Abstract
Chyluria is secondary to the presence of chyle in the urine. The classical appearance on inspection is of milky white urine, which is caused by a fistulous communication between the lymphatic system and the urinary tract. Worldwide, it is most commonly associated with the parasite Wuchereria bancrofti, which is prevalent in Asia, most extensively in India but also China and Taiwan. However, in the United Kingdom, Europe and North America, where the condition is rare, non-parasitic aetiologies predominate. Chyluria is occasionally associated with other urinary tract symptoms including infection, loin pain and haematuria. It may also cause hypoproteinaemia, weight loss and cachexia. Management is based on identifying the aetiology and depends on the severity of the chyluria and presence of associated symptoms. Given its predominate symptom being urinary, cases in the West can fall under the care of the urologist. The aim of this article is to provide an overview and summary of the aetiology, assessment and management of chyluria based on the most up-to-date evidence available. This was achieved through a non-systematic review of world literature.
Keywords: chyluria, infection, kidney, lymphangiography, sclerotherapy
Introduction
In contemporary practice, an increasing numbers of cases of chyluria are being seen. This clinical condition is typically managed by a number of different specialities including nephrology, general medicine, infectious diseases and urology.1 Whilst still uncommon in the United Kingdom (UK) and the Western world, it is useful to understand the pathophysiological abnormalities that result in chyluria, in order to be able to formulate a practical approach to its diagnosis and management. The objective of our review is to provide such as understanding.
Methods
The authors performed a non-systematic review of world literature in order to identify relevant studies on chyluria. All article types were considered and no time restrictions were placed. Bibliographic databases searched included Pubmed/medline, Scopus, CINAHL and Google scholar.
Epidemiology
Chyluria remains endemic in parts of Asia, especially India and also Sub-Saharan Africa.2 Lymphatic filariasis, which is estimated to affect 120 million people worldwide, is often associated with this parasite, and chyluria can be a late manifestation of this disease in around 10% of cases.3 Non-parasitic causes of chyluria are reported to affect men and women in equal distribution; however, parasitic causes affect the former in up to 86% of cases.3 Chyluria is more prevalent in the rural population and is associated with lower socioeconomic status.2 However, cases caused by Wuchereria bancrofti are transmitted by Culex mosquitoes, which in areas of Sri Lanka are found in more urbanised areas.4
Aetiology
Chyluria can be broadly classified into parasitic and non-parasitic causes; 95% of parasitic causes are attributed to W. bancrofti, with the remaining 5% secondary to Taenia echinococcus, Taenia nana, Ankylostomiasis, Trichiniasis and the malarial parasite. Non-parasitic causes are rarer and these are almost always non tropical.3,4 Aetiological factors include blunt or penetrating trauma, a complication of surgery such as partial nephrectomy or aorto-iliac bypass, infection, malignancy, lymphatic malformation, radiation, abscess, congenital abnormalities, pregnancy and stenosis of the thoracic duct.5–9 Acute myeloid leukaemia and testicular cancer are the most commonly described malignancies, which are associated with chyluria10 (Table 1).
Table 1.
Common causes of chyluria.
| Parasitic | Non-parasitic |
|---|---|
|
• Wuchereria bancrofti
(95%) • Taenia echinococcus • Taenia nana • Ankylostomiasis • Trichiniasis • Malaria |
• Trauma • Surgery - Partial nephrectomy - Aorto-iliac bypass • Infections • Malignancy • Lymphatic malformation • Radiation • Abscess • Congenital abnormalities, • pregnancy • Lymphangioma of kidney or bladder • Stenosis of thoracic duct |
In addition to this, there exists an abundance of case reports in the world literature, which reveal a multitude of rare presentations and attributed causes. For example, chyluria has also been reported as a complication of retroperitoneal lesions in lymphangiomatosis and in children secondary to a congenital fistula between lymphatics and the bladder.11 Chyluria in children is very rare and under-reported, with the majority being described in case reports.12 In such cases there are no guidelines for management so it is likely the treatment will be tailored on a case-by-case basis.12 Chylous ascites and chyluria have been reported in nephrotic syndrome with focal segment glomerulosclerosis and nephrotic syndrome due to retroperitoneal lymphangioma.13 The authors identified one case report of chyluria presenting post-renal transplant with spontaneous resolution after 15 days.14
The cause: a tale of two theories
Chyle is composed of albumin, emulsified fat and fibrin.15 Chyle enters the urinary tract subsequent to the rupture of lymphatic channels into the urinary tract leading to a lympho-urinary fistula. This occurs most commonly at the renal pelvis. However, the exact mechanism by which chyluria develops is unclear.16 It may be caused either by regurgitation of chyle from the lymphatic system into the renal tract or as a result of obstruction of the lymphatic system in the vicinity of the urinary tract resulting in the development of a fistula.9,17 The regurgitation theory is based upon either the build-up of toxic metabolites or an inflammatory immune reaction secondary to parasitic infestation resulting in the development of lymphatic ectasia and varicosities that rupture, releasing chyle from dilated intestinal lymphatics into the renal system. In the obstructive theory, there is obliterative lymphangitis secondary to the parasitic infections. This leads to collaterals and varicosities and eventually the backflow of chyle.18
Presentation
In 70% of cases of chyluria, patients present with the worrying appearance of milky white urine.4 Other signs and symptoms reported at the time of presentation include dysuria, urgency and urinary retention secondary to chylous-clot haematuria. Clot colic can also occur. Systemic symptoms include weight loss, chills and peripheral oedema.9 When associated with a parasitic cause, cases can present with concomitant genital manifestations, 25% with lymphatic obstruction in limbs, and 5% with cellulitis, abscesses and haematuria.10
Parasitic chyluria usually occurs between the ages of 20 and 40. However, it has been reported in children as young as 5 years.7 Prior to diagnosis, symptoms may have been present up to 11 years in some reported cases.19 It can follow a relapsing and remitting course.
Those cases without a parasitic aetiology can appear at any age but tend to present later in life.12 The classical presentation is often milky white urine, with or without the associated features mentioned previously, which depends on the severity of the infection. When presenting as a complication of surgical intervention to the kidney, chyluria has been reported to present as late as 2 years following the procedure.4 Miller et al. carried out a retrospective review of all computerised tomography (CT) imaging, which patients (n = 125) had undergone following partial nephrectomy20; 3.2% of patients had incidental findings of chyluria as a result of lymphatic injury. Of note, none of these patients required intervention for this. The only symptom reported in this patient population was intermittent, cloudy urine.
Grading
There exists no universally accepted grading system for chyluria and a number of grading scales that are in current use have been described in world literature.21 Chyluria may be graded mild, moderate or severe (Table 2). The grading takes into account the degree of chyluria, associated symptomatology, episode frequency and the extent of calyceal involvement.17 Mild chyluria (34–50% of cases) comprises intermittent milky urine with no other symptoms. Retrograde pyelograms studies show involvement of only a single calyx. Moderate chyluria (33–40% of cases) is defined as intermittent continuous milky urine with occasional additional symptoms including clot colic or chylous coagulum, but not retention or weight loss. Retrograde studies reveal involvement of two or more calyces. Severe chyluria is characterised by continuous milky urine with the presence of one or more of the following: urinary retention, haematochyluria and systemic symptoms such as unintentional weight loss. Retrograde studies show involvement of most of the calyces with possible ureteric involvement. Of note, there is no defined amount of weight loss to qualify for this. Rather, it rests with the clinicians’ discretion.
Table 2.
Grading of chyluria.
| Mild chyluria | Moderate chyluria | Severe chyluria | |
|---|---|---|---|
| Clinical feature(s) | Intermittent episodes milky urine | Intermittent episodes milky urine +/– clot colic | Persistent milky urine AND One or more of the following: • Urinary retention • Haematochyluria • Systemic symptoms for example, weight loss |
| Retrograde study feature(s) | Involvement of only a single calyx | Involvement of 2 or more calyces | Involvement of most of the calyces with possible ureteric involvement |
A more recently developed alternative scale classifies chyluria Grade 1–3.22 Grade 1 consists of no more than one episode a year and no associated chylous clot(s). Grade 2 is where is also no more than one episode of chyluria a year but there is associated chylous clot and/or clot retention. For Grade 3 is when there has been two or more episodes a year and there is associated chylous clot and/or anaemia.
Management
Investigations
Laboratory
The macroscopic appearance of chyluria is typically milky and may contain a semisolid gel, sometimes blood and fibrin clots.15 A post-prandial specimen of urine is recommended where possible.23 When left to settle, it separates into distinct layers of fat, fibrin and cellular debris, whilst after centrifugation it remains turbid. With the addition of a fat solvent such as ether, the urine should become clear, whilst the addition of Sudan III stain results in a superficial layer of red-stained fatty particles lying on clear urine after resting. An alternative method to this is to mix Sudan III with butter and for the patient to consume it.16 A positive test results in production of orange-pink urine. Estimation of urinary chylomicrons is the most specific and sensitive test for chyluria.17 Small lymphocytes, either individually or as a clump, can be seen with 1–2 drops of 1:1500 methylene blue. Of note, while phosphaturia has the same macroscopic appearance, it clears when a few drops of 5–10% acetic acid is added to a sample.4 Dalela et al. found that triglycerides are present in 100% of chyluria samples.9 Levels >15 mg/dl are indicative for chyluria. Higher levels of triglyceride resulted in greater haziness in the appearance of the patient’s urine. Some studies have found high urinary triglyceride and cholesterol levels a predictive factor for a worse response to sclerotherapy treatment.9,24
For suspected parasitic causes, filarial antigen detection in the urine and serum can be done with immunochromatography or standard ELISA testing.12,25,26 The ELISA tests have a specificity of 85% and a sensitivity of 95%.9
Radiological
Ultrasonography can be used to identify any renal abnormalities as well as blood clots with a chylous component.10 Intravenous pyelography is not usually used as intra renal/ureteric pressure is usually required in order to identify dilated para-calyceal lymphatics. CT can identify fat within the bladder and possible dilated lymphatics. Magnetic resonance imaging is most useful in cases where there is the possibility of a fistula in the lower ureter or bladder as it helps to delineate the anatomy more accurately.27 Renal biopsy can be used to diagnose glomerulonephritis if this is suspected to be part of the disease process.20
Lymphangiography is the pre-operative imaging procedure of choice because it provides information on the site, size and the number of fistulous communications and has a sensitivity of 90%.15,28,29 It may also identify dysplasia of the lymphatic vessels, beading of the thoracic duct, dilated cisterna chylii, and abnormal lymphatics surrounding the ureters. It is not however routinely used because it is invasive, technically challenging and time consuming. Dong et al. recently published their experience and method of diagnostic lymphangiography followed by CT imaging within 60 min, which provided for assessment of redistribution of contrast medium. They determined this allowed for enhanced identification of lymphatic anomalies and the distribution of collateral lymphatic vessels.30
Lymphangioscintography allows for road mapping of the lymphatic transport and drainage system.29 It typically uses Tc99 human serum albumin sulphur colloid, is a safe and non-invasive way of diagnosing the site of a fistula. It also provides an assessment of the degree of reflux and is useful for detecting any possible recurrence. No side effects have been reported to date.9
Endoscopy
Endoscopy can serve as an important part of the diagnostic process although is not necessary in every case and can be used in a more therapeutic role rather than just diagnostic. Endoscopic visualisation of the urinary tract via can help identify any obvious fistula. Furthermore, ureteric catheter(s) may be used to obtain split urinalysis to identify the affected side. Cystoscopy can also sometimes reveal an affected side where the milky urine is visualised as it effluxes from an ureteric orifice.31 Retrograde pyelography can be used to identify lymphatic backflow; however, the specificity associated with this is low as similar images can be seen with contrast when injected under pressure. The role of endoscopy including retrograde pyelography is decisive in patients who have not responded to conservative or medical therapy.21
Diagnostic pathways
In the UK there is no clear consensus on the investigation protocol or algorithm for cases of chyluria. This is most likely due to its rarity, and the pathway adopted usually depends on the speciality overseeing the patient’s care, the resources available and the suspected cause. However, it is likely that after blood and urinary investigations the first line radiological investigations include a CT urogram and retrograde studies, largely owing to their availability.31 For non-parasitic chyluria, on-going management would be tailored based on the suspected cause and discussion with specialist centres can be sought.
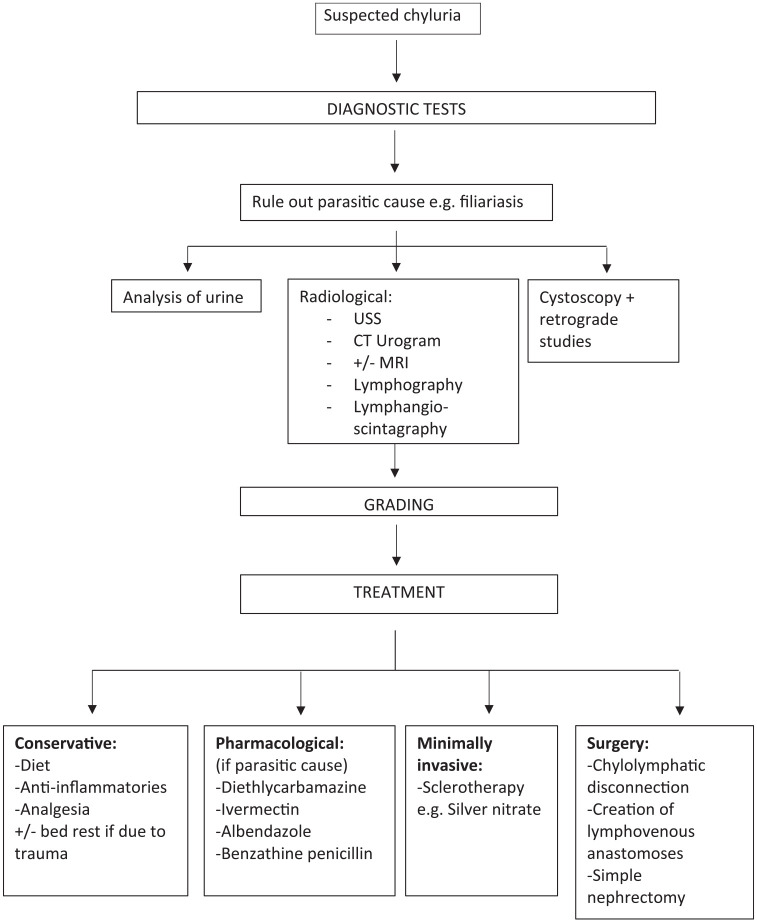
Treatment
The management of chyluria ranges from conservative measures such as dietary changes to invasive management including surgery (Figure 1). It depends on the severity of symptoms and the underlying cause.32
Figure 1.
Diagnostic tests for suspected chyluria.
CT, computerised tomography; MRI, magnetic resonance imaging; USS, ultrasound scan.
Conservative
The natural history of chyluria is unknown and up to 50% of cases may resolve spontaneously.4 Conservative management has a success rate of more than 70%.3 A high fluid intake is recommended and in mild cases, a modified diet such as high protein, multivitamins (A, D, E, K) and leafy green vegetables with fat restriction of <25 g/day can be sufficient.24 Fat-free diets are recommended where possible. An alternative is a low fat, medium-chain triglyceride (<12 carbon atoms) diets for example, using coconut oil for cooking, are recommended as these are absorbed directly through the portal system and bypass the intestinal lymphatics, which does not occur with long chain fatty acids.15 Sharma et al. proposed the concept of total parenteral nutrition in cases of intractable chyluria.26 There is limited information on the recommended length of conservative treatment. Limiting factors would be severity of symptoms and any concern regarding vitamin deficiencies.
In patients with lymphadenitis, bed rest, anti-inflammatories and analgesics are used for symptom control. Chylous clot can be managed with manual bladder washouts in the first instance. However, the presence of such clot often indicates that more than conservative treatment alone will be needed.
For those patients with suspected filiarial infection, medication is used in combination with dietary modifications. Diethlycarbamazine (DEC), ivermectin, albendazole and benzathine penicillin are the most commonly pharmacotherapeutic options. The treatment protocol for DEC is 6 mg/kg/day for 21 days. This drug acts against the microfilaria, the larval phase of the parasite.21 Longer courses of DEC can be required and/or the treatment course can be repeated after 8 weeks if the response is unsatisfactory. Side effects can include itching, painful glands and skin rash. Febrile reaction can occur if the microfilaremia burden is very high and so an antihistamine can be prescribed alongside the treatment course.4 Ivermectin is given as a one off dose of 6–12 mg, which is then repeated after 3 weeks.26 Albendazole is a 10- to 14-day course of 400–800 mg/day and benzathine penicillin is taken for 12 weeks, 1.2 million units weekly. Combination drug treatments have also been applied and success is reported.5 In endemic areas treatment can be given without demonstrating filarial antigen if it is suspected. Bed rest and abdominal binders are also recommended. The latter serve raise the intra-abdominal pressure and thereby lower lympho-urinary reflux. However, the evidence supporting use of abdominal binders is weak (level of evidence 5).4
Minimally invasive
The majority of minimally invasive techniques involve sclerotherapy and this is indicated in patients that have failed conservative or medical treatments. Many different agents having been used, such as silver nitrate 0.1–3%, 0.2% povidone iodine, 1–25% sodium iodide, 10–25% potassium bromide, 50% dextrose and hypertonic saline.4 Currently, 1% silver nitrate is the sclerosant of choice. Sclerotherapy, when instilled into the renal pelvis, reaches the lymphatics via the fistulous communication.26 From here, it induces a chemical lymphangitis with the oedema causing blockage of lymphatics and resulting in immediate relief. As fibrosis of lymphatics subsequently takes place, the relief is lasting. The procedure can be undertaken under local anaesthetic or sedation. A ureteric catheter is passed into the renal pelvis and 7–10 ml of sclerosant is injected until the patient reports pain. With regards to procedural timings, 3 days of instillations occurring every 8 h has been found to be superior to a once weekly instillation for 6 weeks.27 It is advised to only do one side at a time due to the risk of acute tubular necrosis. If both renal units are affected, a waiting period of at least 2 months is recommended before the contralateral side is treated.25
Povidone iodine is a non-ionic and water soluble surfactant polymer, which can achieve an septic sclerosing and obliterative inflammatory reaction.26 It can also be used in combination with 50% dextrose. Singh et al. recently reported details of their sclerotherapy regimen.33 They prepared 0.2% povidone-iodine (5% povidone iodine 10 ml + distilled water 40 ml) and injected 5 ml of this solution into the renal pelvis. After 1 min, this was allowed to drain out. This was repeated until all the agent had been administered. The retrograde ureteric catheter, which had been used to deliver this sclerosing substance, was left in situ and this process was carried out again twice more, at 48 h and 96 h after the initial procedure respectively. A total of three sessions are performed in their method and antibiotic cover was administered over the treatment period. Sclerotherapy can be associated with minor and self-limiting adverse effects such as nausea, flank pain and haematuria.16 These should resolve within 48 h. Several isolated reports do exist of more serious sequelae including renal failure, anuria with pelvi-calyceal cast formation and acute necrotizing ureteritis.34,35
Goel et al. carried out a prospective, randomised study which compared 1% silver nitrate, 0.2% povidone iodine and 50% dextrose.36 While the 50% dextrose arm was discontinued early on due to a very poor success, no significant difference in efficacy was found between povidone iodine and silver nitrate. The authors highlighted that silver nitrate is arguably associated with more disadvantages such as its susceptibility to light, water insolubility and need for autoclaving. The latter can cause water evaporation and therefore presents challenges in quality assurance and maintaining an accurate concentration. In contrast povidone iodine is more readily available, cheaper, easier to reconstitute and stable at room temperature.35 Purkait et al. added to the body evidence supporting endoscopic sclerotherapy and performed a prospective study using dimercaptosuccinic acid renal imaging to evaluate if there is any adverse effect on renal function associated with this treatment method. The authors concluded that relative renal function does not deteriorate by more than 5%.37
Other minimally invasive techniques reported on in literature include heat-clearing and endoscopic coagulation. Pavlinec et al. recently described their method of using flexible ureteroscopy to pinpoint a fistulous opening in the renal pelvis after retrograde studies had revealed an irregularity at this site.38 This pyelolympahitc fistula had presented 4 weeks after robotic pyeloplasty. The authors then used endoscopic fulgaration to successfully treat the patient.
Invasive
Truly invasive management of chyluria is reserved for those with significant symptoms and in whom medical or minimally invasive treatment options have failed.39 The latter include patients who have not responded to two or more courses of sclerotherapy. Overall surgical techniques have a reported 95% success rate.15 The operative techniques described for chyluria include chylolymphatic disconnection and creation of lymphovenous anastomoses, which may be retroperitoneal lymphovenous anastomosis, transinguinal spermatic lymphovenous anastomosis or inguinal lymph node-saphenous venous anastomosis.17 Other surgical procedures include auto-transplantation or nephrectomy. Open surgical methods are now possible laparoscopically or robotically, which offer the advantages of reduced morbidity, duration of stay and pain.40 The process of lympho-venous disconnection was first described in the 1950s. This is still the most frequently performed operation and a modified technique involves only performing medial lymphatic disconnection, reducing operating time, morbidity and excess dissection with good results.41,42 Pre-operatively, the patient eats a high fat diet then undergoes lymphangiography (+/– methylene blue injection) to identify the abnormal lymphatic channels. It typically involves the following steps: nephron-lympholysis, hilar stripping, uretero-lympholysis, fasciectomy and nephropexy. Overall success rates for chylo-lymphatic disconnection have been reported as high as 98%.15
In order to avoid post-operative complications of lymphatic drainage such as lymphocele formation, recurrence, fibrosis and adhesions, a technique of omental wrapping after stripping of the renal vessels was introduced in 2004 by Delela et al.43 Simple nephrectomy is only considered in patients with a non-functioning kidney and after conventional lymphovenous disconnection.
Another surgical intervention involves a more physiological approach by creating lymphovenous anastomosis with the aim of diverting lymph flow and reducing intra-lymphatic pressure, however a 14% failure rate has been reported.44 Sites of anastomosis include groin to the saphenous vein. In elderly patients, more superficial anastomoses can be made with the inguinal region being preferable in men and the dorsum of the foot, leg or thigh being used in women. This microsurgery requires good magnification. Patency is essential, and it can take up to 6 months for the full effects to become apparent. With the advancement of laparoscopic and robotic surgery the complications associated with surgical techniques are in decline.40 However, complications do occur and can include lymphatic leak, injury to the inferior vena cava or renal segmental artery and delayed wound healing as a result invasive surgical treatment should only be used if conservative of minimally invasive measures fail.
Recurrence
Recurrence of chyluria after initial clearance has been reported in up to 80% of patients managed conservatively.45 For sclerotherapy this is improved significantly to 13–41%. However, primary treatment failure with sclerotherapy is between 10 and 20%.9 Recurrence of chyluria post intervention is usually due to incomplete stripping, reflux from contralateral side, and reflux from bladder.26 Methods to prevent recurrence involve the use of an omental wrap, or use of augmented visualisation techniques such as magnifying loops or an operating microscope. Not all cases of recurrence warrant invasive treatment such as surgery.
Pregnancy
Whilst chyluria is less common in females (9:1 male to female ratio) and rare in pregnancy, it is not uncommon in endemic areas.46 It most commonly presents in the second trimester (74.4%).22 The causes of nonparasitic chyluria are the same as previously listed and the clinical presentation is the same. Observation of the disease’s natural history have commented that the condition severity can worsen as pregnancy progresses.47
The overall management is more challenging as the pharmacotherapeutic options are more limited. Anti-filarial drugs have a potentially teratogenic risk and are advised against by the World Health Organisation (WHO) and Federal Drug Agency (FDA).48 However, Gyapong et al. reported outcomes from a community filariasis program where they found pregnant patients had been inadvertently treated with ivermectin and albendazole. There was no significant association with greater risk of birth defect or congenital malformations in these child bearing women.49 The mainstay of treatment for these patients is conservative and supportive. Sclerotherapy has been used but there is no universal treatment protocol which is largely due to the lack of studies in this area. Mahmood et al. reported their tertiary unit’s experience of managing chyluria in pregnant patients; 13/43 and 19/43 experienced resolution after one and two sclerotherapy treatments respectively.4 Patients undergoing this treatment are acknowledged to be at greater risk of infectious complications.22 Where possible, treatment can be deferred until after the birth. Patients who are near term can be considered for planned caesarean section.
Conclusion
While extremely rare in the UK, clinicians need an understanding of the natural history of chyluria and how to investigate and manage it accordingly. The mainstay of investigation lies in confirming the diagnosis of chyluria via analysis of urine for chylomicrons and determining the site of the fistula using lymphangioscintigraphy, retrograde studies or endoscopic visualisation of the urinary tract. Medical management alone is successful in up to 70% of cases, severe cases may require intervention with sclerotherapy and refractory cases with surgical intervention with a 99% success rate.36
Footnotes
Author contributions: VS contributed to conception, design, literature search and draft. PJ contributed to draft and revision and literature search. SOJ and RB to critical review, revision and expertise. AH supervised projected, conceived idea and helped revise each version.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Guarantor: AH
ORCID iD: Patrick Jones  https://orcid.org/0000-0003-4253-1283
https://orcid.org/0000-0003-4253-1283
Contributor Information
Victoria Stainer, Department of Urology, Great Western Hospital, Swindon, UK.
Patrick Jones, Department of Urology, Great Western Hospital, Tremona Rd, Southampton, Swindon SO16 6YD, UK.
Siri Øvereng Juliebø, Department of Gynaecology, Sykehuset Telemark, Skien, Norway.
Rupert Beck, Department of Urology, Great Western Hospital, Swindon, UK.
Amr Hawary, Department of Urology, Great Western Hospital, Swindon, UK.
References
- 1. Tan Y. Chyluria in non-filarial endemic areas: an internist’s perspective. BMJ Case Rep 2017; 2017: bcr2017220772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones RT. Non-endemic cases of lymphatic filariasis. Trop Med Int Health 2014; 19: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 3. Sinha RK, Ranjan N, Singh N, et al. Chyluria: a scourge of our region. BMJ Case Rep 2015; 2015: bcr2014209188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abeygunasekera AM, Sutharshan K, Balagobi B. New developments in chyluria after global programs to eliminate lymphatic filariasis. Int J Urol 2017; 24: 582–588. [DOI] [PubMed] [Google Scholar]
- 5. Komeya M, Sahoda T, Sugiura S, et al. Chyluria after partial nephrectomy: a rare but considerable complication. Int J Urol 2013; 20: 242–245. [DOI] [PubMed] [Google Scholar]
- 6. Yamauchi S. Chyluria: clinical, laboratory and statistical study of 45 personal cases observed in Hawaii. J Urol 1945; 54: 318–347. [Google Scholar]
- 7. Onyeije CI, Sherer DM, Trampert J. Nonfilarial chyluria during pregnancy. Obstet Gynecol 1997; 90: 699–700. [DOI] [PubMed] [Google Scholar]
- 8. Yu HH, Ngan H, Leong CH. Chyluria—a 10-year Follow-up. Br J Urol 1978; 50: 126–133. [DOI] [PubMed] [Google Scholar]
- 9. Dalela D. Issues in etiology and diagnosis making of chyluria. Indian J Urol 2005; 21: 18–23. [Google Scholar]
- 10. Panchal VJ, Chen R, Ghahremani GG. Non-tropical chyluria: CT diagnosis. Abdom Imaging 2012; 37: 494–500. [DOI] [PubMed] [Google Scholar]
- 11. Guillonneau B, Bouchot O, Buzelin F, et al. Lymphangiomyomatosis: an exceptional cause of chyluria. Report of a case. Prog Urol 1993; 3: 484–489. [PubMed] [Google Scholar]
- 12. Stalens JP, Falk M, Howmann-Giles R, et al. Milky urine – a child with chyluria. Eur J Pediatr 1992; 151: 61–62. [DOI] [PubMed] [Google Scholar]
- 13. Lewsuwan S, Kanjanabuch T, Avihingsanon Y, et al. A rare case of chylous ascites and chyluria in an adult nephrotic syndrome with focal segmental glomerulosclerosis. J Med Assoc Thai 2006; 89(Suppl. 20): S253–S256. [PubMed] [Google Scholar]
- 14. Yilmaz VT, Dinc B, Ay N, et al. Chyluria and delayed graft function in early port–transplant period in renal transplant patient. Am J Med Sci 2010; 340: 169–172. [DOI] [PubMed] [Google Scholar]
- 15. Singh I, Darhan P, Sharma N. Chyluria – a clinical and diagnostic stepladder algorithm with review of literature. Indian J Urol 2004; 20: 79–85. [Google Scholar]
- 16. Wiggelinkhuizen J, Landman C, Greenberg E. Chyluria. Am J Dis Child 1972; 124: 99–101. [DOI] [PubMed] [Google Scholar]
- 17. Aye UT, Aung ST. Chyluria. Clin Rad 1975; 26: 237–242. [DOI] [PubMed] [Google Scholar]
- 18. Goel TC, Goel A. (eds). Chyluria. In Lymphatic Filariasis. Singapore: Springer, 2016, pp.273–300. [Google Scholar]
- 19. Tandon V, Singh H, Dwivedi US, et al. Filarial chyluria: long-term experience of a university hospital in India. Int J Urol 2004; 11: 193–198. [DOI] [PubMed] [Google Scholar]
- 20. Miller F, Keppke A, Yaghmai, et al. CT diagnosis of chyluria after partial nephrectomy. AJR 2007; 188: W25–W28. [DOI] [PubMed] [Google Scholar]
- 21. Gutilla A, Beltrami P, Bettin L, et al. Chyluria: the state of the art. Urologia 2017; 84: 65–70. [DOI] [PubMed] [Google Scholar]
- 22. Purkait B, Garg G, Singh M, et al. Chyluria in pregnancy: etiology, diagnosis, and management perspective. Saudi J Kidney Dis Transpl 2019; 30: 309–314. [DOI] [PubMed] [Google Scholar]
- 23. Guttilla A, Beltrami P, Bettin L, et al. Non-parasitic chyluria: our experience with sclerotherapy with solution of povidone-iodine and destrose and a review of the literature. Urol Case Rep 2016; 8: 28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahman MM. Easy method of detection of chyle in urine. Indian J Nephrol 2012, 22: 147–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta VK, Lohar H, Banerjee BK, et al. Surgical filariasis; immunoscreening for filarial IgG antibodies using Wuchereria bancrofti microfilarial excretory-secretory antigen. J Commun Dis 1999; 31: 35–40. [PubMed] [Google Scholar]
- 26. Sharma S, Hemal AK. Chyluria – an overview. Int J Nephrol Urol 2009; 1: 14–26. [Google Scholar]
- 27. Arrivé L, Monnier-Cholley L, El Mouhadi S. Use of unenhanced MR lymphography to characterize idiopathic chyluria. AJR Am J Roentgenol 2018; 211: W200. [DOI] [PubMed] [Google Scholar]
- 28. Ngan H, Leong CH. A lymphographic study of chyluria. Br J Radiol 1977; 50: 863–870. [DOI] [PubMed] [Google Scholar]
- 29. Pui MH, Yueh TC. Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. J Nucl Med 1998; 39: 1292–1296. [PubMed] [Google Scholar]
- 30. Dong Jian, Xin J, Shen W, et al. Unipedal diagnostic lymphangiography followed by sequential CT examinations in patients with idiopathic chyluria: a retrospective study. AJR Am J Roentgenol 2018; 210: 792–798. [DOI] [PubMed] [Google Scholar]
- 31. de Araújo PSR, Júnior VRS, Souza A, et al. Chyluria in a lymphatic filariasis endemic area. BMC Res Notes, 2018; 11: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uzu T, Takamori K, Fujino Y, et al. Idiopathic chyluria with nephrotic-range proteinuria and hypothyroidism. Intern Med 2019; 58: 1307–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh H, Singla A, Jain A. Chyluria-a review of literature and a modified sclerotherapy regimen. Turk J Urol 2019; 45(Suppl. 1): S174–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garg M, Dalela D, Goel A. Devastating complication of silver nitrate instillation for the treatment of chyluria. BMJ Case Rep 2013; 2013: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitsunari K, Imasato Y, Tsurusaki T. Preliminary study of a single instillation of low-concentration high-volume silver nitrate solution for chyluria: is >10 ml instillation an absolute contraindication in the real world? Trop Med Infect Dis 2019; 4: pii: E128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goel S, Mandhani A, Srivastava A, et al. Is povidone iodine an alternative to silver nitrate for renal pelvic instillation sclerotherapy in chyluria? BJU Int 2004; 94: 1082–1085. [DOI] [PubMed] [Google Scholar]
- 37. Purkait B, Goel A, Deswal S, et al. Does endoscopic sclerotherapy in filarial chyluria affect renal function and morphology? A prospective study using dimercaptosuccinic acid renal scan. Asian J Urol 2019; 6: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pavlinec J, Rabley A, Kwenda E, et al. Endoscopic management of chyluria due to pyelolymphatic fistula after robot-assisted laparoscopic pyeloplasty. J Urol 2020; 203(Suppl. 4): e473–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin TP, Hsu YS, Chen KK, et al. Chyluria – experience of Taipei veterans general hospital. J Chin Med Assoc 2003; 66: 109–112. [PubMed] [Google Scholar]
- 40. McGuinness LA, Prasad Rai B. Robotics in urology. Ann R Coll Surg Engl 2018; 100(Suppl. 6): 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. You W, Luan B, Cheng T, et al. The efficacy and safety of retroperitoneoscopic renal pedicle ligation of lymphatic disconnection versus open surgery in the treatment of chyluria: a systematic review and meta-analysis. Clin Nephrol 2019; 91: 211–221. [DOI] [PubMed] [Google Scholar]
- 42. Tung JYM, Chen K, Sim ASP. Indocyanine green - guided laparoscopic renal pedicle lymphatic disconnection: a novel, targeted treatment for chyluria. Int Braz J Urol 2019; 45: 1075. [DOI] [PubMed] [Google Scholar]
- 43. Dalela D, Gupta VP, Goel A, et al. Omental wrap around the renal pedicle: an adjunctive step to minimiza morbidity and recurrence after lymphorenal disconnection for chyluria. BJU Int 2004; 94: 673–674. [DOI] [PubMed] [Google Scholar]
- 44. Hou LQ, Liu QY, Kong QY, et al. Lymphonodovenous anastomosis in the treatment of chyluria. Chin Med J (Engl) 1991; 104: 392–394. [PubMed] [Google Scholar]
- 45. Núñez CM, Cárcamo PV, Kabani MH, et al. Recurrent nonparasitic chyluria. Arch Esp Urol 1998; 51: 932–934. [PubMed] [Google Scholar]
- 46. Mount P, Thong M. Perirenal lymphatic filariasis presenting as chyluria during pregnancy. Kidney Int 2006; 69: 2115. [DOI] [PubMed] [Google Scholar]
- 47. Mahmood K, Ahmad A, Kumar K, et al. Chyluria in pregnancy – a decade of experience in a single tertiary care hospital. Nephrourol Mon 2015; 7: e26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crompton DW. Preventive chemo-therapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Switzerland: World Health Organization, 2006. [Google Scholar]
- 49. Gyapong JO, Chinbuah MA, Gyapong M. Inadvertent exposure of pregnant women to ivermectin and albendazole during mass drug administration for lymphatic filariasis. Trop Med Int Health 2003; 8: 1093–1101. [DOI] [PubMed] [Google Scholar]