Abstract
Background & objectives:
Nearly 5,500 tests for coronavirus disease 2019 (COVID-19) had been conducted on March 31, 2020 across the Indian Council of Medical Research (ICMR)-approved public and private laboratories in India. Given the need to rapidly increase testing coverage, we undertook an exercise to explore and quantify interventions to increase the daily real-time reverse transcription-polymerase chain reaction (qRT-PCR)-based testing capacity over the next few months. The objective of this exercise was to prepare a potential plan to scale-up COVID-19 testing in India in the public sector.
Methods:
Potential increase in daily testing capacity of the existing public laboratories was calculated across the three base scenarios of shifts (9, 16 and 24 h). Additional testing capacity was added for each shift scenario based on interventions ranging from procurement of additional qRT-PCR machines, leveraging spare capacity on available qRT-PCR machines not drafted into COVID-19 testing, to in-laboratory process optimization efforts.
Results:
Moving to a 24 h working model in the existing approved laboratories can enhance the daily testing capacity to 40,464 tests/day. The capacity can be further bolstered by leveraging qRT-PCR and nucleic acid amplification test (NAAT)-based machines available with the Multidisciplinary Research Units (MRUs), National AIDS Control Organisation (NACO) and National Tuberculosis Elimination Programme (NTEP). Using combination/multiplex kits, and provision of automated RNA extraction platforms at all laboratories could also optimize run time and contribute to capacity increase by 1.5-2 times.
Interpretation & conclusions:
Adopting these interventions could help increase public sector's daily testing capacity to nearly 100,000-120,000 tests/day. It is important to note that utilization of the scaled-up testing capacity will require deployment of additional workforce, procurement of corresponding commodities for testing and scale-up of sample collection and transportation efforts.
Keywords: Capacity, laboratory, real-time reverse transcription-polymerase chain reaction test
Coronavirus disease 2019 (COVID-19), first detected in China, has spread to more than 200 countries across the world1. The WHO declared the disease a pandemic on March 11, 20202. Since January 2020, India has undertaken several measures to contain and manage the spread of the disease including international and domestic travel restrictions, rational screening and mandatory quarantines3. One of the key strategies for containing the disease across the world, is to undertake widespread testing for COVID-19 followed by isolation and treatment of confirmed cases and containment measures for clusters of confirmed cases4. The WHO recommends the real-time reverse transcription-polymerase chain reaction (qRT-PCR) diagnostic panel for the detection of 2019 novel coronavirus, a strategy that India has also adopted5. As on March 31, 2020, the daily testing was close to approximately 5,500 tests across public and private laboratories6. Delays in testing can lead to large disease cluster forming, unchecked progression of severe cases and overburdening of the health system with critically ill patients. The present study explores and quantifies interventions to scale-up qRT-PCR-based testing capacity per day across public laboratories in India. The interventions range from optimizing the existing capacity of manual qRT-PCR instruments through multiple shifts and reduction in laboratory-level manual RNA extraction effort; deploying additional manual and automated machines from other public institutes and research organizations and procuring automated high-throughput instruments. The objective of this exercise was to prepare a potential plan to scale-up COVID-19 testing in India in the public sector.
Material & Methods
The various options for scaling up testing facilities were discussed. The potential daily testing capacity calculated for various scenarios, as follows:
-
(i)
Batch size = 36 of 45 possible samples (20% of slots blocked for confirmatory tests); run time/batch with manual extraction = 5 h/batch; run time/batch with automated extraction = 3 h/batch (1/4th of the public laboratories already use automated RNA extraction); no down time on machines.
-
(ii)
Batch size = 36 of 45 possible samples (20% of slots blocked for confirmatory tests); run time/batch with automated extraction = 3 h/batch (all public laboratories use automated RNA extraction); no down time on machines.
-
(iii)
Batch size = 45 of 45 possible samples (no slots blocked for confirmatory tests); run time/batch with manual extraction = 5 h/batch; run time/batch with automated extraction = 3 h/batch (1/4th of the public laboratories already use automated RNA extraction); no down time on machines.
-
(iv)
A total of 42 additional manual qRT-PCR machines deployed from medical and research units; batch size = 36 of 45 possible samples (20% of slots blocked for confirmatory tests); run time/batch with manual extraction = 5 h/batch (all additional machines to run on manual RNA extraction); no down time on machines.
-
(v)
About 10-20 per cent of available nucleic acid amplification test (NAAT)-based point-of-care (POC) testing platforms under the National Tuberculosis Elimination Programme (NTEP)7 can be considered for COVID-19 testing; batch size = 4/run; run time/batch = 2 h/batch; 25 per cent of load reduction in the existing tuberculosis (TB) case load due to reduced footfall; no down time on machines.
-
(vi)
Seventy to hundred per cent of the available automated qRT-PCR-based platforms under the National AIDS Control Organisation (NACO)8 can be considered for COVID-19 testing; batch size = 90/run; run time/batch = 8 h/batch; 90 per cent of spare capacity available due to reduced patient footfall and potential deferral of non-diagnostic HIV viral monitoring; no down time on machines.
-
(vii)
Twelve high-throughput automated qRT-PCR-based platforms to be deployed by the Indian Council of Medical Research (ICMR) (two already in country); daily capacity = 1400 samples in 24 h; 80 per cent of utilization/day; no down time on machines.
An item-wise projection was also made for commodities required to activate the scaled-up testing capacity under various assumptions on the start dates of different interventions (Table I).
Table I.
Assumptions on start dates of different interventions
| Intervention | Intervention subtype | Number of machines (approx.) | Start date |
|---|---|---|---|
| Increase in working hours | Move to 16 h shifts | All | April 10 |
| Move to 24 h shifts | All | April 20 | |
| Redeploy qRT-PCR machines in MRUs | qRT-PCR machines in co-located MRUs | 60 per cent | April 10 |
| qRT-PCR machines in the remaining MRUs | 40 per cent | May 3 | |
| Leverage qRT-PCR machines under NACO | Co-located machines | 65 per cent | May 15 |
| Remaining functional machines | 35 per cent | June 1 | |
| Leverage NAAT POC machines under NTEP | 30 per cent of ~100 machines | 30 | May 15 |
| 30 per cent of ~100 machines | 30 | May 21 | |
| 40 per cent of ~100 machines | 40 | May 31 | |
| Additional machines installed with BSL-2 | 150 | June-December | |
| Automated high-throughput platform to be deployed or procured by the ICMR | Delhi 1 machine | 1 | April 5 |
| Bhubaneswar 1 machine | 1 | April 20 | |
| Accenture 1 machine | 1 | May 15 | |
| 4-10 newly procured machines installed in tranches | 4-10 | June 1 | |
| Automated RNA extraction machines | 30 laboratories per week till all laboratories | 33 per cent | May 8 |
| 67 per cent | May 15 | ||
| 100 per cent | May 23 | ||
| Combination kits | Some laboratories | 50 per cent | May 1 |
| All laboratories | 100 per cent | June 1 |
qRT-PCR, real-time reverse transcription-polymerase chain reaction; ICMR, Indian Council of Medical Research; TB, tuberculosis; NTEP, National Tuberculosis Elimination Programme; POC, point-of-care; NAAT, nucleic acid amplification testing; NACO, National AIDS Control Organisation; MRUs, Multidisciplinary Research Units; BSL-2, biosafety level 2
Results
Several options have been proposed to be enacted upon in the short and medium term. Short-term measures can be implemented with the existing diagnostic equipment already present in the public sector. These include:
-
(i)
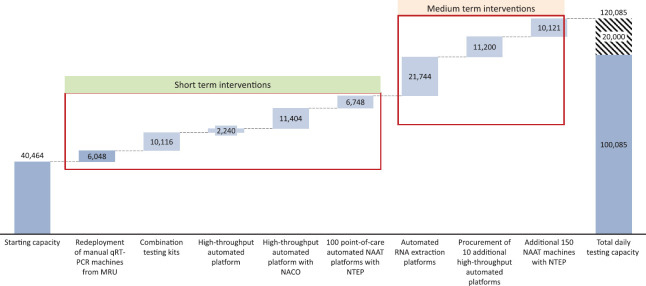
Optimization of starting capacity: The existing 216 manual qRT-PCR machines in approved laboratories can be enhanced from one shift (9 h scenario) to two shifts (16 h scenario) and further to three shifts (24 h scenario), thereby increasing the capacity to 40,464 tests per day (Figs. 1-3).
-
(ii)
Redeployment of manual qRT-PCR machines: There are 42 manual qRT-PCR machines in Multidisciplinary Research Units (MRUs). These can be redeployed and allocated for COVID-19 testing.
-
(iii)
Procurement of combination testing kits that can do both screening and confirmatory tests in one machine run.
-
(iv)
High-throughput automated platform: Operationalizing high-throughput platforms capable of conducting up to 1400 tests/day/machine. Two machines have already been installed in the country at National Institute of Biologicals (NIB), Noida, Uttar Pradesh and Regional Medical Research Center (RMRC), Bhubaneshwar, Odisha.
-
(v)
High-throughput automated platform with NACO: Leveraging spare capacity available due to reduced footfall in lockdown situation on functional automated high-throughput qRT-PCR latforms under NACO; these platforms have U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for COVID-19 testing. Currently, these are used for early infant diagnosis and HIV viral load monitoring. Two third of the functional machines have been co-located in the existing ICMR-approved laboratories.
-
(vi)
Point-of-care NAAT-based automated platform with NTEP: The existing public sector laboratories approved by the ICMR cover only 114 of the 736 districts in the country; the laboratory network needs to be decentralized to increase coverage and ease sample transportation concerns. About 100 of the operational POC NAAT-based machines across the 725 districts in the country, used for TB diagnosis, are biosafety level 2 (BSL-2) approved and can be considered for capacity sharing.
Fig. 1.
Impact on overall daily testing capacity by intervention type in nine working hours (conservative). qRT-PCR, real-time reverse transcription-polymerase chain reaction; NAAT, nucleic acid amplification test; MRU, Multidisciplinary Research Unit; POC, point-of-care; NACO, National AIDS Control Organisation; NTEP, National Tuberculosis Elimination Programme.
Fig. 3.
Impact on overall daily testing capacity by intervention type in 24 working hours (aggressive). Grey area is the expected additional tests (20,000) that can be conducted if all the laboratory resources operate at full efficiency.
Fig. 2.
Impact on overall daily testing capacity by intervention type in 16 working hours (moderate).
For platforms under the NACO and NTEP, supply of corresponding reagents and cartridges from international suppliers will have to be secured. The ICMR has already approved some of the closed platforms with these programmes for COVID-19 diagnosis. Medium-term measures involve a lag time depending on supply-side contingencies. These include:
-
(i)
Procurement and installation of automated RNA extraction platforms along with the procurement of requisite extraction kits: Currently, only about 25 per cent of the laboratories (29 laboratories) have automated RNA extraction capability, while the remaining conduct time-consuming and cumbersome manual extraction. Installation and/or operationalization of automated RNA extraction platforms supported by requisite extraction kits at the remaining 75 per cent of the laboratories could increase testing capacity by 1.5-2 times within the same operating hours.
-
(ii)
High-throughput automated platform: Ten additional high-throughput automated machines may be procured and deployed in the country.
-
(iii)
Point-of-care automated NAAT platform with NTEP: An additional 150 machines under the NTEP can be considered for capacity sharing after getting the required BSL-2 approvals.
Table II outlines the total commodities that would be required to utilize the scaled-up testing capacity.
Table II.
Total commodities required in order to activate scaled-up testing capacity as per timelines in Table I
| Items Commodities |
Projected requirement | Total | |||||
|---|---|---|---|---|---|---|---|
| April 1-15 | April 16-30 | May 1-15 | May 16-30 | June 1-15 | June 16-30 | April 1 - June 30 | |
| VTM (with two swabs) (commercially available) | 5,50,000 | 6,50,000 | 11,15,000 | 14,60,000 | 19,75,000 | 19,75,000 | 77,25,000 |
| RNA extraction kitsa,b | 5,00,000 | 5,00,000 | 8,80,000 | 12,25,000 | 14,00,000 | 14,00,000 | 59,05,000 |
| Testing kits (complete) | |||||||
| For manual qRT-PCR (combination kits)c | 0 | 0 | 2,80,000 | 4,20,000 | 14,00,000 | 14,00,000 | 35,00,000 |
| For automated qRT-PCR (ICMR) | 50,000 | 1,50,000 | 1,00,000 | 1,00,000 | 2,00,000 | 2,00,000 | 8,00,000 |
| For automated qRT-PCR (NACO) | 0 | 0 | 75,000 | 75,000 | 1,75,000 | 1,75,000 | 5,00,000 |
| For POC automated NAAT (NTEP) | 0 | 0 | 60,000 | 60,000 | 2,00,000 | 2,00,000 | 5,20,000 |
| Testing kit (probe, primer and MasterMix separately) | |||||||
| For manual qRT-PCR | 5,00,000 | 5,00,000 | 6,00,000 | 8,05,000 | 0 | 0 | 24,05,000 |
aOnly 1/4th of the laboratories out of the existing approved laboratories have automated RNA extraction capability. Additional procurement of automated RNA extraction equipment is planned. Prospectively, only automated RNA extraction kits should be procured, which are compatible with the RNA extraction machines procured, bThe RNA extraction kits required are one per manual qRT-PCR test, cProjection for requirement of combination kits (screening + confirmatory). VTM, viral transport medium
Discussion
A potential plan to scale-up COVID-19 testing in the public sector was prepared in India. By implementing all the above mentioned interventions, public sector's daily testing capacity could be scaled-up to nearly 100,000-120,000 tests/day (depending on conservative, moderate or aggressive operating scenarios), which can help enhance readiness for worst-case scenario. In order to utilize the scaled-up testing capacity, increased workforce, adequate testing commodities and collection of enough samples/day would be critical. Commodities required to operationalize the plan include viral transport medium (with two swabs) commercially available, RNA extraction kits, testing kits for manual qRT-PCR (combination kits), automated qRT-PCR kits and platforms and testing kits (probe, primer and Master Mix separately) for manual qRT-PCR. Additionally, all private and government medical colleges may be urged to eventually create the state-of-the-art virology laboratories and support the country's fight against COVID-19. Furthermore, sample collection and transportation efforts will have to keep pace with the increased available testing capacity at the laboratories.
Footnotes
Financial support & sponsorship: None
Conflicts of Interest: None.
References
- 1.World Health Organization. Coronavirus (COVID-19) WHO; 2020. [accessed on March 29, 2020]. Available from: https://whosprinklrcom/ [Google Scholar]
- 2.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. WHO; 2020. [accessed on March 29, 2020]. Available from: https://wwwwhoint/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 . [Google Scholar]
- 3.Ministry of Health and Family Welfare, Government of India. COVID-19 India. [accessed on March 31, 2020]. Available from: https://www.mohfw.gov.in/
- 4.World Economic Forum. WHO coronavirus briefing: Isolation, testing and tracing comprise the 'backbone' of response World Economic Forum. 2020. [accessed on March 31, 2020]. Available from: https://wwwweforumorg/agenda/2020/03/testing-tracing-backbone-who-coronavirus-wednesdays-briefing/
- 5.World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: Interim guidance, 2 March 2020. WHO; 2020. [accessed on March 28, 2020]. Available from: https://appswhoint/iris/handle/10665/331329 . [Google Scholar]
- 6.Indian Council of Medical Research. COVID-19 public labs testing data as on Mar 31, 2020. [accessed on March 31, 2020]. Available from: https://icmrnicin/content/covid-19 .
- 7.Central TB Division. Revised National Tuberculosis Control Programme Annual Report. New Delhi: Ministry of Health & Family Welfare, Government of India; 2019. [Google Scholar]
- 8.National AIDS Control Organisation. National Guidelines for HIV-1 Viral Load Laboratory Testing. New Delhi: Ministry of Health & Family Welfare, Government of India; 2018. [Google Scholar]