Abstract
Results from the epidemiologic studies on the relationship between hormone replacement therapy (HRT) and the risk of kidney cancer in women were not completely consistent. This meta-analysis aimed to evaluate the relationship between HRT and risk of kidney cancer in women. We performed a meta-analysis of observational studies to assess this association. The PubMed and Embase databases were searched from their inception to January 29, 2020, to identify relevant studies that fit the pre-stated inclusion criteria; reference lists from the retrieved articles were also been reviewed. Relative risks (RRs) with corresponding 95% CIs were extracted and combined using random effects models. Furthermore, dose–response, sensitivity analyses, publication bias, and subgroup analysis by study design, regional location, and exposure assessment method were conducted. Thirteen articles involving 6 cohort studies and 8 case–control studies were included in our meta-analysis. Overall, 4194 women were diagnosed with kidney cancer among 648 107 participants. The pooled RR for kidney cancer was 1.08 (95% CI: 0.96-1.22) in those who were administered HRT compared to those who had not. Subgroup analysis indicated the overall result was not influenced by study type, regional location, or adjusted variables. Dose–response analysis showed a nonlinear relationship between HRT and kidney cancer (P = .0021) and the risk of kidney cancer decreased by 15% to 28% with 12 to 18 years of HRT use. No evidence of publication bias was found (P for Egger =.111). Our meta-analysis showed that HRT use is inversely associated with kidney cancer risk in a dose–dependent fashion.
Keywords: hormone replacement therapy, kidney cancer, meta-analysis
Introduction
Kidney cancer is the 16th commonest cancer type worldwide, representing 2.2% of all cases.1 It includes renal cell carcinoma originating from the renal parenchyma and accounting for over 90% cases and renal pelvis cancers arising from transitional cells.2 Evidence regarding the etiology of kidney cancer is limited. Established risk factors include cigarette smoking, obesity, a history of hypertension, and chronic kidney disease.3
Epidemiological studies have shown kidney cancer is more common in men than in women, with age-standardized incidence and mortality rates in males being 8.1 per 100 000 and 1.8 per 100 000, respectively; this is approximately 2 to 3 times higher than the rates in women.1 The biologic differences between men and women suggest that female sex hormones may be involved in the etiology of kidney cancer, with the exception of lifestyle differences, such as cigarette smoking, which likely account for some of the disparities. Additionally, experimental studies have demonstrated that the expression of estrogen and progesterone receptors in both normal and neoplastic renal cell tissue4 and estrogens is associated with the development of renal cell tumors in animals.5,6
Hormone replacement therapy (HRT) is the most widely used and effective way to treat menopausal symptoms in both postmenopausal women and young women with early menopause due to surgery, radiotherapy, or chemotherapy. Its potential benefits and risks have attracted people’s attention. Previous reviews have shown that HRT use is associated with a decreased risk of osteoporotic fractures, colorectal cancer, liver cancer and glioma, but an increased risk of stroke and thromboembolic events.7-9 The existence of a relationship between HRT and kidney cancer is uncertain; although many studies have assessed the association between HRT and kidney cancer risk.10-22 The results have been conflicting. Thus, we performed a meta-analysis of case–control and cohort studies to clarify the association between HRT use and risk of kidney cancer.
Materials and Methods
This meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23
Selection Criteria
The inclusion criteria were as follows used: the relationship between HRT and kidney cancer was addressed through describing a case–control or cohort study; the relative risk (RR) estimates (odd ratio, risk ratio, or hazard ratio) with corresponding 95% CIs or the provision of enough data to evaluate them should be provided in the article; the exposure variables in the article should be HRT use (ie, ever/current/past HRT use vs no HRT use). If the same subject was described in 2 or more articles, we used only the most informative study. Conference abstracts that did not report on the availability of the original data, as well as commentaries, meta-analyses, case reports, and reviews were excluded.
Search Strategy
We systematically searched the PubMed and Embase databases for relevant articles (up to January 29, 2020) using the following search terms: “kidney cancer,” “kidney tumor,” “renal cell carcinoma,” “renal cell cancer,” hormone, estrogen, progesterone, and “reproductive factors.” The detailed search strategy is shown in Supplemental Table S1. We also searched the references lists of the identified articles that evaluated the relationship between HRT and kidney cancer. The search was conducted without language restriction.
Data Extraction
Two authors independently extracted the following data from each eligible article: author and publication year, country, study design, age at baseline, follow-up year, sample size, methods used for data collection, exposure variables, and RRs ratios with corresponding 95% CIs (or the raw data to calculate them). We used the Newcastle-Ottawa Scale (NOS)24 to assess the risk of bias of these studies (range, 0-9 points). A final score of >6 was regarded as high quality.
Statistical Analysis
Since the prevalence of kidney cancer is low, we deemed the odds ratios and hazard ratios (HRs) equivalent to RRs.25 In this meta-analysis, we used RR with corresponding 95% CIs to measure the association between HRT use and kidney cancer. For the studies that reported risk estimates separately for different states of HRT use (current use and past use vs no history of HRT use), we calculated a combined risk estimates using a fixed effect model to represent a comparison of ever use versus no history of use. This method is widely used in meta-analyses and systematic reviews.26-28
Heterogeneity was assessed by the Q statistical test and the I 2 test.29,30 Heterogeneity was considered statistically significant when the P value was < .1 and I 2 was >50%. To explore the variability across studies, we pooled the RRs with 95% CIs using the random effect model.31 Subgroup analysis was performed according to the study type, study location, and adjusted variables. To assess the stability of the results, sensitivity analysis was conducted by excluding one study at a time. Another method of sensitivity analysis that combined the RRs and 95% CIs with a fixed-effects model was also performed. Publication bias was estimated by funnel plots and the Egger test (P < .05 was considered indicative of statistical significance).32 The potential dose–response effect of HRT use on kidney cancer was assessed using robust-error meta-regression (REMR), as described by Doi.33 Briefly, REMR is a one-step procedure that considers each study as a cluster within the whole sample and establishes a study-level weighted (inverse variance) least square regression between HRT and the risk of kidney cancer. The nonreference effects were correlated within each cluster and addressed using the Huber-White robust errors. We used this model since it only requires at least 2 levels of exposure and corresponding risk estimates with 95% CIs, while the group sample information for each exposure level is unnecessary. A detailed theoretical description and the Stata commands can be found in the methodological paper of Xu and Doi.33 A restricted cubic spline function with 3 adaptive knots was used to approximate the potential nonlinear relationship. The Wald test was used to determine whether a nonlinear relationship exists by treating the nonlinear term as zero (null hypothesis) and a P value < .05 indicated a strong evidence of nonlinearity (leading to rejection of the null hypothesis). All of the methodological tips of the dose–response meta-analysis were based on the recommendations proposed by Xu et al.34 All P values were 2-sided. We performed the all statistical analysis using STATA version 14.0 (STATA Corporation).
Results
Literature Search
Figure 1 shows a flow diagram of the process used for the literature search and study selection. We retrieved 533 articles from PubMed database and 753 articles from Embase database. Two case–control studies were found by reviewing the references of identified articles.20,21 After reading the titles and abstracts, 497 duplicates articles and 765 articles unrelated with our research were excluded. Of the 24 publications identified for full-text assessment, 2 did not provide any available data,35,36 1 was a commentary,37 2 were abstracts,38,39 1 was a report with data overlapping with another publicaion,40 and 5 were reviews41-45; there were all therefore excluded. This left 13 articles for inclusion in this meta-analysis.10-22
Figure 1.
Flow diagram of the process used for the literature search and relative study selection.
Study Characteristics
Table 1 shows the characteristics of the 6 cohort and 8 case–control studies reported in 13 articles. All of the articles were published in English. Of these 12 articles, the earliest study was published in 1992, and the study published in 2013 was the latest one. A total of 648 107 participants were recruited, and 4194 were diagnosed with kidney cancer. Most of the included studies were performed in North America, 3 were performed in Europe, and 2 in Asia-Pacific region. Six of the included studies assessed the relationship between estrogen therapy and kidney cancer risk12,13,15,18-20 and 2 reported estrogen plus progesterone use.12,13 We used the NOS to assess the risk of bias of these studies. Based on the NOS, 7 of these studies10,12,13,15,16,19,20 were scored >6 points, and 6 studies 11,14,18,17 , 21,22 scored ≤6 points. All studies provided adjusted risk estimates for multiple variables, such as body mass index, educational level, race, smoking status, sex, age, study center, hypertension, and family history of kidney cancer, menopausal status, alcohol intake, period of interview, number of births, oral contraceptives (OC) use, oophorectomy, hysterectomy, fruit intake, and vegetable intake.
Table 1.
Characteristics of the Included Studies.
| References | Location | Study design | Age at baseline | Follow-up, year | No. of cases | No. of participants | Exposure assessment | Exposure cat | RR (95% CI) | Type of HRT | Risk of bias (points) | Adjusted variables or control factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karami et al10 NIH-AARP study | United States | Cohort study | 50-71 | 11.2 | 601 | 210 300 | Self- administered questionnaire | Ever vs never | 0.83 (0.69-0.99) | HRT | 7 | BMI, educational level, race, and smoking status |
| ≤4/≤5 vs never | 0.9 (0.71-1.13) | BMI, educational level, race and smoking status | ||||||||||
| 5-9/6-9 vs never | 0.66 (0.49-0.89) | BMI, educational level, race, and smoking status | ||||||||||
| >10 vs never | 0.86 (0.68-1.07) | BMI, educational level, race, and smoking status | ||||||||||
| Karami et al10 PLCO. study | United States | Cohort study | 55-74 | 14.2 | 191 | 73652 | Self-administered questionnaire | Ever vs never | 1.30( 0.93-1.80) | HRT | BMI, educational level, race, and smoking status | |
| ≤4 /≤5 vs never | 1.46 (1.02-2.10) | BMI, educational level, race, and smoking status | ||||||||||
| 5-9/6-9 vs never | 1.42 (0.87-2.33) | BMI, educational level, race, and smoking status | ||||||||||
| >10 vs never | 1.00 (0.65-1.55) | HRT | BMI, educational level, race, and smoking status | |||||||||
| Purdue et al11 | United States | Case– control study | ≥55 | NA | 497 | 1043 | Interview | Ever vs never | 0.8 (0.5-1.1) | 5 | Sex, age, study center, education, smocking status, BMI, hypertension, and family history of kidney cancer | |
| ≤5 vs never | 0.8 (0.5-1.3) | Sex, age, study center, education, smocking status, BMI, hypertension, and family history of kidney cancer | ||||||||||
| 6-10 vs never | 0.5 (0.3-1.1) | Sex, age, study center, education, smocking status, BMI, hypertension, and family history of kidney cancer | ||||||||||
| 11-15 vs never | 1.0 (0.5-2.0) | Sex, age, study center, education, smocking status, BMI, hypertension, and family history of kidney cancer | ||||||||||
| >15 vs never | 0.6 (0.4-1.1) | Sex, age, study center, education, smocking status, BMI, hypertension, and family history of kidney cancer | ||||||||||
| Setiawan et al12 | United States | Cohort study | 45-75 | 10.6 | 229 | 106036 | Self-administered questionnaire | Former vs never | 1.28 (0.86-1.91) | HRT, ERT, EPT | 7 | Area, race/ethnicity, menopausal status, age, BMI, smoking status, hypertension, alcohol intake, and diuretic use |
| Current vs never | 1.29 (0.78-2.14) | Area, race/ethnicity, menopausal status, age, BMI, smoking status, hypertension, alcohol intake, and diuretic use | ||||||||||
| Zucchetto.et al14 | Italy | Case–control study | 24-79 | NA | 273 | 819 | Interview | Ever vs never | 1.23 (0.72–2.11) | HRT | 5 | Period of interview, years of education, smoking habits, history of hypertension (No, Yes), family history of kidney cancer in first-degree relatives, and BMI at age 30 years |
| ≤5 vs never | 1.1 (0.6–2.0) | Period of interview, years of education, smoking habits, history of hypertension (No, Yes), family history of kidney cancer in first-degree relatives, and BMI at age 30 years | ||||||||||
| ≥5 vs never | 1.7 (0.6–4.7) | Period of interview, years of education, smoking habits, history of hypertension (No, Yes), family history of kidney cancer in first-degree relatives, and BMI at age 30 years | ||||||||||
| Kabat et al16 | Canada | Cohort study | 40-59 | 16.4 | 172 | 89835 | Self-administered questionnaire | Ever vs never | 0.98 (0.69-1.41) | HRT | 7 | Age, pack-years, body mass index, menopausal status, education, study center, and randomisation group |
| ≤1 vs never | 1.02 (0.56–1.85) | Age, pack-years, body mass index, menopausal status, education, study center, and randomisation group | ||||||||||
| 1-5 vs never | 0.98 (0.53–1.82) | Age, pack-years, body mass index, menopausal status, education, study center, and randomisation group | ||||||||||
| ≥5 vs never | 0.91 (0.51–1.63) | Age, pack-years, body mass index, menopausal status, education, study center, and randomisation group | ||||||||||
| Lindblad et al19 | Australia | Case–control study | 20-79 | NA | 608 | 1374 | Interview | Ever vs never | 1.0 (0.8-1.4) | ERT | 7 | Age, center, tobacco use, BMI, number of births, age at first birth, age at menarche, duration of menstrual life, OC use, ERT use, and oophorectomy only /hysterectomy only /both; |
| ≤0.5 vs never | 1.1 (0.7-1.8) | Age, center, tobacco use, BMI, number of births, age at first birth, age at menarche, duration of menstrual life, OC use, ERT use, and oophorectomy only /hysterectomy only /both; | ||||||||||
| 0.6-2.9 vs never | 0.9 (0.6-1.6) | Age, center, tobacco use, BMI, number of births, age at first birth, age at menarche, duration of menstrual life, OC use, ERT use, and oophorectomy only /hysterectomy only /both; | ||||||||||
| 3.0-7.0 vs never | 0.8 (0.5-1.5) | Age, center, tobacco use, BMI, number of births, age at first birth, age at menarche, duration of menstrual life, OC use, ERT use, and oophorectomy only /hysterectomy only /both; | ||||||||||
| >7 vs never | 1.2 (0.7-2.0) | Age, center, tobacco use, BMI, number of births, age at first birth, age at menarche, duration of menstrual life, OC use, ERT use, and oophorectomy only /hysterectomy only /both; | ||||||||||
| Molokwu et al15 | United States | Cohort study | 55-69 | 18 | 165 | 37440 | Self-administered questionnaire | Past vs never | 1.56 (1.13-2.17) | ERT | 6 | Age, BMI, WHR, alcohol use, and history of hypertension |
| Current vs never | 1.17 (0.6-2.02) | Age, BMI, WHR, alcohol use, and history of hypertension | ||||||||||
| Lee et al13 | United States | Cohort study | 30-55 | 28 | 247 | 118219 | Self-administered questionnaire | Past vs never | 0.91 (0.63-1.30) | HRT, ERT, EPT | 6 | History of hypertension, BMI;, smoking status, fruit intake vegetable intake, and alcohol intake |
| <5 vs never | 1.05 (0.72-1.54) | History of hypertension, BMI;, smoking status, fruit intake vegetable intake, and alcohol intake | ||||||||||
| 5–<10 vs never | 1.06 (0.68-1.67) | History of hypertension, BMI;, smoking status, fruit intake vegetable intake, and alcohol intake | ||||||||||
| ≥10 vs never | 0.9 (0.58-1.40) | History of hypertension, BMI;, smoking status, fruit intake vegetable intake, and alcohol intake | ||||||||||
| Current vs never | 1.01 (0.70-1.45) | History of hypertension, BMI;, smoking status, fruit intake vegetable intake, and alcohol intake | ||||||||||
| Chow et al20 | United States | Case–control study | 20-79 | NA | 165 | 392 | Interview | Ever vs never | 1.8 (1.1-3.0) | ERT | 6 | Age, cigarette smoking, and body mass index |
| <0.8 vs never | 2.4 (1.0-5.8) | Age, cigarette smoking, and body mass index | ||||||||||
| 0.8-2 vs never | 1.3 (0.5-3.1) | Age, cigarette smoking, and body mass index | ||||||||||
| 3-6 vs never | 1.8 (0.7-4.8) | Age, cigarette smoking, and body mass index | ||||||||||
| >7 vs never | 2.0 (0.8-5.0) | Age, cigarette smoking, and body mass index | ||||||||||
| Gago- Dominguez et al18 | United States | Case–control study | 25-74 | NA | 422 | 844 | Interviewer | Ever vs never | 1.0 (0.7-1.3) | ERT | 5 | Education, and history of hysterectomy. |
| Fernandez et al17 | Italy | Case–control study | 45-79 | NA | 102 | 7078 | Interview | Ever vs never | 1.3 (0.7-2.4) | HRT | 6 | Age, study center, year of interview, education, smoking, drinking, type of menopause, age at menopause and BMI |
| <2 vs never | 1.3 (0.6-2.8) | Age, study center, year of interview, education, smoking, drinking, type of menopause, age at menopause and BMI | ||||||||||
| ≥2 vs never | 0.9 (0.3-3.2) | Age, study center, year of interview, education, smoking, drinking, type of menopause, age at menopause and BMI | ||||||||||
| McLaughlin et al21 | China | Case–control study | 35-74 | NA | 154 | 311 | Interview | Ever vs never | 1.9 (0.4-8.3) | HRT | 5 | Age |
| Mellemgaard et al22 | Denmark | Case–control study | 20-79 | NA | 368 | 764 | Interview | <10 vs never | 1.0 (0.6-1.8) | HRT | 5 | Age, BMI, smoking, and socio-economic status |
| ≥10 vs never | 0.8 (0.3-2.3) | Age, BMI, smoking, and socio-economic status |
Abbreviations: CI, confidence interval; BMI, body mass index; EPT, estrogen-progesterone therapy; ERT, estrogen replacement therapy; HRT, hormone replacement therapy; OC, oral contraceptive; RR, relative risk.
The Overall Relationship Between HRT and Kidney Cancer Risk
Figure 2 shows the pooled outcomes for ever hormone users. Of all 13 studies, two15,20 showed a significantly positive association between HRT use and kidney cancer, and 1 study10 showed an inverse relationship between HRT and kidney cancer. When pooling these studies revealed statistically significant heterogeneity in this meta-analysis (P = .075, I 2 = 38.9%). The pooled RR was 1.08 (95% CI: 0.96-1.22). Six of the included studies assessed the relationship between estrogen therapy and kidney cancer risk,12,13,15,18-20 where the pooled RR was 1.21 (95% CI: 1.02-1.45). Three studies reported kidney cancer risk according to the state of HRT use.12,13,15 The pooled RRs were 1.15 (95% CI: 0.93-1.41) for current users and 1.30 (95% CI: 1.02-1.64) for past users.
Figure 2.
Forest plots for association between hormone replacement therapy use and kidney cancer. The squares represent the risk estimate for each individual study, with the area reflecting the weight assigned to the study. The horizontal line across each square represents the 95% CI. The diamond represents the pooled risk estimate, with width representing 95% CI.
Outcome of Subgroup Analysis
We performed a subgroup analysis by study type, geographic area, and adjusted variables. Table 2 shows the results of the subgroup analysis. The RRs and 95% CIs were 1.08 (0.89-1.31) and 1.07 (0.91-1.26) for cohort studies and case–control studies, respectively. When subgroup analyses were performed by geographical location (North American, Europe, Asia-Pacific), the RR ranged from 1.02 (95% CI: 0.78-1.34) to 1.15 (95% CI: 0.84-1.57). In subgroup analyses adjusted for smoking, hypertension, BMI, alcohol, or all confounders listed above, similar results were found (Table 2).
Table 2.
Summary Risk Estimates of the Association Between HRT and Kidney Cancer Risk.
| Group | No. of included studies | RR and 95% CI | I 2% | P Q | P for difference between subgroups |
|---|---|---|---|---|---|
| Overall | 13 | 1.08 (0.96-1.22) | 38.9% | 0.075 | |
| Study design | |||||
| Cohort study | 5 | 1.08 (0.89-1.31) | 65.8% | 0.020 | .798 |
| Case–control | 8 | 1.07 (0.91-1.26) | 11.1% | 0.343 | |
| Geographic area | |||||
| North America | 8 | 1.08 (0.92-1.27) | 61.2% | 0.012 | |
| Europe | 3 | 1.15 (0.84-1.57) | 0.0% | 0.771 | .819 |
| Asia-Pacific | 2 | 1.02 (0.78-1.34) | 0.0% | 0.414 | |
| Variables adjustment | |||||
| Smoking | |||||
| Yes | 9 | 1.05 (0.91-1.21) | 39.0% | 0.108 | .169 |
| No | 4 | 1.16 (0.91-1.47) | 35.1% | 0.202 | |
| Hypertension | |||||
| Yes | 5 | 1.13 (0.92-1.40) | 53.0% | 0.074 | .098 |
| No | 8 | 1.01 (0.89-1.16) | 16.4% | 0.301 | |
| BMI | |||||
| Yes | 11 | 1.09 (0.95-1.25) | 47.2% | 0.041 | .911 |
| No | 2 | 1.03 (0.76-1.39) | 0.0% | 0.416 | |
| Alcohol | |||||
| Yes | 4 | 1.22 (0.99-1.49) | 39.7% | 0.174 | .02 |
| No | 9 | 0.99 (0.87-1.12) | 13.5% | 0.321 | |
| Smoking, hypertension, BMI, alcohol | |||||
| Yes | 2 | 1.11 (0.84-1.47) | 58.0% | 0.123 | .462 |
| No | 10 | 1.07 (0.93-1.24) | 40.1% | 0.081 |
Abbreviations: BMI, body mass index; HRT, hormone replacement therapy; No., number.
Sensitivity Analysis
To investigate whether the pooled results were influenced by individual studies, we excluded one single study at one time to conduct the sensitivity analysis. We found the overall result was not influenced by any of the studies (Table 3). Another method of sensitivity analysis that combined the RRs and 95% CIs with a fixed effects model was also performed, with a pooled RR of 1.04 (95% CI: 0.96-1.14).
Table 3.
Results of Sensitivity Analyses.
| Study omitted | Pooled results | |
|---|---|---|
| Combined RR and 95% CI | P | |
| Karami et al10 | 1.12(0.99-1.26) | .082 |
| Purdue et al11 | 1.10(0.97-1.25) | .130 |
| Lee et al13 | 1.10(0.96-1.26) | .176 |
| Setiawan et al12 | 1.05(0.93-1.20) | .415 |
| Zucchetto et al14 | 1.07(0.95-1.22) | .272 |
| Kabat et al16 | 1.09(0.96-1.24) | .199 |
| Molokwu et al15 | 1.03(0.92-1.15) | .597 |
| Fernandez et al17 | 1.07(0.95-1.22) | .274 |
| Gago-Dominguez et al18 | 1.09(0.95-1.25) | .205 |
| Chow et al20 | 1.05(0.94-1.17) | .421 |
| Lindblad et al19 | 1.09(0.95-1.25) | .203 |
| Mellemgaard et al22 | 1.09(0.95-1.24) | .214 |
| McLaughlin et al21 | 1.08(0.95-1.22) | .247 |
Abbreviation: RR, relative risk.
Publication Bias
As shown in Figure 3, the funnel plots appear to be symmetrical. The Egger linear regression test suggested no evidence of publication bias (P = .111).
Figure 3.
Begg funnel plot of publication bias.
Dose–Response Analysis
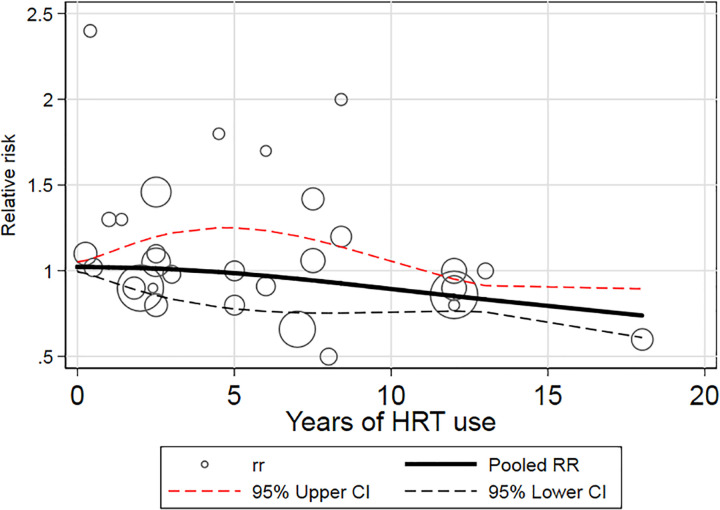
Nine reports involving 4 cohort and 6 case–control studies 10,11,13,14,16,17,19,20,22 can be included in the dose–response analysis, according to the REMR model. Figure 4 showed the dose–response relationship between kidney cancer and years of HRT use. A nonlinear relationship was observed (P = .0021). As shown in Figure 4, the risk of kidney cancer decreased by approximately 15% to 28% with up to 12 to 18 years of HRT use.
Figure 4.
Dose–response relationships between HRT and kidney cancer. HRT indicates hormone replacement therapy.
Discussion
To the best of our knowledge, this is the first meta-analysis to evaluate the relationship between HRT use and kidney cancer risk in women. Our study indicates that a dose–response inverse relationship exists between HRT use and kidney cancer risk. A significant benefit in terms of kidney cancer risk reduction was obtained in women with a history of more than 12 years of HRT use.
There are several lines of evidence supporting the notion that female hormones may prevent kidney tumorigenesis. Estrogens can exert their biological effect through 2 major endoplasmic reticulum (ER) subtypes, ER-α, and in particular, ER, which is highly expressed in normal tissues, but at low levels in kidney cancer tissues,46,47 and has antiproliferative and apoptosis-inducing functions.48 When estrogen binds to ER-β, AKT, ERK, NF-κB, MMP 9, and JAK signaling pathway among growth hormone downstream signaling activation was reduced and activation of the apoptotic cascade was improved by estrogen complex, promoting apoptosis of kidney cancer cells.
Two challenging issue related to the state of HRT use and the type of hormones. Regarding the state of HRT use, past use and current use were the most common exposure types studied and 3 studies addressed this issue. Our pooled results showed that an increased risk of kidney cancer was observed in past users, but not in current users of HRT. Different exposure times prior to the date the study took place should be taken into account, and therefore, these results need to be treated with caution. With regard to the type of hormones, 6 studies described the relationship between estrogen replacement therapy and kidney cancer risk.12,13,15,18-20 The pooled results indicated that an increased risk was found for kidney cancer. Two studies assessed the association between kidney cancer risk and estrogen plus progestin use.12,13 Among 106 036 female participants from Hawaii-Los Angeles Multiethnic Cohort with an average 10.6 years of follow-up, the current user of estrogen plus progestin had a nonsignificant 27% elevation in kidney cancer risk.12 Another cohort with 118 219 US nurses showed there was a nonsignificant tend toward a decreased risk of kidney cancer for users of estrogen plus progestin.13 The significance of these findings are unclear, indicating the need for further high-quality studies addressing the relationship between specific types of female hormones and kidney cancer.
Our findings have potential implications for clinical practice. As HRT is the most common therapy in menopausal females, and young females with artificial menopause, many are concerned about the safety profile and adverse effects of HRT use, such as the promotion of tumorigenesis. Our meta-analysis indicates HRT use can decrease the risk of kidney cancer after HRT use up 12 to 18 years, suggesting that HRT may have a protective effect on the development of kidney cancer. Similarly, previous studies suggested that women using HRT had a decreased risk of colorectal cancer, liver cancer, esophageal cancer, lung cancer, and glioma.7-9 Current evidence, however, also suggests that HRT is associated with an increased risk of cardiovascular disease, stroke, breast cancer, meningioma, ovarian cancer, and cholecystitis.7,49 Taken together, this evidence suggests that doctors should balance the benefits and harmful effects of HRT prior to prescribing it. Nevertheless, based on this meta-analysis, concerns that HRT use is associated with an increased risk of kidney cancer may, at least in part, be eliminated.
To our knowledge, it is the first meta-analysis to comprehensively evaluate the relationship between HRT use and the risk of kidney cancer. A total of 13 studies, including 648 107 participates, were included. Statistical power was greatly enhanced to detect significant association. Therefore, our study provided more reliable estimates. Another strength of our study was that we evaluated the presence of a dose–response relationship between HRT use and kidney cancer, finding a nonlinear relationship between HRT use and kidney cancer.
Of course, several limitations of our study should be mentioned. First of all, the presence of residual confounding factors is a major concern in observational studies. Women with HRT use were more likely to adopt other healthy lifestyles. Although all studies reported risk estimates adjusted for a wide range of potential confounders, we cannot still rule out the possibility that measured or inadequately measured factors have biased the true association. Second, the use of different methods to collect data may influence the results. Both face-to-face interviews and self-administered questionnaires were used to assess HRT exposure in included studies, and participants may have different attitudes in response to the assessment using the 2 different methods, and they may be unable to understand a question included in questionnaires correctly. This could influence the accuracy of the data. Third, recall and selective biases were also a concern in this meta-analysis, as most of included studies were retrospective. Forth, some heterogeneity was observed in our meta-analysis. Sources of heterogeneity involved variations of study type, study design, population characteristics, and residual confounders. Finally, potential publication bias could affect the robustness of our results, although no evidence for publication bias was found.
In conclusion, our meta-analysis indicated a dose–response relationship between HRT use and kidney cancer risk. Women with more than 12 years of HRT use were at a decreased risk of developing kidney cancer during the follow-up period of the included studies. Additional well-designed prospective studies with (ie, with lager simple sizes, longer follow-up, specific types and doses of female hormones addressed and well-controlled confounding factors) are needed to confirm our finding.
Supplemental Material
Supplemental Material, M-track_changes for The Relationship Between Hormone Replacement Therapy and Risk of Kidney Cancer in Women: A Meta-Analysis by Xiaojun Zhang, Yuelin Du, Xiaojun Tan, Hui Wang, Yunxiang Li, Zongping Zhang and Anguo Wang in Cancer Control
Supplemental Material, PRISMA_2009_checklist for The Relationship Between Hormone Replacement Therapy and Risk of Kidney Cancer in Women: A Meta-Analysis by Xiaojun Zhang, Yuelin Du, Xiaojun Tan, Hui Wang, Yunxiang Li, Zongping Zhang and Anguo Wang in Cancer Control
Supplemental Material, Table_1-track_changes for The Relationship Between Hormone Replacement Therapy and Risk of Kidney Cancer in Women: A Meta-Analysis by Xiaojun Zhang, Yuelin Du, Xiaojun Tan, Hui Wang, Yunxiang Li, Zongping Zhang and Anguo Wang in Cancer Control
Footnotes
Authors’ Note: As our study is a meta-analysis, ethics statement is not required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anguo Wang  https://orcid.org/0000-0002-8638-7415
https://orcid.org/0000-0002-8638-7415
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(2):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. Jama. 1999;281(17):1628–1631. [DOI] [PubMed] [Google Scholar]
- 3. Scelo G, Larose TL. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 2018:Jco2018791905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka Y, Sasaki M, Kaneuchi M, Fujimoto S, Dahiya R. Estrogen receptor alpha polymorphisms and renal cell carcinoma—a possible risk. Mol Cell Endocrinol. 2003;202(5):109–116. [DOI] [PubMed] [Google Scholar]
- 5. Cavalieri EL, Kumar S, Todorovic R, Higginbotham S, Badawi AF, Rogan EG. Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: implications for estrogen-induced initiation of renal tumors. Chem Res Toxicol. 2001;14(6):1041–1050. [DOI] [PubMed] [Google Scholar]
- 6. Li JJ, Li SA. Estrogen carcinogenesis in hamster tissues: a critical review. Endocr Rev. 1990;11(3):524–531. [DOI] [PubMed] [Google Scholar]
- 7. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(2):872–881. [DOI] [PubMed] [Google Scholar]
- 8. Zhong GC, Liu Y, Chen N, et al. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematic review and dose-response meta-analysis of observational studies. Hum Repro Update. 2016;23(10):126–138. [DOI] [PubMed] [Google Scholar]
- 9. Qi ZY, Shao C, Zhang X, Hui GZ, Wang Z. Exogenous and endogenous hormones in relation to glioma in women: a meta-analysis of 11 case-control studies. PLoS One. 2013;8:e68695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karami S, Daugherty SE, Schonfeld SJ, et al. Reproductive factors and kidney cancer risk in 2 US cohort studies, 1993-2010. Am J Epidemiol. 2013;177(1):1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purdue MP, Colt JS, Graubard B, et al. A case-control study of reproductive factors and renal cell carcinoma among black and white women in the United States. Cancer Causes Control. 2011;22(8):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Setiawan VW, Kolonel LN, Henderson BE. Menstrual and reproductive factors and risk of renal cell cancer in the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2009;18(5):337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JE, Hankinson SE, Cho E. Reproductive factors and risk of renal cell cancer. Am J Epidemiol. 2009;169(1):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zucchetto A, Talamini R, Dal Maso L, et al. Reproductive, menstrual, and other hormone-related factors and risk of renal cell cancer. Int J Cancer. 2008;123(1):2213–2216. [DOI] [PubMed] [Google Scholar]
- 15. Molokwu JC, Prizment AE, Folsom AR. Reproductive characteristics and risk of kidney cancer: Iowa Women’s health study. Maturitas. 2007;58:156–163. [DOI] [PubMed] [Google Scholar]
- 16. Kabat GC, Navarro Silvera SA, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer. 2007;96(4):845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer. 2003;105(3):408–412. [DOI] [PubMed] [Google Scholar]
- 18. Gago-Dominguez M, Castelao JE, Yuan JM, Ross RK, Yu MC. Increased risk of renal cell carcinoma subsequent to hysterectomy. Cancer Epidemiol Biomarkers Prev. 1999;8(11):999–1003. [PubMed] [Google Scholar]
- 19. Lindblad P, Mellemgaard A, Schlehofer B, et al. International renal-cell cancer study. V. reproductive factors, gynecologic operations and exogenous hormones. Int J Cancer. 1995;61(2):192–198. [DOI] [PubMed] [Google Scholar]
- 20. Chow WH, McLaughlin JK, Mandel JS, Blot WJ, Niwa S, Fraumeni JF., Jr Reproductive factors and the risk of renal cell cancer among women. Int J Cancer. 1995;60(3):321–324. [DOI] [PubMed] [Google Scholar]
- 21. McLaughlin JK, Gao YT, Gao RN, et al. Risk factors for renal-cell cancer in Shanghai, China. Int J Cancer. 1992;52(4):562–565. [DOI] [PubMed] [Google Scholar]
- 22. Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal-cell carcinoma in Denmark. III. Role of weight, physical activity and reproductive factors. Int J Cancer. 1994;56(1):66–71. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(3):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells GA, Beverley JS, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp (accessed January 29, 2020).
- 25. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 26. Aune D, Lau R, Chan DS, et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23(1):37–45. [DOI] [PubMed] [Google Scholar]
- 27. Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58(13):1378–1385. [DOI] [PubMed] [Google Scholar]
- 28. Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;10(343):d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1545. [DOI] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Health. 2018;16(3):138–144. [DOI] [PubMed] [Google Scholar]
- 34. Xu C, Liu Y, Jia PL, et al. The methodological quality of dose-response meta-analyses needed substantial improvement: a cross-sectional survey and proposed recommendations. J Clin Epidemiol. 2019;107:1–11. [DOI] [PubMed] [Google Scholar]
- 35. Talamini R, Barón AE, Barra S, et al. A case-control study of risk factor for renal cell cancer in northern Italy. Cancer Causes Control. 1990;1(2):125–131. [DOI] [PubMed] [Google Scholar]
- 36. Sun L, Chao F, Luo B, et al. Impact of estrogen on the relationship between obesity and renal cell carcinoma risk in women. EBioMedicine. 2018;34:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boorjian S. Commentary on “Reproductive factors and kidney cancer risk in 2 US cohort studies, 1993-2010.” Urol Oncol. 2014;32(6):932–933. [DOI] [PubMed] [Google Scholar]
- 38. Karami S, Daugherty SE, Park Y, Hollenbeck AR, Purdue MP. Kidney cancer and female reproductive factors in two prospective cohorts. Can Res. 2011;71(18):B25. [Google Scholar]
- 39. Schouten LJ, Kviatkovsky MJ, Verhage BAJ, et al. Reproductive and hormonal factors and the risk of renal cell cancer in women: A prospective cohort study. Can Prevent Res. 2010;3(12): B97. [Google Scholar]
- 40. Nicodemus KK, Sweeney C, Folsom AR. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Can. 2004;108(1):115–121. [DOI] [PubMed] [Google Scholar]
- 41. Milne R, Vessey M. The association of oral contraception with kidney cancer, colon cancer, gallbladder cancer (including extrahepatic bile duct cancer) and pituitary tumours. Contraception. 1991;43(6):667–693. [DOI] [PubMed] [Google Scholar]
- 42. Possinger K, Wagner H, Mergenthaler HG, et al. Renal cell carcinomas. Incidence, diagnosis and systemic treatment in advanced stages: baseline situation. Munchener Medizinische Wochenschrift. 1994;136(8):32–35. [Google Scholar]
- 43. Robles JE, Rosell D, Zudaire JJ, Berián JM. Epidemiology of tumors of the renal parenchyma. Revista de medicina de la Universidad de Navarra. 1999;43(2):68–76. [PubMed] [Google Scholar]
- 44. Lipworth L, Tarone RE, Lund L, McLaughlin JK. Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol. 2009;1(8):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Troisi R, Bjørge T, Gissler M, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Int Med. 2018;283(5):430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu CP, Ho JY, Huang YT, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PLOS One. 2013;8:e56667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ronchi E, Pizzocaro G, Miodini P, Piva L, Salvioni R, Di Fronzo G. Steroid hormone receptors in normal and malignant human renal tissue: relationship with progestin therapy. J Steroid Bio Chem. 1984;21(5):329–335. [DOI] [PubMed] [Google Scholar]
- 48. Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469(7331):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi ZY, Shao C, Huang YL, Hui GZ, Zhou YX, Wang Z. Reproductive and exogenous hormone factors in relation to risk of meningioma in women: a meta-analysis. PLoS One. 2013;8(12):e83261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, M-track_changes for The Relationship Between Hormone Replacement Therapy and Risk of Kidney Cancer in Women: A Meta-Analysis by Xiaojun Zhang, Yuelin Du, Xiaojun Tan, Hui Wang, Yunxiang Li, Zongping Zhang and Anguo Wang in Cancer Control
Supplemental Material, PRISMA_2009_checklist for The Relationship Between Hormone Replacement Therapy and Risk of Kidney Cancer in Women: A Meta-Analysis by Xiaojun Zhang, Yuelin Du, Xiaojun Tan, Hui Wang, Yunxiang Li, Zongping Zhang and Anguo Wang in Cancer Control
Supplemental Material, Table_1-track_changes for The Relationship Between Hormone Replacement Therapy and Risk of Kidney Cancer in Women: A Meta-Analysis by Xiaojun Zhang, Yuelin Du, Xiaojun Tan, Hui Wang, Yunxiang Li, Zongping Zhang and Anguo Wang in Cancer Control