/PD-L1 monotherapy in published clinical trials. In addition, we investigated the role of PD-L1 expression status as a predict biomarker, and quantified the potential differences in the incidences of tumor response, progress-free survival (PFS), and OS among a variety of cancer types and treatment strategies.
Methods
Search strategy and study selection
A comprehensive search of PubMed, Embase, and Cochrane databases from inception to July 2019 was performed to identify prospective clinical trials with PD-1/PD-L1 blockade monotherapy. The major search keywords and medical subject headings used were: atezolizumab, avelumab, cemiplimab, durvalumab, nivolumab, pembrolizumab, checkpoint inhibitors, PD-1, programmed cell death receptor 1, PD-L1, programmed cell death 1 ligand 1, clinical trial, and phase. The references of published studies and reviews were also examined for additional eligible trials. All the authors independently carried out the initial search, screened the title and abstract for potentially relevant studies, and identified trials as included, excluded, and uncertain. For uncertain studies, the full-texts were reviewed for the confirmation of eligibility. Any discrepancy was resolved by discussion and unanimous agreement.
Both inclusion and exclusion criteria were pre-specified. To be eligible, trials had to meet the following criteria: (a) population: prospective phase I, II, or III trials recruiting adult subjects (>18 years old) with cancer; for patients with reported PD-L1 expression status, the cutoff values for PD-L1 positivity/negativity and PD-L1 status were extracted from the original studies directly; (b) intervention: patients were treated with PD-1/PD-L1 inhibitor (avelumab, atezolizumab, cemiplimab, durvalumab, nivolumab, and pembrolizumab) monotherapy irrespective of dosage and duration in at least one arm; (c) outcomes: available information on ORR, PFS and OS. Trials published online before print were included, but meeting abstracts were excluded. In addition, studies were excluded if they were retrospective trials. Other publications, including review articles, basic research, case reports, letters, comments, correspondences, editorials, and cost effectiveness analyses were also excluded. When multiple publications of the same trial occurred, only the most recent, and/or most complete, reporting articles was selected. Any discrepancies were settled by discussion until unanimous agreement was reached. All the included articles represented unique studies.
Data extraction
For each study, the following items were exacted: name of study, first author and year of publication, clinical phase, line of treatment, cancer type, PD-L1 detection assay, name of the PD-1 or PD-L1 inhibitor, dosing schedule, number of patients recruited, median progression-free survival (PFS), median OS, median treatment duration (range), median follow up (range), number of patients for efficacy analysis, median time to response (range), median duration of response (range), number of complete response (CR), number of partial response (PR), number of objective response (OR), number of PD-L1 positive patients for efficacy analysis, number of CR in PD-L1 positive patients, number of PR in PD-L1 positive patients, number of OR in PD-L1 positive patients, number of PD-L1 negative patients for efficacy analysis, number of CR in PD-L1 negative patients, number of PR in PD-L1 negative patients, and number of OR in PD-L1 negative patients. All authors independently carried out the data extraction.
Risk of bias
To evaluate the methodological quality of eligible studies, the seven-item Jadad ranking system including randomization, double blinding, and the flow of recruited subjects (withdrawals and dropouts) were applied.5 As previously described, a controlled study could achieve a Jadad score of between 5 (optimal methodological quality) and 0 (poor methodological quality). Any disagreements were resolved by discussion and unanimous agreement.
Statistical analysis
The primary purpose of this study was to investigate the overall incidence and corresponding 95% confidence interval (CI) of objective response rates (ORRs) in cancer patients treated by PD-1/PD-L1 inhibitors. ORR referred to the percentage of patients whose tumor shrunk (partial response) or disappeared (complete response) after treatment. To calculate the incidence, the number of subjects for efficacy analysis and the number of responses were extracted from every study. We compared the tumor response in PD-L1 positive patients and PD-L1 negative patients by calculating the odds ratios in each trial. To investigate PFS and OS, we derived the hazard ratios (HRs) and their 95% CI from each trial, separately for PD-L1 positive patients and PD-L1 negative patients.
Statistical heterogeneity across studies was evaluated by Cochrane’s Q statistic. The I2 statistic was calculated to assess the extent of inconsistency contributable to the heterogeneity across different studies.6 The assumption of homogeneity was considered invalid for I2 > 25% and p < 0.05. Summary ORs and incidences were calculated using fixed-effects model or random-effects model depending on the heterogeneity. The heterogeneities of PFS and OS between PD-L1 positive patients and PD-L1 negative patients were assessed by an interaction test and expressed as P for interaction.
Potential publication bias was assessed by visual inspection of a funnel plot, and also evaluated using the tests of Egger et al. and Begg et al.7,8 Two-sided p < 0.05 were considered statistically significant.
Results
Search results
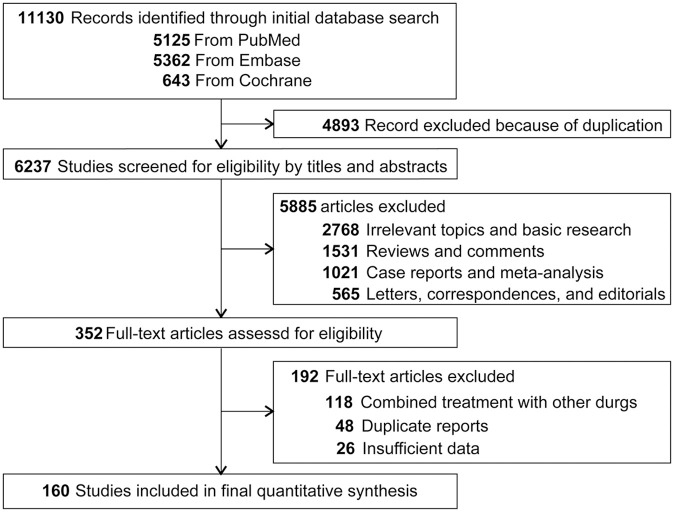
We identified a total of 11,130 potentially relevant publications from the initial search, including 5125 from PubMed, 5362 from Embase, and 643 from Cochrane; 4893 records were removed due to duplication. After screening of the titles and abstracts, 5885 articles did not meet our inclusion criteria. Additional eligibility assessing the full-texts of the remaining studies, 160 prospective clinical trials involving 28,304 cancer patients were included in the final analysis.9–168 Among these patients, 22,165 subjects were treated by PD-1/PD-L1 inhibitors, the rest 6139 patients were included in the control arms. A flow chart presenting the study selection is shown in Figure 1.
Figure 1.
Flow-chart diagram of selected trials included in this study.
Study characteristics
The baseline characteristics of the eligible trials included in this study were demonstrated in Supplemental Table S1. The median treatment duration of PD-1/PD-L1 inhibitors lasted between 1.4 months and 11.9 months, and median follow up ranged from 2.6 months to 49.9 months. The median time to response in immunotherapy is relatively stable, with most response occurred within 3 months after treatment (range, 1.4 months to 4.5 months). In some studies, several different types of tumors or different clinical phases were investigated and reported in one article. Accordingly, 185 arms of PD-1/PD-L1 blockade immunotherapy arms were found in the 160 eligible records. Among these 185 arms, the agents administrated were nivolumab (n = 68, 6403 patients), pembrolizumab (67, 8124), atezolizumab (22, 4094), avelumab (17, 1785), durvalumab (9, 1674), and cemiplimab (2, 85). The responses to PD-1/PD-L1 inhibitors were examined in lung cancer (n = 40, 7265 patients), melanoma (21, 3501), urothelial cancer (14, 3443), gastric or gastro-esophageal junction cancer (10, 1265), lymphoma (10, 757), head and neck cancer (9, 1166), renal cancer (9, 915), breast cancer (7, 652), ovarian cancer (6, 573), mesothelioma (5, 200), sarcoma (5, 154), and other types of cancers (49, 2274).
Of all the eligible 185 arms, 108 arms (58.38%) revealed or partly revealed the responses to immunotherapy based on PD-L1 expression status (Supplemental Table S2). In most cases, the cutoff value for PD-L1 positivity or negativity was that PD-L1 stained cells accounted for 1% of cancer cells, or cancer and immune cells, assayed by immunohistochemistry staining techniques. However, the thresholds were 5% in 17 studies,10,17–20,24,26,35,40,47,56,61,69,78,88,96,168 10% in 3 articles,31,127,159 25% in 5 studies,43–45,48,49 and 50% in 3 records.121,131,155 In addition, only PD-L1 positive cancer patients were recruited in 17 studies.19,24,50,110,123,125,128,132,136,137,145,146,152,155,158,161,164 In the present study, we extracted the PD-L1 expression status directly from the original records.
ORR
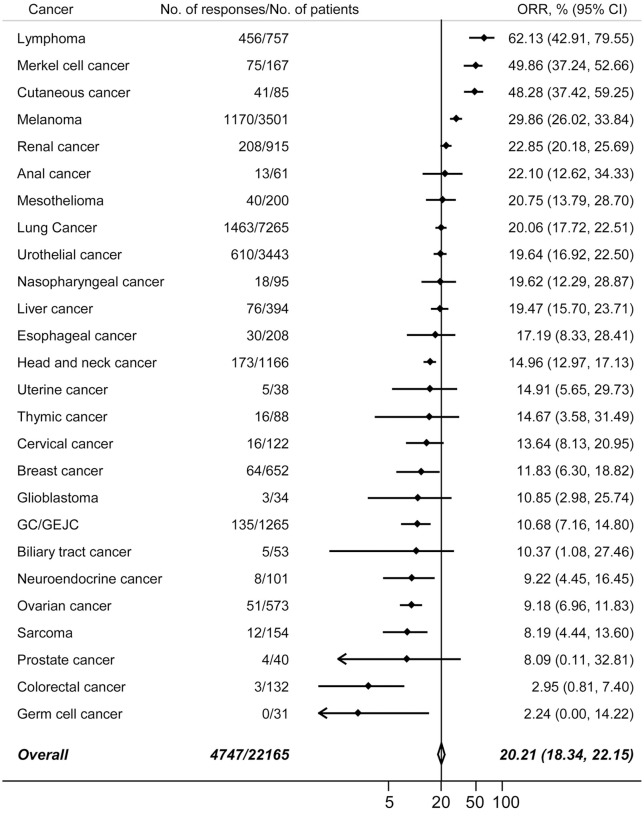
Overall, the eligible 160 studies recorded 4747 responses to immunotherapy. The summary ORRs in patients receiving PD-1/PD-L1 inhibitors was 20.21% (95% CI, 18.34–22.15%). 26 types of tumors were investigated in more than two trials. Next, we calculated the pooled ORRs in these cancers. The incidences of responses, in decreasing order, were shown in Figure 2. As expected, the overall response rates varied significantly by tumor types, with the highest anti-cancer activities were reported in carcinogen-induced tumors or malignancies driven by viral infections, such as classic Hodgkin’s lymphoma, desmoplastic melanoma, and the virally induced Merkel cell carcinoma of the skin. The second subgroup of tumors with relatively high ORRs were cancer with a relative high immunogenicity,1 such as melanoma and lung cancer, liver cancer and renal cancer. Some tumors, such as prostate cancer, colorectal cancer, and ovarian cancer, only had limited T-cell infiltration, and were considered immune excluded.12 Accordingly, the response rates in these tumors were relatively low.
Figure 2.
The pooled ORRs in 26 different types of cancers. Vertical line indicates the overall mean incidence of tumor responses calculated from all 22,165 patients in 160 eligible trials.
CI, confidence interval; GC/GEJC, gastric or gastro-esophageal junction cancer; ORR, objective response rate.
A total of 862 complete responses were observed in 19,418 cancer patients, and the overall incidence of CRs was 3.85% (95% CI, 3.06–4.73%). Moreover, the incidences of CR in 25 tumors are listed in Supplemental Figure S1. It was noted that the incidences were relatively low in most types of tumors. In addition, 3400 partial responses were reported and the pooled incidence of PR was 15.83% (95% CI, 14.36–17.35%). The incidences of partial responses from 25 tumors were presented in Supplemental Figure S2. The most frequent PRs were found in cutaneous cancer, Merkel cell cancer, and lymphoma.
Impact of clinicopathological characteristics on ORR
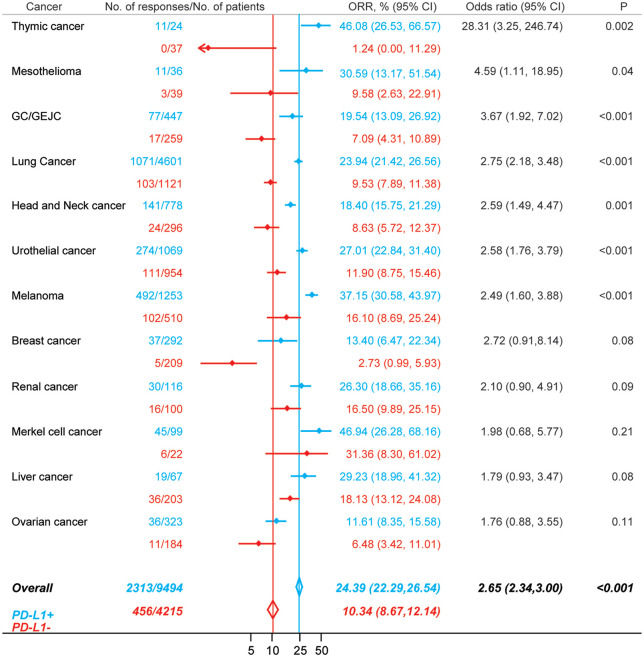
We further conducted the efficacy analysis based on PD-L1 expression status (Figure 3). Among 9494 patients that were PD-L1 positive, 2313 responses were observed (ORR, 24.39%; 95% CI, 22.29–26.54%); whereas in 4215 PD-L1 negative patients, 456 responses were recorded (ORR, 10.34%; 95% CI, 8.67–12.14%). Overall, there was a significant difference in ORRs between PD-L1 positive patients and PD-L1 negative patients (odds ratio, 2.65; 95% CI, 2.34–3.00; p < 0.001). We further examined the impact of PD-L1 expression status on tumor responses in 12 types of cancers (Figure 3). The magnitudes of efficacy of immunotherapy were greater for PD-L1 positive patients than for PD-L1 negative patients in all types of tumors. However, the differences were insignificant in breast cancer, renal cancer, Merkel cell cancer, liver cancer, and ovarian cancer.
Figure 3.
Comparison of the ORRs between patients that were PD-L1 positive (blue) and patients that were PD-L1 negative (red) in PD-1 or PD-L1 blockade monotherapy.
CI, confidence interval; GC/GEJC, gastric or gastro-esophageal junction cancer; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1.
Next, we investigated the incidences of CR and PR based on PD-L1 expression status, respectively. In total, 276 CRs were reported among 6904 PD-L1 positive cancer patients, and the overall incidence was 4.03% (95% CI, 2.86–5.40%). In contrast, only 84 CRs occurred in 2883 PD-L1 negative patients, and the incidence was 2.37% (95% CI, 1.52–3.40%). PD-L1 positive patients had a higher incidence of CRs compared with PD-L1 negative patients (odds ratio, 2.22; 95% CI, 1.69–2.91; p < 0.001) (Supplemental Figure S3). The pooled incidence of PR in PD-L1 positive patients was 18.48% (95% CI, 16.61–20.43%) as 1310 partial responses were discovered in 6845 subjects. By contrast, 224 PRs occurred in 2732 PD-L1 negative patients, and the incidence was 7.41% (95% CI, 5.91–9.05%). The overall incidence of PR in PD-L1 positive patients and PD-L1 negative patients was significantly different (odds ratio, 2.41; 95% CI, 2.02–2.86; p < 0.001) (Supplemental Figure S4).
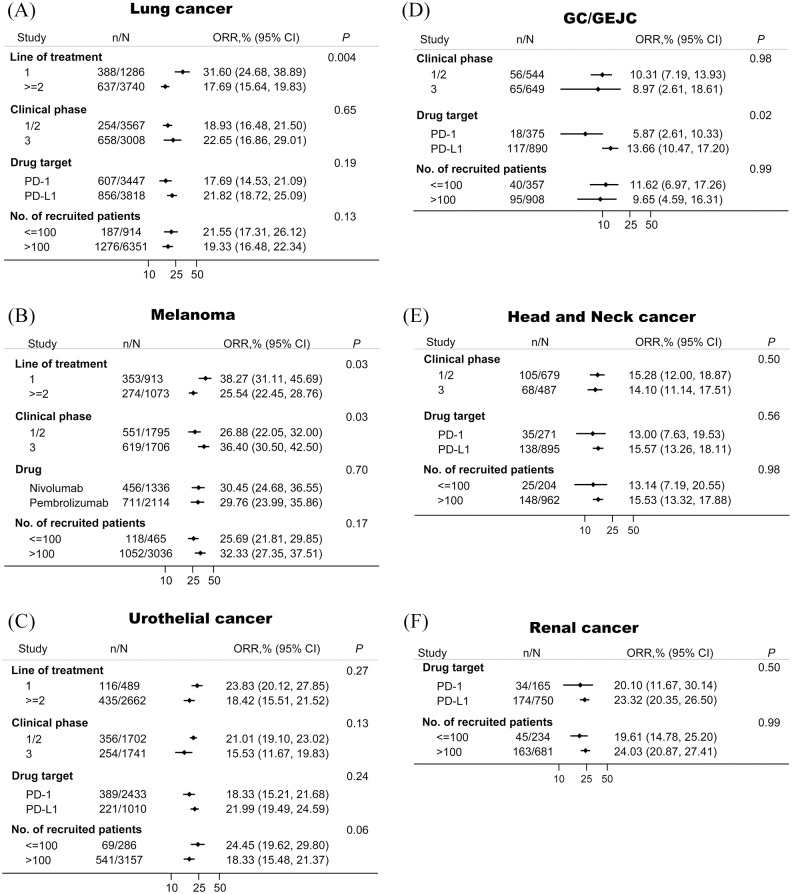
We also examined the association between tumor response and some other features including line of treatment, clinical phase, drug target, and numbers of recruited patients in several major tumors (Figure 4). Line of treatment was an important contributor to ORRs in lung cancer and melanoma, but not in urothelial cancer. In all cancers except melanoma, the ORRs in phase I/II trials were similar compared with the ORRs in phase III trials. In GC/GEJC, patients treated with PD-L1-targeted agents showed higher ORRs than those treated with PD-1-targeted agents. The impact of numbers of recruited patients on the efficacies was insignificantly in all examined types of tumors.
Figure 4.
The association between tumor responses and several major clinicopathological characteristics in lung cancer (A), melanoma (B), urothelial cancer (C), GC/GEJC (D), head and neck cancer (E), and renal cancer (F).
CI, confidence interval; GC/GEJC, gastric or gastro-esophageal junction cancer; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1.
Immunotherapy versus conventional treatment
Next, we compared the efficacies of PD-1/PD-L1 blockade immunotherapy versus conventional treatment in patients that were PD-L1 positive and PD-L1 negative (Figure 5). In total, 26 randomized, controlled trials (RCTs) including 13,899 patients, were eligible (Supplemental Table S3). Among them, 21 studies were phase III RCTs, four studies were phase II RCT, and one study was phase II/III RCT. In all, 12 studies were conducted in lung cancer, 4 in melanoma, 3 in GC/GEJC, 2 each in urothelial cancer, renal cancer, and head and neck cancer, and 1 in colorectal cancer. All studies were performed in solid tumors, and 6993 (50%) of 13,899 patients had lung cancer. Patients in the intervention arms received nivolumab in nine studies, pembrolizumab in eight studies, atezolizumab in six studies, avelumab in two studies, and durvalumab in one study. The methodological qualities of the eligible trials were generally moderate to good. Randomized treatment allocation sequence generated in all trials. The main issue affecting quality was lack of blinding.
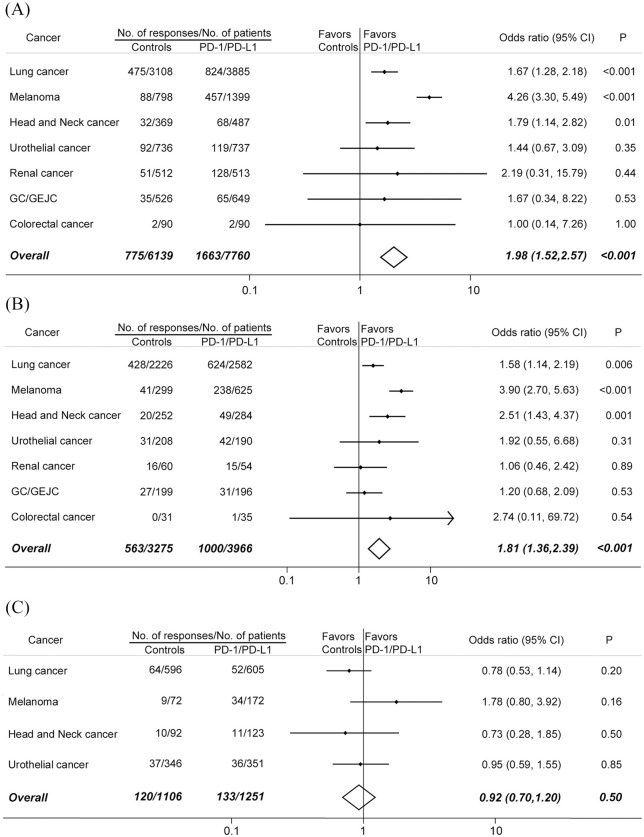
Figure 5.
Comparison of the ORRs between PD-1 or PD-L1 blockade monotherapy and conventional therapy in (A) all recruited patients, (B) patients who were PD-L1 positive, and (C) patients who were PD-L1 negative.
CI, confidence interval; GC/GEJC, gastric or gastro-esophageal junction cancer; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1.
Among 7760 patients treated with PD-1/PD-L1 inhibitors, 1663 tumor responses occurred, whereas in 6139 patients treated with conventional agents, 775 responses were recorded. The difference was significant (odds ratio, 1.98; 95% CI, 1.52–2.57; p < 0.001). Comparisons between immunotherapy and controls in seven types of tumors were shown in Figure 5A.
For PD-L1 positive patients,1000 responses were reported from 3966 patients in the PD-1/PD-L1 blockade arms; 563 responses occurred in 3275 patients in the control arms (Figure 5B). More PD-L1 positive patients responded to PD-1/PD-L1 inhibitors than to conventional agents (odds ratio, 1.81; 95% CI, 1.36–2.39; p < 0.001). On the other hand, 2357 patient who had PD-L1 negative disease from 10 RCTs were included in our analysis. PD-1/PD-L1 blockade immunotherapy did not increase the tumor responses compared with conventional treatment (odds ratio, 0.92; 95% CI, 0.70–1.20; p = 0.50) (Figure 5C).
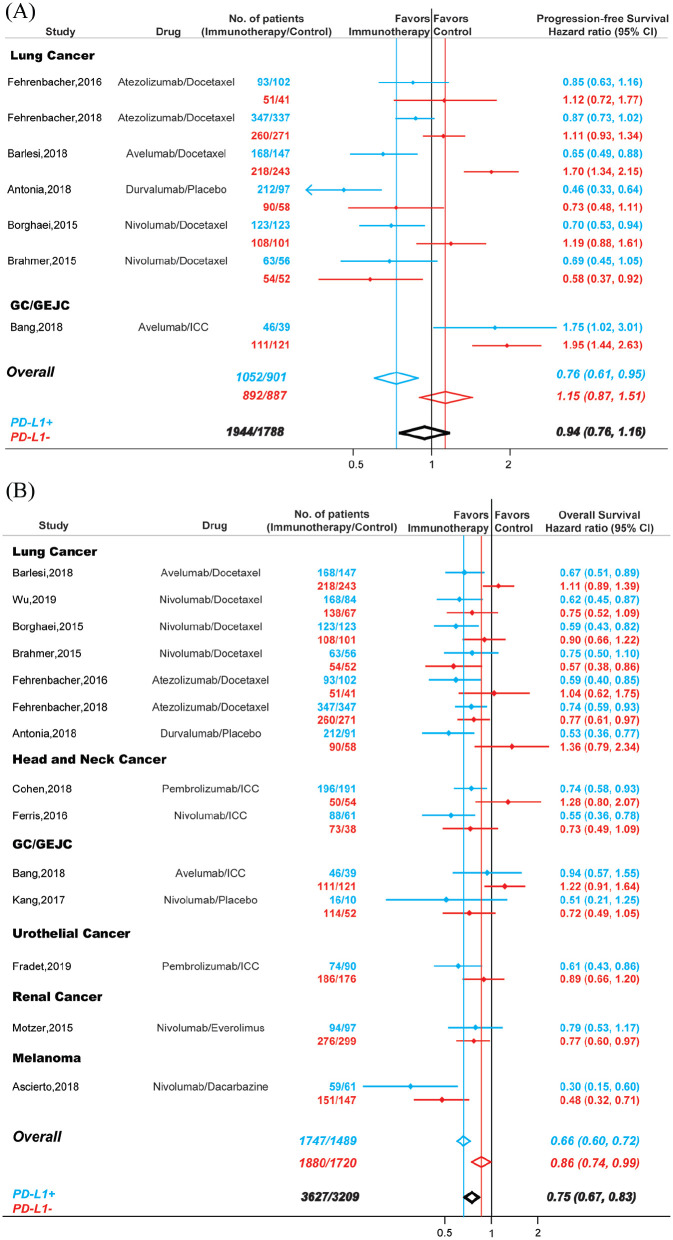
It was well known that some non-responders also derived significant benefit from immunotherapy.1,2 Accordingly, we evaluated PFS and OS in patients treated with PD-1/PD-L1 inhibitors and explored their association with PD-L1 expression status (Figure 6). Seven RCTs with 1944 PD-L1 positive patients and 1788 PD-L1 negative patients were included in PFS analysis (Figure 6A). Compared with controls, PD-1/PD-L1 blockade immunotherapy could significantly improved PFS in patients that were PD-L1 positive (HR,0.76; 95% CI, 0.61–0.95), but not in patients that were PD-L1 negative (HR, 1.15; 95% CI, 0.87–1.51), and the difference between these two groups were statistically significant (Pinteraction < 0.001). Overall, the PFS values were similar in patients treated with immunotherapy and patients treated with conventional therapy (HR, 0.94; 95% CI, 0.76–1.16).
Figure 6.
Comparison of PFS (A) and OS (B) between PD-1 or PD-L1 blockade monotherapy and conventional therapy. Blue, patients that were PD-L1 positive; red, patients that were PD-L1 negative; black, all recruited patients.
CI, confidence interval; GC/GEJC, gastric or gastro-esophageal junction cancer; PFS, progression-free survival; ORR, objective response rate; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1.
For OS, 14 studies including 3627 PD-L1 positive patient and 3209 PD-L1 negative patients were investigated (Figure 6B). Compared with conventional therapy, PD-1/PD-L1 blockade immunotherapy decreased the risk of death by 25% in cancer patients (HR, 0.75; 95% CI, 0.67–0.83). However, the OS in patients that were PD-L1 positive (HR, 0.66; 95% CI, 0.60–0.72) and patients that were PD-L1 negative (HR, 0.86; 95% CI, 0.74–0.99) were significantly different (Pinteraction < 0.001).
Publication bias
Potential publication biases were assessed by Begg’s funnel plots and Begg’s rank correlation. Visual inspection of the Begg’s funnel plot did not identify substantial asymmetry in all the analysis conduct in this study. The Begg’s rank correlation test also indicated no evidence of publication bias.
Discussion
To our knowledge, this is the largest and most comprehensive meta-analysis investigating the clinic benefit in cancer patients treated with PD-1/PD-L1 blockade monotherapy. Previous studies included fewer trials and focused mainly on some specific cancers, such as non-small cell lung cancer,169 advanced urothelial carcinoma,170 and malignant melanoma.171 Here, with published data from over 28,000 subjects in 160 perspective clinical trials, we report a meta-analysis evaluating the association between PD-1/PD-L1 blockade monotherapy and tumor response, PFS, and OS in cancer patients. Such a global overview of clinical efficacies is important, as the results constitute a critical reference for clinicians, drug developers, and basic scientists, and complementary information in drafting the clinical practice guidelines.
The development of immunotherapy has revolutionized the treatment of cancer and are increasingly being used in earlier disease settings and in combination with other therapies.172,173 A key unmet need in immunotherapy is the development of predictive biomarkers, which can identify patients that are likely to respond to PD-1/PD-L1 inhibitors and therefore reduce exposure and risk of toxicities for those patients with little potential for benefit of response. Given the high-cost of immunotherapy, in the era of value-based cancer care this becomes even more important. However, predictive biomarkers to PD-1/PD-L1 blockade immunotherapy are difficult to develop. The potential markers are usually functional targets rather than stable oncogenic targets; hence the expression is context-dependent and transient, and can be influenced by various factors in the microenvironment. In addition, clinical issues in evaluating any reliable biomarkers are often associated with some inherent characteristics of immunotherapy such as limitations to evaluate tumor responses with standard imaging methods, clinical response and survival benefit are not necessarily related.
Because PD-L1 participates in suppressing immunogenicity and is a direct or indirect target of PD-1/PD-L1 antibodies, it was the first and most popular biomarker routinely used for cancer patients treated with immunotherapy in clinical trials. Indeed, accumulating evidences has revealed that PD-L1 expression was associated with the efficacy of immunotherapy and clinical outcomes.2,173 Moreover, several companion IHC assays for PD-L1 expression has been approved by FDA (22C3 for pembrolizumab; SP142 for atezolizumab).174 However, some major pitfalls still remain for PD-L1 expression evaluation such as different antibodies (22C3 for pembrolizumab, 28-8 for nivolumab, SP142 for atezolizumab, and SP263 for durvalumab) and various labelling threshold for positivity/negativity. In 2017, the Blueprint PD-L1 IHC Assay Comparison Project showed that these assays had different performances: PD-L1 expression were comparable for 22C3, 28-8, and SP263, but not for SP142.175 Furthermore, despite similar analytical performance of 28-8, 22C3, and SP263, interchanging assays and thresholds still led to “misclassification” of PD-L1 status for some patients. Linear epitope mapping experiments revealed different binding features for these four PD-L1 antibody clones. It could cause particular staining patterns depending on PD-L1 conformation or isoform expression. Besides analytical consideration, other issues exist for PD-L1 evaluation. For example, biopsies cannot represent the entire tumor due to spatial heterogeneity. In addition, PD-L1 expression was regulated at transcriptional, post-transcriptional, and protein levels.1,172 In clinical practice, conventional treatments such as chemotherapy and radiotherapy are also considered as potential regulators of PD-L1 expression as well as the antitumor immunity.176 In the present study, considering the original researchers knows their trials better than anybody else, we extracted and illustrated the IHC assay and cutoff values from every eligible trial, and classified the PD-L1 positivity and negativity based on the results from the original manuscripts. Hence, our study is subject to any biases or errors of the original investigators, and the results are generalizable only to the patient groups eligible for these trials.
Given the limitations of PD-L1 expression evaluation, new strategies are developing to select stable and reliable genomic biomarkers. Research in this field mostly focused on TMB as a surrogate marker for tumor immunogenicity. For most tumors, it is reported that higher TMB was association with improved OS in patients receiving PD-1/PD-L1 inhibitors.177 Moreover, a study across 27 tumors revealed that a correlation between TMB and immunotherapy response rate by linear regression analysis.178 However, TMB has not been approved as a companion biomarker because the ideal quantification methods and threshold values have not been defined for clinical application (i.e. sequencing approach, tumor tissue or blood TMB, whole-exome sequencing or selected gene panel, and sequencing depth). On the other hand, tumor with MSI showed impressive clinical benefit to immunotherapy.179 In addition, mutations in DNA mismatch repair (MMR) genes such as MSH2, MSH6, MLH1, and PMS2 were also associated with durable response to PD-1/PD-L1 inhibitors.180 These studies led to the first tissue-agnostic approval for PD-1/PD-L1 blockade treatment across tumors with dMMR/MSI-H.181 However, it should be note that the frequency of dMMR/MSI-H in tumors not within colorectal, endometrial, or gastric cancer is only 0.8% based on one study including 11,139 cancer samples from 39 cancer types.182 Accordingly, all the available single biomarkers showed some limitations in the real-world clinical application. Currently, it is believed that combinable biomarkers and/or algorithms using multiplex ways and the support of artificial intelligence will be more successful.174 For example, in OAK trial,183 a so-called “Teff signature” (IFN-γ, PD-L1, CXCL9) was applied as a surrogate for pre-existing immunity. This signature was correlated with PD-L1 expression but was more sensitive than PD-L1 in predicting OS from atezolizumab.
Consist with previous findings,2,169,184 our analysis here showed that there was a statistically significant difference between PD-L1 positive and PD-L1 negative patients in terms of ORR, PFS, and OS. PD-L1 significantly increased the chance of tumor response in melanoma, urothelial cancer, head and neck cancer, lung cancer, GC/GEJC, mesothelioma, and thymic cancer. In addition, compared with conventional treatment, the ORRs were significantly higher in PD-L1 positive patients but not in PD-L1 negative patients only in lung cancer, melanoma, and head and neck cancer. These results suggested PD-L1 could be a valuable predictive biomarker in selected tumors. However, about 10% of cancer patients who were PD-L1 negative showed tumor responses, and PD-1/PD-L1 monotherapy could decreased the risk of death by 14% for PD-L1 negative patients. These data suggested that PD-L1 expression status neither guaranteed nor precluded response to PD-1/PD-L1 monotherapy in all cases. Recently, Davis et al. also evaluated the role of PD-L1 expression as a predictive biomarker in 45 FDA approvals of immune checkpoint inhibitors across 15 tumor types.184 It should be noted that there were several differences between these two studies. First, our primary aim was to assess the ORRs in cancer immunotherapy. Many clinicopathological characteristics could impact the tumor responses including tumor type, PD-L1 expression status, clinical phase, line of treatments. We further examined the association between ORRs and these factors in our study, while Davis et al. focused mainly on the predictive value of PD-L1 expression because nine FDA approvals linked to a specific PD-L1 threshold and companion diagnostic biomarker. Second, our study included 160 perspective clinical trials with over 28,000 patients. With the increased statistical power, our meta-analysis was the most up-to-date and comprehensive study, and should be more reliable and solid. In addition, the assessment of the response to immunotherapy is insufficient regarding clinical value, it has been proven that patients treated with immunotherapy have a significant benefit even if they do not respond to treatment.2 Accordingly, we also examined PFS and OS in both PD-L1 positive patients and PD-L1 negative patients. Our results revealed that PD-1/PD-L1 blockade monotherapy significantly decrease the risk of death by 34% for PD-L1 positive patients and by 14% for PD-L1 negative patients. It should be acknowledged that the survival benefit between cancer patients who were PD-L1 positive and those who were PD-L1 negative were significantly different.
The molecular basis underlying tumor resistance to immune checkpoint inhibitors is not yet clear; it may be due partly to inadequate cancer specific T cells function,185 insufficient anti-cancer T cell generation,186 and development of T-cell memory impairments.186 Lack of sufficient or suitable neoantigens, or impaired neoantigen processing or presentation of neoantigens, can lead to dysfunctional impairments during the development of cancer-reactive T cells.187 It is well established that efficacious anti-cancer response followed by PD-1/PD-L1 inhibitors need clonal-proliferation and reactivation of antigen-experienced T cells in the tumor micro-environments, and eventually secret the cytolytic effector to kill the cells showing tumor-associated antigen.186,187 Accordingly, it seems more critical to understand whether the PD-1/PD-L1 pathway was active rather than focusing solely on the expression of PD-L1 during cancer immunotherapy. Thereby, predicting tumor responses to PD-1/PD-L1 blockade immunotherapy remains the greatest challenge, and considerable efforts should be made to profile the complex and dynamic factors governing the strength and duration of immune response in the immunotherapy, making treatment decisions on a personalized basis.
From the standpoint of patient counseling, several results derived from this meta-analysis are important. Although approximately one-fifth of patients respond to PD-1/PD-L1 monotherapy in clinical trials, the tumor responses vary significantly across different cancers and PD-L1 expression. These numbers could be critical to share with patients before they began PD-1/PD-L1 blockade immunotherapy. In addition, PD-L1 expression status can inform the proper selection of patients who have a significantly increased chance of tumor responses in lung cancer, melanoma, and head and neck cancer.
This study is restricted by some limitations. First, this meta-analysis relied on published results rather than on individual patients’ data. Accordingly, we cannot exclude the fact that other clinicopathological characteristics can affect the response and influence our results. Second, the majority of studies are phase I/II trials, which add heterogeneity to our analysis. We adjusted this heterogeneity by conducting random-effects models to achieve the overall ORRs. Furthermore, we examined the ORRs in different phases in several major tumors. In most examined cancers, ORRs in phase III are similar compared with ORRs in phase I/II. Even so, it might underestimate the real incidences of tumor responses given that trials with limited numbers of patients received disproportional weight in calculation. Third, some included studies were open-label trials. Even for the double-blinded RCTs, skillful clinicians can identify the tumor responses induced by PD-1/PD-L1 antibodies, which may cause potential bias. Fourth, these included studies are conducted at various medical centers by different researches, and may have some subjectivities in recording clinical outcomes. Our analysis is subject to any errors or biases of the original researchers, and the conclusions are generalizable only to recruited patients included in these trials.
Conclusion
With 28,304 cancer patients from 160 perspective trials, our comprehensive analysis revealed the clinical benefit to PD-1/PD-L1 monotherapy in cancer patients. This global overview can be used as a reference and may guide clinical practice and drug development.
Supplemental Material
Supplemental material, supplement for Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials by Bin Zhao, Hong Zhao and Jiaxin Zhao in Therapeutic Advances in Medical Oncology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by National Natural Science Foundation of China (No. 31571417), Postdoctoral Science Foundation of China (No. 2018M641862 and No. 2019T120282), and Wenzhou Municipal Science and Technology Bureau (No. Y20180086). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Bin Zhao  https://orcid.org/0000-0002-5990-1773
https://orcid.org/0000-0002-5990-1773
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bin Zhao, The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, 109 Xueyuan West Rd, Wenzhou, 325035, China.
Hong Zhao, The Cancer Center of the Fifth Affiliated Hospital, Sun Yat-Sen University, Zhuhai, China.
Jiaxin Zhao, The Second Affiliated Hospital & Yuying Children’s Hospital, Wenzhou Medical University, Wenzhou, China; Zhuhai People’s Hospital, Zhuhai Hospital Affiliated With Jinan University, Zhuhai, China.
References
- 1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 2018; 362: k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17: e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arora S, Velichinskii R, Lesh RW, et al. Existing and emerging biomarkers for immune checkpoint immunotherapy in solid tumors. Adv Ther 2019; 36: 2638–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 6. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 9. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol 2018; 29: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 11. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019; 5: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019; 20: 849–861. [DOI] [PubMed] [Google Scholar]
- 13. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 14. Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol 2018; 13: 1156–1170. [DOI] [PubMed] [Google Scholar]
- 15. Horn L, Gettinger SN, Gordon MS, et al. Safety and clinical activity of atezolizumab monotherapy in metastatic non-small-cell lung cancer: final results from a phase I study. Eur J Cancer 2018; 101: 201–209. [DOI] [PubMed] [Google Scholar]
- 16. Liu JF, Gordon M, Veneris J, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol 2019; 154: 314–322. [DOI] [PubMed] [Google Scholar]
- 17. McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016; 34: 833–842. [DOI] [PubMed] [Google Scholar]
- 18. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018; 24: 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol 2017; 35: 2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petrylak DP, Powles T, Bellmunt J, et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol 2018; 4: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 22. Pujol JL, Greillier L, Audigier-Valette C, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol 2019; 14: 903–913. [DOI] [PubMed] [Google Scholar]
- 23. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spigel DR, Chaft JE, Gettinger S, et al. FIR: efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J Thorac Oncol 2018; 13: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol 2019; 76: 73–81. [DOI] [PubMed] [Google Scholar]
- 26. Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol 2017; 35: 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018; 29: 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018; 19: 1468–1479. [DOI] [PubMed] [Google Scholar]
- 29. Chung HC, Arkenau HT, Lee J, et al. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer 2019; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Angelo SP, Russell J, Lebbe C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol 2018; 4: e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat 2018; 167: 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Disis ML, Taylor MH, Kelly K, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 2019; 5: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doi T, Iwasa S, Muro K, et al. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer. Epub ahead of print 4 December 2018. DOI: 10.1007/s10120-018-0903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017; 18: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hassan R, Thomas A, Nemunaitis JJ, et al. Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 2019; 5: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heery CR, O’Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017; 18: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ⩾1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keilholz U, Mehnert JM, Bauer S, et al. Avelumab in patients with previously treated metastatic melanoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer 2019; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mego M, Svetlovska D, Chovanec M, et al. Phase II study of avelumab in multiple relapsed/refractory germ cell cancer. Invest New Drugs 2019; 37: 748–754. [DOI] [PubMed] [Google Scholar]
- 40. Le Tourneau C, Hoimes C, Zarwan C, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer 2018; 6: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379: 341–351. [DOI] [PubMed] [Google Scholar]
- 42. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–2350. [DOI] [PubMed] [Google Scholar]
- 43. Antonia SJ, Balmanoukian A, Brahmer J, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol 2019; 14: 1794–1806. [DOI] [PubMed] [Google Scholar]
- 44. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016; 34: 3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Necchi A, Giannatempo P, Raggi D, et al. An open-label randomized phase 2 study of durvalumab alone or in combination with tremelimumab in patients with advanced germ cell tumors (APACHE): results from the first planned interim analysis. Eur Urol 2019; 75: 201–203. [DOI] [PubMed] [Google Scholar]
- 47. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 2017; 3: e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Segal NH, Ou SI, Balmanoukian A, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer 2019; 109: 154–161. [DOI] [PubMed] [Google Scholar]
- 49. Siu LL, Even C, Mesia R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019; 5: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ⩾25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019; 107: 142–152. [DOI] [PubMed] [Google Scholar]
- 51. Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018; 24: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol 2019; 37: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016; 17: 883–895. [DOI] [PubMed] [Google Scholar]
- 55. Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 2018; 36: 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019; 5: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ben-Ami E, Barysauskas CM, Solomon S, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: results of a phase 2 study. Cancer 2017; 123: 3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carneiro BA, Konda B, Costa RB, et al. Nivolumab in metastatic adrenocortical carcinoma: results of a phase II trial. J Clin Endocrinol Metab 2019; 104: 6193–6200. [DOI] [PubMed] [Google Scholar]
- 63. D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol 2018; 19: 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375: 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Flippot R, Dalban C, Laguerre B, et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: results of the GETUG-AFU 26 NIVOREN multicenter phase II study. J Clin Oncol 2019; 37: 2008–2016. [DOI] [PubMed] [Google Scholar]
- 67. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378: 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fujimoto D, Yomota M, Sekine A, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer 2019; 134: 274–278. [DOI] [PubMed] [Google Scholar]
- 69. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015; 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015; 33: 4015–4022. [DOI] [PubMed] [Google Scholar]
- 72. Hida T, Nishio M, Nogami N, et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci 2017; 108: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1480–1492. [DOI] [PubMed] [Google Scholar]
- 74. Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 2018; 36: 2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 76. Katsuya Y, Horinouchi H, Seto T, et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019; 113: 78–86. [DOI] [PubMed] [Google Scholar]
- 77. Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017; 18: 631–639. [DOI] [PubMed] [Google Scholar]
- 78. Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018; 36: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee JS, Lee KH, Cho EK, et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer 2018; 122: 234–242. [DOI] [PubMed] [Google Scholar]
- 80. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016; 34: 2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018; 19: 672–681. [DOI] [PubMed] [Google Scholar]
- 82. Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic phase 2 consortium (NCI-9742). J Clin Oncol 2018; 36: 1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ma Y, Fang W, Zhang Y, et al. A phase I/II open-label study of nivolumab in previously treated advanced or recurrent nasopharyngeal carcinoma and other solid tumors. Oncologist 2019; 24: 891–e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maruyama D, Hatake K, Kinoshita T, et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci 2017; 108: 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015; 33: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morris VK, Salem ME, Nimeiri H, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2015; 33: 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open 2016; 1: e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Okada M, Kijima T. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase 2 study in malignant pleural mesothelioma (MERIT). J Clin Cancer Res 2019; 25: 5485–5492. [DOI] [PubMed] [Google Scholar]
- 91. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol 2018; 20: 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ornstein MC, Wood LS, Hobbs BP, et al. A phase II trial of intermittent nivolumab in patients with metastatic renal cell carcinoma (mRCC) who have received prior anti-angiogenic therapy. J Immunother Cancer 2019; 7: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol 2018; 13: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 95. Ramchandren R, Domingo-Domenech E, Rueda A, et al. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 study. J Clin Oncol 2019; 37: 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019; 20: 239–253. [DOI] [PubMed] [Google Scholar]
- 98. Sharma P, Siefker-Radtke A, de Braud F, et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol 2019; 37: 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017; 18: 312–322. [DOI] [PubMed] [Google Scholar]
- 100. Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 2017; 12: 1798–1805. [DOI] [PubMed] [Google Scholar]
- 101. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 2019; 4: 611–621. [DOI] [PubMed] [Google Scholar]
- 104. Voorwerk L, Slagter M, Horlings HM. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019; 25: 920–928. [DOI] [PubMed] [Google Scholar]
- 105. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013; 31: 4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res 2016; 4: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14: 867–875. [DOI] [PubMed] [Google Scholar]
- 108. Yamamoto N, Nokihara H, Yamada Y, et al. Phase I study of nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs 2017; 35: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a phase II study. Cancer Sci 2017; 108: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019; 30: 405–411. [DOI] [PubMed] [Google Scholar]
- 111. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019; 30: 397–404. [DOI] [PubMed] [Google Scholar]
- 112. Adra N, Einhorn LH, Althouse SK, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier Cancer Research Network Study GU14-206. Ann Oncol 2018; 29: 209–214. [DOI] [PubMed] [Google Scholar]
- 113. Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017; 18: 623–630. [DOI] [PubMed] [Google Scholar]
- 114. Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34: 3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Armand P, Chen YB, Redd RA, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood 2019; 134: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 117. Barta SK, Zain J, MacFarlane AW, et al. Phase II study of the PD-1 inhibitor pembrolizumab for the treatment of relapsed or refractory mature T-cell lymphoma. Clin Lymphoma Myeloma Leuk 2019; 19: 356–364.e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol 2017; 35: 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: a randomised clinical trial. Eur J Cancer 2018; 101: 236–243. [DOI] [PubMed] [Google Scholar]
- 120. Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017; 35: 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol. Epub ahead of print 15 June 2018. DOI: 10.1200/jco.2017.77.3184. [DOI] [PubMed] [Google Scholar]
- 122. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2019; 37: 1470–1478. [DOI] [PubMed] [Google Scholar]
- 123. Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol 2018; 41: 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019; 393: 156–167. [DOI] [PubMed] [Google Scholar]
- 125. Doi T, Piha-Paul SA, Jalal SI, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 2018; 36: 61–67. [DOI] [PubMed] [Google Scholar]
- 126. Feun LG, Li YY, Wu C, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 2019; 125: 3603–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019; 30: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol 2017; 35: 4035–4041. [DOI] [PubMed] [Google Scholar]
- 129. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4: e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol 2018; 13: 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018; 19: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017; 86: 37–45. [DOI] [PubMed] [Google Scholar]
- 134. Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019; 30: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 2018; 29: 1807–1813. [DOI] [PubMed] [Google Scholar]
- 136. Herbst RS, Baas P, Perez-Gracia JL, et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol 2019; 30: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol 2017; 35: 4050–4056. [DOI] [PubMed] [Google Scholar]
- 138. Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019; 25: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol 2019; 37: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Leighl NB, Hellmann MD, Hui R, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med 2019; 7: 347–357. [DOI] [PubMed] [Google Scholar]
- 141. Levy BP, Giaccone G, Besse B, et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur J Cancer 2019; 108: 120–128. [DOI] [PubMed] [Google Scholar]
- 142. Long GV, Dummer R, Hamid O, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 2019; 20: 1083–1097. [DOI] [PubMed] [Google Scholar]
- 143. Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase 2 KEYNOTE-100 study. Ann Oncol 2019; 30: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 144. Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018; 119: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 146. Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016; 17: 717–726. [DOI] [PubMed] [Google Scholar]
- 147. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 2016; 34: 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol 2019; 37: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nishio M, Takahashi T, Yoshioka H, et al. KEYNOTE-025: phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1-positive advanced non-small-cell lung cancer. Cancer Sci 2019; 110: 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ott PA, Piha-Paul SA, Munster P, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol 2017; 28: 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 2017; 35: 3823–3829. [DOI] [PubMed] [Google Scholar]
- 152. Ott PA, Bang YJ, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol 2017; 35: 2535–2541. [DOI] [PubMed] [Google Scholar]
- 153. Ott PA, Bang YJ, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 2019; 37: 318–327. [DOI] [PubMed] [Google Scholar]
- 154. Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017; 18: 212–220. [DOI] [PubMed] [Google Scholar]
- 155. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37: 537–546. [DOI] [PubMed] [Google Scholar]
- 156. Ribrag V, Avigan DE, Green DJ, et al. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br J Haematol 2019; 186: e41–e44. [DOI] [PubMed] [Google Scholar]
- 157. Rugo HS, Delord JP, Im SA. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res 2018; 24: 2804–2811. [DOI] [PubMed] [Google Scholar]
- 158. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17: 956–965. [DOI] [PubMed] [Google Scholar]
- 159. Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol 2019; 5: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shimizu T, Seto T, Hirai F, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs 2016; 34: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018; 392: 123–133. [DOI] [PubMed] [Google Scholar]
- 162. Si L, Zhang X, Shu Y, et al. A phase Ib study of pembrolizumab as second-line therapy for chinese patients with advanced or metastatic melanoma (KEYNOTE-151). Transl Oncol 2019; 12: 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017; 18: 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Varga A, Piha-Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol 2019; 152: 243–250. [DOI] [PubMed] [Google Scholar]
- 165. Yamazaki N, Takenouchi T, Fujimoto M, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol 2017; 79: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 167. Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017; 130: 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018; 19: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wang S, Hao J, Wang H, et al. Efficacy and safety of immune checkpoint inhibitors in non-small cell lung cancer. Oncoimmunology 2018; 7: e1457600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ghate K, Amir E, Kuksis M, et al. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: a meta-analysis. Cancer Treat Rev 2019; 76: 51–56. [DOI] [PubMed] [Google Scholar]
- 171. Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Invest Dermatol 2017; 10: 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 2018; 48: 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kythreotou A, Siddique A, Mauri FA, et al. PD-L1. J Clin Pathol 2018; 71: 189–194. [DOI] [PubMed] [Google Scholar]
- 174. Bellesoeur A, Torossian N, Amigorena S, et al. Advances in theranostic biomarkers for tumor immunotherapy. Curr Opin Chem Biol 2020; 56: 79–90. [DOI] [PubMed] [Google Scholar]
- 175. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 2017; 12: 208–222. [DOI] [PubMed] [Google Scholar]
- 176. Melosky B, Chu Q, Juergens RA, et al. Breaking the biomarker code: PD-L1 expression and checkpoint inhibition in advanced NSCLC. Cancer Treat Rev 2018; 65: 65–77. [DOI] [PubMed] [Google Scholar]
- 177. Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019; 51: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Le DT, Durham JN. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 2016; 22: 813–820. [DOI] [PubMed] [Google Scholar]
- 182. Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017; 2017: 10.1200/PO.17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019; 7: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med 2005; 201: 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. O’Donnell JS, Long GV, Scolyer RA, et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017; 52: 71–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplement for Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials by Bin Zhao, Hong Zhao and Jiaxin Zhao in Therapeutic Advances in Medical Oncology