Abstract
Background:
Hepatic fibrosis is the result of chronic liver injury that can progress to cirrhosis and lead to liver failure. Nevertheless, there are no anti-fibrotic drugs licensed for human use. Here, we investigated the anti-fibrotic activity of GNS561, a new lysosomotropic molecule with high liver tropism.
Methods:
The anti-fibrotic effect of GNS561 was determined in vitro using LX-2 hepatic stellate cells (HSCs) and primary human HSCs by studying cell viability, activity of caspases 3/7, autophagic flux, cathepsin maturation and activity, HSC activation and transforming growth factor-β1 (TGF-β1) maturation and signaling. The contribution of GNS561 lysosomotropism to its anti-fibrotic activity was assessed by increasing lysosomal pH. The potency of GNS561 on fibrosis was evaluated in vivo in a rat model of diethylnitrosamine-induced liver fibrosis.
Results:
GNS561 significantly decreased cell viability and promoted apoptosis. Disrupting the lysosomal pH gradient impaired its pharmacological effects, suggesting that GNS561 lysosomotropism mediated cell death. GNS561 impaired cathepsin activity, leading to defective TGF-β1 maturation and autophagic processes. Moreover, GNS561 decreased HSC activation and extracellular matrix deposition by downregulating TGF-β1/Smad and mitogen-activated proteine kinase signaling and inducing fibrolysis. Finally, oral administration of GNS561 (15 mg/kg per day) was well tolerated and attenuated diethylnitrosamine-induced liver fibrosis in this rat model (decrease of collagen deposition and of pro-fibrotic markers and increase of fibrolysis).
Conclusion:
GNS561 is a new potent lysosomotropic compound that could represent a valid medicinal option for hepatic fibrosis treatment through both its anti-fibrotic and its pro-fibrolytic effects. In addition, this study provides a rationale for targeting lysosomes as a promising therapeutic strategy in liver fibrosis.
Keywords: autophagy, cathepsin, GNS561, liver fibrosis, lysosomotropism, TGF-β1
Introduction
Chronic liver diseases are a major global health issue causing approximately two million deaths per year worldwide.1 Hepatic fibrogenesis results from repeated liver injury due to a large variety of factors, such as alcohol abuse, chronic viral infections, metabolic syndromes and genetic disorders.1 When injury persists, fibrosis progresses into liver cirrhosis and is usually accompanied by a broad spectrum of complications such as liver failure, hepatocellular carcinoma (HCC) and death.1
Activation of hepatic stellate cells (HSCs) is a central event in fibrogenesis and liver fibrosis progression.2 In normal liver tissue, HSCs are quiescent; however, in injured liver tissue, quiescent HSCs transdifferentiate into activated HSCs, which exhibit a myofibroblast-like phenotype and generate cytokines and growth factors, such as transforming growth factor-β1 (TGF-β1).2 Sustained HSC activation and proliferation lead to excessive accumulation of extracellular matrix (ECM) components. Therefore, inhibition of the activated HSC accumulation by modulating either their activation and/or proliferation and/or by promoting HSC apoptosis is an interesting therapeutic strategy for the resolution and the treatment of liver fibrosis.2–4
To date, the most effective approach to prevent and treat liver fibrosis is to remove or ameliorate the causative agents, such as alcohol abstinence after alcoholic liver diseases.4 However, such treatments remain unrealistic for patients with liver fibrosis due to other causes, such as genetics, autoimmune liver diseases or advanced non-alcoholic steatohepatitis.
Although many advances have been made in understanding the pathogenesis of hepatic fibrosis, there are no specific Food and Drug Administration-approved anti-fibrotic drugs and scarcely any effective treatments.5 The major encountered problem in treating liver fibrosis is hepatic fibrosis and cirrhosis progression over a long period of time, which modifies liver vascularization, ECM composition and drug metabolism. Therefore, any anti-fibrotic treatment should be tolerable and specifically target the liver;6 however, none of the current therapeutic arsenal address these issues. Consequently, there is an urgent need for new, effective and safe therapies.
GNS561, a new orally available anticancer drug with high liver tropism,7 is currently being assessed in patients with advanced liver cancer in a global clinical phase Ib/IIa clinical trial,8 but its effect on liver fibrosis has never been addressed. In this study, we evaluated the anti-fibrotic activity of GNS561 both in vitro and in vivo and investigated its underlying mechanism.
Materials and methods
Details of the materials and employed methods are provided in the Supplemental Material online.
Cell culture
LX-2 cells (an immortalized human HSC) were supplied by Merck Millipore (Burlington, MA, USA). Primary human HSCs, liver-specific mesenchymal cells, were supplied by iXCells Biotechnologies (San Diego, CA, USA). These primary human HSCs are low passaged-cells and were used only at early passages, below 5. LX-2 were cultured using DMEM high glucose with stable glutamine (Dutscher, Brumath, France) and human HSCs were cultured using DMEM high glucose (Life Technologies, Carlsbad, USA) supplemented with 1% L-glutamine (Life Technologies). Both media were supplemented with 1% penicillin-streptomycin (Dutscher) and 10% fetal bovine serum (HyClone, Logan, UT, USA). Cells were maintained at 37°C in the presence of 5% CO2 and 95% air in a humidified incubator.
Rat model
All animals received humane care in accordance with the Guidelines on the Humane Treatment of Laboratory Animals (Directive 2010/63/EU), and experiments were approved by the animal Ethics Committee: GIN Ethics Committee n°004. Fourteen 6-week-old Fischer 344 male rats (Charles River, Wilmington, MA, USA) were housed in the Plateforme de Haute Technologie Animale animal facility (Jean Roget, University of Grenoble-Alpes, France). All rats were treated weekly with intra-peritoneal injections of 50 mg/kg of diethylnitrosamine (DEN) (Sigma-Aldrich), which were diluted in olive oil to obtain a cirrhotic liver with hepatocellular carcinoma after 14 weeks.9 After 6 weeks of treatment with GNS561 or vehicle, animals were anesthetized with isoflurane and euthanized with vena cava blood sampling.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.2.1 (GraphPad Software Inc., La Jolla, CA, USA). For small datasets (<5, such as western blotting and real-time polymerase chain reaction (PCR) datasets), median and 95% confidence interval were calculated and used to compare different groups. For datasets (>5) with normal distribution, means were compared using one-way ANOVA with Dunnett’s post hoc analysis. The parametric paired t-test was used to compare two paired groups of data with normal distribution. The Mann–Whitney two tailed test was used to compare two unpaired groups of data without normal distribution. Data are presented as the mean values ± standard error of the mean (SEM), except for small data sets (<5, such as western blotting and real-time PCR datasets), for which data are presented as median values surrounded by upper and lower confidence limits (95%). Each p-value is adjusted to account for multiple comparisons. Statistical significance was defined as p-values < 0.05.
Results
GNS561 decreases cell viability and induces apoptosis in HSC
The human HSC line LX-2 has been widely characterized. It is a low-passaged human HSC line derived from normal human HSCs that are spontaneously immortalized.10 The cells exhibit the typical key features of hepatic stellate cytokine signaling, retinoid metabolism and fibrogenesis, making it a very suitable model of human hepatic fibrosis. In addition, the phenotype of this cell line is most similar to that of ‘activated’ cells in vivo.10,11
To explore the effects of GNS561, a new potent lysosomotropic compound on liver fibrosis, we first conducted in vitro studies using LX-2 cells. We showed that GNS561 induced death of LX2 cells in a concentration-dependent manner [Figure 1(a), left] after 24 h of treatment. This effect of GNS561 on cell viability was confirmed in primary human HSCs isolated directly from human liver tissues (Figure 1(a), right). The half maximal inhibitory concentration (IC50) at 72 h was determined to be 2.29 ± 0.11 µM in LX-2 cells (Supplemental Figure S1).
Figure 1.
GNS561 decreases cell viability and induces apoptosis via caspase activation. (a) Viability percent of LX-2 cells (left) and primary human hepatic stellate cells (HSC) (right) against vehicle condition after 24 h of treatment with GNS561 (mean of three experiments + SEM). (b) Immunoblotting of the cleaved and non-cleaved forms of poly (ADP-ribose) polymerase (PARP) after 24 h of GNS561 treatment on LX-2 cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoblotting was used as a loading control. (c) Cell viability percent (full line) and fold change of activation of caspases 3/7 (dotted line) against vehicle condition after 8 h (left), 24 h (middle) and 30 h (right) of treatment with GNS561 of LX-2 cells (mean of three experiments + SEM).
As HSC apoptosis is described as a vital mechanism that contributes to recovery from hepatic fibrosis,3 we investigated apoptosis in the LX-2 cell line after GNS561 treatment. Specifically, we performed western blotting analyses of cleaved poly(ADP-ribose) polymerase (PARP) [Figure 1(b)], which is commonly used as a marker of cells undergoing apoptosis.12 Specific bands corresponding to full length PARP were clearly detected in all tested conditions, while cleaved PARP was not visible under the control condition (0 µM GNS561) and after 1.5 µM GNS561 treatment. In contrast, cleaved PARP was undoubtedly detected in cells treated during 24 h with 3 µM and 6 µM GNS561. Next, we further examined whether GNS561-induced apoptosis was related to caspase activation. After 8 h of cell exposure, GNS561 had little or no effect on the activity of caspases 3/7 or on cell viability [Figure 1(c), left]. In contrast, GNS561 induced the activation of caspases 3/7 after 24 h of treatment [Figure 1(c), middle], and this activation was sustained at 30 h [Figure 1(c), right]. The activation of these apoptotic executioner caspases was concomitant with a decrease in cell viability [Figure 1(c)]. In addition, we quantified reactive oxidative species (ROS) after GNS561 treatment of LX-2 cells during 24 h. We did not detect a significant change in ROS levels after GNS561 treatment (Supplemental Figure S2), demonstrating that the pro-apoptotic activity of GNS561 was not associated with the induction of intracellular oxidative stress in LX-2 cells.
The anti-fibrotic effect of GNS561 depends on its lysosomotropism in LX-2 cells
Previously, we showed that the antitumor activity of GNS561 against intrahepatic cholangiocarcinoma was related to its lysosomotropism.7 Here, we examined whether its lysosomotropism contributed to its anti-fibrotic properties on HSC. For this purpose, LX-2 cells were pre-treated for 2 h with a specific vacuolar-type H+-ATPase inhibitor (bafilomycin) or with a weak base, NH4Cl, then treated with GNS561 during 24 h. Therefore, disrupting the lysosomal pH gradient by either bafilomycin or by NH4Cl partially protected LX-2 against GNS561-induced cell death (Figure 2). These results imply that the GNS561-mediated anti-fibrotic properties on HSC is at least partially caused by its lysosomotropic properties.
Figure 2.

GNS561-induced cell death depends on its lysosomotropism in LX-2 cells. Cell viability percent against vehicle condition after 24 h of treatment with GNS561 in the presence or absence of bafilomycin A1 (Baf) (a) or NH4Cl (b) (mean + SEM of three experiments).
****p < 0.0001 (parametric paired t-tests).
GNS561 inhibits cathepsin activity, which leads to impairment of autophagic flux and TGF-β1 maturation in LX-2 cells
The lysosomal-dependent cell death induced by GNS561 prompted us to evaluate the capacity of GNS561 to modulate lysosomal functions. We therefore examined the enzymatic activity of the two prominent and ubiquitous lysosomal cysteine proteinases cathepsin B (CTSB) and cathepsin L (CTSL), and one aspartic proteinase, cathepsin D (CTSD). After 24 h of treatment, GNS561 significantly decreased the proteolytic activity of cathepsins in a dose-dependent manner [Figure 3(a)].
Figure 3.
GNS561 inhibits cathepsin activity, leading to defective autophagic flux and transforming growth factor-β1 (TGF-β1) maturation impairment in LX-2 cells. LX-2 cells were treated with vehicle (0 µM) or with the indicated concentrations of GNS561 for 24 h. (a) Peptidase activity of cysteine cathepsins (including both cathepsins B and L) (CTSB/L), cathepsin B (CTSB) and cathepsin D (CTSD). Fold change of the peptidase activity was calculated in comparison with vehicle condition (mean + SEM of three experiments). **p < 0.01 and ****p < 0.0001 significant versus vehicle condition (Dunnett’s test). Western blotting of procathepsin B (immature form) and mature CTSB (b), of procathepsin L (immature form) and mature CTSL (c) and of procathepsin D, intermediate and mature CTSD (d). (e) Western blotting analysis of light chain 3 phosphatidylethanolamine conjugate (LC3-II) levels in the presence or absence of bafilomycin A1 (Baf) (100 nM, 2 h). In each lane, the autophagic flux, determined as the ratio between the LC3-II level normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) level (Norm LC3-II) with Baf and without Baf is presented. (f) Western blotting analysis of latency-associated peptide (LAP) in its native pro-TGF-β1 (upper bands) and cleaved LAP (lower bands) forms. The LAP cleavage ratio was determined as the ratio between the LAP level and the pro-TGF-β1 level for each condition in comparison with the ratio obtained for the vehicle condition. The data are presented as median values of three separate experiments surrounded by upper and lower confidence limits (95%). For all blots, GAPDH immunoblotting was used as a loading control.
Cathepsins are synthesized as inactive zymogens, which are converted to their active mature forms by other proteases or by autocatalytic processing.13,14 As depicted in Figure 3(b), GNS561 did not impact CTSB maturation while it impaired the maturation of CTSL and CTSD [Figure 3(c) and (d)]. Thus, the abnormal accumulation of pro-cathepsins following treatment with GNS561 suppressed normal processing of lysosomal enzymes and lysosomal degradation.
As GNS561 induced lysosomal dysfunction, the effect of GNS561 on the autophagic process was investigated. GNS561-induced accumulation of light chain 3 phosphatidylethanolamine conjugate was not enhanced in the presence of bafilomycin [Figure 3(e)], supporting the ability of GNS561 to inhibit degradation of the autophagic content.
TGF-β1, an important pro-fibrogenic cytokine, is synthesized in a precursor form that is modified intracellularly prior to secretion.15,16 One of the most relevant intracellular modifications is the cleavage of the C-terminal pro-region, referred to as the latency-associated peptide (LAP), from the N-terminal portion of the protein, which is thus called mature TGF-β1. It has been reported that CTSB is implicated in the maturation of intracellular pro-TGF-β1.17 Therefore, we investigated the effect of GNS561 treatment on pro-TGF-β1 processing. As seen in Figure 3(f), GNS561 treatment of LX-2 led to a significant decrease in the intensity of the bands migrating at 32.5 kDa, corresponding to LAP. The results indicated that GNS561 downregulated the pro-TGF-β1 processing and the level of mature TGF-β1.
GNS561 prevents HSC stimulation and ECM deposition
It is well described that inhibition of HSC activation can be achieved by blockage of the autophagic process.18–20 As GNS561 inhibited autophagic flux, we next assessed HSC activation in the presence of GNS561. To explore whether GNS561 treatment suppressed the TGF-β1-induced fibrogenic response,21–26 we analyzed the mRNA and protein levels of phenotypic markers of HSC activation including alpha smooth muscle actin (α-SMA) and collagen type I alpha 1 chain (COL1A1) by real-time quantitative PCR (RT-qPCR) and western blotting in serum-starved LX-2 cells treated with GNS561 for 24 h and stimulated with TGF-β1 for 22 h. As expected, TGF-β1 increased LX-2 activation, as indicated by enhanced mRNA expression of α-SMA and COL1A1, while this activation was suppressed in the presence of GNS561 [Figure 4(a) and (c)]. A similar effect was observed at the protein level in LX-2 cells: GNS561 decreased TGF-β1-induced α-SMA and COL1A1 protein synthesis in a dose-dependent manner [Figure 4(b) and (d)]. This drop was observed to a larger and significant extent for COL1A1. This effect of GNS561 on HSC activation was confirmed using primary human HSCs isolated directly from human liver tissues (Supplemental Figure S3). Interestingly, we showed that GNS561 was able to decrease basal activation of LX-2 cells, as indicated by a drop of mRNA expression of α-SMA and COL1A1 after treatment with GNS561 (Supplemental Figure S4).
Figure 4.
GNS561 inhibits hepatic stellate cell stimulation and ECM deposition in LX-2. Cell lysates were prepared after both GNS561 treatment (24 h) and transforming growth factor-beta 1 (TGF-β1) stimulation (22 h, 5 ng/μL) and analyzed by real-time polymerase chain reaction (PCR) and western blotting. mRNA (a and c) and protein (b and d) fold changes of alpha smooth muscle actin (α-SMA) (a and b) and collagen type I alpha 1 chain (COL1A1) (c and d) were measured in comparison with the untreated condition (neither GNS561 nor TGF-β1 stimulation). The mRNA levels of TGF-β1 (e) and metalloproteinases (MMPs) 2 and 9 (f) and tissue inhibitors of MMP (TIMP) 1 and 3 (f) were analyzed using real-time PCR and compared against the untreated condition (neither GNS561 nor TGF-β1 stimulation). The data are presented as median values of three separate experiments surrounded by upper and lower confidence limits (95%). For all blots, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoblotting was used as a loading control.
As it is known that TGF-β1 is a key pro-fibrogenic cytokine from both paracrine and autocrine sources25–27 we investigated whether GNS561 treatment impacted TGF-β1 mRNA expression in LX-2 cells. As seen in Figure 4(e), GNS561 decreased TGF-β1 mRNA level.
As TGF-β1 provokes the remodeling and accumulation of the ECM by modulating downstream target genes such as matrix metalloproteinases (MMPs) and tissue inhibitors of MMP (TIMPs),16,22,28 we investigated MMP-2, MMP-9, TIMP-1 and TIMP-3 mRNA expression levels following GNS561 treatment [Figure 4(f)]. We found that TGF-β1 stimulated MMP-2, TIMP-1 and TIMP-3 mRNA levels, but it had no effect on MMP-9 mRNA level. In contrast, the addition of GNS561 significantly decreased MMP-2 and TIMP-3 mRNA levels and induced a biphasic response for MMP-9 and TIMP-1 mRNA expression: a maximum mRNA level increase was observed with a 1.5 µM treatment, but these levels then decreased with higher concentration treatments [Figure 4(f)].
GNS561 decreases TGF-β1-inducted pathways
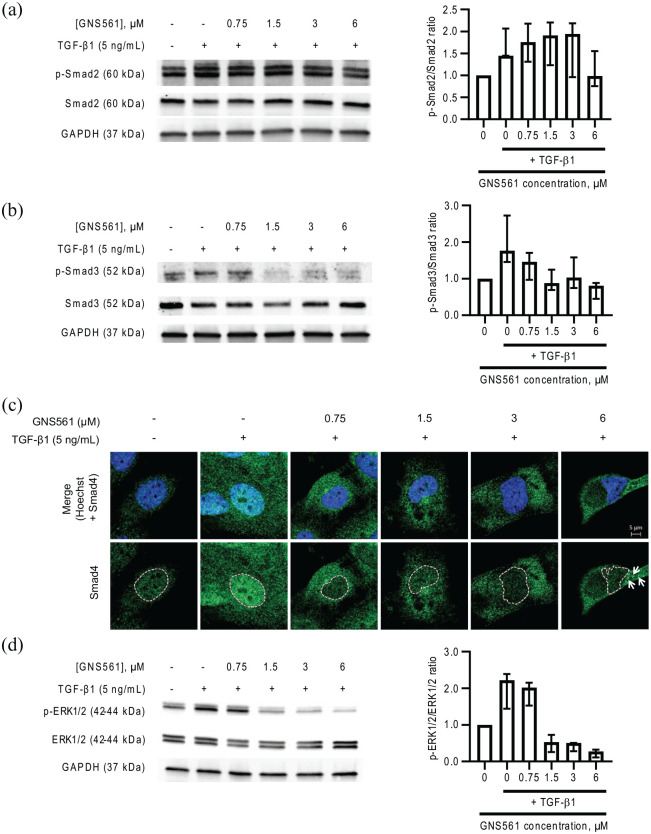
We observed that GNS561 reduced the mRNA/protein expression of several hepatic fibrogenic markers (α-SMA, COL1A1, TGF-β1, TIMP and MMP), implying that its inhibitory effect may lie upstream of such gene transcription. Thus, to elucidate the mechanism underlying the impairment of HSC activation by GNS561, we investigated the effect of GNS561 on TGF-β1-induced pathways. As Smad proteins are the major transducers of TGF-β1 signaling through receptor-associated phosphorylation,27 we measured the Smad2/3 phosphorylation after TGF-β1 treatment in the presence or absence of GNS561. As indicated in Figure 5(a) and (b), TGF-β1 treatment induced an increase in phosphorylated Smad2 and phosphorylated Smad3 (p-Smad3). These increases were attenuated by pre-treatment with GNS561, which was ob-served to a larger and significant extent for p-Smad3 (Figure 5b).
Figure 5.
GNS561 downregulates transforming growth factor-beta 1 (TGF-β1)/Smad and mitogen-activated protein kinase signaling in LX-2 cells. Cell lysates were prepared after both GNS561 treatment (24 h) and TGF-β1 stimulation (22 h, 5 ng/μL). Phosphorylated (p-Smad2) and total Smad2 (a) and phosphorylated (p-Smad3) and total Smad3 (b) and phosphorylated (p-Erk2/3) and total Erk2/3 (d) were analyzed by western blotting. For all blots, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoblotting was used as a loading control. The p-Smad/Smad and p-ERk1/2/Erk1/2 ratios were determined as the ratio between the phosphorylated protein and total protein level for each condition in comparison with the ratio obtained for the untreated condition (neither GNS561 nor TGF-β1 stimulation). The data are presented as median values of four separate experiments surrounded by upper and lower confidence limits (95%). (c) Smad4 localization was determined using immunofluorescence assay. Nucleus was stained with Hoechst. Arrows show cytosolic clumps of Smad4. Scale bar represents 5 µm.
Localization of Smad4 and its ability to bind directly to target gene promoters are crucial for TGF-β1 signaling. Indeed, once Smad2 and Smad3 are phosphorylated, they both form a complex with Smad4 which translocates to the nucleus.22 As GNS561 decreased Smad2 and Smad3 phosphorylation, we monitored Smad4 localization in LX-2 cells after GNS561 treatment and TGF-β1 activation using immunofluorescence assays. As seen in Figure 5(c), TGF-β1 stimulation increased Smad4 nuclear level compared with the untreated condition. In contrast, in GNS561 treated cells, the TGF-β1-induced Smad4 nuclear localization decreased in a dose-dependent manner. Concomitantly, an increase of Smad4 in cytosolic level can be observed in these conditions. For the highest concentration of GNS561, Smad4 was detected in cytosolic clumps [see arrows in Figure 5(c)]. Thus, our results confirm that TGF-β1/Smad signaling was influenced by GNS561 treatment.
Canonical Smad-mediated TGF-β1 signaling does not always explain all observed effects of TGF-β1. Other signaling pathways, such as mitogen-activated protein kinase (MAPK) signaling, could be also implicated.29 Thus, we explored the MAPK activation by analyzing p44/42 MAPK (Erk1/2) phosphorylation after TGF-β1 treatment in the presence or absence of GNS561 in LX-2 cells. The results clearly show that GNS561 decreased MAPK activation induced by TGF-β1 stimulation in a dose-dependent manner [Figure 5(d)].
All these findings indicate that GNS561 prevents TGF-β1-induced HSC activation by disruption of the TGF-β1/Smad signaling pathway but also the MAPK signaling pathway.
GNS561 attenuates DEN-induced fibrosis in rats
One of the well-established models that reproduces human cirrhosis and that can be employed for studying the molecular mechanism of fibrogenesis is DEN-injured rats.9,30 Therefore, we used the DEN-induced fibrosis rat model to test the anti-fibrotic effects of GNS561. Food consumption did not differ between the control and GNS561-treated (15 mg/kg) groups during the last 6 weeks of the experiment, and no body weight loss was observed in the GNS561-treated rats. GNS561 (15 mg/kg) was thus considered well tolerated in these fibrotic rats.
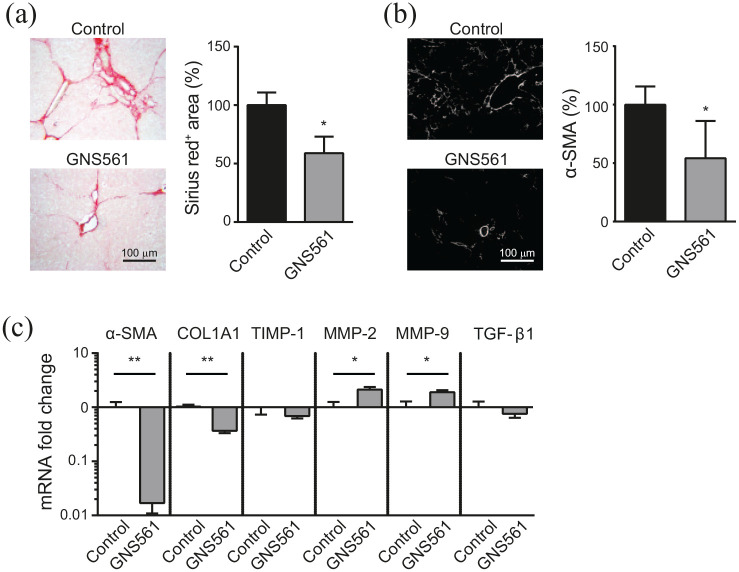
Liver fibrosis was first analyzed by staining of collagen fibers using Sirius red and by α-SMA staining. As shown in Figure 6(a), the fibrotic tissue area was reduced by 41% in the GNS561-treated group compared with the control group. The decrease of α-SMA staining in the GNS561-treated group compared with the control group indicated a reduction of activation status of HSC in the liver. These results were confirmed by the determination of gene expression changes using RT-qPCR analysis [Figure 6(c)]. As expected, GNS561 significantly reduced the α-SMA and COL1A1 mRNA expression levels compared with the control group. To explore the potential mechanisms of the protective effect of GNS561 in liver fibrosis, we investigated the effect of GNS561 on MMP-2 and MMP-9 mRNA expression, which participate in the regression of liver fibrosis through cleavage of the fibrillar ECM. Both MMP-2 and MMP-9 were significantly upregulated in the GNS561-treated group compared with the control group. Accordingly, TIMP-1 was slightly decreased compared with the control group. This effect on the matrix pathway in the liver was accompanied by a weak decrease of TGF-β1 level in the GNS561-treated group, but this decrease was not significant. Therefore, GNS561 significantly reduced hepatic collagen deposition and expression of pro-fibrogenic markers, as well as increased pro-fibrolytic proteins. All these GNS561 effects led to an improvement of liver fibrosis in these DEN-induced fibrotic rats.
Figure 6.
Effect of GNS561 treatment on liver fibrosis in a diethylnitrosamine (DEN)-injured rat model of fibrosis. DEN-injured rats were treated over 6 weeks by daily oral gavages of GNS561 (15 mg/kg of GNS561, GNS561 group) or vehicle (control group) (n = 8/group). (a) Representative histological images of livers stained with Sirius red (left). Data are presented as percent of Sirius red positive area compared with the control group (right). (b) Representative images of livers stained with alpha smooth muscle actin (α-SMA) (left). Data are presented as percent of α-SMA positive area compared with the control group (right). (c) Fold change of α-SMA, collagen type I alpha 1 chain (COL1A1), tissue inhibitor of matrix metalloproteinase 1 (TIMP-1), matrix metalloproteinase (MMP) 2 and 9 and transforming growth factor-beta 1 (TGF-β1) mRNA against the control group in liver tissue (mean + SEM). n = 7/group. *p < 0.05 and **p < 0.01 (Mann–Whitney two tailed tests).
Discussion
In this study, we demonstrated that GNS561 inhibited liver fibrosis in vitro by inducing HSC cell death and by preventing HSC activation in the LX-2 cell line and in primary human HSCs. The anti-fibrotic effect of GNS561 was validated in vivo in DEN-induced fibrosis rats, a well-established cirrhosis model that can be employed for studying the molecular mechanism of fibrogenesis.31,32 In this model, GNS561 induced a reduction in collagen deposition, as well as diminished expression of pro-fibrogenic markers and increased pro-fibrolytic proteins level. In addition, GNS561 was also shown to be safe at the active dose in this in vivo model. One limitation of this in vivo model is that chronic exposure to low doses of DEN, a liver carcinogen, causes hepatocellular damage and chronic liver injury that lead to HCC development.9,30,33 So, since we demonstrated that GNS561 has anti-neoplastic actions in intrahepatic cholangiocarcinoma,7 another rodent model, such as CCl4-induced liver fibrosis model, could be used to confirm anti-fibrotic activity of GNS561 in vivo and to dissociate the GNS561 effect on fibrosis and on tumor.
Abolition of GNS561-induced cell death by disruption of the lysosomal pH gradient confirmed that GNS561 lysosomotropism is responsible for its anti-fibrosis effect. This lysosomal-dependent cell death prompted us to evaluate the effects of GNS561 on lysosomal related processes. Therefore, we demonstrated that GNS561 induced an impairment of the peptidase activity of three major lysosomal proteinases, CTSB, CTSL, and CTSD. The abnormal accumulation of the zymogen forms of CTSL and CTSD following treatment with GNS561 could explain the impairment of their activity. Further studies are required to determine the causes of the decrease of CTSB activity.
Several studies have demonstrated that autophagy is closely linked with hepatic fibrosis.34,35 Specifically, it was reported that autophagy promotes fibrogenesis through the degradation of lipid droplets and that the release of lipids in HSCs provides energy for HSC activation.18 So, our findings that GNS561 inhibited the autophagic process combined with those from previous reports indicate that GNS561 may inhibit HSC activation through autophagic flux impairment.
TGF-β1 is secreted by activated HSC, which in turn stimulates the activation of HSC and promotes fibrosis progression.21–26 Here, we found that GNS561 inhibited the pro-TGF-β1 processing. This result is coherent with previous studies showing that CTSB is implicated in the maturation of the intracellular pro-TGF-β117,36 and our present results showing that GNS561 led to the impairment of CTSB activity.
The main downstream mediators of the TGF-β1 canonical pathway are Smad2 and Smad3.27 Whereas both Smad2 and Smad3 are strongly activated in hepatic fibrosis,19 only Smad3 appears to be a main factor responsible for TGF-β1-induced fibrosis by driving the expression of key ECM genes, especially in the induction of collagen expression.22,37 According to our results, GNS561 decreased Smad2/Smad3 phosphorylation, al-though this change was greater for Smad3. Smad3 is primarily responsible for the TGF-β1 autocrine production;38 therefore, the Smad3 phosphorylation impairment by GNS561 treatment could explain the downregulated GNS561-induced expression of TGF-β1 mRNA. Thus, the inhibitory effect of GNS561 in fibrotic liver tissue may be closely related to a decrease in TGF-β1 autocrine synthesis and mature TGF-β1 generation. The observed decrease of TGF-β1-induced Smad4 nuclear localization in GNS561-treated cells confirmed that canonical TGF-β1/Smad signaling was influenced by GNS561 treatment. Moreover, we showed that GNS561 also decreased the activation of MAPK, a non-canonical TGF-β1 pathway. These findings indicate that GNS561 prevents TGF-β1-induced HSC activation by disruption of the TGF-β1/Smad signaling pathway but also the MAPK signaling pathway.
So, based on our results, we could hypothesize that GNS561 might partly counteract hepatic fibrosis through a decrease in cathepsin activity, leading to: (1) weaker TGF-β1 maturation and the subsequent downregulation of the TGF-β1/Smad and MAPK signaling pathways and (2) defective autophagy flux and a lack of energy. All these GNS561-induced events lead to HSC activation impairment characterized by GNS561-induced expression decrease of pro-fibrogenic markers in vitro and in vivo. Consistent with this hypothesis, studies in several hepatic fibrosis experimental models concluded that cathepsins have a critical role in HSC activation.39–41 Indeed, it was previously shown that CTSB and CTSD silencing attenuates liver damage, reduces scarring and decreases HSC proliferation and fibrogenic gene levels (e.g. TGF-β1 and α-SMA). Moreover, in lung fibroblasts, pharmacological inhibition and genetic silencing of CTSB led to an accumulation of intracellular pro-TGF-β1, downregulated α-SMA expression and Smad2/3 phosphorylation and delayed fibroblast TGF-β1-driven differentiation.17
A main factor in progressive fibrosis is the ongoing loss in the ability to degrade the increased interstitial or scar matrix. Therefore, the balance between MMP and TIMP is critical in sustaining the intrahepatic homeostasis of the ECM.42–45 Upon HSC activation, MMP and TIMP levels change, leading to increased degradation of the normal liver matrix (non-fibrillar collagens) and decreased degradation of fibrillar collagens.46 During liver fibrosis resolution, the pattern reverses. Hence, in vitro, we found that GNS561 treatment significantly decreased MMP-2 and TIMP-3 mRNA levels and induced a biphasic response in MMP-9 and TIMP-1 mRNA expression. In the DEN-induced fibrosis model, both MMP-2 and MMP-9 mRNA were significantly upregulated in the GNS561-treated group compared with the control group. Accordingly, TIMP-1 was slightly decreased compared with the control group. These results suggest that GNS561 resolves fibrosis in part by inducing fibrolysis. The differences observed at the mRNA level between the in vitro and in vivo models could be explained by the dynamic regulation of MMP during various stages of liver fibrosis.46 We speculate that chronic treatment of animals leads to more advanced liver fibrosis resolution in DEN-induced fibrosis rats than the acute effects of GNS561 treatment in TGF-β1-stimulated LX-2 cells. Further studies are required to determine the effects of GNS561 on this dynamic MMP/TIMP process.
Another part of fibrosis resolution is the elimination of HSC by apoptosis.3 Thus, our study indicated that GNS561 induced the activation of pro-apoptotic caspases 3/7 concomitantly with a decrease in cell viability in the LX-2 cell line and primary human HSCs. Moreover, TIMP-1, MMP-2 and MMP-9 proteins have also been implicated in the regulation of HSC apoptosis: while TIMP-1 can inhibit HSC apoptosis, MMP-2 and MMP-9 can promote this process.28,47,48 Thus, GNS561 effects of MMP-2, MMP-9 (increase) and TIMP-1 (decrease) level may indirectly contribute to GNS561 anti-fibrotic activity by accelerating HSC apoptosis. In addition, it was reported that HSC apoptosis induces MMP-2 activation,49 which emphasizes the importance of this MMP in GNS561-induced fibrolysis.
Therefore, our results demonstrated that GNS561 has both anti-fibrotic and pro-fibrolytic effects (Figure 7). So, not only does GNS561 induce the apoptosis of HSC, but also GNS561 prevents HSC activation and decreases ECM deposition, by impairing cathepsin activity and autophagic flux and by disrupting TGF-β1 maturation and TGF-β1/Smad and MAPK signaling. GNS561 mediates a reduction in ECM deposition by both directly reducing type I collagen synthesis and indirectly removing the scar tissue through an increase of the net matrix protease activity (decreased TIMP-1 level and increased MMP-2 and MMP-9 levels). Then, this study provides a rationale for considering GNS561 as a potential option for hepatic fibrosis treatment and for targeting lysosomes as a promising therapeutic strategy in liver fibrosis. Moreover, results showing that GNS561 impairs TGF-β1 (maturation and signaling) and recent publications reporting that TGF-β blockage improves anti-PD-1- and anti-PD-L1-induced tumor regression50,51 strongly suggest that the combination of GNS561 with immunotherapy should be explored as a cancer therapy.
Figure 7.
GNS561 has both anti-fibrotic and pro-fibrolytic effects. In injured liver tissue, sustained hepatic stellate cell (HSC) proliferation and transforming growth factor-β1 (TGF-β1)-mediated activation of HSCs leads to excessive accumulation of extracellular matrix (ECM) components and fibrosis progression. GNS561 treatment induces the apoptosis of HSC, prevents HSC activation and decreases ECM deposition, by impairing cathepsin activity and autophagic flux and by disrupting TGF-β1 maturation and TGF-β1/Smad signaling; this leads to fibrosis regression.
Supplemental Material
Supplemental material, Supp_material_Bestion_et_al._review for GNS561 acts as a potent anti-fibrotic and pro-fibrolytic agent in liver fibrosis through TGF-β1 inhibition by Eloïne Bestion, Zuzana Macek Jilkova, Jean-Louis Mège, Marie Novello, Keerthi Kurma, Seyedeh Tayebeh Ahmad Pour, Gilles Lalmanach, Lise Vanderlynden, Lionel Fizanne, Firas Bassissi, Madani Rachid, Jennifer Tracz, Jérôme Boursier, Jérôme Courcambeck, Cindy Serdjebi, Christelle Ansaldi, Thomas Decaens, Philippe Halfon and Sonia Brun in Therapeutic Advances in Chronic Disease
Footnotes
Conflict of interest statement: EB, MN, FB, MR, JT, JC, CS, CA, PH and SB are employees of Genoscience Pharma. FB, CS, CA, PH and SB are shareholders of Genoscience Pharma. FB, MR, JC, PH and SB are co-inventors of a pending patent in fibrosis. The other authors declare that they have no conflicts of interest to report.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sonia Brun  https://orcid.org/0000-0001-8981-8957
https://orcid.org/0000-0001-8981-8957
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Eloïne Bestion, Genoscience Pharma, Marseille, France, IRD, MEPHI, IHU Méditerranée Infection, Aix Marseille Université, Marseille, France.
Zuzana Macek Jilkova, Institute for Advanced Biosciences, Research Center UGA/Inserm U 1209/CNRS 5309, La Tronche, France Université Grenoble Alpes, Faculté de médecine, France, Clinique Universitaire d’Hépato-gastroentérologie, Pôle Digidune, CHU Grenoble, France.
Jean-Louis Mège, IRD, MEPHI, IHU Méditerranée Infection, Aix Marseille Université, Marseille, France.
Marie Novello, Genoscience Pharma, Marseille, France.
Keerthi Kurma, Institute for Advanced Biosciences, Research Center UGA/Inserm U 1209/CNRS 5309, La Tronche, France Université Grenoble Alpes, Faculté de médecine, France, Clinique Universitaire d’Hépato-gastroentérologie, Pôle Digidune, CHU Grenoble, France.
Seyedeh Tayebeh Ahmad Pour, Institute for Advanced Biosciences, Research Center UGA/Inserm U 1209/CNRS 5309, La Tronche, France Université Grenoble Alpes, Faculté de médecine, France, Clinique Universitaire d’Hépato-gastroentérologie, Pôle Digidune, CHU Grenoble, France.
Gilles Lalmanach, INSERM, UMR1100, Centre d’Etude des Pathologies Respiratoires, Equipe «Mécanismes Protéolytiques dans l’Inflammation», Tours, France, Université de Tours, Tours, France.
Lise Vanderlynden, INSERM, UMR1100, Centre d’Etude des Pathologies Respiratoires, Equipe «Mécanismes Protéolytiques dans l’Inflammation», Tours, France, Université de Tours, Tours, France.
Lionel Fizanne, Laboratoire HIFIH, UPRES EA 3859, Université d’Angers, Angers, France.
Firas Bassissi, Genoscience Pharma, Marseille, France.
Madani Rachid, Genoscience Pharma, Marseille, France.
Jennifer Tracz, Genoscience Pharma, Marseille, France.
Jérôme Boursier, Laboratoire HIFIH, UPRES EA 3859, Université d’Angers, Angers, France.
Jérôme Courcambeck, Genoscience Pharma, Marseille, France.
Cindy Serdjebi, Genoscience Pharma, Marseille, France.
Christelle Ansaldi, Genoscience Pharma, Marseille, France.
Thomas Decaens, Institute for Advanced Biosciences, Research Center UGA/Inserm U 1209/CNRS 5309, La Tronche, France Université Grenoble Alpes, Faculté de médecine, France, Clinique Universitaire d’Hépato-gastroentérologie, Pôle Digidune, CHU Grenoble, France.
Philippe Halfon, Genoscience Pharma, 10 Rue d’Iéna, Marseille, 13006, France.
Sonia Brun, Genoscience Pharma, 10 Rue d’Iéna, Marseille, 13006, France.
References
- 1. Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019; 70: 151–171. [DOI] [PubMed] [Google Scholar]
- 2. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017; 121: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 2001; 21: 437–451. [DOI] [PubMed] [Google Scholar]
- 4. Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: it is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int 2018; 17: 192–197. [DOI] [PubMed] [Google Scholar]
- 5. Manka P, Zeller A, Syn WK. Fibrosis in chronic liver disease: an update on diagnostic and treatment modalities. Drugs 2019; 79: 903–927. [DOI] [PubMed] [Google Scholar]
- 6. Dietrich CG, Gotze O, Geier A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol 2016; 22: 72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brun S, Bassissi F, Serdjebi C, et al. GNS561, a new lysosomotropic small molecule, for the treatment of intrahepatic cholangiocarcinoma. Invest New Drugs. Epub ahead of print 20 February 2019. DOI: 10.1007/s10637-019-00741-3. [DOI] [PubMed] [Google Scholar]
- 8. ClinicalTrials.gov. Study of GNS561 in patients with liver cancer, https://clinicaltrials.gov/ct2/show/NCT03316222 (2018, accessed 18 December 2018).
- 9. Jilkova ZM, Kuyucu AZ, Kurma K, et al. Combination of AKT inhibitor ARQ 092 and sorafenib potentiates inhibition of tumor progression in cirrhotic rat model of hepatocellular carcinoma. Oncotarget 2018; 9: 11145–11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu L, Hui AY, Albanis E, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 2005; 54: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taimr P, Higuchi H, Kocova E, et al. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology 2003; 37: 87–95. [DOI] [PubMed] [Google Scholar]
- 12. Oliver FJ, de la Rubia G, Rolli V, et al. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem 1998; 273: 33533–33539. [DOI] [PubMed] [Google Scholar]
- 13. Kramer L, Turk D, Turk B. The future of cysteine cathepsins in disease management. Trends Pharmacol Sci 2017; 38: 873–898. [DOI] [PubMed] [Google Scholar]
- 14. Masson O, Bach AS, Derocq D, et al. Pathophysiological functions of cathepsin D: targeting its catalytic activity versus its protein binding activity? Biochimie 2010; 92: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 15. Khalil N. TGF-beta: from latent to active. Microbes Infect 1999; 1: 1255–1263. [DOI] [PubMed] [Google Scholar]
- 16. Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors 2011; 29: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kasabova M, Joulin-Giet A, Lecaille F, et al. Regulation of TGF-beta1-driven differentiation of human lung fibroblasts: emerging roles of cathepsin B and cystatin C. J Biol Chem 2014; 289: 16239–16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 2012; 142: 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu L, Zhang Q, Mo W, et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-beta1/Smads and PI3K/Akt pathways. Sci Rep 2017; 7: 9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thoen LF, Guimaraes EL, Dolle L, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol 2011; 55: 1353–1360. [DOI] [PubMed] [Google Scholar]
- 21. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016; 12: 325–338. [DOI] [PubMed] [Google Scholar]
- 22. Xu F, Liu C, Zhou D, et al. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem 2016; 64: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res 2012; 347: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabregat I, Moreno-Caceres J, Sanchez A, et al. TGF-beta signalling and liver disease. FEBS J 2016; 283: 2219–2232. [DOI] [PubMed] [Google Scholar]
- 25. Gressner AM, Weiskirchen R, Breitkopf K, et al. Roles of TGF-beta in hepatic fibrosis. Front Biosci 2002; 7: d793–d807. [DOI] [PubMed] [Google Scholar]
- 26. Friedman SL. Hepatic fibrosis – overview. Toxicology 2008; 254: 120–129. [DOI] [PubMed] [Google Scholar]
- 27. Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007; 56: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hemmann S, Graf J, Roderfeld M, et al. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol 2007; 46: 955–975. [DOI] [PubMed] [Google Scholar]
- 29. Dewidar B, Meyer C, Dooley S, et al. TGF-beta in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells 2019; 8: 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiffer E, Housset C, Cacheux W, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 2005; 41: 307–314. [DOI] [PubMed] [Google Scholar]
- 31. Uehara T, Pogribny IP, Rusyn I. The DEN and CCl4 -induced mouse model of fibrosis and inflammation-associated hepatocellular carcinoma. Curr Protoc Pharmacol 2014; 66: 14.30.1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delire B, Starkel P, Leclercq I. Animal models for fibrotic liver diseases: what we have, what we need, and what is under development. J Clin Transl Hepatol 2015; 3: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yanguas SC, Cogliati B, Willebrords J, et al. Experimental models of liver fibrosis. Arch Toxicol 2016; 90: 1025–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thoen LF, Guimaraes EL, Grunsven LA. Autophagy: a new player in hepatic stellate cell activation. Autophagy 2012; 8: 126–128. [DOI] [PubMed] [Google Scholar]
- 35. Allaire M, Rautou PE, Codogno P, et al. Autophagy in liver diseases: time for translation? J Hepatol 2019; 70: 985–998. [DOI] [PubMed] [Google Scholar]
- 36. Lalmanach G, Saidi A, Marchand-Adam S, et al. Cysteine cathepsins and cystatins: from ancillary tasks to prominent status in lung diseases. Biol Chem 2015; 396: 111–130. [DOI] [PubMed] [Google Scholar]
- 37. Roberts AB, Tian F, Byfield SD, et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 2006; 17: 19–27. [DOI] [PubMed] [Google Scholar]
- 38. Piek E, Ju WJ, Heyer J, et al. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem 2001; 276: 19945–19953. [DOI] [PubMed] [Google Scholar]
- 39. Moles A, Tarrats N, Fernandez-Checa JC, et al. Cathepsin B overexpression due to acid sphingomyelinase ablation promotes liver fibrosis in Niemann-Pick disease. J Biol Chem 2012; 287: 1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Canbay A, Guicciardi ME, Higuchi H, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest 2003; 112: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moles A, Tarrats N, Fernandez-Checa JC, et al. Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology 2009; 49: 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015; 44–46: 247–254. [DOI] [PubMed] [Google Scholar]
- 43. Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol 2006; 21(Suppl. 3): S84–S87. [DOI] [PubMed] [Google Scholar]
- 44. Roeb E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol 2018; 68–69: 463–473. [DOI] [PubMed] [Google Scholar]
- 45. Zbodakova O, Chalupsky K, Tureckova J, et al. Metalloproteinases in liver fibrosis: current insights. Metalloproteinases In Medicine 2017; 2017: 25–35. [Google Scholar]
- 46. Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol 2006; 21(Suppl. 3): S88–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou X, Murphy FR, Gehdu N, et al. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem 2004; 279: 23996–24006. [DOI] [PubMed] [Google Scholar]
- 48. Hartland SN, Murphy F, Aucott RL, et al. Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int 2009; 29: 966–978. [DOI] [PubMed] [Google Scholar]
- 49. Preaux AM, D’Ortho MP, Bralet MP, et al. Apoptosis of human hepatic myofibroblasts promotes activation of matrix metalloproteinase-2. Hepatology 2002; 36: 615–622. [DOI] [PubMed] [Google Scholar]
- 50. Principe DR, Park A, Dorman MJ, et al. TGFbeta blockade augments PD-1 inhibition to promote T-cell-mediated regression of pancreatic cancer. Mol Cancer Ther 2019; 18: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018; 554: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supp_material_Bestion_et_al._review for GNS561 acts as a potent anti-fibrotic and pro-fibrolytic agent in liver fibrosis through TGF-β1 inhibition by Eloïne Bestion, Zuzana Macek Jilkova, Jean-Louis Mège, Marie Novello, Keerthi Kurma, Seyedeh Tayebeh Ahmad Pour, Gilles Lalmanach, Lise Vanderlynden, Lionel Fizanne, Firas Bassissi, Madani Rachid, Jennifer Tracz, Jérôme Boursier, Jérôme Courcambeck, Cindy Serdjebi, Christelle Ansaldi, Thomas Decaens, Philippe Halfon and Sonia Brun in Therapeutic Advances in Chronic Disease