Abstract
Aims:
Gastric cancer (GC) is the third leading cause of cancer death worldwide, but the burden of disease is not distributed evenly. GC screening routinely occurs in some high-incidence regions/countries and is generally cost-effective, which is attributed largely to the associated GC mortality reduction. In regions of low–intermediate incidence, less is known about the outcomes of GC screening and gastric precancer surveillance, including cost-effectiveness, since there are no comparative clinical studies. Decision analytic studies are informative in such instances where logistical limitations preclude “gold standard” study designs. We therefore aimed to conduct a systematic review of decision model analyses focused on endoscopic GC screening or precancer surveillance.
Methods:
We identified decision model analyses, including cost effectiveness and cost utility studies, of GC screening or preneoplasia surveillance. At minimum, articles were evaluated for: study country; analytic design; population and health states; time horizon; model assumptions; outcomes; threshold value(s) for “cost-effective” determination; and sensitivity analyses. Quality appraisal was performed using a modified Drummond’s analytic scoring system. Data sources were PubMed, Web of Science, Embase, and the Cochrane Library
Results:
We identified 17 studies (8 screening, 4 surveillance, and 5 screening and surveillance) that met full inclusion criteria. Endoscopic screening in countries of high GC incidence was cost-effective across all studies; targeted screening of high-risk populations within otherwise low-intermediate incidence countries was also generally cost-effective. Surveillance of gastric precancer, including atrophic gastritis or gastric intestinal metaplasia, was generally cost-effective. Most studies had high appraisal scores, with 4 (24%) studies achieving perfect scores on the Drummond scale.
Conclusion:
Decision model analyses offer a unique mechanism with which to efficiently explore the cost benefit of various prevention and early detection strategies. Based on this comprehensive systematic review, upper endoscopy for GC screening and gastric precancer surveillance might be cost-effective depending on the population and protocol. Focused efforts are especially needed not only to define the optimal approach, but also to define the populations within otherwise low-intermediate regions/countries who might benefit most.
Keywords: cost effective analysis, cost utility analysis, decision model analyses, gastric cancer, screening, surveillance, systematic review
Introduction
As the fifth most common cancer and the third leading cause of cancer-related death worldwide, gastric cancer (GC) remains a major health burden, with an estimated 1 million new cases and 780,000 related deaths occurring in 2018 alone.1–5 There is marked global variation in disease incidence, with Asian-Pacific countries accounting for approximately 50% of all new cases, followed by Central/Latin American and Eastern European countries. The United States (US) is considered a low–intermediate incidence country with 27,510 new GC cases diagnosed in 2019.2 Even though approximately 10,000 more cases of GC occur annually compared with esophageal cancer, screening occurs for esophageal cancer in selected US populations but not for GC, even among identifiable high-risk populations. Indeed, certain populations, such as non-white racial/ethnic minorities and certain immigrant groups, experience a disproportionately and significantly higher burden of GC, although this is not well recognized.3,4
In countries with established GC screening protocols, particularly Japan and South Korea, at least 60% of all GCs are diagnosed in a stage where resection can be curative.5,6 By contrast, in the US, where screening does not occur regularly, GC is more often diagnosed at advanced stages when there are limited therapeutic options, as reflected in the dismal <32% 5-year overall survival. GC, especially when diagnosed in a non-curative stage, incurs not only massive personal and healthcare costs, but also societal costs related to lost life and productivity.7
Generally speaking, intestinal-type noncardia GC is the most common form of GC. The strongest known risk factor for intestinal-type noncardia GC is chronic Helicobacter pylori infection, and approximately 89% of all non-cardia GC has been attributed at least in part to H. pylori infection.8 Chronic H. pylori gastritis can progress over time to atrophic gastritis, gastric intestinal metaplasia (GIM), and, in a small proportion of people, to gastric neoplasia, including dysplasia and cancer. GIM is generally considered to be the first irreversible histopathological change, and is associated with a baseline 0.16% annual risk of incident GC, although this might be higher in some groups.9 GIM is therefore one way to identify individuals at higher risk who might benefit from endoscopic surveillance in an effort to diagnose gastric neoplasia at a stage when resection is curative.9,10 However, the recently published evidenced-based guidelines on GIM surveillance in the US recommended against routine endoscopic surveillance of GIM in all-comers, given the potential cumulative associated harms and costs when considering the prevalence of GIM.11 Consistent with international guidelines, a more personalized approach is recommended, such that GIM surveillance is considered for select high-risk populations who have the highest likelihood of benefit.10,12,13
To our knowledge, there are no direct comparative studies of endoscopy for GC screening versus no screening in low intermediate incidence countries such as the US, although studies in Asian-Pacific populations have demonstrated that compared with no screening, endoscopic screening is associated with a 40% statistically significant reduction in GC-related mortality.14 Countries with an overall lower incidence have relevant logistical barriers to such comparative studies including cost, procedural risk, and a long time interval until GC or related outcomes occur. For these same reasons, studies directly comparing the outcomes of endoscopic surveillance of gastric preneoplasia versus no surveillance with respect to patient-important outcomes such as GC-related mortality are similarly limited; in fact, one recent comprehensive systematic review and meta-analysis did not identify any direct comparative studies of endoscopic surveillance versus no surveillance of GIM.9
Considering these logistical limitations of direct clinical comparative studies, indirect evidence from decision analyses, such as cost-effectiveness and cost-utility analyses, that simulate GC screening and/or preneoplasia surveillance may be valuable for informing the effectiveness (or lack thereof) of these interventions. That said, the outcomes of decision analyses are driven by the quality and selection of data inputs, model algorithms, and model assumptions. Indeed, heterogeneity due to variability in these parameters must be considered when interpreting and extrapolating the findings of such studies to the clinical and public health area. We therefore aimed primarily to systematically review and qualitatively analyze GC screening and surveillance decision analysis studies, and, secondarily, to appraise the quality of these studies using a standardized approach.
Methods
Search strategy and selection criteria
We conducted a systematic search of four databases – PubMed, Web of Science, Embase, and the Cochrane Library – from their respective dates of inception through 13 January 2020 to identify decision analysis studies of GC screening or preneoplasia surveillance. We searched key words: cost effectiveness; cost analysis; utility; cost benefit; gastric cancer; gastric neoplasm; neoplasia; preneoplasia; gastric intestinal metaplasia; atrophic gastritis; screening; surveillance; early cancer detection; and Markov model. The full search strategies are provided in the supplemental material. Non-clinical, non-human, non-English, and studies restricted to pediatric populations were excluded. We intended to be broad in our inclusion and thus included studies if they were clearly stated to be cost-effectiveness analyses (CEAs), cost-utility analyses (CUAs), or other decision analyses and economic evaluations of endoscopic GC screening or endoscopic surveillance of gastric preneoplasia (atrophic gastritis or gastric intestinal metaplasia). Studies analyzing only non-endoscopic modalities were excluded, although hybrid studies of endoscopic and non-endoscopic modalities were included. Studies focused only on the cost effectiveness of preventative or early detection strategies for recurrent GC (i.e., secondary prevention) were excluded. We also manually searched references from included articles and relevant review articles.
Study selection
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines for this study.15 All study titles and abstracts were independently evaluated for inclusion by two reviewers (AC, EP), with adjudication by a third reviewer (SCS) in discrepant cases, with a similar process of eligibility evaluation for full-text articles.
The following predefined data elements were abstracted from included full-text articles: study authors and publication date, study country, study objective, study design and decision model, population and/or base-case description, selected health and transition states, comparator arms, time horizon, model assumptions, outcome measures [e.g., incremental cost effectiveness ratio (ICER)], threshold value for “cost-effective” determination, predefined study outcomes, sensitivity, and secondary analyses. We also recorded the methods used for identifying data inputs and transition probabilities for the model (e.g. systematic review of the literature). Whenever appropriate and relevant, we reached out to the corresponding study authors for additional or clarifying information; if unsuccessful, or if the data were otherwise not available, we stated “no details provided”.
Studies were categorized as (1) “screening” if subjects were asymptomatic and had no additional risk factors for GC above general demographic factors such as age and race/ethnicity; (2) “surveillance” if a person was already identified as higher risk based on additional non-demographic factors such as presence of gastric preneoplasia; or (3) “screening and surveillance” if both criteria were met. Studies were additionally categorized based on whether the study was conducted in a country of high versus low-intermediate GC incidence. This designation was based on publicly available data of GC incidence for that country, and in accordance with the International Agency for Research on Cancer (IARC) definition of high- and low-intermediate incidence countries, that is, countries with an age-standardized rate (ASR) of GC greater than 10 cases per 100,000 are high-incidence, while countries with an ASR of 10 cases or less per 100,000 are considered low-intermediate incidence.1,16
Study quality assessment and appraisal
Quality assessment and appraisal of each study was conducted according to previously established guidance by Drummond et al. and using a modified comprehensive 27-item quality assessment tool.17 We elected to use this assessment tool since it is the most commonly used and has been utilized in a modified format for other systematic reviews of decisions analyses.18,19 From the original 35-item assessment tool, 8 items were not applicable or were deemed to have sufficient overlap with other items on the checklist, and were thus removed for the study appraisal used for this qualitative analysis. Each criterion was evaluated as a binary “yes” (1-point) or “no” (0-points) depending on whether it was completely met or not. Each criterion is weighted equally per the Drummond assessment tool. There is no threshold that is universally agreed upon for categorizing decision analyses as high versus low quality based on the current literature. Instead, it is largely accepted that the higher the number of criteria met on the Drummond checklist, then the higher the quality of the study.20
Results
Based on our search, we identified 6725 citations. After removal of duplicate studies (3270) and removal of studies based on irrelevant title/abstract (3425), a total of 30 articles remained for full-text review. Of these, one article was unable to be accessed as full text despite also reaching out to the study authors. Thus, 29 articles were reviewed as full texts, of which 12 were excluded for the following reasons: editorial or review (5)21–25; did not include endoscopic arm (3)26–28; ineligible study design (1)29; or analyzed only a specialized population, such as patients with pernicious anemia or gastric ulcers (3) (Figure 1).30–32
Figure 1.
PRISMA flow diagram of study selection.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
A total of 17 articles were ultimately included for qualitative analysis; 8 studies were decision analyses of endoscopic screening for GC,33–40 4 were decision analyses of endoscopic surveillance of gastric preneoplasia,41–44 and 5 were decision analyses of both screening and surveillance.45–49 Whereas 6 studies were from high-incidence countries,34,35,37–40 11 were from low–intermediate incidence countries33,36,41–49 as defined by IARC. Details of each of these studies are provided in Tables 1–3.
Table 1.
Characteristics of gastric cancer screening studies.
| Study | Study design | Population and/or base-case description | Health states | Study arms | Outcomes |
|---|---|---|---|---|---|
| Countries of High GC Incidence † | |||||
| Cho et al.35 South Korea | CEA, Non-simulated model based on real world
data Sensitivity analysis: One way Source of data inputs: • Systematic review of literature: No • Real world data: Yes |
Age: ⩾40 yo Sex: Males and Females Base Case Population: 17,044,987 subjects from the 2002–2003 NCSP and Korea Central Cancer Registry databases |
No health states provided | 1) No screening 2) EGD (interval not stated) 3) UGIS Follow up: 7-year period |
WTP Threshold: No ICER threshold provided; Outcomes examined cost per cancer deaths averted and cost per life year saved. 1USD=1088 Korean Won Age-Adjusted ICER costs for EGD: 119,099,000–178,700,000 Korean won/survival Age-Adjusted ICER for survival UGIS: 260,201,000 –371,011,000 won/survival Model was sensitive to endoscopy related costs for males and females |
| Chang et al.34 South Korea | CUA, Markov model, 1-year cycle Sensitivity analysis: One way Source of data inputs: • Systematic Review of literature: Yes • Real world data: No |
Age: 30–80 yo Sex: Males and females |
Pre-neoplasia: None Neoplasia: Early or advanced GC +/– symptoms Death |
No screening compared with 12 Strategies, with two available
screening methods: EGD and UGIS Initiation: age 30, 40, or 50 years End: 80 years Screening intervals (1 and 2 years) Time horizon: Lifetime or up to the age of 99 |
WTP Threshold: $19,162/QALY (based on 2008 International
Monetary Fund data) Annual EGD (males): Age 50–80 $4,979 Age 40–80 $20,490 Age 30–80 $81,294 Annual EGD (females) Age 50–80 $12,188 Age 40–80 $22,283 Age 30–80 $50,033 Biennial EGD (males): Age 50–80 $5,116 Age 40–80 Dominated Age 30–80 Dominated Biennial EGD (females) Age 50–80 $11,378 Age 40–80 $21,014 Age 30–80 Dominated Annual UGIS: Dominated for both sexes, all age groups Biennial UGIS: Dominated for both sexes, all age groups Model was sensitive to cost of endoscopic screening or UGI series and the distribution of cancer stage at screening |
| Lee et al.38 South Korea | Direct cost analysis, Non-simulated model based on real world
data for which cost effectiveness was defined as the cheapest
strategy of the compared arms Sensitivity Analysis: None Source of data inputs: • Systematic review of literature: No • Real world data: Yes |
Age: ⩾ 40 yo Sex: Males and Females Base Case Population: 1,503,646 subjects from the 2002 to 2004 NCSP |
Included patients who underwent GC screening by UGIS or EGD in Korea between 2002 and 2004 | Model I: Additional cost following abnormal screening result Model II: Included only direct screening costs Follow Up: 1-year |
WTP Threshold: No ICER threshold available. Model I
versus Model II compared Model I: UGIS: $53,094 EGD: $16,900 Model II UGIS: $44,445 EGD: $16,900 |
| Kowada37 Japan | CEA, Markov Model Sensitivity analysis: One way, multiway, probabilistic Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: 50–80 yo Sex: Males and females, aggregated |
“Healthy”: with or without HP Pre-neoplasia None Neoplasia: GC stages I-IV Death |
1) HP screening 2) EGD (annual) 3) UGI Follow up: 1-year cycle length, Lifetime horizon |
WTP Threshold: $50,000/QALY HP screening: 50 yo: $1,218 60 yo $1,888 70 yo $2,503 80 yo $2,691 EGD: 50 yo $6,670 60 yo $5,939 70 yo $5,224 80 yo $4,225 UGI: 50 yo $10,522 60 yo $8,956 70 yo $7,289 80 yo $5,394 Model was sensitive to HP screening |
| Saito et al.39 Japan | CEA, Markov model Sensitivity analysis: One way, probabilistic Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: ⩾ 50 yo Sex: Males and females, aggregated Base case Population: Subjects with no history of HP eradication therapy. ‡ABC method was used to group subjects based on seropositivity and gastric atrophy |
“Healthy”: seronegative or seropositive (as defined by presence
of HP or PG) Pre-neoplasia: None Neoplasia: GC stages I-IV Death |
Compared only two strategies: 1) Annual EGD 2) ABC method Follow up: 30-year period |
WTP Threshold: $50,000/QALY ABC method dominated annual EGD Model was not sensitive to input variables |
| Tashiro et al.40 Japan | Direct cost analysis. Non-simulated model based on real world
data Sensitivity analysis: None Source of data inputs: • Systematic Review of literature: No • Real world data: Yes |
Age: ⩾ 40 yo Sex: Males and females, aggregated Base case population: 106,246 subjects from the GC screening program in Niigata City, Japan 2002– 2004 |
(No health states detailed) | 1) EGD 2) UGI 3) Photofluorography Follow up: 1-year follow up based on screening program registry |
WTP Threshold: not applicable; the costs of identifying one case
of GC were calculated based on the total expense for each
screening program. Cost-effectiveness was defined as the cheapest strategy of the compared arms EGD: 1,608,000 Japanese yen UGI: 4,177,000 Japanese yen Photofluorography: 3,290,000 Japanese yen Exchange rate from yen to USD was not provided. |
| Countries of Intermediate GC Incidence † | |||||
| Dan et al.36 Singapore | CUA, Markov model Sensitivity analysis: One and Two way Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: 50–70 yo Sex: Males and females Base case Population: Singapore population and high-risk subgroup Singaporean Chinese men |
Pre-neoplasia: None Neoplasia: GC stages I-IV various stage Death |
1) No screening 2) EGD q 2 years Follow up: Lifetime, until death of all subjects (up to 99 yo) |
WTP Threshold: $50,000/QALY was adopted and rationalized to
$28,000 (based on Singapore’s 2003 GNI per capita (USD 21,230)
to that of US (USD 37,610). Screening EGD: Total population $45,982 Males $38,435 Females $63,298 Chinese Males: $26,836 Chinese Males, HP+: $21,800 Chinese Males, HP+ with premalignant lesion on first EGD: $19,900 Model was sensitive to GC incidence, cost of EGD, and the distribution of cancer stage at screening |
| Areia et al.33 Portugal | CUA, Markov model Sensitivity analysis: Deterministic, Probabilistic Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: 50–75 yo Sex: Males and females, aggregated |
Pre-neoplasia: None Neoplasia: GC Stages I–IV Post-treatment follow up Death |
1) No Screening 2) EGD q 5 years 3) EGD (q 5–10 years) & Colonoscopy if fecal occult positive 4) Biennial PG screening followed by EGD if positive Follow up: 2, 5 or 10 years based on study arm |
WTP Threshold: Euros 37,000/QALY EGD q 5 years: Euros 70,396 Screening EGD & Colonoscopy: Euros 15,407–30,908 PG q 2 years & EGD: Euros 143,344 Model was sensitive to endoscopy costs, number of endoscopies per patient over the screening age range, and ASR of GC |
AG, Atrophic gastritis; ASR, age standardized rate; BE, Barrett’s Esophagus; CEA, cost effective analysis; CUA, cost utility analysis; EC, Esophageal Cancer; EGD, Esophagogastroduodenoscopy; EMR, endoscopic mucosal resection; GC, Gastric cancer; GDP, gross domestic product; HP, Helicobacter pylori; ICER, incremental cost-effectiveness ratio; IM, Intestinal Metaplasia; NCGA, noncardia gastric adenocarcinoma; NCSP, National Cancer Screening Program; NHB, Non-Hispanic black; NHW, Non-Hispanic white; OLGA, operative link of gastritis; PG, serum pepsinogen; q, every; QALY, quality-adjusted life years; UGIS, Upper Gastrointestinal Series; WTP, willingness-to-pay; yo, years old.
High-incidence countries defined as countries with an age standardized rate (ASR) in males and females greater than 10 per 100,000 of the world standard population, whereas low-incidence countries were defined as an ASR less than 10.15
ABC method (i.e. presence of anti-Helicobacter pylori IgG antibody (HPA) and serum pepsinogen (PG)); then classify individuals into four groups: negative for both HPA and gastric atrophy (group A); seropositive for H. pylori but negative for gastric atrophy (group B); positive for both HPA and gastric atrophy (group C); gastric atrophy with HPA concentrations below the cut-off value (group D).
Table 2.
Characteristics of gastric precancer surveillance studies.
| Study | Study design | Population and/or base-case description | Health states | Study arms | Outcomes |
|---|---|---|---|---|---|
| Countries of Low–Intermediate GC Incidence † | |||||
| Areia et al.41 Portugal | CUA, Markov model sensitivity analyses: One way, probabilistic Source of data inputs: • Systematic Review of literature: Yes • Real world data: No |
Age: 50 yo Sex: Males and females, aggregated |
Pre-neoplasia: AG or IM (without distinguishing
subtype) Neoplasia: GC stage I–IV Post-cancer death |
1) No surveillance 2) EGD surveillance every 3, 5 or 10 years Follow up: 25 years |
WTP adopted threshold: Euros 36,574/QALY
(corresponding to USD $50,000, based on 2013 exchange rate) EGD surveillance: every 3 years: Euros 18,336 every 5 years Dominated every 10 years Dominated |
| Lahner et al.43 Italy | Direct cost analysis. Non-simulated model based on real
world data from Italy Sensitivity analyses: None Source of data inputs: • Systematic Review of literature: No • Real world data: yes |
Age: Median 55 yo, range 22–84 Sex: Males and females (67% female), aggregated post hoc analysis, prospective cohort of 200 Italian subjects with AG between 1992 and 2009 |
No health states (all started at AG) | Following diagnosis of AG, patients underwent surveillance
EGD at 4-year intervals Follow up: Mean 7.5 years (range 4–23.4 years) |
WTP Threshold: n/a Overall cost in all 200 patients was related to a total of 361 surveillance EGDs, corresponding to Euros 55,955. Cost adopted from the Italian Society of Digestive Endoscopy Subgroup: Pernicious anemia Total Costs: Euros 29,915 Cost per Neoplastic lesion detected Euros 2139; Costs per Gastric malignancy detected Euros 7471 Extensive atrophy Total Costs: Euros 19,530 Cost per Neoplastic lesion detected Euros 2170 Costs per Gastric malignancy detected Euros 9765 Pernicious Anemia & OLGA 3–4 Total Costs Euros 4185 Cost per Neoplastic lesion detected Euros 837 Costs per Gastric malignancy detected Euros 2092 Pernicious Anemia, age > 50 & OLGA 3–4 Total Costs Euros 3720 Cost per Neoplastic lesion detected Euros 930 Costs per Gastric malignancy detected Euros 1876 |
| Yeh et al.44 USA | CUA, Markov model sensitivity analyses: One way probabilistic Source of data inputs: • Systematic Review of literature: Yes • Real world data: No |
Age: ⩾ 50 yo Sex: Males Base Case population: Subjects with gastric precancerous lesion Subgroup for specific race/ethnicity models (i.e. Age 40 versus 60, Hispanics, and Asian immigrants) |
Pre-neoplasia: Gastritis, Atrophy, IM,
dysplasia Neoplasia: GC (stage not stated) Death |
1) No surveillance or treatment 2) Surveillance and treatment with EMR, followed by varying surveillance intervals (none, every 1, 5 or 10 years) Follow up: 50 yo to death |
WTP Threshold: $50,000/QALY Dysplasia, EMR with Surveillance: 10 years: $18,600 5 years: $20,900 1 year: $39,800 1 year and post treatment surveillance every 10 years: $1,048,000 IM, EMR with Surveillance: 10 years: $544,500 10 years and post treatment surveillance every 10 years: $25,930,000 Subgroup Analysis Hispanic, Dysplasia EMR with Surveillance: 10 years: $27,700 5 years: $32,200 1 years: $70,100 1 year and post treatment surveillance every 10 years: $15,397,000 Asian Immigrant, Dysplasia EMR with Surveillance: 10 years: $19,700 5 years: $20,700 1 year: $36,200 1 year and post treatment surveillance every 10 years: $1,614,400 Hispanic, IM EMR with Surveillance: 10 years: $1,374,500 Asian Immigrant, IM EMR with Surveillance: 10 years: $60,400 Model was sensitive to the risk of surgical mortality and likelihood of complete removal of dysplastic lesions |
| Hassan et al.42 USA | CEA, Markov model sensitivity analyses: Two-way, probabilistic Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: ⩾ 60 yo Sex: males and females, aggregated |
Pre-neoplasia: IM Neoplasia: GC (stage not stated) Death |
1) No surveillance 2) Surveillance EGD every year over a 10-year period Follow up: 10-year period |
WTP Threshold: $100,000/QALY Surveillance: $72,519 Model was sensitive to cancer incidence and the rate of GC downstaging (i.e. corresponding to a 44% downstaging from regional to local stage and 64% downstaging from distant to regional) |
High-incidence countries defined as countries with an age standardized rate (ASR) in males and females greater than 10 per 100,000 of the world standard population, whereas low-incidence countries were defined as an ASR less than 10.15
Table 3.
Characteristics of gastric (pre)cancer screening and surveillance studies.
| Study | Study design | Population and/or base-case description | Health states | Study arms | Outcomes |
|---|---|---|---|---|---|
| Countries of Low-Intermediate GC Incidence † | |||||
| Wu et al.48 Singapore | CEA, Markov model Sensitivity analyses: Deterministic, Probabilistic Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: 50–69 yo Sex: Males and females Ethnicity: Singaporean Chinese |
No endoscopy-detectable GC Pre-neoplasia: AG, IM and dysplasia Also, included patients with ulcer in surveillance category Neoplasia: GC stage I–IV Death |
1) No EGD 2) One-time EGD screening followed by annual surveillance when preneoplasia was identified 3) Biennial EGD screening Follow up: Until the age of 82 or death |
WTP Threshold: $44,000/QALY Based on gross national income of $42,930 per capita in 2011 and US threshold of $50,000 Whole Cohort: Annual surveillance: $34,200 Biennial Screening: $525,900 Surveillance Males: 50–54 years: $27,668 55–59 years: $22,535 60–64 years: $18,502 65–69 years: $15,531 Screening Males: 50–54 years: $66,920 55–59 years: $60,370 60–64 years: $44,442 65–69 years: $38,275 Surveillance Females: 50–54 years: $57,693 55–59 years: $49,156 60–64 years: $41,287 65–69 years: $35,276 Screening Females: 50–54 years: $145,440 55–59 years: Dominated 60–64 years: Dominated 65–69 years: Dominated Model was sensitive to: Discount rate, age starting surveillance, proportion of program cost, cost of EGD/biopsy, odds ratio of precancerous lesions, utility of GC stage I |
| Zhou et al.49 Singapore | CEA, Markov model sensitivity analyses: deterministic, Probabilistic Source of data inputs: • Systematic Review of literature: Yes • Real world data: No |
Age: 50–69 yo Sex: Males and females, aggregated Ethnicity: Singaporean Chinese |
Pre-neoplasia: asymptomatic state of low risk and high risk
subjects Neoplasia: GC stages I–IV Death |
1) No Screening 2) Yearly EGD surveillance of patients with precancerous lesions 3) 2-yearly EGD surveillance of patients with precancerous lesions 4) 2-yearly EGD screening 5) 2-yearly screening plus annual surveillance Authors did not specify which premalignant lesions should be surveyed.Follow up:Not stated |
WTP Threshold: $46,200/QALY (Based on Singapore GDP for the year 2011) Annual Surveillance: $44,098 2-yearly EGD surveillance: $25,949 2-yearly Screening: $79,673 2-yearly Screening & Surveillance: $59,565 Model was sensitive to the discount rate, age of starting surveillance, cost of follow-up EGD and proportion of program cost, the odds |
| ratio for GC of the high-risk group, prevalence of premalignant lesions, utility of GC Stage 1 and early detection effect in the interval years of the 2-yearly surveillance program. | |||||
| Saumoy et al.47 USA | CEA, Markov model Sensitivity Analysis: One way, probabilistic Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: 50 yo Sex: Males and females, aggregated Base Case Description: 50 yo undergoing screening colonoscopy for colorectal cancer screening, otherwise healthy Ethnicity Subgroups: 1) NHW 2) NHB 3) Hispanic/Latino 4) Asian |
Pre-neoplasia: Gastritis (+/– HP), AG, IM,
Dysplasia Neoplasia: local, regional or metastatic NCGA Death |
1) No screening 2) EGD with biopsy and continued surveillance of IM 3) EGD with biopsy every 2 years irrespective of pathology Follow up 30 years |
WTP Threshold: $100,000/QALY Screening EGD with continued surveillance if indicated NHW: $122,428 NHB: $80,278 Hispanic/Latino: $76,070 Asian: $71,451 Biennial screening strategy: dominated Model was sensitive to IM prevalence, transition rates from IM to dysplasia to local and regional cancer, cost of endoscopy, and cost of endoscopic or surgical resection. |
| Yeh et al.46 USA | CEA, Markov model, Mathematical simulation model of
intestinal-type NCGA Sensitivity Analysis: One Way, Probabilistic Source of data inputs: • Systematic Review of literature: Yes • Real world data: No |
Age: 50 yo ⩾ Sex: Males Subgroup analyses based on smoking status (never, current or former) |
Pre-neoplasia: Gastritis, Atrophy, GIM,
Dysplasia Neoplasia: Localized, regional or distance NCGA Death |
1) No screening 2) PG* 3) EGD screening** 4) HP screening & treatment with triple therapy *positive test followed by EGD and random gastric biopsies **positive EGD for dysplasia or asymptomatic localized cancer underwent EMR to remove lesions. All subjects treated with EMR returned for surveillance endoscopy in 10 years Follow up: To death |
WTP Threshold: $100,000/QALY PG: 50 yo Males $105,400 Subgroup analysis (50 yo males) $76,000 current smokers $94,500 former smokers $137,800 never smoker Model was sensitive to H. pylori prevalence, screening age, and serum pepsinogen test sensitivity |
| Gupta et al.45 USA | CUA, Markov model sensitivity analysis: One way Source of data inputs: • Systematic review of literature: Yes • Real world data: No |
Age: 50 yo Sex: Males and females, aggregated Base case population: Subjects already undergoing screening colonoscopy |
Pre-neoplasia: AG, IM, low- and high-grade gastric
dysplasia Neoplasia: GC (stages not specified) Death Health states for BE and various stages of EC are not included in this table |
1) No Screening 2) Screening (no interval) EGD at time of colonoscopy This study also included BE screening/surveillance as well Follow up: 30 years or until death |
WTP Threshold: $100,000/QALY No EGD Screening: $115,664 EGD Screening at time of colonoscopy: $95,559 Of note, the results did not differentiate between GC and EC Model was sensitive to the cost of endoscopy with biopsies |
High-incidence countries defined as countries with an age standardized rate (ASR) in males and females greater than 10 per 100,000 of the world standard population, whereas low-incidence countries were defined as an ASR less than 10.15
Decision analyses for GC screening
Of the 8 decision analyses for GC screening, 6 were conducted in high-incidence countries (South Korea and Japan), which notably also have national GC screening programs, and 2 were from low-intermediate incidence countries (Singapore, Portugal); 3 were CEAs35,37,39 and 3 were CUAs.33,34,36 Of the CEAs and CUAs, five used Markov models, and one summated costs from real world data. The sources of data inputs for the non-simulated studies included five with systematic reviews and three with real-world data (Table 1). Of the simulated Markov models, three studies included H. pylori testing and treatment. None of the studies considered gastric preneoplastic mucosal changes in the “health states”.
The three CEAs from South Korea all demonstrated that endoscopic screening for GC is a cost-effective strategy, as defined by costs and effectiveness outcomes compared with no screening or non-endoscopic intervention arms [e.g., upper GI series (UGIS)].34,35,38 Each of these studies had different base case populations, including age of screening initiation, compared different screening strategies, and had distinct comparator groups, with only two studies including a no screening arm (Table 1).34,35 Two of the studies leveraged population-based data from the cancer registry linked Korean National Cancer Screening Program (NCSP) and conducted a non-simulated cost analysis using real-world data to calculate direct costs associated with the screening or no screening strategies.35,38 Both of these studies demonstrated that endoscopic GC screening was more cost-effective than radiographic screening. The study by Chang et al., which was the only simulated analysis from South Korea, compared the cost effectiveness of endoscopic screening versus radiographic screening versus a no screening strategy among the general South Korean population. In their model, the authors varied the age of screening initiation (age 30 years, 40 years, or 50 years), screening interval (1 versus 2 years), and the modality of screening, and evaluated the effect on the output.34 The authors’ predetermined willingness-to-pay (WTP) threshold was based on the South Korean society economic valuation and set at US$19,162. Based on this threshold, annual endoscopic screening (ICER $4979) was the cost-effective strategy for South Korean males aged 50–80 years, while biennial endoscopic screening (ICER $11,378) was the cost-effective strategy for females aged 50–80 years. Endoscopic screening for individuals younger than age 50 years was not cost-effective irrespective of the interval. Radiographic screening with upper GI fluoroscopy either annually or biennially was not cost-effective for any age group, irrespective of sex. Two of the studies34,35 performed sensitivity analyses, which demonstrated that the models were most sensitive to the cost of the screening strategy and the distribution of cancer stage at screening.
Three CEAs of endoscopic GC screening were from Japan.37,39,40 Two of these studies37,39 included Markov models, while the third analyzed non-simulated real-world data from a population-based screening cohort.40 While both of the Markov model studies included an annual endoscopy arm, the conclusions differed. In the study by Kowada, annual upper endoscopy [US$6671, 19.5850 quality-adjusted life-years (QALYs)] incurred a lower overall cost and slightly higher associated QALY value compared with annual upper GI series (US$10,522, 19.5658 QALYs) initiated at age 50 years old for males and females, although the total costs were slightly lower if screening began at age 60, 70, or 80.37 While endoscopic screening was more cost-effective compared with upper GI series, this study concluded that endoscopy was less cost-effective compared with the H. pylori test and treat strategy (Table 1).37 Importantly, though, this study did not consider the presence of gastric preneoplastic mucosal changes in the model, including among those with confirmed H. pylori exposure. The decision analysis by Saito et al. included a Markov model that compared annual endoscopy to a risk-stratification approach for guiding the GC screening modality.39 Risk-stratification was based on the “ABCD method”, which was developed in Japan. This method classifies people into low and high risk based on H. pylori status and serum pepsinogen (PG) levels as a surrogate for gastric atrophy. Group A (H. pylori negative, normal PG) is lowest risk, and individuals in this category were not followed up in the study. Groups B (H. pylori positive, normal PG), C (H. pylori positive, positive PG), and D (H. pylori negative, positive PG) are all higher risk for GC, in ascending order. In addition to being treated for H. pylori, individuals in Group B underwent endoscopy for GC screening at different predetermined intervals. Compared with annual esophagogastroduodenoscopy (EGD), total costs slightly favored the ABCD method ($64,074 versus $64,489). GC related costs were reduced by 35% in the ABCD method versus annual EGD ($3214 versus $4826). The third CEA compared the cost-effectiveness of endoscopic versus radiographic screening (UGIS or photofluorography) for GC initiated at age 40 years old using real-world data from a Japanese population-based cohort from Nigata City (2002–2004).40 The investigators reported that 55% more localized GCs were diagnosed on endoscopy compared with radiography, and thus concluded that endoscopy was more cost-effective than either of two radiographic strategies.
The two other decision analyses were CUAs from intermediate incidence countries (Singapore and Portugal), although the study from Singapore specifically analyzed a high-risk population (Chinese males aged 50–70 years) residing in the country.33,36 This study demonstrated that, compared with a no screening strategy, biennial endoscopic screening was cost-effective only for Chinese men (ASR for GC: 25.9/100,000, versus 16/100,000 for Singaporean men versus 11/100,000 for Singaporean women), particularly those who were H. pylori positive (ICER: $26,836/QALY) and who had gastric precancer diagnosed on the index endoscopy (ICER: $19,900/QALY); biennial endoscopy was not cost-effective for the overall Singaporean population (ICER, males: $38,435/QALY; ICER, females: $63,298).36 Notably, the WTP threshold for this study was strict ($28,000/QALY) and was derived from an adopted and rationalized threshold of $50,000 USD based on Singapore’s 2003 gross national income.
Areia et al. analyzed the cost utility of adding an upper endoscopy for upper GI malignancy screening at the time of colonoscopy for either diagnostic purposes [positive fecal occult blood test (FOBT)] or colorectal cancer screening.33 Among a simulated Portuguese population aged 50–75 years, compared with no screening, only EGD every 5–10 years at the time of a diagnostic colonoscopy for a positive FOBT was cost-effective, while stand-alone endoscopy every 5 years and biennial serum PG with endoscopy were not. Based on sensitivity analyses, this screening strategy was only cost-effective if the ASR for GC exceeded 10/100,000 persons.
Decision analyses of endoscopic surveillance of gastric preneoplasia
Of the four decision analyses of gastric preneoplasia surveillance, all were conducted in low-intermediate areas (Portugal, Italy, US).41–44 The two European studies demonstrated that endoscopic surveillance of gastric preneoplasia, which included extensive AG or GIM, was cost-effective but depended in part on the endoscopic surveillance interval and the population (Table 2).41,43 In the study by Areia et al., a surveillance strategy of EGD with biopsies every 3 years among Portuguese people aged 50 years or older was cost-effective compared with a no surveillance strategy (ICER: 18,336 Euros/QALY) at the WTP threshold of 36,574 Euros/QALY, but not if performed every 5 or 10 years (both dominated).41
The non-simulated model based on real-world data from Italy by Lahner et al., conducted a post hoc analysis of 200 patients with AG who underwent endoscopic surveillance, at least 4 years after diagnosis, with a mean follow up of 7.5 years.43 Over the course of the study, 19 lesions (4 GC, 8 gastric carcinoid, 7 dysplasia) were detected which corresponded to a cost of 2945 Euros per lesion detected and a number-needed-to-screen of 19. In a subgroup analysis restricting surveillance to patients with pernicious anemia, the number-needed-to-screen decreased to 13.8 and the associated cost decreased to 2139 Euros per lesion detected. Restricting surveillance even further to patients with pernicious anemia and Operative Link on Gastritis Assessment (OLGA) stage 3–4 (high risk) was associated with a number-needed-to-screen of only 5.4, and a cost per lesion of 837 Euros.
The two surveillance studies42,44 conducted in a US population also demonstrated that endoscopic surveillance might be cost-effective. Yeh et al. analyzed the cost-effectiveness of endoscopic mucosal resection (EMR) followed by interval endoscopic surveillance (annually, every 5 years, or every 10 years) compared with no endoscopic treatment/surveillance in 50-year-old males with gastric precancer (i.e., AG, GIM, or dysplasia).44 At a predetermined WTP threshold of $50,000/QALY, EMR of dysplasia with ongoing annual endoscopic surveillance was cost-effective (ICER: $39,800/QALY) and was associated with 94.7% reduction in lifetime risk of GC. EMR of dysplasia followed by extended interval endoscopic surveillance every 5 years or every 10 years was also cost-effective, with lower ICERs ($20,900/QALY and $18,600/QALY, respectively), but with 92.4% and 89.2% lifetime reductions in GC risk, respectively. A subgroup analysis of endoscopic surveillance of dysplasia according to race/ethnicity demonstrated that, among Hispanics, EMR of dysplasia followed by every 5-year surveillance (ICER: $32,200/QALY) or every 10-year surveillance (ICER: $27,700/QALY) was cost-effective, but annual surveillance (ICER: $70,100/QALY) exceeded the WTP threshold. Among Asians, EMR of dysplasia was cost-effective at all intervals ($19,700/QALY, $20,700/QALY, $36,200/QALY for every 10-year, 5-year, or 1-year interval, respectively). By comparison, the authors concluded that none of these endoscopic surveillance strategies were cost-effective for gastric preneoplastic changes (AG and GIM) among men aged 50-years or older; indeed, most strategies were dominated, that is, they were more costly and less effective compared with the no treatment/surveillance arm. It must be emphasized, though, that EMR was included in all of these modeled strategies despite the fact that AG and GIM are typically mucosal changes that occur in the absence of discrete lesions, and, thus, are usually not even amenable to EMR.
The decision analysis by Hassan et al. was conducted among patients diagnosed with GIM and compared yearly surveillance endoscopy over a 10-year period for males and females aged 60 years old, with a no surveillance strategy.42 This study reported that the surveillance strategy was cost-effective (ICER: $72,519/QALY), with a WTP threshold of $100,000/QALY.42 A subsequent sensitivity analysis demonstrated that the 10-year interval surveillance program for patients 50 years old was also cost-effective (ICER: $52,361/QALY), but not for individuals who were 70 years or older (ICER: $112,984/QALY).
Decision analyses of combined GC screening and gastric preneoplasia surveillance
Five studies meeting inclusion criteria consisted of study designs that included endoscopic screening followed by endoscopic surveillance if indicated, with two studies conducted in an overall intermediate incidence country (Singapore)48,49 and three studies in an overall low incidence country (US).45–47 Four were CEAs46–49 and one was a CUA.45 All used Markov models. Both CEAs conducted in Singapore analyzed a higher risk sub-population of Singaporean Chinese males and females aged 50–69 years old.48,49 Wu et al. compared three screening strategies: EGD for screening every 2 years, EGD with screening and annual surveillance only if patients were identified to have an ulcer or a precancerous mucosal changes (AG, GIM, or dysplasia), and no screening. Using an adapted WTP threshold of $44,000/QALY, they reported that the one-time screening with select annual surveillance was cost-effective (ICER: $34,000/QALY) while biennial screening was not cost-effective (ICER: $525,900/QALY).48 Table 3 details additional model comparisons of various base case age and sex combinations, all of which consistently demonstrated that screening with annual surveillance if indicated generated lower ICERs compared with the biennial strategy. Subgroup analysis, however, demonstrated that cost-effectiveness was heterogenous across combinations of age group and sex. When analyzing the whole cohort or males only, screening with annual surveillance was cost-effective for both age groups; however, when analyzing females only, annual surveillance was cost-effective for age group 60–69 years but not the age group 50–59 based on the WTP threshold.
The other CEA conducted in Singapore, by Zhou et al., compared five intervention strategies: no screening, yearly EGD surveillance for patients with precancerous mucosal changes, biennial EGD surveillance of patients diagnosed with precancerous mucosal changes, biennial EGD screening, and biennial screening plus annual surveillance.49 The authors did not specify which premalignant changes were being surveyed. With a Singaporean WTP threshold of $46,200/QALY, annual EGD surveillance was the optimal strategy (ICER: $44,098/QALY), while biennial surveillance was the most cost-effective (ICER: $25,949/QALY).49 Biennial EGD screening (ICER: $79,673/QALY) and biennial screening plus annual surveillance (ICER $59,565/QALY) were not cost-effective. A sensitivity analysis determining the threshold odds ratio for GC in a healthy person was conducted to evaluate the optimal time interval for endoscopic surveillance. Based on this analysis, annual EGD surveillance was favored as long as the odds of GC exceeded 5.5 times that of a healthy person, while biennial surveillance was favored if the subpopulation had between 2.4 to 5.5 times higher likelihood of GC compared with a healthy person.
In a decision analysis model from the US, Saumoy et al. demonstrated that performing one-time endoscopic screening for GC at the time of colonoscopy performed for colorectal cancer screening among patients aged 50 years, with ongoing upper endoscopic surveillance only if gastric preneoplasia was diagnosed (specifically GIM), was cost-effective compared with biennial upper endoscopy for GC screening or compared with no GC screening (current standard of care in the US); however, this strategy was only cost-effective for non-white races/ethnicities and not non-Hispanic whites (Table 3).47 The biennial screening strategy, which was continued even if there no abnormal histologic findings, dominated in this study.
Gupta et al. analyzed the cost-effectiveness of performing an upper endoscopy for both esophageal and GC screening at the time of screening colonoscopy for the general US population starting at age 50 years old.45 Of note, the authors made no separate considerations for sex, race/ethnicity or other risk factors. Additionally, while esophageal lesions were resected endoscopically in the model, this was not the case for gastric lesions, whereby resectable GC was modeled only as surgical resection (resection of gastric dysplasia not mentioned/modeled). Compared with the no screening strategy, a one-time screening endoscopy at age 50 years old for upper GI cancers was not cost-effective (ICER: $115,664/QALY) at the WTP threshold of $100,000/QALY. While their primary analysis did not analyze esophageal and gastric cancer separately, the sensitivity analysis demonstrated that the prevalence of esophageal adenocarcinoma, esophageal squamous cell cancer, and GC in the general US population would have to increase by 654%, 1948% and 337%, respectively, to result in an ICER below $50,000/QALY.45
Another US-based simulation study compared endoscopic and non-endoscopic GC screening strategies only among males aged 50 years or older and stratified by smoking status.46 The authors compared a no screening strategy to one of the following one-time screening strategies with subsequent endoscopic surveillance determination based on the type of screening test conducted and whether this screening test was positive: serum PG, EGD, and H. pylori, with treatment of the latter if positive. The only strategy that was cost-effective at the WTP threshold of $100,000/QALY was serum PG testing followed by EGD with random biopsies if serum PG was abnormal (no further testing if normal serum PG) among current smokers (ICER: $76,000/QALY), but not former/never smokers (ICER: $105,400/QALY). If EGD with biopsies confirmed dysplasia or asymptomatic localized cancer, then patients underwent EMR to remove the lesions, while those with a negative EGD and biopsies had a follow-up EGD at 10 years. Endoscopy without serum PG screening and H. pylori test and treat strategies were not cost-effective, irrespective of smoking status. Yeh et al. also modeled the GC risk reduction by each strategy when screening the general population at age 50 years. Screening reduced the lifetime risk of GC by 26.4% with serum PG testing, 21.2% by endoscopy and EMR, and by 0.2% with H. pylori screening and eradication therapy if indicated. This model also showed that targeting screening for current smokers would additionally reduce lifetime GC risk by 30.8%, 25.5%, and 0.1%, respectively.46
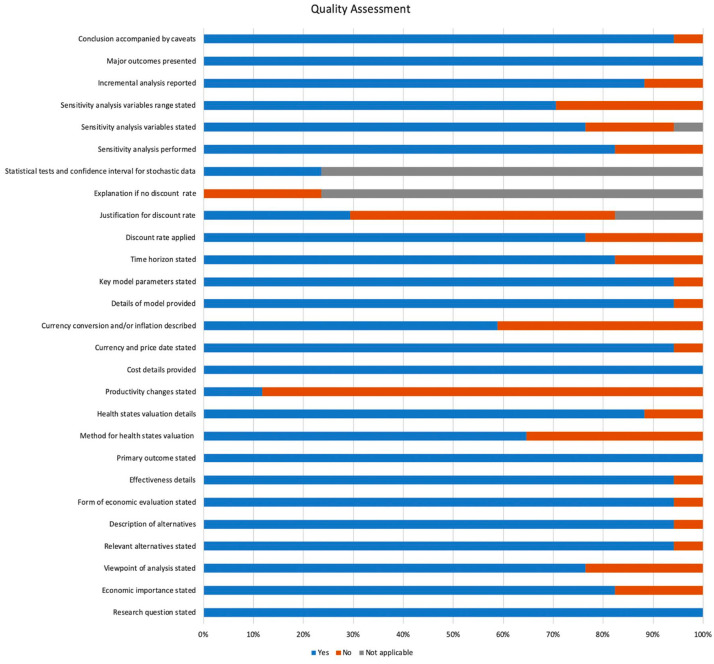
Study quality appraisal
All reviewed articles were independently assessed for quality appraisal.17 Figure 2 illustrates the analyzed metrics of interest using the 27-item modified Drummond’s assessment tool. The median number of criteria fulfilled by each study was 22, and for each criterion the median number of studies which fulfilled it was 15. Four criteria were met by 100% of the included studies. By contrast, a separate four criteria were met by less than 50% of the studies. Of the included studies, 18% did not perform a sensitivity analysis or state the time horizon of the study, while 24% did not apply a discount rate or state the variables included in sensitivity analysis. Of note, discount rates are not applicable to studies using non-simulated real-world data or for those studies of only 1-year duration.
Figure 2.
Systematic quality assessment of included studies based on a modified Drummond scoring system. The maximum score for the modified checklist was 27. From the original 35-item checklist, 8 items were not scored for the present qualitative analysis based on irrelevance or redundancy with other checklist items. These 8 items included: the choice of form of economic evaluation is justified in relation to the questions addressed; the source of effectiveness estimates used are stated; details of the method of synthesis or meta-analysis of estimates are given; details of the subjects from whom valuations were obtained are given; the relevance of productivity changes to the study question is discussed; quantities of resources are reported separately from their unit costs; relevant alternatives are compared; the answer to the study question is given.
Discussion
Based on a comprehensive systematic review of the literature, we identified 17 decision analysis studies analyzing the cost effectiveness or cost utility of endoscopy for gastric (pre)cancer screening and surveillance, with data inputs derived from either real-world (e.g. NCSP) or simulated data. Our study extends the current literature since it is the only systematic review and qualitative analysis, to our knowledge, that is focused on endoscopic strategies for GC screening and preneoplasia surveillance. While an earlier study by Areia et al. included CEAs up to 2012, their synthesis had a strong focus on H. pylori “test-and-treat” strategies,21 as opposed to endoscopic strategies. In addition, our quality appraisal identified areas for improvement and standardization in decision models related to endoscopic GC screening/surveillance moving forward, including the need to consider preneoplastic transition states. In contrast to Japan and South Korea, where endoscopy for GC screening is associated with reduced GC-related mortality compared with no screening, there are no studies establishing whether or not endoscopic screening for GC is associated with reduced GC-related mortality in countries with low-intermediate GC incidence, even among identifiable high-risk groups in these regions. The reasons are multiple, but predominantly relate to financial and logistical challenges of randomized-controlled trials and direct clinical comparative studies of endoscopic screening/surveillance versus no screening/surveillance; in this context, rigorously designed decision analyses hold unique value. Additional clinical data will ideally help to further refine these models and better define the higher-risk groups so that we may enhance our approach to GC prevention and early detection interventions, particularly in countries/regions such as the US that have a grossly unequal distribution of disease burden across subpopulations.
CEAs and CUAs ideally incorporate a systematic review of the literature to inform model inputs including transition probabilities from one disease state to another, economic data, and probabilities of adverse outcomes; as we have demonstrated, the consistency of this “best practice” varies. When interpreting decision analyses, close scrutiny of the design, the inputs, and assumptions, and the ability to identify the origins of each is critical since any differences, however minute, can result in alternate outcomes and conclusions. It is also important that a decision analysis define the perspective and thresholds for cost-effectiveness or cost-utility determination since countries differ in their WTP thresholds – for example, the threshold for considering an intervention to be cost-effective is less than $100,000/QALY in the US, but in other countries this threshold might be $50,000/QALY or lower.50–52 This has direct implications for generalizability since health care infrastructures and their cost ceilings vary widely. To this end, geographic variations in clinical practice are also highly relevant. For example, Asian-Pacific countries most often diagnose gastric preneoplasia non-invasively instead of histologically based on biopsies and also less often use procedural sedation, which is distinct contrast to Western countries, especially the US. Such variations again highlight the need for region- and population-specific decision analyses.
Structured screening and surveillance programs are widely used as one strategy for reducing the cancer burden for those cancers in which early cancer detection or intervention on precancerous conditions positively and meaningfully impacts patient-important outcomes, particularly cancer-specific incidence and related mortality. In general, our qualitative analysis demonstrated that endoscopic screening and surveillance were favorable based on decision analyses, but there was wide heterogeneity across studies, particularly with respect to model design, data inputs, cost valuations, and thresholds. Indeed, this underscores the need for region- and population-specific GC screening and gastric preneoplasia surveillance recommendations, as opposed to a ‘one-size-fits-all’ approach. The majority (65%) of studies that we identified were conducted in regions of low-intermediate GC incidence, specifically the US, Singapore, Italy, and Portugal. Many but not all of these studies attempted to identify higher risk populations within these regions for whom endoscopic screening and surveillance might be more cost-effective compared with the background general population; for example, according to race/ethnicity, smoking status, or the ABCD method of risk-stratification. All but one decision analysis that we identified analyzed GC-related mortality as the final outcome.40
Endoscopy for GC screening is consistently associated with improved GC-related mortality in high-incidence countries where GC screening routinely occurs,14 driven primarily by earlier detection of neoplasia and the opportunity for preventative/curative resection. A recent meta-analysis of over 342,000 individuals from Japan, South Korea, or China encompassing 6 prospective cohort and 4 nested case-control studies demonstrated that, compared with no screening and with radiography-based screening, endoscopy for GC screening was associated with a 42% and 67% significant reduction in GC-related mortality, respectively.14 However, GC screening does not reliably occur among high risk populations in other countries/regions. This is even the case in those areas (1) with an established, stable infrastructure for endoscopy for gastrointestinal cancer screening – such as the US, where upper endoscopy and colonoscopy are common, approved modalities for esophageal and colorectal cancer screening, respectively; and (2) where resources and expertise exist such that cancer diagnosis in an earlier stage will generally translate to improved outcomes, and, ideally, even cure,50 for example, endoscopic or surgical resection of early stage GC. The reasons for this incongruency are multifactorial, including an under-recognition of populations at disproportionately higher risk for GC in otherwise low–intermediate incidence countries.4 Additionally, there are no comparative studies of GC screening or preneoplasia surveillance versus no screening/surveillance, which are fundamental for evaluating the clinical benefit (or lack thereof) of these interventions. The greatest challenges to performing such studies include study cost, the long sojourn time to gastric cancer, the variable population risk, and the small but still present procedural risk, to name but a few.
We elected to focus this systematic review on endoscopic screening and surveillance strategies since endoscopy offers several advantages over non-endoscopic modalities, such as radiographic or serological (e.g. serum PG). First, at least based on evidence from high-incidence countries with national GC screening programs, endoscopy has a higher sensitivity and specificity compared with radiography for GC screening, with the former more often able to diagnose early stage disease14,53; extrapolating the performance of radiography for GC screening to countries/regions where it is rarely used, such as the US, is also problematic. Endoscopy also allows the opportunity for curative resection if early stage disease is diagnosed. While not GC screening modalities per se, some strategies such as H. pylori testing and serum PG have been used to identify individuals at higher risk. These each have significant limitations, however. The H. pylori testing and treatment strategy will not identify people who have gastric preneoplastic mucosal changes who remain at significantly increased risk for GC despite H. pylori eradication.9 Thus, even though this strategy is cost-effective, it still risks missing the opportunity for early cancer detection and improved outcomes. Additionally, despite the large body of evidence supporting a “test and treat” strategy for GC prevention in high-incidence countries where H. pylori was previously endemic, the performance of this strategy for GC prevention in low-intermediate incidence areas, or in areas with now much lower H. pylori prevalence compared with prior time periods (e.g. Japan, South Korea), has not been established. Along similar lines, while serum PG has demonstrated adequate test characteristics for predicting the presence of AG in some Asian-Pacific populations, this has not been adequately validated in the majority of other populations, namely Western populations, and is affected by several factors such as smoking, and is also less able to reliably predict more advanced preneoplastic or neoplastic changes. Moreover, serum PG testing is not available commercially in the US.
The most reliable method for diagnosing gastric preneoplasia is via endoscopy with biopsies or, where available, advanced endoscopic imaging techniques.4 Gastric preneoplasia is important for risk stratification given that it is associated with a 0.16% annual baseline risk of incident intestinal-type GC, with some groups at even higher risk.9 Based on this risk, the majority of GI societies advocate endoscopic surveillance for gastric preneoplasia.9,10,13,54,55 However, there is variability with respect to details of the surveillance recommendation and reflects in large part the heterogeneous literature and lack of studies directly comparing patient-important outcomes between endoscopic surveillance versus no surveillance.9 As noted, and as confirmed by decision analyses conducted in both high and low–intermediate incidence countries, the “benefit” of, and cost saving associated with, systematic screening surveillance and screening in at-risk individuals is diagnosing gastric neoplasia in a curable stage and preventing advanced staged disease, for which there is no cure and which therefore incurs significant societal, healthcare, and personal costs including mortality that overwhelm any perceived benefit.47 Notably, the framework of endoscopy for GC screening and preneoplasia surveillance overlaps with screening/surveillance for other GI neoplasia – specifically esophageal and colorectal – in that the identification of preneoplasia [e.g., Barrett’s esophagus and colorectal adenoma(s)] dictates subsequent surveillance and intervals.
Because of both between-country and within-country variation of GC risk,1,3 a targeted screening approach as opposed to universal approach is considerably more palatable for GC screening in low-intermediate incidence countries when considering population health and resource allocation, as demonstrated by several decision analyses identified in our systematic review.44,46–49 In fact, in the US, some non-white groups have up to 10-fold higher GC incidence compared with non-Hispanic whites,56 with rates of GC even exceeding rates of colorectal cancer in the average-risk population recommended for routine colorectal cancer screening in the US. These groups also have significantly higher rates of gastric preneoplasia.11 The screening and surveillance intervals, however, should be dictated by the population of interest and healthcare infrastructure of the specific country, given the different cost and economic considerations, particularly for countries that are overall low–intermediate incidence but have identifiable higher-risk populations such as immigrants from countries of high GC incidence.3
Another consideration, and one on that receives more scrutiny in the current era of exploding healthcare costs, is evaluating whether the costs of case finding – which include both diagnostic costs as well as downstream implications related to medical, surgical, and other adjunctive cancer therapeutics – are appropriately economically balanced with the value of cancer screening/surveillance from the society/healthcare perspective and individual quality life years gained.50 In this setting, studies using decision model analyses are useful surrogate approaches for comparing clinical strategies. Indeed, decision analyses allow essentially real-time evaluation of how alteration in one strategy or associated transition probabilities and cost considerations affect the outcome(s). While decision analyses cannot exactly simulate clinical outcomes, they nevertheless provide valuable information about whether a strategy may be a suitable clinical option for a given population. The majority of cost-effectiveness and cost-utility studies identified by our comprehensive search were independently appraised as high-quality.
As noted, this is the first systematic review focused on endoscopic strategies for GC screening and preneoplasia surveillance. Despite several strengths of our qualitative analysis, there are a few limitations that must be acknowledged. First, although not a limitation of our study per se, we acknowledge that cost-effectiveness does not equate to clinical efficacy; that is, even though an intervention might be cost-favorable based on a model and under ideal circumstances, this does not imply that the intervention will be clinically beneficial. This underscores the need for clinical comparative studies, ideally randomized controlled clinical trials, analyzing patient-important outcomes (specifically, GC-related mortality) in those who undergo endoscopy for gastric (pre)cancer screening/surveillance versus no screening/surveillance; such studies have not been performed outside of few Asian-Pacific countries (e.g., Japan and South Korea). Secondly, heterogeneity of the studies precluded direct comparison and also limited generalizability. For this reason, we provided a detailed categorization of each of the studies in tabular format to facilitate contextual interpretation of the studies and their respective conclusions. We are hopeful such a format will be informative when designing and implementing future studies, particularly clinical comparative studies that also analyze objective clinical outcomes, such as GC-related mortality, and economic outcomes associated with endoscopic screening/surveillance versus no screening/surveillance.
In summary, systematic screening and surveillance strategies are paramount to reducing GC morbidity, mortality, and societal costs given that early detection is directly associated with improved mortality and even the opportunity for cure. For populations in which screening and surveillance do not routinely occur, GC is most often diagnosed at an advanced stage when symptoms present and there are limited if any therapeutic options, none of which are curative.57 Because of logistical difficulties in conducting direct comparative clinical studies, decision analyses offer a unique mechanism for both clinicians and health policy makers to model and explore costs and outcomes of various GC reduction strategies efficiently with essentially real-time evaluation of how altering certain parameters might affect the predicted outputs. Moving forward, high-quality decision analyses might best serve high incidence but resource limited countries in order to inform resource allocation and motivate discovery into lower cost interventions, as well as low-to-intermediate incidence countries in order to better define the high-risk subgroups who might benefit most from GC screening and preneoplasia surveillance.
Supplemental Material
Supplemental material, Supplemental_Material for Decision model analyses of upper endoscopy for gastric cancer screening and preneoplasia surveillance: a systematic review by Andrew Canakis, Ethan Pani, Monica Saumoy and Shailja C. Shah in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors would like to acknowledge and thank David Flynn and the Boston University School of Medicine Library for assisting with the literature search.
Footnotes
Author contributions: AC: literature search, data acquisition, analysis and interpretation of data, drafting of manuscript; EP: literature search, data acquisition, review of manuscript; MS: analysis and interpretation of data, critical revision of the manuscript for important intellectual content; SCS: study concept and design, analysis and interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; guarantor of article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: SCS is funded by the Agency for Healthcare Research (AHRQ) and Quality and Patient-Centered Outcomes Research Institute (PCORI) under award number K12 HS026395, a 2019 American Gastroenterological Association Research Scholar Award, and Veterans Affairs Career Development Award under award number ICX002027A. The content is solely the responsibility of the listed authors and does not necessarily represent the official views of the funding agencies listed.
ORCID iD: Shailja C. Shah  https://orcid.org/0000-0002-2049-9959
https://orcid.org/0000-0002-2049-9959
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Andrew Canakis, Department of Medicine, Boston University Medical Center, Boston, Massachusetts, USA.
Ethan Pani, Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Monica Saumoy, Division of Gastroenterology and Hepatology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Shailja C. Shah, Division of Gastroenterology, Hepatology, and Nutrition, Vanderbilt University Medical Center, 2215 Garland Avenue, Medical Research Building IV, Room 1030-C (mail), Nashville, TN 37232-0252, USA; Section of Gastroenterology, Veterans Affairs Tennessee Valley Health System, Nashville, TN, USA.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Surveillance, Epidemiology, and End Results. Cancer of the stomach - cancer stat facts, https://seer.cancer.gov/statfacts/html/stomach.html (accessed 13 April 2020).
- 3. Pabla BS, Shah SC, Corral JE, et al. Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020; 18: 347–359e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah SC, Nunez H, Chiu S, et al. Low baseline awareness of gastric cancer risk factors amongst at-risk multiracial/ethnic populations in New York City: results of a targeted, culturally sensitive pilot gastric cancer community outreach program. Ethn Health 2020; 25: 189–205. [DOI] [PubMed] [Google Scholar]
- 5. Hamashima C. Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 2018; 48: 673–683. [DOI] [PubMed] [Google Scholar]
- 6. Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology 2017; 152: 1319–1328.e7. [DOI] [PubMed] [Google Scholar]
- 7. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 8. Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015; 136: 487–490. [DOI] [PubMed] [Google Scholar]
- 9. Gawron AJ, Shah SC, Altayar O, et al. AGA Technical review on gastric intestinal metaplasia—natural history and clinical outcomes. Gastroenterology 2020; 158: 705–731.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta S, Li D, Serag HBE, et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology 2020; 158: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altayar O, Davitkov P, Shah SC, et al. AGA technical review on gastric intestinal metaplasia-epidemiology and risk factors. Gastroenterology 2020; 158: 732–744.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European society of gastrointestinal endoscopy (ESGE), European helicobacter and microbiota study group (EHMSG), European society of pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019; 51: 365–388. [DOI] [PubMed] [Google Scholar]
- 13. Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019; 68: 1545–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology 2018; 155: 347–354e9. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J 2014; 55: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ economic evaluation working party. BMJ 1996; 313: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luhnen M, Prediger B, Neugebauer EAM, et al. Systematic reviews of health economic evaluations: a structured analysis of characteristics and methods applied. Res Synth Methods 2019; 10: 195–206. [DOI] [PubMed] [Google Scholar]
- 19. Watts RD, Li IW. Use of checklists in reviews of health economic evaluations, 2010 to 2018. Value Health 2019; 22: 377–382. [DOI] [PubMed] [Google Scholar]
- 20. Jefferson T, Smith R, Yee Y, et al. Evaluating the BMJ guidelines for economic submissions: prospective audit of economic submissions to BMJ and The Lancet. JAMA 1998; 280: 275–277. [DOI] [PubMed] [Google Scholar]
- 21. Areia M, Carvalho R, Cadime AT, et al. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter 2013; 18: 325–337. [DOI] [PubMed] [Google Scholar]
- 22. Matsuda A, Saika K, Tanaka R, et al. Simulation models in gastric cancer screening: a systematic review. Asian Pac J Cancer Prev 2018; 19: 3321–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sossai P, Barbazza R. Is it useful to follow-up intestinal metaplasia in the stomach? Analysis of cost/effectiveness. J Clin Gastroenterol 1992; 14: 356–357. [DOI] [PubMed] [Google Scholar]
- 24. Rugge M, Cassaro M, Pennelli G, et al. Pathology and cost effectiveness of endoscopy surveillance for premalignant gastric lesions. Gut 2003; 52: 453–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shao YC, Zhang N, Wang JL, et al. Current situation of screening for upper gastrointestinal cancer and the research progress in economic evaluation. Chin J Cancer Prev Treat 2018; 25: 681–684. [Google Scholar]
- 26. Li D, Yuan Y, Sun LP, et al. Health economics evaluation of a gastric cancer early detection and treatment program in China. Asian Pac J Cancer Prev 2014; 15: 5133–5136. [DOI] [PubMed] [Google Scholar]
- 27. Lee YC, Lin JT, Wu HM, et al. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomark Prev 2007; 16: 875–885. [DOI] [PubMed] [Google Scholar]
- 28. Tsuji I, Fukao A, Sugawara N, et al. Cost-effectiveness analysis of screening for gastric cancer in Japan. Tohoku J Exp Med 1991; 164: 279–284. [DOI] [PubMed] [Google Scholar]
- 29. Zhou H, Zhu F. A six-year cost analysis of hospital-based endoscopic surveillance programme for gastric cancer. Ann Oncol 2011; 22: v55. [Google Scholar]
- 30. Borch K. Epidemiologic, clinicopathologic, and economic aspects of gastroscopic screening of patients with pernicious anemia. Scand J Gastroenterol 1986; 21: 21–30. [DOI] [PubMed] [Google Scholar]
- 31. Vakil N, Talley N, van Zanten SV, et al. Cost of detecting malignant lesions by endoscopy in 2741 primary care dyspeptic patients without alarm symptoms. Clin Gastroenterol Hepatol 2009; 7: 756–761. [DOI] [PubMed] [Google Scholar]
- 32. Yeh JM, Ho W, Hur C. Cost-effectiveness of endoscopic surveillance of gastric ulcers to improve survival. Gastrointest Endosc 2010; 72: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Areia M, Spaander MC, Kuipers EJ, et al. Endoscopic screening for gastric cancer: a cost-utility analysis for countries with an intermediate gastric cancer risk. United Eur Gastroenterol J 2018; 6: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang HS, Park EC, Chung W, et al. Comparing endoscopy and upper gastrointestinal X-ray for gastric cancer screening in South Korea: a cost-utility analysis. Asian Pac J Cancer Prev 2012; 13: 2721–2728. [DOI] [PubMed] [Google Scholar]
- 35. Cho E, Kang MH, Choi KS, et al. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev 2013; 14: 2533–2540. [DOI] [PubMed] [Google Scholar]
- 36. Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol 2006; 4: 709–716. [DOI] [PubMed] [Google Scholar]
- 37. Kowada A. Cost-effectiveness of Helicobacter pylori test and eradication versus upper gastrointestinal series versus endoscopy for gastric cancer mortality and outcomes in high prevalence countries. Scand J Gastroenterol 2019; 54: 685–689. [DOI] [PubMed] [Google Scholar]
- 38. Lee HY, Park EC, Jun JK, et al. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol 2010; 16: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saito S, Azumi M, Muneoka Y, et al. Cost-effectiveness of combined serum anti-Helicobacter pylori IgG antibody and serum pepsinogen concentrations for screening for gastric cancer risk in Japan. Eur J Health Econ 2018; 19: 545–555. [DOI] [PubMed] [Google Scholar]
- 40. Tashiro A, Sano M, Kinameri K, et al. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol 2006; 12: 4873–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Areia M, Dinis-Ribeiro M, Goncalves FR. Cost-utility analysis of endoscopic surveillance of patients with gastric premalignant conditions. Helicobacter 2014; 19: 425–436. [DOI] [PubMed] [Google Scholar]
- 42. Hassan C, Zullo A, Di Giulio E, et al. Cost-effectiveness of endoscopic surveillance for gastric intestinal metaplasia. Helicobacter 2010; 15: 221–226. [DOI] [PubMed] [Google Scholar]
- 43. Lahner E, Hassan C, Esposito G, et al. Cost of detecting gastric neoplasia by surveillance endoscopy in atrophic gastritis in Italy: a low risk country. Dig Liver Dis 2017; 49: 291–296. [DOI] [PubMed] [Google Scholar]
- 44. Yeh JM, Hur C, Kuntz KM, et al. Cost-effectiveness of treatment and endoscopic surveillance of precancerous lesions to prevent gastric cancer. Cancer 2010; 116: 2941–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta N, Bansal A, Wani SB, et al. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest Endosc 2011; 74: 610–624.e2. [DOI] [PubMed] [Google Scholar]
- 46. Yeh JM, Hur C, Ward Z, et al. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: a cost-effectiveness analysis. Gut 2016; 65: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saumoy M, Schneider Y, Shen N, et al. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology 2018; 155: 648–660. [DOI] [PubMed] [Google Scholar]
- 48. Wu JT, Zhou J, Naidoo N, et al. Determining the cost-effectiveness of endoscopic surveillance for gastric cancer in patients with precancerous lesions. Asia Pac J Clin Oncol 2016; 12: 359–368. [DOI] [PubMed] [Google Scholar]
- 49. Zhou HJ, Dan YY, Naidoo N, et al. A cost-effectiveness analysis evaluating endoscopic surveillance for gastric cancer for populations with low to intermediate risk. PLoS One 2013; 8: e83959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shah SC, Kayamba V, Peek RM, et al. Cancer control in low- and middle-income countries: is it time to consider screening? J Glob Oncol 2019; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Braithwaite RS, Meltzer DO, King JT, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008; 46: 349–356. [DOI] [PubMed] [Google Scholar]
- 52. Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996; 276: 1253–1258. [PubMed] [Google Scholar]
- 53. Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in korea: a population-based study. PLoS One 2012; 7: e50041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fock KM, Talley N, Moayyedi P, et al. Asia–Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol 2008; 23: 351–365. [DOI] [PubMed] [Google Scholar]
- 55. Shah SC, Gawron AJ, Li D. Surveillance of gastric intestinal metaplasia. Am J Gastroenterol 2020; 115: 641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang RJ, Sharp N, Talamoa RO, et al. One size does not fit all: marked heterogeneity in incidence of and survival from gastric cancer among Asian American subgroups. Cancer Epidemiol Biomark Prev 2020; 29: 903–909. [DOI] [PubMed] [Google Scholar]
- 57. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018; 10: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Decision model analyses of upper endoscopy for gastric cancer screening and preneoplasia surveillance: a systematic review by Andrew Canakis, Ethan Pani, Monica Saumoy and Shailja C. Shah in Therapeutic Advances in Gastroenterology