Abstract
Gastrointestinal complications in critically ill patients during the COVID-19 pandemic pose a diagnostic and treatment dilemma. We present a case of a 74-year-old male who was brought to our emergency department with worsening shortness of breath, fever, and dry cough and was found to have COVID-19 pneumonia. Early in his hospital course, he was admitted to the intensive care unit, and was found to have significant abdominal distension with large amounts of simple fluid on bedside ultrasound. Bedside paracentesis returned succus and enteric feeds, and a methylene blue test confirmed a likely gastrointestinal perforation. The patients’ family refused surgical intervention and the patient underwent bedside drainage. This case represents several critical dilemmas clinicians faced during the recent surge of the COVID-19 pandemic.
Keywords: COVID-19, general surgery, critical care, duodenal perforation
Introduction
Patients infected with SARS-CoV-2, the causative virus behind the Corona Virus Disease–19 (COVID-19) pandemic, have been increasing rapidly in New York City. At last count, approximately 45% of deaths from COVID-19 in the United States were in New York City.1 Our medical center, a tertiary academic medical center serves three of the top seven zip codes in New York City for COVID-19 incidence. Due to the large surge of COVID-19 patients, we expanded our intensive care unit bed capacity and re-assigned faculty, residents, and staff from the Department of Surgery to care for some of these patients in an intensive care setting. The gastrointestinal tract is a key site of infection in SARS-CoV-2. Data on the surgical complications of SARS-CoV-2 infection in the gastrointestinal tract are limited to few case reports.2,3 We present a case of gastrointestinal perforation in a patient undergoing treatment for COVID-19. This case highlights the diagnostic barriers and management dilemmas of acute gastrointestinal complication in critically ill patients during the COVID-19 pandemic.
Case presentation
A 74-year-old male was referred to our emergency department with 8–10 days of worsening shortness of breath, fever, and dry cough. The patient had no prior medical history, took no medications at home, and had never had an endoscopy. Physical examination was significant for respiratory distress with bilateral crackles and oxygen saturation of 70% on room air requiring the initiation of high flow nasal cannula for oxygen support. Chest x-ray on presentation demonstrated diffuse bilateral ground glass opacities and a left-sided consolidation consisted with viral pneumonia secondary to COVID-19. SARS-CoV-2 reverse transcription–polymerase chain reaction (RT-PCR) viral testing was positive. His initial electrolytes, kidney functions, and complete blood count were within the normal range, serum albumin on presentation was 2.3 mg/dL. He was placed on hydroxychloroquine, azithromycin, and ceftriaxone empirically. The patient was admitted to an intensive care unit. Of note, he reported significant hunger and asked several times for additional food.
On the second hospital day, the patient became increasingly hypoxic despite maximal non-invasive ventilatory support. He was intubated and placed on mechanical ventilation. He was placed on pantroprazole for GI prophylaxis.
Throughout hospital days 2 and 3, the patient showed signs of acute kidney injury with metabolic acidosis. Vasopressors were administered as well as Bicarbonate drip. The patient gradually improved, requiring fewer vasopressors and ventilator support. Tube feeds were started on the fourth hospital day, and the patient appeared to tolerate feeding without issue.
On the evening of the fifth hospital day, the patient began to require higher levels of oxygenation to maintain saturation. His abdomen was noted to be acutely markedly distended. White blood cell count was elevated to 15,900 (94.4% Neutrophils). An abdominal x-ray remonstrated no evidence of air or stool in the rectum, a paucity of intestinal gas, and no evidence of obstruction (Figure 1). Chest x-ray demonstrated a small amount of pneumoperitoneum which was not present in the previous day chest x-ray. As the patient had worsening hypoxia with maximal ventilator support, CT scan was deferred. A bedside ultrasound was performed which demonstrated significant amounts of hypoechoic fluid. Bedside abdominal paracentesis was performed under ultrasound guidance, and a catheter was placed in the right lower quadrant. One liter of cloudy, milky-appearing fluid was aspirated immediately, and the catheter was left in place connected to a drainage bag. The fluid was sent for gram stain and culture. Analysis of this fluid revealed no neutrophils, 4000 red blood cells (pHF), lactate dehydrogenase of 3791 (U/L), and an amylase of 871 (U/L).
Figure 1.
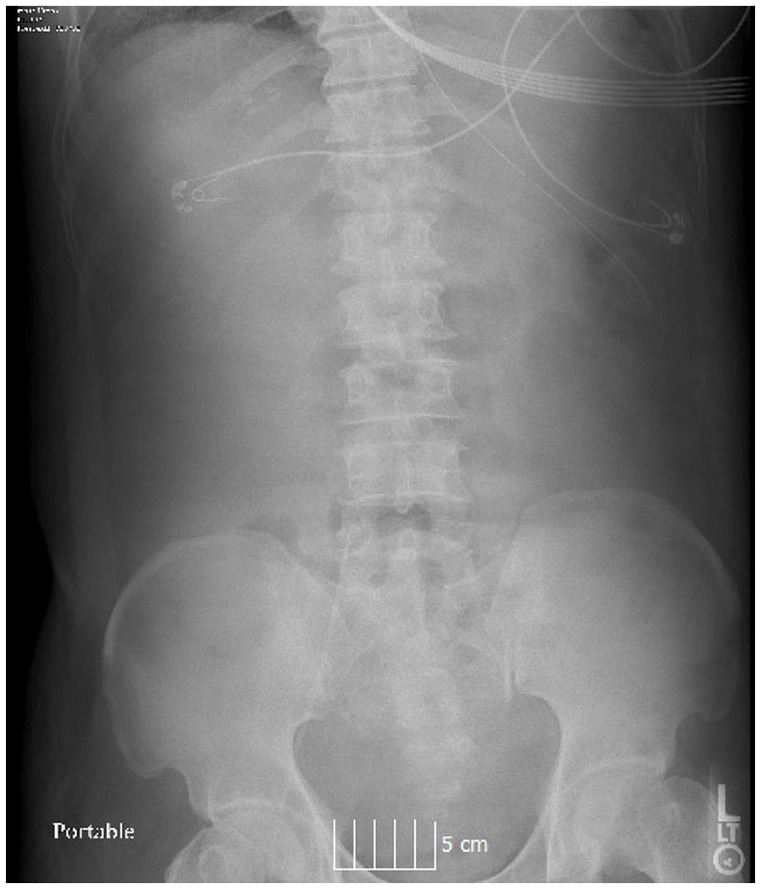
Flat plate abdominal radiograph demonstrating collapsed bowel without evidence of intestinal obstruction.
Due to the appearance of the fluid, which was similar to the tube feeds the patient had started the day before, and the fact that the fluid did not contain white blood cells or bacteria, it was suspected that the patient may have proximal gastrointestinal perforation. For confirmation, methylene blue was administered via the nasogastric tube (NGT) and was noted to appear in the drainage bag rapidly. The patient was made NPO (nothing by mouth), and broad spectrum antibiotic therapy was initiated in order to cover for any enteric anaerobic bacteria and fungi. Given the patients critical condition, we elected to offer the patient’s family surgical exploration for diagnosis and treatment of gastrointestinal perforation. However, the patient’s family elected to proceed with non-operative management. Following the drainage and the conservative treatment, over the next day, the patient’s vasopressor and oxygen requirements improved dramatically.
On hospital days 6 and 7, the amount of fluid noted in the drainage bag decreased. Bile was noted in the NGT. Repeated bedside ultrasound did not demonstrate re-accumulation of fluid. The patient remained stable. Unfortunately, over the next several days, the patient’s respiratory status began to decline and he demonstrated a clinical picture typical to severe COVID-19 infection with extreme respiratory failure. He further required renal replacement therapy, and expired on hospital day 9. Due to hospital policy during the pandemic surge, we could not perform autopsy.
Discussion
Surgical complications of gastrointestinal disorders may be challenging to diagnose in critically ill intubated patients. This report highlights several factors pertaining to the diagnosis and management of upper gastrointestinal perforation during the recent COVID-19 pandemic.
Etiology
Despite a primary diagnosis of COVID-19 associated acute respiratory failure, our patient presented with a surgical complication of presumed gastric or duodenal ulcer, which likely worsened and perforated on the evening of his fifth day of admission. Several contributing factors are present which may have influenced his eventual pathology. Stress-related mucosal damage, traditionally referred to as stress ulceration or ICU ulceration is significantly worsened in the absence of enteral feeding.4 It has been anecdotally observed that patients with COVID-19 infection become significantly constipated in the intensive care unit, which may contribute to increased intraluminal pressure. Although the patient suffered from a presumed complication of ulcer disease, our patient’s death was likely due to severe acute respiratory distress syndrome (ARDS) secondary to a systemic infection with SARS-CoV-2. We suggest that conservative management of his perforated viscous may have briefly ameliorated his initial presentation; however, it further deteriorated his COVID-19 pneumonia–associated multi-organ failure.
The gastrointestinal tract is a key site of infection in SARS-CoV-2. A key mechanism to infection in the GI tract is high expression of the angiotensin converting enzyme (ACE) 2 receptor. SARS-CoV-2 binds to this receptor in order to gain entrance to host cells.5 The highest staining of this receptor was found in one study to be in the epithelium of the stomach, duodenum, and rectum.6 Diarrhea is a common symptom of mild to moderate COVID-19, and several studies have demonstrated the detectable presence of coronavirus RNA in stool of infected patients.7,8 Henry et al.,9 in a systemic review and meta-analysis, found that severity of gastrointestinal symptoms may be correlated to severity of COVID-19. The authors suggest that this increase may be due to more rapid replication of SARS-CoV-2 in intestinal epithelium. Data on surgical complication of gastrointestinal disease during the COVID-19 pandemic are limited. DeNardi et al.3 report a case of patient with colonic perforation presumed to be secondary to over-distension of bowel. Lin et al.2 reported a case of esophageal erosions and bleeding in a small series of six patients undergoing endoscopy for upper GI symptoms. The study noted that SARS-CoV-2 RNA was detected in the epithelium of the esophagus, stomach, duodenum, and rectum of two patients with “severe” symptoms.
Diagnosis management challenges
The diagnosis of perforated ulcer is typically based on history and physical exam. Often, radiographic evidence of pneumoperitoneum in the setting of a clinical finding of peritonitis, and a typical history, is considered sufficient for diagnosis. Treatment typically consists of surgical exploration with abdominal washout and either a graham or thal patch to close the defect. Current World Society of Emergency Surgery (WSES) guidelines recommend against non-operative management of perforated ulcer except in situations where spontaneous sealing of the perforation is documented by upper gastrointestinal contrast imaging.10 Due to the instability of the patient, a CT scan could not be safely performed. In accordance with WSES for resource limited settings where CT is not available, we performed both a flat plain abdominal x-ray and a diagnostic ultrasound. Due to constraints on resources available at the height of the COVID-19 pandemic in our institution at the epicenter, additional tests, such as upper GI series or small bowel follow-through oral contrast imaging were limited. We chose to perform a methylene blue test to confirm a perforation as it could be easily performed at the bedside and can contribute in diagnosing upper gastrointestinal perforations. As the family elected to proceed with non-operative management, we elected to drain the patient at the bedside and proceed with non-operative management.
Non-operative management of a perforated ulcer was described first in 1946 by Taylor, who found that mortality was 14% in patients treated with NGT decompression and bowel rest versus 20% in laparotomy.11 More recent studies have found lower mortality, approximately 5% in both groups.12 However, in some stable patients with a contained perforation or an absolute contraindication to surgery, non-operative management may be attempted.10 Few reports describe endoscopic management of perforated duodenal ulcer accompanied by percutaneous drainage, however the practice has not been widely accepted and this patient would not be considered a candidate for endoscopy given the presence of COVID-19.13
In the light of limitations on diagnostic tests and the limited resources in the setting of the COVID-19 pandemic, this case highlights the value of history and clinical exam in the diagnosis of acute surgical pathology in the critically ill intubated patient. This patient’s initial desire for food may have been consistent with an underlying ulcer, as classically discomfort associated with a duodenal ulcer is said to improve after meals. In addition, the presence of sudden abdominal distension with a temporal relation to the initiation of feeds, coupled with hypoechoic fluid on bedside ultrasound, prompted a quick and simple intervention to safely achieve source control, as evidenced by the patients’ clinical improvement in the following days. The co-existence of abdominal distension in many of the COVID-19 critically ill patients who are sedated and paralyzed makes the diagnosis of abdominal catastrophe extremely difficult. Clinicians should be aware of these conditions and trust their clinical judgment as frequently they will need to base the diagnosis on simple bedside procedures given the acuity of these patients as well as the limited resources during the COVID-19 pandemic.
Conclusion
While likely rare, the co-incidence of emergency surgical pathology such as a perforated ulcer and COVID-19 infection presents a unique challenge to the clinician. While unfortunately our patient ultimately expired, early recognition of a surgical pathology and swift, creative intervention led to a temporary, but significant improvement in his clinical condition.
Acknowledgments
The authors wish to acknowledge the extraordinary devotion of the staff, nurses, residents, and faculty of Maimonides Medical Center, who have risen to answer the call of duty in service of the people of Brooklyn.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written Informed consent for patient information to be published in this article was obtained retroactively from legally authorized representative, as patient had expired and thus was not able to give consent directly.
ORCID iD: Aaron Kangas-Dick  https://orcid.org/0000-0003-1264-2054
https://orcid.org/0000-0003-1264-2054
References
- 1. Worldometers. COVID-19 pandemic. https://www.worldometers.info/coronavirus/ (Accessed May 21, 2020).
- 2. Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020; 69(6): 997–1001. [DOI] [PubMed] [Google Scholar]
- 3. De Nardi P, Parolini DC, Ripa M, et al. Bowel perforation in a Covid-19 patient: case report. Int J Colorectal Dis. Epub ahead of print 27 May 2020. DOI: 10.1007/s00384-020-03627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plummer MP, Blaser A, Deane AM. Stress ulceration: prevalence, pathology and association with adverse outcomes. Crit Care 2014; 18(2): 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. Epub ahead of print 25 March 2020. DOI: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 6. Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. Epub ahead of print 12 March 2020. DOI: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. Epub ahead of print 19 February 2020. DOI: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020; 9(1): 386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henry BM, de Oliveira MHS, Benoit J, et al. Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Intern Emerg Med. Epub ahead of print 17 April 2020. DOI: 10.1007/s11739-020-02329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarasconi A, Coccolini F, Biffl WL, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg 2020; 15: 3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor H. Perforated peptic ulcer treated without operation. Lancet 1946; 248(6422): 441–444. [DOI] [PubMed] [Google Scholar]
- 12. Crofts TJ, Park KGM, Steele RJC, et al. A randomized trial of nonoperative treatment for perforated peptic ulcer. N Engl J Med 1989; 320(15): 970–973. [DOI] [PubMed] [Google Scholar]
- 13. Bergström M, Vázquez J, Park P-O. Self-expandable metal stents as a new treatment option for perforated duodenal ulcer. Endoscopy 2012; 45(3): 222–225. [DOI] [PubMed] [Google Scholar]


