Abstract
Background
Increased volume of extra-axial cerebrospinal fluid (EA-CSF) is associated with autism spectrum disorder diagnosis in young children. However, little is known about EA-CSF development in typically developing children or in children at risk for schizophrenia.
Methods
3T magnetic resonance imaging scans were obtained in typically developing children (TD; N=105) and in children at risk for schizophrenia (SCZHR; N=38) at ages 1 and 2 years. EA-CSF volume and several measures of brain structure were generated, including global tissue volumes, cortical thickness (CT), and surface area (SA). Cognitive and motor abilities at 1 and 2 years were assessed using the Mullen Scales of Early Learning.
Results
In the TD children, EA-CSF volume was positively associated with total brain volume, gray and white matter volumes, and total surface area at ages 1 and 2 years. In contrast, EA-CSF volume was negatively associated with average CT. Lower motor ability was associated with increased EA-CSF volume at age 1. EA-CSF was not significantly increased in SCZHR children compared to TD children.
Conclusions
EA-CSF volume is positively associated with overall brain size and cortical surface area, but negatively associated with CT. Increased EA-CSF is associated with delayed motor development at 1 year of age, similar to studies of children at risk for autism, suggesting that increased EA-CSF may be an early biomarker of abnormal brain development in infancy. Infants at risk for schizophrenia did not exhibit significantly increased EA-CSF, suggesting that increased EA-CSF could be specific to neurodevelopmental disorders with an earlier onset, such as autism.
Keywords: infancy, MRI, early brain development, high-risk, schizophrenia, extra-axial cerebrospinal fluid
Introduction
Cerebrospinal fluid (CSF) provides mechanical and immunological protection to the brain by playing a vital role in brain homeostasis through the transportation of water, ions, and proteins (1). CSF, produced in the choroid plexuses, flows through the ventricles, cisterns, and into the subarachnoid space, delivering growth factors, peptides, and proteins (2). CSF flow also plays a critical role in the elimination of toxic waste; therefore, an abnormality in CSF absorption can be damaging to the developing brain (3, 4). Proper development of the neocortex is highly dependent upon proper CSF production and absorption (5, 6). Extra-axial CSF (EA-CSF), or excessive CSF in the subarachnoid space, tends to be associated with accelerated head growth (7) and is thought to be caused by a disruption in CSF absorption during the first year of life when CSF production is increased (7,8). Enlarged volume of the EA-CSF space has been found in high risk infants and toddlers who develop autism, a finding that has been replicated three times (6, 7, 9). While there is evidence that EA-CSF volume is associated with risk for autism, nothing is known about the normal variation of EA-CSF volume in typically developing (TD) children and its relation to brain structure. Although ASD has a stronger genetic liability, ASD and schizophrenia have similar underlying pathogenic mechanisms are present at different times and are influenced by similar genetic and environmental factors (10–13). Patients with schizophrenia often have increased CSF associated with increased lateral ventricles and widened sulci but such changes later in life may be related to degenerative changes (14, 15). It is unknown whether EA-CSF volume is increased in infants at risk for schizophrenia (SCZHR) at ages 1 and 2 years.
Our study had two objectives. First, we examined the relationship of EA-CSF volume to structural brain development, and cognitive development in a cohort of typically developing children at ages 1 and 2 years. Second, based on our previous finding that male neonates at risk for schizophrenia have enlarged lateral ventricles, suggesting that ventricle enlargement arises during prenatal brain development and maybe an early structural biomarker of risk (16), we hypothesized that EA-CSF would be increased in infants at risk for schizophrenia and would be associated with deficits in cognitive development.
Methods and Materials
Participants
This study was approved by the biomedical institutional review board of the University of North Carolina at Chapel Hill and is part of the UNC Early Brain Development Study (16–18). Mothers were initially recruited for a fetal ultrasound, and offspring returned after birth and at ages 1 and 2 years for MRI and cognitive assessment visits. Participants were excluded at enrollment due to a fetal abnormality on ultrasound, major medical illness or pregnancy complication, or active substance use during pregnancy at the time of enrollment. Infants were excluded from this analysis if they had any major medical illness, a neonatal intensive care unit stay of greater than 24 hours, gestational age of birth fewer than 32 weeks, or an abnormality on MRI other than a minor intracranial hemorrhage (ICH), which is common in the neonatal period (19). Mothers with schizophrenia or schizoaffective disorder were also enrolled in a related study (16), and assessed with the Structured Clinical Interview for DSM-IV Axis Disorders (SCID) (20). Typically developing (TD mothers were screened with a modified SCID questionnaire. Each mother's past medical records were reviewed, and a final diagnosis was assigned by two board-certified psychiatrists (author JHG; LFJ). There were 140 TD and 33 SCZHR subjects after excluding subjects who failed quality control procedures (see Methods: Neuroimaging Protocol). Demographic variables for all subjects are reported in Table 1. There was a subsample of 24 TD subjects that with longitudinal data at ages 1 and 2 years.
Table 1.
Demographic variables for all subjects.
| Typically Developing (N=107) | SCZHR (N=33) | t-test / Fisher Exact Test | |||
|---|---|---|---|---|---|
| Continuous Variables | N | Mean (SD) | N | Mean (SD) | pa |
| Birth Weight (g) | 107 | 3445.32 (419.93) | 33 | 3053.79 (605.55) | <.001* |
| Gestational Age at Birth (Days) | 107 | 276.79 (9.28) | 34 | 266.73(13.07) | <.001* |
| Postnatal Age at MRI at age One (Days) | 82 | 381.21 (20.53) | 26 | 388.31 (30.86) | .180 |
| Postnatal Age at MRI at age Two (Days) | 49 | 740.27(17.16) | 18 | 768.00 (35.33) | <.001* |
| APGAR 5 minutes | 107 | 8.85 (0.66) | 33 | 8.76 (0.61) | .472 |
| Total Income ($) | 100 | 72938.12 (44106.61) | 26 | 30529.62 (30926.58) | <.001* |
| Maternal Education (years) | 107 | 16.44 (2.91) | 33 | 10.48 (3.82) | <.001* |
| Paternal Education (years) | 104 | 16.39 (3.32) | 26 | 10.38 (3.54) | <.001* |
| Maternal Age at Birth (years) | 107 | 29.79 (4.94) | 32 | 27.28 (6.62) | .022* |
| Paternal Age at Birth (years) | 104 | 31.24 (5.01) | 25 | 34.40 (10.76) | .031* |
| Categorical Variables | N | n (%) | N | n (%) | pb |
| Sex | 107 | 33 | .692 | ||
| Male | 54 (50.47) | 15 (45.45) | |||
| Female | 53 (49.53) | 18 (54.55) | |||
| Maternal Race | 107 | 33 | <.001* | ||
| African American | 19 (17.76) | 20 (60.61) | |||
| Asian | 4 (3.74) | 1 (3.03) | |||
| Caucasian | 84 (78.50) | 12 (36.36) | |||
| Scanner at age One | 82 | 26 | .730 | ||
| Allegra | 71(86.59) | 24 (92.31) | |||
| Trio | 11 (13.41) | 2 (7.69) | |||
| Scanner at age Two | 49 | 18 | .693 | ||
| Allegra | 43 (87.76) | 15 (83.33) | |||
| Trio | 6(12.24) | 3 (16.67) | |||
t-test p-value
Fisher Exact Test p-value
Cognitive Assessments
The Mullen Scales of Early Learning is composed of five Scales- Gross Motor, Visual Reception, Fine Motor, Receptive Language, and Expressive Language (21). Each scale has developmental tasks to capture the functionality of distinct behavioral skills. The Mullen Early Learning Composite (ELC) is calculated using the sum of the standardized T-scores from four of the Scales, excluding Gross Motor. The Mullen was administered at ages 1 and 2 years by experienced testers who were blind to the high-risk status of subjects. Raw scores were used for the analysis of the independent Scales. The assessment date for each subject was included as a covariate in statistical analyses to account for any sample drift or variability due to changes in test administrators during the 10-year data collection period (22).
Neuroimaging Protocol
MR Image Acquisition
Scans were acquired on Siemens 3T scanners during natural sleep (Allegra scanner and TIM Trio; Siemens Medical Solutions, Erlangen, Germany). Before scanning, infants were swaddled, fed, and fitted with ear protection. Neonatal structural T1-weighted (T1w) a 3-D magnetization prepared rapid gradient echo sequence (N=99) was acquired on Allegra (N=89) and Tim Trio (N=10) scanners during the initial phase of the study (MP-RAGE, TR=1820ms, TE=3.75–4.38ms, Flip Angel=7°, Spatial Resolution=1x1mm2, Slice thickness= 1mm). At ages 1 and 2 years, T1-weighted images were acquired using a 3-D magnetization prepared rapid gradient echo sequence (MP-RAGE, TR=1880–1900ms, TE=3.74–4.38ms, Flip Angel=7°, Spatial Resolution=1x1mm2, Slice thickness= 1mm) on both Allegra (N=153 ) and Tim Trio scanners (N=22). Neonatal proton density and T2-weighted images (T2w) low-angle shot sequence (N=89) were acquired on the Allegra scanner used turbo spin-echo sequences (TSE, TR=5480–6200, TEl=20ms, TE2=119ms, Flip Angel=150°, Spatial Resolution=1x1mm2, Slice thickness= 1.5–2). Neonatal T2 images acquired on the Tim Trio scanner (N=10) with a 3DT2 SPACE protocol (TSE, TR=3200ms, TE=406ms, Flip Angle=120°, Spatial Resolution=1,1mm2, Slice Thickness=l). Additional proton density and T2w images acquired at ages 1 and 2 years on the Allegra scanner (N=153) used turbo spin-echo sequences (TSE, TR=7380–7590ms, TE1=20ms, TE2=119ms, Flip Angle=150°, Spatial Resolution=1.25, 1.25mm2, Slice Thickness=1.5–1.95). T2w images were also acquired on the Tim Trio scanner (N=22) with a 3DT2 SPACE protocol (TSE, TR=3200ms, TE=406ms, Flip Angle=120°, Spatial Resolution=1,1mm2, Slice Thickness=1).
MR Image Processing
Images were processed using a standard protocol which included removing the cerebellum and brain stem from all images (18, 23, 24). All T1w and T2w images were corrected for intensity inhomogeneity by applying N4 based bias correction (25). T2w images were re-sampled to 1x1x1mm3 if necessary. Brain tissue was classified into gray matter, nonmyelinated white matter, myelinated white matter, and CSF in neonates using an automatic, atlas-moderated expectation maximization segmentation tool (18). T2w images were used for the 1 year and 2 years tissue segmentations of CSF, gray and white matter, utilizing an automatic brain tissue segmentation tool similar to the neonatal tissue segmentation (26–28). ITK SNAP, a semi-automated 3D segmentation tool, was used to trace lateral ventricles, yielding 3D segmentations and volumetric outputs for all ages (18). All images were assessed for quality and the presence of motion, utilizing a four-scale quality rating system that was used to determine the suitability of MR images.
We employed an adapted analysis workflow to define EA-CSF in the subarachnoid space using only T2w images due to the improved CSF contrast in the T2w images as compared to T1w images. This quantitative protocol allows us to measure EA-CSF utilizing automatic tissue and CSF segmentation (applied to the T2w data), as well as additional prior masks of the lateral, third, and fourth ventricles. A ventral boundary was also defined to measure the region of the brain that has the most EA-CSF in the subarachnoid space in the dorsal region of the brain, consistent with Shen et al. (9). The volume of the EA-CSF was quantified for each subject. A visual quality check system, based on Shen et al. (2017), was implemented to evaluate the quality of segmentations. The output was visually checked to observe if the subarachnoid space was accurately segmented, meaning there was no overestimation of segmentation into brain tissue or skull and no underestimation where EA-CSF found in the subarachnoid space was not included in the segmentation. All segmentations were given a score of 0–3, where 0 indicated no abnormalities in segmentations, 1 indicated minor under- or overestimation of EA-CSF in one region, 2 indicated moderate under or overestimation in multiple regions, and 3 showed either significant over-estimation or under-estimation of segmentation in the subarachnoid space that could not be manually edited. Only subjects with a score of 0 or 1 were included in this study. At age 1 year, 108 of 115 eligible subjects had a passing rating of 0 or 1; at age 2 years, 67 subjects of 85. Eligible subjects included participants who had global tissue volumes, Mullen Scales scores, passing quality EA-CSF segmentations, and were not excluded for major medical illness or psychiatric history.
Cortical thickness (CT) and surface area (SA) measurements were obtained from an image analysis pipeline described by Li et al. (2016) (29). Topologically corrected white matter segmentations for each hemisphere were tessellated into a triangular surface mesh that is deformed to obtain the inner, central, and outer surfaces under Laplacian field deformation.(26). CT is measured as the average distance of the minimum distance from the inner to middle surfaces and the minimum distance from outer to inner surface. And SA is based on the central cortical surface located in the middle between the inner and outer surfaces. CT and SA anatomical parcellations included 78 cortical regions of interests that were obtained using the Automated Anatomical Labeling atlas (30). The average CT, the mean of all regions, and total SA, the sum of all regions, were calculated for each subject at each time point. Quality control was performed on the surface reconstructions and included manual edits if necessary.
Statistical Analyses
Linear models were used to analyze the relationship between the response variable (EA-CSF) and other brain tissue and cognitive measures and high-risk status. The r-squared, t-statistic (t), degrees of freedom (DF), standard deviation (sd), mean, median, minimum, maximum, and p-values (p) were calculated for all analyses. To observe if gender and high-risk status differences are present, regressions with gender and the interaction of gender with EA-CSF as predictors, and regressions with group and the interaction of group with EA-CSF as predictors were performed. The significance level was set at an alpha of 0.05. FDR correction was performed for associations of EA-CSF with regional CT and SA. Linear model analyses were done with SAS, version 9.4.
Results
Distribution of EA-CSF in TD subjects
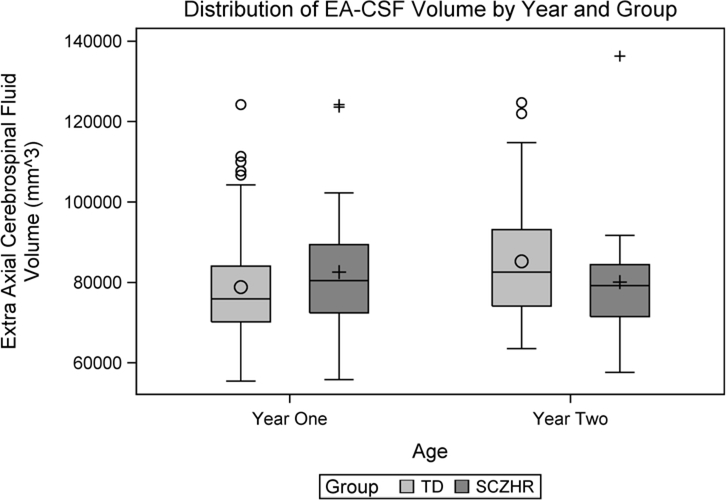
The distribution of EA-CSF at ages 1 and 2 years are shown in Figure 1. The mean volume of EA-CSF increased slightly between 1 and 2 years in the cross-sectional samples (Table 2a). In the longitudinal sub-sample (N=24), there was a similar increase of EA-CSF from 1 year (mean= 78,568 mm3; sd= 12,582) to 2 years (mean= 89,062 mm3; sd= 14,874). EA-CSF at age 1 was highly correlated with EA-CSF at age 2 years (r = 0.585, p = 0.003; Figure S1).
Figure 1.
Distribution of EA-CSF volume at ages 1 and 2 years.
Table 2.
Brain Tissue Relationships to EA-CSF at ages 1 and 2 years in typically developing.
| Continuous Variables | Year One (N=82) | Year Two (N=49) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | R2 | Est. | p | Mean (SD) | R2 | Est. | p | |
| EA-CSF (mm3) | 78830.59 (13491.83) | - | - | - | 85285.73 (14635.93) | - | - | - |
| CSF at Birth (mm3) | 60213.59 (11805.56)a | .309 | 0.584 | <.001* | 59236.32 (11233.64)b | .107 | 0.431 | 0.048* |
| Intracranial (mm3) | 938899.07 (90378.31) | .211 | 0.069 | <.001* | 1061392.36 (108222.17) | .260 | 0.069 | <.001* |
| Gray Matter (mm3) | 639499.57 (56240.98) | .187 | 0.103 | <.001* | 731709.03 (74139.13) | .270 | 0.103 | <.001* |
| White Matter (mm3) | 230625.04 (31376.44) | .136 | 0.159 | <.001* | 258942.39 (28145.69) | .217 | 0.242 | <.001* |
| CSF (mm3) | 68774.46 (10040.55) | .311 | 0.750 | <.001* | 70740.95 (8483.61) | .174 | 0.720 | .002* |
| Total Ventricle (mm3) | 6552.18 (3301.14) | .039 | 0.807 | .075 | 6830.35 (3878.06) | .102 | 1.205 | .025* |
| Average CT (mm) | 2.84 (0.14)c | .129 | 34039.4 | .002* | 2.68 (0.09)d | .001 | −4414.55 | .850 |
| Total SA (mm2) | 141205.33 (12799.35)c | .213 | 0.465 | <.001* | 167593.47 (14747.69)d | .168 | 0.411 | .004* |
n=56
n=37
n=7
n=48.
EA-CSF Relationship to Brain Tissues Volumes in TD subjects
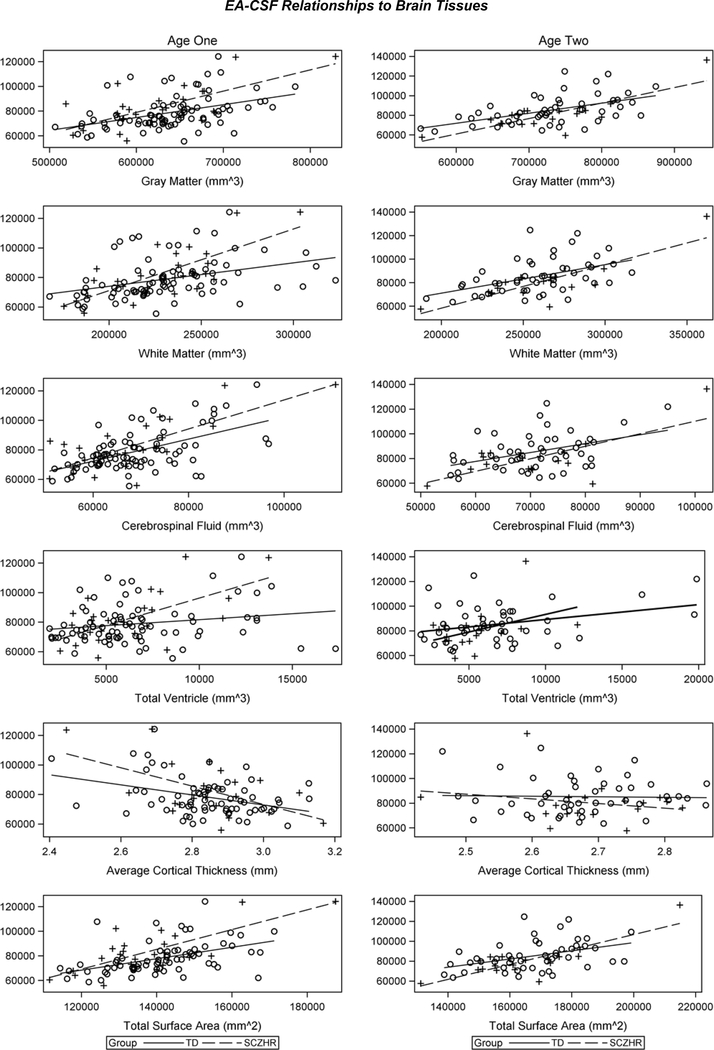
EA-CSF was significantly and positively correlated with brain tissue volumes at each age, with larger EA-CSF associated with overall intracranial volume, gray matter, white matter, total cerebrospinal fluid (Table 2a). EA-CSF was also correlated with total ventricle volumes at 2 years (Table 2a). Since EA-CSF volumes were positively correlated with total CSF at each age, we explored the relationship of EA-CSF to CSF at birth and found that it was positively and significantly related at ages 1 and 2 years (Table 2a). Scatter plots of these associations can be seen in Figure 2.
Figure 2.
EA-CSF volume relationships to gray matter, white matter, cerebrospinal fluid, and total ventricle volumes and average CT and total SA at ages 1 and 2 years.
EA-CSF Relationship to Surface Area and Cortical Thickness in TD subjects
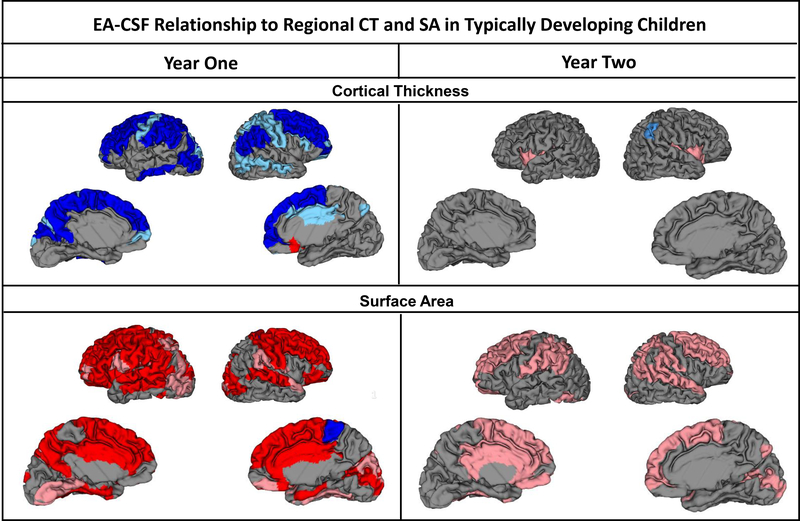
Total SA was positively related to EA-CSF at 1 year and 2 years (Table 2a). EA-CSF had a positive relationship to regional SA, with 50 significant cortical regions at age 1 year (37 remained significant after FDR corrections) and 29 regions at 2 years (0 remained significant after FDR corrections) (Table S1 and Figure 3). In contrast, EA-CSF had a significant relationship with average CT at 1 year, with larger EA-CSF associated with smaller cortical thickness (Table 2a). Similarly, 35 of the 36 significant regions had significantly smaller CT correlated with larger EA-CSF at 1 year (25 remained significant after FDR corrections; Table S2 and Figure 3). There were a few regional CT relationships present at 2 years and none remained significant after FDR correction.
Figure 3.
Regional relationships of EA-CSF volume to CT and SA at 1 and 2 years. Pink represents a significant positive association, Red is significant after FDR correction. Light blue represents a significant negative association, Dark blue is significant after FDR correction.
EA-CSF Relationship to Mullen Scales of Early Learning in TD subjects
Larger EA-CSF was significantly related to lower gross motor scores at age 1 year (p=0.005; F(3,78)=4.68) (Table S3, Figure S2). Interactions of gender with EA-CSF revealed female subjects had the same significant relationship and the same pattern of lower scores associated with larger EA-CSF volume at age 1 year (p=0.001; t(78)=−3.38) (Table S3). Male subjects exhibited the same pattern as the overall and female groups, though the relationship was not significant (Table S3). At age 2 years, there was no significant relationship between EA-CSF and any Mullen score (Table S3). Scatter plots of these relationships can be seen in Figure S3.
Since CT and SA measures are correlated to EA-CSF development, we also explored whether EA-CSF predicted motor scores at age 1 beyond CT and SA and found that EA-CSF did significantly predict GM at age 1 beyond CT and SA. Also, EA-CSF also predicted fine motor scores beyond SA (Table S4).
EA-CSF Relationship to High-Risk Status
Figure 1 shows the distribution of EA-CSF at ages 1 and 2 years in the SCZHR group. EA-CSF was not related to risk for schizophrenia at age 1 (mean (SD) TD: 78,831 mm3 (13,492); SCZHR: 82,574 mm3 (17,146) t(106) = −1.15; p = 0.252) or age 2 (TD: 85,286 mm3 (14,636); SCZHR: 80,104 mm3 (16,543) t(65) = 1.24; p = 0.219). At years 1 and 2, EA-CSF was significantly and positively correlated with brain tissue volumes in the SCZHR, with larger EA-CSF associated with overall intracranial volume, gray matter, white matter, total cerebrospinal fluid, cerebrospinal fluid at birth and total surface area (Table 3). Average cortical thickness was negatively related at age 1 year (Table 3). These associations can be observed in scatterplots (Figure 2).
Table 3.
Brain tissue relationships to EA-CSF at ages 1 and 2 years in SCZHR.
| Continuous Variables | Year One (N=26) | Year Two (N =18) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | R2 | Est. | p | Mean (SD) | R2 | Est. | p | |
| EA-CSF (mm3) | 82573.58 (17144.59) | - | - | - | 80104.17 (16543.21) | - | - | - |
| CSF at Birth (mm3) | 62477.00 (18298.30)a | .676 | 0.886 | <.001* | 64178.60 (13715.61)b | .617 | 1.250 | .007* |
| Intracranial (mm3) | 917274.98 (108687.54) | .540 | 0.116 | <.001* | 1051756.09(129475.77) | .643 | 0.102 | <.001* |
| Gray Matter (mm3) | 621378.09 (68767.12) | .475 | 0.172 | <.001* | 722166.33 (83084.18) | .629 | 0.158 | <.001* |
| White Matter (mm3) | 227646.10 (30648.47) | .556 | 0.417 | <.001* | 259146.74(36889.80) | .678 | 0.369 | <.001* |
| CSF (mm3) | 68250.79 (12971.67) | .553 | 0.983 | <.001* | 70443.02 (11168.81) | .469 | 1.015 | .002* |
| Total Ventricle (mm3) | 6298.62 (2814.94) | .367 | 3.690 | .001* | 5394.44 (2398.89) | .171 | 2.850 | .088 |
| Average CT (mm) | 2.84 (0.15)c | .293 | 62050.8 | .006* | 2.69 (0.09) | .046 | −37746.3 | .394 |
| Total SA (mm2) | 137128.94(15848.73)c | .556 | 0.803 | <.001* | 164607.69 (18029.59) | .668 | 0.750 | <.001* |
n=18
n=10
n=24.
EA-CSF relationships with brain structure in SCZHR
To determine if the SCZHR group had significantly different EA-CSF/brain structure relationships compared to the TD group, we used a linear model with group, brain tissue, and the interaction as predictors. At age 1 year, there were highly significant relationships EA-CSF with CSF at birth, gray matter, CSF, Average CT, intracranial volume, and total SA in the overall (TD and SCZHR), TD, and SCZHR separately (Table S5). There is no significant group dependence of these relationships. EA-CSF with total ventricular volume was highly significant in the SCZHR group but only trending in the TD group with a trend towards group dependence of this relationship. WM is highly significant in each group and overall, and there is significant group dependence of this relationship.
At age 2 years, intracranial volume, gray matter, white matter, CSF, total SA, and average CT had highly significant relationships to EA-CSF in each group and the overall group (Table S5). There were no significant group differences. There is a significant group dependence of the relationship of EA-CSF with CSF at birth relationship with a stronger relationship in the SCZHR group (Table S5).
EA-CSF Relationship to Neonatal Intracranial Hemorrhage
We explored the association of neonatal intracranial hemorrhage (ICH) with EA-CSF and found no significant relationships. In TD children, neonatal ICH was not associated with EA-CSF at 1 year (ICH=19 of 82; r=−0.143, p=0.199) or 2 years (ICH= 13 of 49; r=−0.268, p=0.063). In the SZCHR group, neonatal ICH was not associated with increased EA-CSF at 1 year (ICH=2 of 26; r=−0.313, p=0.119). At age 2 years, no SCZHR subjects had neonatal ICH.
Discussion
This is the first study of EA-CSF volume in the developing brain of TD children. We found that EA-CSF was highly and positively correlated to overall brain size, including cerebrospinal fluid, gray, and white matter volumes. Increased EA-CSF is also associated with reduced cortical thickness at age 1 year. Cortical thinning has also been found in the frontal lobe of school-age children with mild to moderate cases of increased EA-CSF, and widespread thinning has been associated with severely increased EA-CSF when compared to control subjects (32). We found that increased EA-CSF is associated with delayed gross motor development at 1 year of age, a relationship also observed in children at risk for autism (7, 9). The relationship with reduced cortical thickness and gross motor development was not present at age 2 years, suggesting that in TD children, the association is age-dependent. While increased EA-CSF is present in children with autism and at risk for autism, we did not find increased EA-CSF in SCZHR children. Additionally, group dependences of EA-CSF volume relationships to brain tissue volumes were non-significant except for white matter at age 1 and CSF at birth at age 2. This suggests that increased EA-CSF is not a general feature of all neuropsychiatric disorders and may be specific to those with earlier onset (e.g., autism) rather than those with later onset (e.g., schizophrenia), though our study is limited by small SCZHR sample size and additional studies are necessary.
Increased EA-CSF has been linked to many terms including benign enlargement of the subarachnoid space, external hydrocephalus, benign subdural hydrocephalus, enlargement of the subarachnoid spaces, and extra axial fluid collections (33). This condition is distinct from frank hydrocephalus and is often found in children who have a head circumference that exceeds the expected growth rate. Neuroimaging studies of clinically defined EA-CSF typically find enlarged subarachnoid space, enlarged brain, and sometimes mildly enlarged ventricles (34–38). The etiology of this increase in EA-CSF is unknown, though a common theory suggests immature arachnoid villi cause a disruption in the absorption of CSF (35). These villi tend to mature around 18 months, and interestingly, growth and development of these infants tend to normalize around 2 years of age. This increase is often described as benign, and most patients are untreated because symptoms tend to resolve spontaneously in childhood (33). However, in some cases of gross hydrocephalus, surgical procedures are often necessary to relieve pressure. Hydrocephalus presents in a heterogeneous manner, which is probably a result of its heterogeneous etiology. Four genes have been linked to hydrocephalus that are associated with different cellular processes, including cell adhesion and cell polarity. However, the mechanisms related to these genes do not provide a clear understanding of the mechanisms driving the disorder (37). There are also non-genetic causes of increased CSF in the subarachnoid space like infection, trauma or hemorrhage occurring perinatally or prenatally.
We found that increased EA-CSF had opposite relationships with CT and SA measures; greater EA-CSF was related to larger SA at ages 1 and 2 years and smaller CT at age 1 year. Gray matter measures can be parsed into CT and SA measures. However, CT and SA have distinct patterns of development within the first year of life (39). CT growth reaches approximately 97% of adult values at age 2 years, while surface expansion is robust and accounts for the majority of cortical volume growth after the first year of life. Additionally, thinner CT is observed in many regions of the frontal lobe in children with longer gestational periods, while larger SA is seen in later-born infants (40). These distinct patterns of development between how CT grows and SA and gray matter grow suggest the presented relation differences between these measures and EA-CSF. This suggests that the distribution of EA-CSF reflects overall brain size and that atrophy was not found in subjects with increased EA-CSF. Further research is needed to understand the possible genetic and environmental contributions affecting these relationships.
We found gross motor deficits were present with increased EA-CSF at age 1 year in our sample of TD subjects. Increased EA-CSF has been related to ataxia and seizures in infants from < 6 months to 2 years old (33, 41). Increased EA-CSF has also been related to motor deficits during infancy from approximately 5 to 12 months that tend to resolve around 1 to 3 years (33, 34, 42). Shen et al. (2017) found poorer motor ability in subjects with increased EA-CSF in infancy and decreased fine motor abilities at ages 2–4 years in children with autism, and we found a similar effect in TD infants.
Our study revealed no relationship to being at risk for schizophrenia and having increased EA-CSF. Although increased EA-CSF has been replicated in children diagnosed with autism, this is the first study to evaluate whether this biomarker is specific to autism or present in other disorders like schizophrenia (6, 7, 9). Patients with schizophrenia often have increased CSF associated with increased lateral ventricles, widened sulci, and increased subarachnoid spaces (14, 15). Researchers have found that the presentation of schizophrenia and measures of patients with the disorder is heterogeneous. Studies of high risk youth find cognitive deficits that progress with the onset of psychosis (43). Studies of the association of schizophrenia with dementia are inconsistent, though it is likely that abnormalities during early neurodevelopment can cause alterations in later development and aging (44). We previously found that male SCZHR neonates had enlarged lateral ventricles, suggesting that CSF distribution could be altered in this at-risk population (16). In this study, we did not find larger EA-CSF spaces in our sample of SCZHR, SCZHR showed a normal distribution of EA-CSF with no major outliers of increased or decreased EA-CSF volume, however, the sample size was small and in need of replication.
Historically, ASD and schizophrenia were thought to share similar mechanisms that present at different times during brain development (10). Theories suggest both of these disorders have neurodevelopmental origins. We know that genetic factors influence both disorders; ASD and schizophrenia share copy number variants, rare genetic abnormalities, and candidate genes (10, 13). This indicates that ASD and schizophrenia may have some similar underlying pathogenic mechanisms that present at different times. Our observations of EA-CSF occurred at ages 1 and 2 years, after the 6month time-point of Shen's et al. (2013; 2017; 2018) studies. Future studies should determine if enlarged EA-CSF is present at 6 months of age in children at risk for schizophrenia, though our finding that neonatal CSF volume, highly correlated with EA-CSF in 1 year olds, is not significantly larger in high risk children, suggests that enlarged EA-CSF is not associated with risk for schizophrenia at early ages.
While this study has many strengths, there are several limitations that must be considered when interpreting results. It should be noted that the method to segment EA-CSF was not done exactly as Shen et al. (2013, 2017), though measures were taken to ensure the segmentations used in these analyses were done similarly and assessed for a high standard of quality. The analysis of children at high risk for schizophrenia had a small sample size and should be considered preliminary until replicated. Attrition occurred from 1 year to the 2-year-old imaging, and the imaging success rate is less at these ages, so the sample at each age was somewhat different; this may have contributed to age-related imaging findings.
In summary, we find that EA-CSF is associated with overall brain size in TD children, and is associated with reduced CT and lower gross motor scores at age 1 year, but not 2 years. EA-CSF was not increased in children at risk for schizophrenia, suggesting that increased EA-CSF is not present in infancy in all neurodevelopmental disorders.
Supplementary Material
Acknowledgments
The authors thank participating families and staff of the UNC MRI Research Center, the UNC Neuro Image Research and Analysis Laboratories, the UNC Early Brain Development Program, and the FPG Infant and Child Assessment Lab.
Role of Funding
This work was supported by the National Institutes of Health (MH064065, MH070890, and HD053000 to J.G., MH091645, HD-003110, and HD079124 to M.S, K12-HD001441 to M.D.S., and T32 NS007431, R25GM055336, MH070890 to VM.)
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008): Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapoor KG, Katz SE, Grzybowski DM, Lubow M (2008): Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull. 77: 327–34. [DOI] [PubMed] [Google Scholar]
- 3.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. (2012): A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 4: 147ralll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Wu X, Cui J, Li H, Yan X (2013): Increase ICAM-1 and LFA-1 expression by cerebrospinal fluid of subarachnoid hemorrhage patients: involvement of TNF-α. Brain Res. 1512: 89–96. [DOI] [PubMed] [Google Scholar]
- 5.Lehtinen MK, Walsh CA (2011): Neurogenesis at the Brain-Cerebrospinal Fluid Interface. Annu Rev Cell Dev Biol. 27: 653–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen MD, Nordahl CW, Li DD, Lee A, Angkustsiri K, Emerson RW, et al. (2018): Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2–4 years: a case-control study. The Lancet Psychiatry.. doi: 10.1016/S2215-0366(18)30294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, et al. (2017): Increased Extra-axial Cerebrospinal Fluid in High-Risk Infants who Later Develop Autism. Biol Psychiatry.. doi: 10.1016/j.biopsych.2017.02.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda T, Tomita T, McLone DG, Donovan M (2002): Measurement of cerebrospinal fluid output through external ventricular drainage in one hundred infants and children: correlation with cerebrospinal fluid production. Pediatr Neurosurg. 36: 22–8. [DOI] [PubMed] [Google Scholar]
- 9.Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, et al. (2013): Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 136: 2825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lacy N, King BH (2013): Revisiting the Relationship Between Autism and Schizophrenia: Toward an Integrated Neurobiology. Annu Rev Clin Psychol. 9: 555–587. [DOI] [PubMed] [Google Scholar]
- 11.Stahlberg O, Soderstrom H, Rastam M, Gillberg C (2004): Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. J Neural Transm. Ill: 891–902. [DOI] [PubMed] [Google Scholar]
- 12.Mouridsen SE, Rich B, Isager T, Nedergaard NJ (2008): Psychiatric Disorders in Individuals Diagnosed with Infantile Autism as Children: A Case Control Study. J Psychiatr Pract. 14: 5–12. [DOI] [PubMed] [Google Scholar]
- 13.Carroll LS, Owen MJ (2009): Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. 1: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota M, van Haren NEM, Haijma S V, Schnack HG, Cahn W, Hulshoff Pol HE, Kahn RS (2015): Association of IQ Changes and Progressive Brain Changes in Patients With Schizophrenia. JAMA psychiatry. 72: 803–12. [DOI] [PubMed] [Google Scholar]
- 15.Narr KL, Sharma T, Woods RP, Thompson PM, Sowell ER, Rex D, et al. (2003): Increases in regional subarachnoid CSF without apparent cortical gray matter deficits in schizophrenia: modulating effects of sex and age. Am J Psychiatry. 160: 2169–80. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore JH, Kang C, Evans DD, Wolfe HM, Smith JK, Lieberman JA, et al. (2011): Prenatal and Neonatal Brain Structure and White Matter Maturation in Children at High Risk for Schizophrenia. 167:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knickmeyer RC, Xia K, Lu Z, Ahn M, Jha SC, Zou F, et al. (2016): Impact of Demographic and Obstetric Factors on Infant Brain Volumes: A Population Neuroscience Study. Cereb Cortex. 27: 5616–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, et al. (2008): A structural MRI study of human brain development from birth to 2 years. J Neurosci. 28: 12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, Gilmore JH (2007): Intracranial Hemorrhage in Asymptomatic Neonates: Prevalence on MR Images and Relationship to Obstetric and Neonatal Risk Factors. Radiology. 242: 535–541. [DOI] [PubMed] [Google Scholar]
- 20.Kubler U (2013): Structured Clinical Interview for DSM-IV (SCID). Encycl Behav Med. New York, NY: Springer New York, pp 1919–1920. [Google Scholar]
- 21.Mullen E (1995): Mullen scales of early learning (AGS ed.) [Manual]. Circle Pines: American Guidance Service Inc. Retrieved February 13, 2017, from https://books.google.com/books/about/Mullen_Scales_of_Early_Learning_Manual.html?id=ZYYQAQAAMAAJ. [Google Scholar]
- 22.Girault JB, Langworthy BW, Goldman BD, Stephens RL, Cornea E, Steven ReznickJ, et al. (2018): The predictive value of developmental assessments at 1 and 2 for intelligence quotients at 6. Intelligence. 68: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, et al. (2007): Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 27: 1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha SC, Xia K, Ahn M, Girault JB, Li G, Wang L, et al. (2018): Environmental Influences on Infant Cortical Thickness and Surface Area. Cereb Cortex.. doi: 10.1093/cercor/bhy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC (2010): N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 29: 1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Nie J, Wu G, Wang Y, Shen D (2012): Consistent reconstruction of cortical surfaces from longitudinal brain MR images. Neuroimage. 59: 3805–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D (2011): Infant brain atlases from neonates to 1and 2-year-olds. PLoS One. 6: el8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Shi F, Yap P-T, Lin W, Gilmore JH, Shen D (2013): Longitudinally guided level sets for consistent tissue segmentation of neonates. Hum Brain Mapp. 34: 956–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, Wang L, Shi F, Lyall AE, Ahn M, Peng Z, et al. (2016): Cortical thickness and surface area in neonates at high risk for schizophrenia. Brain Struct Funct. 221: 447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y (1995): Controlling The False Discovery Rate - A Practical And Powerful Approach To Multiple Testing. J R StatSoc, Ser B. 57: 289–300. [Google Scholar]
- 32.Zhang S, Ye X, Bai G, Fu Y, Mao C, Wu A, et al. (2017): Alterations in Cortical Thickness and White Matter Integrity in Mild-to-Moderate Communicating Hydrocephalic School-Aged Children Measured by Whole-Brain Cortical Thickness Mapping and DTI. Neural Plast. 2017: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahl SM, Egge A, Helseth E, Wester K (2011): Benign external hydrocephalus: a review, with emphasis on management. Neurosurg Rev. 34: 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez LA, Maytal J, Shinnar S (1986): Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics. 77: 901–7. [PubMed] [Google Scholar]
- 35.Barlow CF (1984): CSF dynamics in hydrocephalus--with special attention to external hydrocephalus. Brain Dev. 6: 119–27. [DOI] [PubMed] [Google Scholar]
- 36.Maytal J, Alvarez LA, Elkin CM, Shinnar S (1987): External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. AJR Am J Roentgenol. 148: 1223–30. [DOI] [PubMed] [Google Scholar]
- 37.Odita JC (1992): The widened frontal subarachnoid space. Child's Nerv Syst. 8: 36–39. [DOI] [PubMed] [Google Scholar]
- 38.Sahar A (1978): Pseudohydrocephalus-Megalocephaly, Increased Intracranial Pressure and Widened Subarachnoid Space. Neuropediatrics. 9: 131–139. [DOI] [PubMed] [Google Scholar]
- 39.Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. (2014): Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb Cortex. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jha SC, Xia K, Schmitt JE, Ahn M, Girault JB, Murphy VA, et al. (2018): Genetic influences on neonatal cortical thickness and surface area. Hum Brain Mapp.. doi: 10.1002/hbm.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roshan K, Elizabeth C, Chacko A, Rajendra J, Gururaj A, Dilip S (1998): External hydrocephalus - a report of 16 cases from Oman. J Trop Pediatr. 44: 153–156. [DOI] [PubMed] [Google Scholar]
- 42.Laubscher B, Deonna T, Uske A, Van Melle G (1990): Primitive megalencephaly in children: natural history, medium term prognosis with special reference to external hydrocephalus. EurJ Pediatr. (Vol. 149), Springer: Retrieved February 14, 2019, from https://link-springercom.libproxy.lib.unc.edu/content/pdf/10.1007%2FBF01959405.pdf. [DOI] [PubMed] [Google Scholar]
- 43.Sommer IE, Bearden CE, van Dellen E, Breetvelt EJ, Duijff SN, Maijer K, et al. (2016): Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2: 16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bora E (2015): Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol Med. 45: 1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.