Abstract
Background:
Achilles tendinopathy (AT) is a common cause of overuse injury in both athletes and nonactive individuals, especially at older ages. Due to the limited number of direct comparisons among interventions, determining the best treatment option can be difficult.
Purpose:
To evaluate the comparative efficacy and tolerability of nonsurgical therapies for midportion AT.
Study Design:
Systematic review; Level of evidence, 1.
Methods:
PubMed, MEDLINE, EMBASE, and Google Scholar were searched from database inception through June 20, 2019. Randomized controlled trials investigating the effect of nonsurgical therapies for midportion AT using the Victorian Institute of Sports Assessment–Achilles (VISA-A) assessment were eligible for inclusion. Primary outcome was mean change in VISA-A score from baseline. Comparisons between interventions were made through use of random-effects network meta-analysis over the short term (≤3 months) and longer term (>3 to <12 months). A safety profile was defined for each intervention by rate of all-cause discontinuation (dropout) during follow-up. Relative ranking of therapies was assessed by the surface-under-the–cumulative ranking possibilities.
Results:
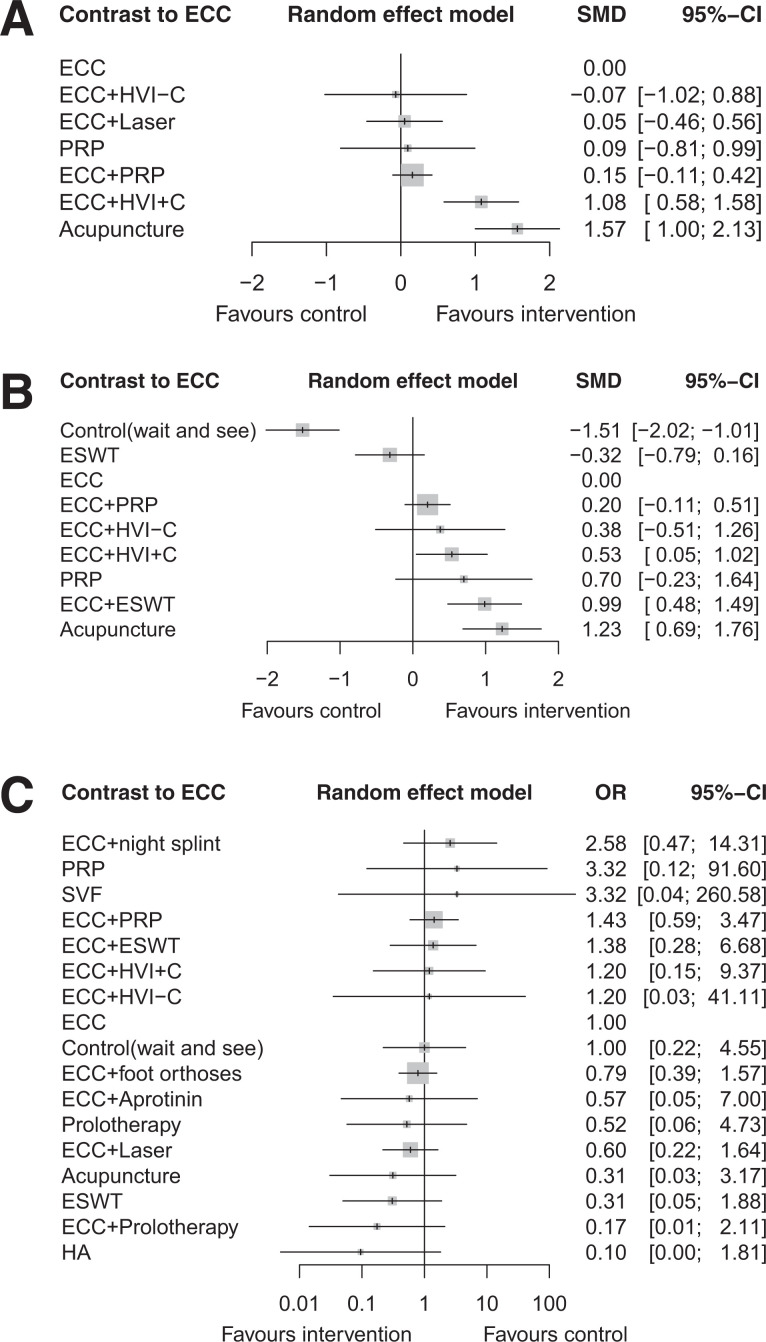
A total of 22 studies with 978 patients met the inclusion criteria. In short-term studies, high-volume injection with corticosteroid (HVI+C) along with eccentric exercise (ECC) significantly improved the change of VISA-A score compared with that of ECC alone (standardized mean difference [SMD], 1.08; 95% CI, 0.58-1.58). Compared with ECC, acupuncture showed benefits over both the short term (SMD, 1.57; 95% CI, 1.00-2.13) and longer term (SMD, 1.23; 95% CI, 0.69-1.76). In longer-term studies, the wait-and-see approach resulted in unfavorable outcomes compared with ECC (SMD, −1.51; 95% CI, −2.02 to −1.01). Improvement was higher when ECC was combined with HVI+C (SMD, 0.53; 95% CI, 0.05-1.02) and extracorporeal shockwave therapy (ESWT) (SMD, 0.99; 95% CI, 0.48-1.49). All interventions had a similar safety profile.
Conclusion:
From available high-level studies, HVI+C and ESWT may be possible interventions to add along with ECC to improve longer-term outcomes.
Keywords: Achilles, intervention, eccentric loading, shockwave, platelet-rich plasma
Achilles tendinopathy (AT) is a common cause of overuse injury in both athletes and nonactive individuals, especially at older ages.35 In the general population, 2.16 per 1000 patients experience AT every year, and AT accounts for 6.2% to 9.5% running-related injuries in athletes.1,36 In the past 3 decades, the incidence of AT has increased owing to greater participation in recreational and competitive sports.36 Patients with AT present with focal tendon pain, morning stiffness, and restricted function.11
The process of tendinopathy is understood to represent a failed healing response characterized by a combination of tendon cell degeneration, collagen fiber disruption, irregular tenocyte proliferation, and resulting noncollagenous matrix.34 Therapies have focused on methods to reduce symptoms and stimulate tendon healing.34 In 1998, Alfredson et al2 demonstrated that eccentric strengthening (ECC) improved long-term symptoms and function, and this treatment remains the cornerstone for treating AT. ECC may affect type I collagen production, leading to increases in tendon volume and tensile strength.30
Exercise regimens, including ECC, may improve symptoms in approximately 60% of patients.35 Surgical treatment may be considered after at least 6 months of nonoperative management38; thus, in addition to ECC, various nonoperative therapies have been proposed, such as high-volume injections with corticosteroid (HVI+C), extracorporeal shockwave therapy (ESWT), and platelet-rich plasma (PRP). However, randomized controlled trials (RCTs) have inconsistently shown benefits in improving pain and functional outcomes for each treatment. The insufficient number of head-to-head trials comparing therapies over different lengths of follow-up creates a challenge to determine the best evidence-based practice.
Despite the effort to find optimal treatment for AT, the number of nonsurgical treatments exceeds 15 interventions. In this situation, network meta-analysis (NMA) may be advantageous to identify the efficacy and safety hierarchy of such numerous interventions. It has been reported that NMA is more likely to provide stronger and earlier evidence than conventional pairwise meta-analysis.47 Furthermore, the World Health Organization has adopted NMA for its decision-making process and encourages the use of NMA in the development of clinical guidelines.25
The purpose of this systematic review with NMA was to evaluate the comparative efficacy of nonsurgical options for the treatment of midportion AT and offer insight into evidence-based clinical practice. To understand the effects of interventions over different time intervals, study results were evaluated over short-term (≤3 months) and longer-term follow-up (>3 to <12 months).
Methods
Systematic Review Registration
The protocol for this systematic review was registered at PROSPERO: CRD42019139369.
Search Strategy and Selection Criteria
We searched PubMed, MEDLINE, EMBASE, and Google Scholar for RCTs published up to June 20, 2019, that evaluated the effect of nonsurgical therapies for the treatment of midportion AT. We also screened EMBASE to search for abstract published in international conferences for the acquisition of the latest data, with a preference for English and other languages that could be translated to English. The literature search process followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.42 Various combinations of terms such as “Achilles,” “tendinopathy,” “tendinosis,” “tendinitis,” “non-insertional,” “injection,” “shockwave,” “eccentric,” “splint,” “orthoses,” “laser,” “sclerotherapy,” “prolotherapy,” “topical glyceryl trinitrate,” and “PRP” were used with “AND” or “OR” commands. The references of relevant review articles were reviewed to search for additional articles that may not have been indexed. Studies involving patients with a diagnosis of midportion AT (also described as “tendinosis,” “tendon pain,” or “tendinitis”) that compared nonsurgical therapies were eligible for inclusion. Exclusion criteria were the following: (1) studies that were not randomized or quasi-randomized; (2) studies that did not specify the type of AT; (3) studies that compared different exercise protocols; (4) studies involving patients younger than 18 years; (5) studies involving patients with insertional AT; (6) studies involving patients with Achilles tendon rupture; (7) studies involving patients who had undergone surgeries to treat AT; (8) studies involving patients who received dietary supplements or oral pharmacotherapies; (9) studies involving patients with inflammatory disease (rheumatoid arthritis, psoriatic arthritis, or inflammatory bowel disease); (10) studies involving patients with AT associated with the use of antibiotics; (11) studies that did not use the Victorian Institute of Sports Assessment–Achilles (VISA-A) scale for primary outcome or those that used the VISA-A scale but had a follow-up period of ≥1 year.
Data Extraction and Quality Assessment
Data extraction was independently conducted by 2 authors (H.C.R. and M.S.K.). Characteristics such as number of patients, mean and median pain duration before therapy, treatment protocols, follow-up periods, and adverse events were manually extracted from each article. The Cochrane PICO (Patient, Population or Problem; Intervention; Comparison; and Outcome) components22 were identified with consensus between the 2 authors, and the articles were reviewed following the PICO consensus. The risk of bias was assessed by 2 authors (S.C. and H.C.R.) using the Cochrane risk of bias tool,22 and a comparison-adjusted funnel plot was constructed to evaluate publication bias.7
Primary Outcome Measure
A previous systematic review reported that the heterogeneity of outcome measures led to difficulty in drawing conclusive results.18 Therefore, only studies that used a validated and reliable outcome scale were included. Currently, the VISA-A is the only valid (P < .01) and reliable (test-retest reliability, r = 0.98) measure to assess pain and function in AT.43,52 Studies that used the American Orthopaedic Foot and Ankle Score were excluded because it was not designed specifically for AT.43 Studies that used the visual analog scale (VAS) or numerical rating scale for pain without also using the VISA-A were excluded because the VAS has been shown to have poor test reliability at rest in AT (r = 0.45).43 Moreover, the relationship between pain and function is intertwined in tendinopathy since symptoms are load-dependent, and thus including a measure of pain without linking it to function may lead to imprecise estimate of the effect.11,12
Additionally, we evaluated tolerability of interventions with all-cause discontinuation (dropout) because loss of follow-up and withdrawal from an intervention may reflect both severe adverse events and lack of efficacy.9,57
Following the recommendations of the Cochrane Collaboration10 and drawing on previous reviews,13,28 we categorized our results into 2 outcome measures for short term (≤3 months) and longer term (>3 to <12 months). For articles with multiple follow-ups, each follow-up period for VISA-A was categorized as short term or longer term for subgroup analysis in the NMA. When multiple short-term follow-ups were provided, the longest period was used for analysis (eg, if the follow-up time points for VISA-A were 1 month, 2 months, and 3 months, the data from the 3-month period were used as short-term results).
Statistical Analysis
Pairwise and network meta-analysis using a random-effects model was performed. The change of mean score for VISA-A from baseline (change-from-baseline scale) was extracted as the primary outcome. The analysis was based on changes from baseline to partially correct for between-person variability.10 Standardized mean difference (SMD) with 95% CI was used to compare effect sizes. The Higgins I 2 statistic and the Cochran Q test were calculated to assess the heterogeneity among the studies.21 A 2-sided P value less than .05 was regarded as statistically significant.
We conducted the random-effects NMA within a frequentist framework using STATA (version 15.0; Stata Corp) and R (Version 3.5.1) software.62 Indirect and mixed comparisons were performed through the mvmeta command and self-programmed routines of STATA7,56 and the netmeta package of R.46 When the outcome was presented as median (interquartile range), it was converted to mean (SD) by calculation.23,61 When variance was reported as 95% CI, it was converted to standard deviation by use of the Revman calculator.10 Restricted maximum likelihood method was used to assess heterogeneity, assuming a common heterogeneity variable for all comparisons (the tau value),37 and I 2 and its 95% CI were computed. Global inconsistencies that represent plausibility of inconsistency in the entire network were evaluated with a design-by-treatment model,6,20 and local inconsistencies that represent plausibility of inconsistency in the loop network were estimated by a loop-specific approach for every closed triangular or quadratic loop and by a node-splitting method.6,56,62 The net heat plot was constructed by the netmeta package of R to visualize the inconsistency matrix and detect specific comparisons that resulted in large inconsistencies.29 The rank of effect estimation for each therapy was calculated by use of the surface-under-the–cumulative ranking (SUCRA) curve of P rank score of R software.55
Results
Eligible Studies and Patient Descriptors
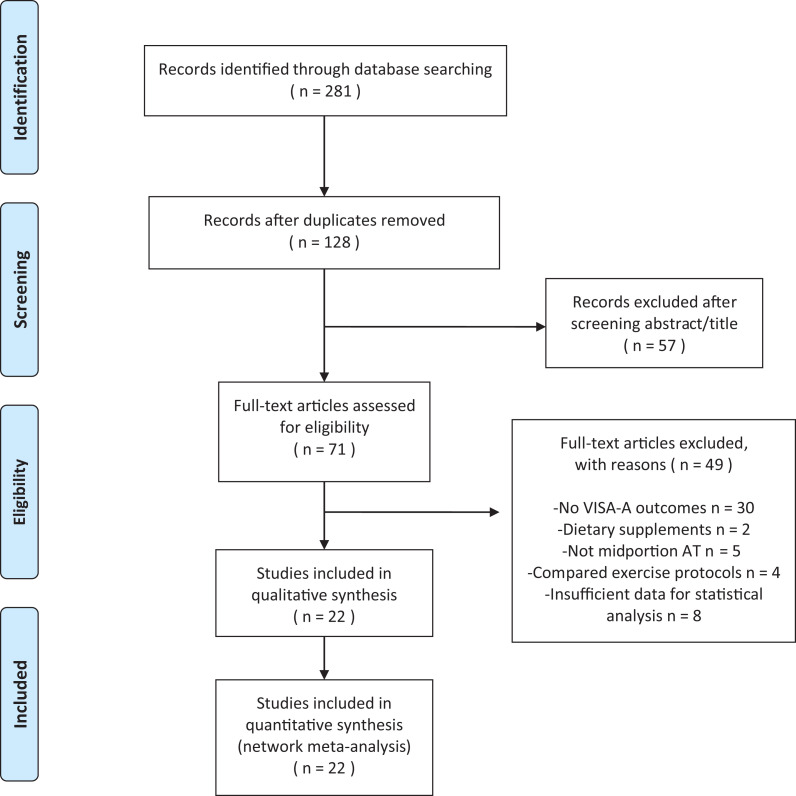
The initial search yielded a total of 281 articles. After reviewing the titles and abstracts, we found 71 studies that were eligible for full-text review. We excluded 49 articles due to unavailable VISA-A outcome scale, not meeting inclusion criteria, and insufficient data for statistical analysis. The resulting 22 RCTs published up to June 20, 2019, were included in the NMA. This process is presented in the PRISMA flowchart (Figure 1). A total of 22 RCTs with 978 participants met our inclusion criteria for the evaluation of nonsurgical treatment options for midportion AT. The mean study sample size was 44 patients (range, 20–140 patients), and 13 studies set inclusion criteria as the minimum duration of symptoms for 3 months. There were 18 studies that explicitly stated mean or median duration of symptoms, which ranged from 6 to 38.6 months. AT was diagnosed clinically in 4 studies, whereas clinical and ultrasonographic diagnosis was made in 18 studies. In 19 studies that used ECC treatment, 8 specifically reported patient compliance with the treatment. The characteristics of individual studies are summarized in Appendix Table A1 (available online as supplemental material).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Meta-Analyses) diagram showing selection of articles for pairwise and network meta-analysis. AT, Achilles tendinopathy; VISA-A, Victorian Institute of Sports Assessment–Achilles.
For both the pairwise meta-analysis and network meta-analysis, there was no evidence of heterogeneity (I 2) and no inconsistency.
Efficacy and Tolerability of Interventions Measured in NMA
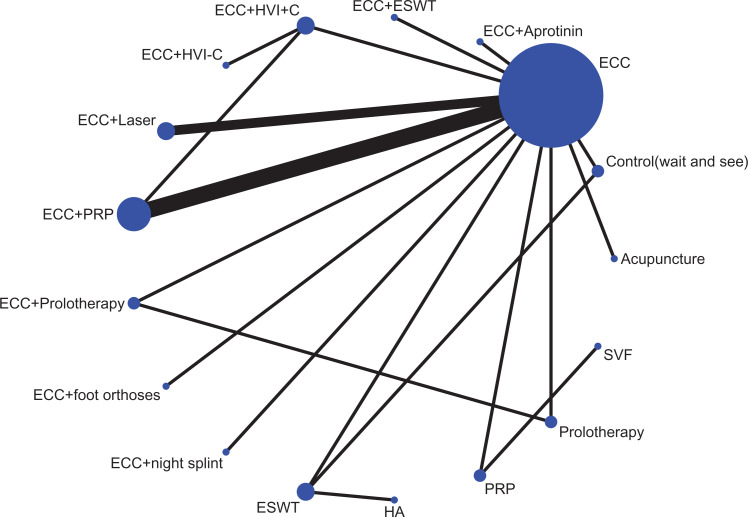
Figure 2 shows the network of eligible comparisons for the nonsurgical treatment options for midportion AT. Data for the pairwise meta-analysis for the primary outcome can be found in Appendix Table A2 (available as supplemental material), and data for network meta-analysis of the primary outcome are presented in Figure 3 and Appendix Table A2.
Figure 2.
Network of eligible comparisons for management of midportion Achilles tendinopathy. Line indicates direct comparison of agents, and thickness of the line corresponds to the number of trials in the comparison. Size of node corresponds to the number of studies that involve the intervention. Control, wait and see; ECC, eccentric training; ESWT, extracorporeal shockwave therapy; Foot orthoses, prefabricated or customized foot orthoses; HA, hyaluronan; HVI+C, high-volume injection (a large volume of saline, steroid, and local anesthetic injection); HVI–C, high-volume injection without corticosteroid; PRP, platelet-rich plasma; SVF, adipose-derived stromal vascular fraction.
Figure 3.
Network meta-analysis of interventions compared with ECC for mean change in Victorian Institute of Sports Assessment–Achilles score from baseline. (A) Short-term outcomes (≤3 months). (B) Longer-term outcomes (>3 to <12 months). (C) All-cause discontinuation (tolerability). Effect estimation is presented as mean difference (SMD) and odds ratio (OR) with 95% CI. For SMD, a CI that does not cross 0 is considered significant; for OR, a CI that does not cross 1 is considered significant. Pharmacological agents are ranked by surface–under–the–cumulative ranking curve value. ECC, eccentric training; ESWT, extracorporeal shockwave therapy; Foot orthoses, prefabricated or customized foot orthoses; HA, hyaluronan; HVI+C, high-volume injection (a large volume of saline, steroid, and local anesthetic injection); HVI–C, high-volume injection without corticosteroid; PRP, platelet-rich plasma; SVF, adipose-derived stromal vascular fraction.
In short-term interventions (Figure 3A), both ECC with HVI+C as well as acupuncture as monotherapy significantly improved the VISA-A score compared with ECC alone (ECC+HVI+C: SMD, 1.08; 95% CI, 0.58-1.58; acupuncture: SMD, 1.57; 95% CI, 1.00-2.13). In longer-term interventions (Figure 3B), the wait-and-see approach resulted in unfavorable outcomes compared with ECC (SMD, −1.51; 95% CI, −2.02 to −1.01). Improvement was significantly higher when ECC was combined with HVI+C (SMD, 0.53; 95% CI, 0.05-1.02) and ESWT (SMD, 0.99; 95% CI, 0.48-1.49). Acupuncture alone was superior to ECC in longer-term outcomes (SMD, 1.23; 95% CI, 0.69-1.76). ESWT was not reported in short-term results because all studies with ESWT had longer-term follow-up time points. There was comparable tolerability among all interventions evaluated (Figure 3C).
Study Quality and Risk of Bias
The risk of bias for studies involved in the analysis is presented in Appendix B (available as supplemental material). The risk of bias was generally considered low for each component of the Cochrane Risk of Bias tool. All studies reported the use or described the methodological details of randomized sequence generation and allocation concealment. Some studies could not blind patients because of obvious difference in treatments such as ESWT versus ECC or ESWT versus injections, but these studies minimized bias by blinding assessors or statisticians. Approximately one-half (13/22) of the included studies registered clinical trials before their studies, and if the preplanned outcomes were reported in the articles, the risk of bias was considered low. Comparison-adjusted funnel plots for the primary outcomes showed a low probability of publication bias (see supplemental material, pages 7, 11, and 16).
Discussion
The purpose of this investigation was to conduct NMA using published RCT results to evaluate nonsurgical treatment options to manage midportion AT. The results introduce the first NMA specific to the midportion AT to identify efficacy rank among the interventions using the VISA-A outcome measure. Both HVI+C with ECC and acupuncture as monotherapy significantly improved VISA-A scores from baseline in both the short term and the longer term. In longer-term results, ESWT combined with ECC showed significant improvement in VISA-A scores, while all treatments maintained comparable tolerability.
AT is commonly treated with exercise-based interventions of ECC, and ECC was found to be beneficial, particularly in the longer term, compared to the wait-and-see approach. There are 6 different exercise protocols commonly reported45: the Alfredson protocol,2 a low-volume version of the Alfredson protocol (“do-as-tolerated”),60 concentric training,39 the Silbernagel protocol,17 heavy slow resistance training,3 and the Stanish protocol.59 A previous review concluded these protocols did not have clinically significant differences.45 A recent meta-analysis by Murphy et al44 identified a mean improvement of 21.1 points on the VISA-A at 12 weeks after the inception of loading protocols in midportion AT. The results of the NMA seem consistent with the findings of Murphy et al, given that an improvement of 18.2 points was observed in the VISA-A score compared with the wait-and-see approach after 3 months (Appendix Table A3, available as supplemental material).
To account for the natural history of AT, the NMA included the effect of a control group defined as a wait-and-see approach. This result is unique, and an important feature of our study is that we reflected the natural course of recovery in the analysis using the indirect comparison and compared it with other interventions. At the same time, we set ECC as a reference group in both the short and longer terms because ECC was ubiquitously used as a control group in most studies, and only 1 study54 had published data that could be used to measure the wait-and-see group at longer term. Even though a previous review cautioned that ECC may improve symptoms in only 60% of patients or fewer,35 it may still be an appropriate initial treatment given its wide availability, low cost, and favorable safety profile.
HVI+C combined with ECC showed a positive effect in both the short and longer terms. High-volume injection uses a mixture of saline, anesthetic, and/or steroid and can be localized to the interface of the Kager fat pad and surrounding tissue adjacent to the midportion of the Achilles tendon.8,24 It is hypothesized that HVI+C has a mechanical effect on neovascular ingrowth and adhesions between the tendon and peritendinous tissues as well as having effects on pain and local sensitization.8,24 To date, 2 studies by Boesen et al4,5 have investigated the effect of HVI+C on midportion AT. The first study compared HVI+C with PRP and sham,4 whereas the later study evaluated the effect of corticosteroid by comparing HVI+C and HVI without corticosteroid (HVI-C).5 The later study found that HVI+C was more effective in the short term (first 12 weeks) than HVI-C, indicating that corticosteroid may play a significant role in improving pain and function during the first weeks after treatment. However, concerns arise regarding the long-term effects of corticosteroids, such as atrophy, pain, and tendon rupture.13,41 In addition, compared with placebo injection, corticosteroid injection has shown a significantly increased relative risk of atrophy for Achilles tendon.16 Many relapses were seen after 6 to 12 weeks with corticosteroid injection alone,16 and compared with exercise, long-term outcomes might be poorer.13,58 Thus, when offering HVI+C as a treatment option, clinicians should inform patients of the anticipated length of benefits from this treatment. Even though the studies by Boesen et al4,5 did not report any adverse events or relapses associated with HVI+C, more research is required to establish the safety of this treatment.
In the longer-term studies, PRP combined with ECC significantly improved VISA-A scores over the wait-and-see approach, but it was not significantly effective compared with ECC alone. Clinically, autologous blood-derived products including autologous whole blood and PRP are commonly used to manage chronic osteoarthritis and tendinopathy.15 Proposed mechanisms of action of PRP include effects of trophic growth factors, including platelet-derived growth factor, transforming growth factor β, and insulin-like growth factors, to stimulate a healing response.19,27,48 PRP can be further subdivided into leukocyte-rich and leukocyte-poor PRP and can be mixed with local anesthetics using a various range of kits.15 Despite subtle differences among products, which may result in heterogeneity, 3 recent meta-analyses showed that neither PRP33,64 nor autologous blood-derived products (including PRP)32 were effective in the management of AT. Although our results indicated that PRP alone and PRP+ECC yielded better outcomes than the wait-and-see approach, our NMA is consistent with previous pairwise meta-analyses in that we found PRP+ ECC may not be effective as ECC alone. Our results did not show a clear benefit of PRP over ECC and reflect the need for further research to show its role or synergy with other interventions in treating midportion AT.
ESWT along with ECC was also shown effective for treatment of midportion AT in the longer term. ESWT has been used in the management of various lower limb soft tissue conditions and may provide benefits of soft tissue healing and inhibition of pain.51,53 A previous meta-analysis showed that ESWT is an effective intervention for greater trochanteric pain syndrome, patellar tendinopathy, and AT.40 Although a pooled meta-analysis suggested that focused shockwave may be superior to radial shockwave for tendinopathy,31 there is currently no consensus on the best form and energy setting of ESWT for AT. Included in our analysis were 2 studies by Rompe et al,53,54 who used a radial shockwave device with an energy flux density of 0.1 mJ/mm2 and applied 2000 pulses at each of 3 sessions 1 week apart. In these studies, low-energy shockwave resulted in no adverse event. In a previous RCT using focused shockwave, 2 older women (ages 62 and 65) experienced Achilles tendon ruptures within 2 weeks of the first treatment session.14 Collective studies evaluating ESWT for AT suggest overall efficacy, but further studies are needed to evaluate efficacy in the short term and beyond 1 year.
The NMA identified that most studies combined interventions (most with ECC) and commonly did not use wait-and-see as a control group (most patients received the ECC program). For example, in both studies investigating the efficacy of HVI+C,4,5 all patients performed the Alfredson protocol2 for ECC, limiting the ability to isolate the true effect of HVI+C compared with no treatment. Except for 1 study that directly compared PRP with ECC,26 most investigations combined either PRP or autologous blood injections (ABI) with ECC in both intervention and placebo groups, so only indirect comparisons could be made with the wait-and-see approach in our NMA. Synergistic effects of ECC combined with interventions may yield superior outcomes. Rompe et al54 evaluated the efficacy of ESWT compared with ECC and a wait-and-see approach. In that study, ECC and ESWT both showed significant results at 4 months over no treatment. A separate investigation showed that ESWT combined with ECC was superior to ECC as monotherapy.53 These studies suggest that ESWT and ECC may have a synergistic effect.53,54 Collective results suggest the value of ECC with interventions such as HVI+C and ESWT to optimize outcomes.
Although this is the first NMA specific to nonsurgical therapies for the management of midportion AT, our study has some limitations. First, while acupuncture showed favorable outcomes in the short and longer terms, these results were measured from a single study.63 Moreover, the results of HVI+C and ESWT were each derived from 2 studies,4,5,53,54 reflecting the value of further studies to substantiate these findings. Second, studies that did not use the VISA-A or that reported outcomes outside of our predefined follow-up time period were excluded from the analysis. Therefore, even though 1 study showed that topical glyceryl trinitrate was effective in reducing pain with activity and at night at 12 weeks,49 the study was excluded because the VAS was used. The same authors used the VISA-A to reassess patients at 3-year follow-up, but that study was excluded because our review limited the follow-up period up to 1 year.50 Third, some interventions in the tolerability analysis (Figure 3C) do not appear in the efficacy analysis because they did not provide sufficient statistical information. Also, even though tolerability may imply severe adverse event or lack of efficacy, there may be other reasons for dropout that could affect interpretation of tolerability. According to our results, however, none of the interventions significantly resulted in higher dropout, indicating that each intervention is comparably tolerable. Fourth, although ABI and PRP may differ in that PRP is blood rich in platelets derived from autologous whole blood,33 these 2 injections were combined in our analysis, as done in the previous meta-analysis.64
Conclusion
Few studies in our NMA demonstrated favorable outcomes for short- and longer-term interventions for midportion AT. Acupuncture demonstrated favorable results in both the short and longer term. Although HVI+C combined with ECC may be effective in studies up to 12 months, clinicians must be aware of potential deleterious effects associated with the use of corticosteroid. Longer-term outcomes were improved with the use of ECC over the wait-and-see approach. Our findings suggest that interventions such as HVI+C and ESWT can be combined with ECC to provide additional benefit. Future studies are required to provide more direct comparisons for relative efficacy or for outcomes longer than 1 year.
Supplemental Material
Supplemental Material, DS_10.1177_2325967120930567 for Comparative Efficacy and Tolerability of Nonsurgical Therapies for the Treatment of Midportion Achilles Tendinopathy: A Systematic Review With Network Meta-analysis by Hye Chang Rhim, Min Seo Kim, Seungil Choi and Adam S. Tenforde in Orthopaedic Journal of Sports Medicine
Footnotes
Final revision submitted February 26, 2020; accepted March 10, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Albers IS, Zwerver J, Diercks RL, et al. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC Musculoskelet Disord. 2016;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26(3):360–366. [DOI] [PubMed] [Google Scholar]
- 3. Beyer R, Kongsgaard M, Hougs Kjær B, et al. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43(7):1704–1711. [DOI] [PubMed] [Google Scholar]
- 4. Boesen AP, Hansen R, Boesen MI, et al. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med. 2017;45(9):2034–2043. [DOI] [PubMed] [Google Scholar]
- 5. Boesen AP, Langberg H, Hansen R, et al. High volume injection with and without corticosteroid in chronic midportion Achilles tendinopathy. Scand J Med Sci Sports. 2019;29(8):1223–1231. [DOI] [PubMed] [Google Scholar]
- 6. Caldwell D, Ades A. Checking consistency in mixed treatment comparison. Stat Med. 2010;29:932–944. [DOI] [PubMed] [Google Scholar]
- 7. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PloS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan O, O’Dowd D, Padhiar N, et al. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil. 2008;30(20-22):1697–1708. [DOI] [PubMed] [Google Scholar]
- 9. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus. 2018;16(4):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Cochrane Collaboration; Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. 2011. [Google Scholar]
- 11. Cook J, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–416. [DOI] [PubMed] [Google Scholar]
- 12. Cook J, Rio E, Purdam CR, et al. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med. 2016;50(19):1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751–1767. [DOI] [PubMed] [Google Scholar]
- 14. Costa M, Shepstone L, Donell S, et al. Shock wave therapy for chronic Achilles tendon pain: a randomized placebo-controlled trial. Clin Orthop Relat Res. 2005;440:199–204. [DOI] [PubMed] [Google Scholar]
- 15. Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45(1):226–233. [DOI] [PubMed] [Google Scholar]
- 16. Fredberg U, Bolvig L, Pfeiffer-Jensen M, et al. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper’s knee and Achilles tendinitis: a randomized, double-blind, placebo-controlled study. Scand J Rheumatol 2004;33(2):94–101. [DOI] [PubMed] [Google Scholar]
- 17. Grävare Silbernagel K, Thomeé R, Thomeé P, et al. Eccentric overload training for patients with chronic Achilles tendon pain—a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001;11(4):197–206. [DOI] [PubMed] [Google Scholar]
- 18. Habets B, Van Cingel R. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25(1):3–15. [DOI] [PubMed] [Google Scholar]
- 19. Hansen M, Boesen A, Holm L, et al. Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scand J Med Sci Sports. 2013;23(5):614–619. [DOI] [PubMed] [Google Scholar]
- 20. Higgins J, Jackson D, Barrett J, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Green S Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2011. [Google Scholar]
- 23. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Humphrey J, Chan O, Crisp T, et al. The short-term effects of high volume image guided injections in resistant non-insertional Achilles tendinopathy. J Sci Med Sport. 2010;13(3):295–298. [DOI] [PubMed] [Google Scholar]
- 25. Kanters S, Ford N, Druyts E, et al. Use of network meta-analysis in clinical guidelines. Bull World Health Organ. 2016;94(10):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kearney R, Parsons N, Costa M. Achilles tendinopathy management: a pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kjær M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19(4):500–510. [DOI] [PubMed] [Google Scholar]
- 28. Korakakis V, Whiteley R, Tzavara A, et al. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: a systematic review including quantification of patient-rated pain reduction. Br J Sports Med. 2018;52(6):387–407. [DOI] [PubMed] [Google Scholar]
- 29. Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol. 2013;13(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langberg H, Ellingsgaard H, Madsen T, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports. 2007;17(1):61–66. [DOI] [PubMed] [Google Scholar]
- 31. Liao C-D, Tsauo J-Y, Chen H-C, et al. Efficacy of extracorporeal shock wave therapy for lower-limb tendinopathy: a meta-analysis of randomized controlled trials. Am J Phys Med Rehabil. 2018;97(9):605–619. [DOI] [PubMed] [Google Scholar]
- 32. Lin M-T, Chiang C-F, Wu C-H, et al. Meta-analysis comparing autologous blood-derived products (including platelet-rich plasma) injection versus placebo in patients with Achilles tendinopathy. Arthroscopy. 2018;34(6):1966–1975. [DOI] [PubMed] [Google Scholar]
- 33. Liu C-J, Yu K-L, Bai J-B, et al. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: a meta-analysis. Medicine. 2019;98(16):e15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc Rev. 2009;17(2):112–126. [DOI] [PubMed] [Google Scholar]
- 35. Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc Rev. 2018;26(1):16–30. [DOI] [PubMed] [Google Scholar]
- 36. Lopes AD, Hespanhol LC, Yeung SS, et al. What are the main running-related musculoskeletal injuries? Sports Med. 2012;42(10):891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313–2324. [DOI] [PubMed] [Google Scholar]
- 38. Maffulli N, Kader D. Tendinopathy of tendo Achillis. J Bone Joint Surg Br. 2002;84(1):1–8. [DOI] [PubMed] [Google Scholar]
- 39. Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9(1):42–47. [DOI] [PubMed] [Google Scholar]
- 40. Mani-Babu S, Morrissey D, Waugh C, et al. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: a systematic review. Am J Sports Med. 2015;43(3):752–761. [DOI] [PubMed] [Google Scholar]
- 41. Metcalfe D, Achten J, Costa ML. Glucocorticoid injections in lesions of the Achilles tendon. Foot Ankle Int. 2009;30(7):661–665. [DOI] [PubMed] [Google Scholar]
- 42. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 43. Murphy M, Rio E, Debenham J, et al. Evaluating the progress of mid-portion Achilles tendinopathy during rehabilitation: a review of outcome measures for self-reported pain and function. Int J Sports Phys Ther. 2018;13(2):283. [PMC free article] [PubMed] [Google Scholar]
- 44. Murphy M, Travers M, Gibson W, et al. Rate of improvement of pain and function in mid-portion Achilles tendinopathy with loading protocols: a systematic review and longitudinal metaanalysis. Sports Med. 2018;48(8):1875–1891. [DOI] [PubMed] [Google Scholar]
- 45. Murphy MC, Travers MJ, Chivers P, et al. Efficacy of heavy eccentric calf training for treating midportion Achilles tendinopathy: a systematic review and meta-analysis. Br J Sports Med. 2019;53(7):1070–1077. [DOI] [PubMed] [Google Scholar]
- 46. Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PloS One. 2014;9(12):e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nikolakopoulou A, Mavridis D, Furukawa TA, et al. Living network meta-analysis compared with pairwise meta-analysis in comparative effectiveness research: empirical study. BMJ. 2018;360:k585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olesen JL, Heinemeier KM, Langberg H, et al. Expression, content, and localization of insulin-like growth factor I in human Achilles tendon. Connect Tissue Res. 2006;47(4):200–206. [DOI] [PubMed] [Google Scholar]
- 49. Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg. 2004;86(5):916–922. [DOI] [PubMed] [Google Scholar]
- 50. Paoloni JA, Murrell GA. Three-year followup study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28(10):1064–1068. [DOI] [PubMed] [Google Scholar]
- 51. Reilly JM, Bluman E, Tenforde AS. Effect of shockwave treatment for management of upper and lower extremity musculoskeletal conditions: a narrative review. PM R. 2018;10(12):1385–1403. [DOI] [PubMed] [Google Scholar]
- 52. Robinson J, Cook JL, Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37(3):463–470. [DOI] [PubMed] [Google Scholar]
- 54. Rompe JD, Nafe B, Furia JP, Muffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35(3):374–383. [DOI] [PubMed] [Google Scholar]
- 55. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shim S, Yoon B-H, Shin I-S, et al. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Slee A, Nazareth I, Bondaronek P, et al. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393(10173):768–777. [DOI] [PubMed] [Google Scholar]
- 58. Smidt N, Van Der Windt DA, Assendelft WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657–662. [DOI] [PubMed] [Google Scholar]
- 59. Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res. 1986;208:65–68. [PubMed] [Google Scholar]
- 60. Stevens M, Tan C-W. Effectiveness of the Alfredson protocol compared with a lower repetition volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44(2):59–67. [DOI] [PubMed] [Google Scholar]
- 61. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu C, Niu Y, Wu J, et al. Software and package applicating for network meta-analysis: a usage-based comparative study. J Evid Based Med. 2018;11(3):176–183. [DOI] [PubMed] [Google Scholar]
- 63. Zhang BM, Zhong LW, Xu SW, Jiang HR, Shen J. Acupuncture for chronic Achilles tendnopathy: a randomized controlled study. Chin J Integr Med. 2013;19(12):900–904. [DOI] [PubMed] [Google Scholar]
- 64. Zhang Y-J, Xu S-Z, Gu P-C, et al. Is platelet-rich plasma injection effective for chronic Achilles tendinopathy? A meta-analysis. Clin Orthop Relat Res. 2018;476(8):1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, DS_10.1177_2325967120930567 for Comparative Efficacy and Tolerability of Nonsurgical Therapies for the Treatment of Midportion Achilles Tendinopathy: A Systematic Review With Network Meta-analysis by Hye Chang Rhim, Min Seo Kim, Seungil Choi and Adam S. Tenforde in Orthopaedic Journal of Sports Medicine