Abstract
Although black (Rattus rattus) and brown (Rattus norvegicus) rats are among the most widespread synanthropic wild rodents, there is a surprising scarcity of knowledge about their ecology in the urban ecosystem. In particular, relatively few studies have investigated their helminth species diversity in such habitat. We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guideline to synthesize the existing published literature regarding the helminth fauna of urban rats in developed countries (North America, Europe, Australia, New Zealand and Japan). We aimed at describing the species diversity and richness of urban rat helminths, the species prevalence and associations, the methods of investigation, the pathological changes observed in the hosts, the risk factors of infection and the public health significance of rat-borne helminthiases. Twenty-three scientific papers published between 1946 and 2019 were reviewed, half of them were conducted in Europe. Twenty-five helminth species and eight genera were described from the liver, digestive tract, lungs and muscles of urban rats. The most commonly reported parasite was Calodium hepaticum. Prevalence and risk factors of helminth infection in urban rats varied greatly between studies. Observed pathological findings in the rat host were generally minor, except for C. hepaticum. Several rat helminths can parasitize humans and are therefore of public health significance. The lack of references to identification keys and the rare use of molecular tools for species confirmation represent the main limitation of these studies. Knowledge gap on this topic and the needs for future research are discussed.
Electronic supplementary material
The online version of this article (10.1007/s00436-020-06776-3) contains supplementary material, which is available to authorized users.
Keywords: Rattus rattus, Rattus norvegicus, Helminth, Urban habitat, City, Review
Introduction
The urbanisation process, together with the climate change and increasing habitat loss and pollution, alters the distribution range, physiology, biology and behaviour of many wildlife species (Prange et al. 2003; Partecke et al. 2006; French et al. 2008; McKinney 2008; Costantini et al. 2014; Meillère et al. 2015). Within the fragmented urban ecosystem, modified habitat structures, increased resource availability, reduced biodiversity and altered trophic interactions affect wildlife population biology, hence the nature and functioning of wildlife communities (Bradley and Altizer 2007; Cavia et al. 2009; Horsák et al. 2013; Aronson et al. 2016). These changes are subsequently affecting host-parasites interactions, parasite transmission and the structure of the parasite communities (Bradley and Altizer 2007; Cable et al. 2017), potentially leading to an augmented risk of spillover of wildlife parasites to humans (Gordon et al. 2016).
Rats (Rattus spp.) benefit from urban sprawl (McKinney 2006) and, over the past centuries, they have become one of the most successful urban wildlife species. Despite their close proximity with humans, the biology and ecology of urban rats have been understudied (Himsworth et al. 2013). If bacterial and viral organisms carried by rats know nowadays an increasing scientific interest (particularly if they are zoonotic) (Himsworth et al. 2013; Desvars-Larrive et al. 2017; Angley et al. 2018; Strand and Lundkvist 2019), studies on the helminth fauna of urban rats remain scarce. Helminths can modify wildlife host population dynamics (Hudson et al. 1998; Albon et al. 2002), communities (Mouritsen and Poulin 2002) and health status (Tompkins et al. 2011). Therefore, knowledge on urban rat helminths could interest (urban) ecologists but also pest management professionals. Moreover, several helminths carried by urban rats are zoonotic (Meerburg et al. 2009; Gordon et al. 2016). Rodent-borne helminthiases are neglected diseases that mostly (but not exclusively) affect people from developing, low-income countries (World Health Organization 2012). They can impact human nutritional status, inducing negative effects at different stages of the human life cycle (Crompton and Nesheim 2002), exacerbate malaria and HIV/AIDS, impair vaccine efficacy (World Health Organization 2012) and enhance the risk of allergy (Sitcharungsi and Sirivichayakul 2013).
This systematic review aimed at compiling and summarizing the peer-reviewed literature on the helminths of urban rats, i.e. the brown rat, Rattus norvegicus (Berkenhout, 1769), and the black rat, Rattus rattus (Linnaeus, 1758). Helminths require relatively specific biotic and abiotic conditions to complete their life cycle (Froeschke and Matthee 2014), therefore, we restricted the scope of this systematic review to urban ecosystems within the so-called developed regions, as defined by the United Nations (i.e. North America, Europe, Australia, New Zealand and Japan) (United Nations 2016). Indeed, urban habitats in developed countries present a common general framework (Johnson and Munshi-South 2017) that includes relatively similar sanitary (e.g. water quality) and climatic conditions, and provides comparable environmental situations to the parasites and their hosts.
In this systematic review, we addressed the following questions:
Which helminth species infect urban rats and what are their prevalence?
What are the methods used to investigate the helminths of urban rats?
What are the risk factors for infection in the rat host?
What are the pathological findings related to helminth infection in the rat host?
What is the veterinary and public health importance of rat-borne helminthiases in cities?
Materials and methods
Search strategy
We performed a systematic search following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline for systematic reviews (Moher et al. 2009). Our search included the databases CAB Direct, JSTOR, Pubmed, Web of Science Core Collection and Scopus. We searched the databases between inception and the cut-off date 23 March 2019. The literature was searched using the following query:
(((“Rattus norvegicus” OR “Norway rat*” OR “brown rat*” OR “Rattus rattus” OR “black rat*” OR “roof rat*”) AND (worm* OR parasite* OR helminth* OR nematode* OR cestode* OR round worm* OR tapeworm*)) AND (urban* OR cities OR city OR municipal* OR suburban OR residential OR metropol*)).
Study selection
References identified by the search were screened for inclusion criteria and relevance to the review question by two of the co-authors (ADL and DG). Discrepancies were resolved by consensus. Studies were selected using the following inclusion criteria: studies published from inception to 23 March 2019, studies in English language, studies published in peer-reviewed journals, studies that investigated wild R. rattus and/or R. norvegicus as hosts, studies that investigated helminth parasites, studies conducted in urban ecosystems and studies conducted in developed countries (United Nations 2016). A major challenge in urban ecology is to clearly delineate the urban ecosystem (McIntyre et al. 2000). Regional- or country-scale differences in urban planning make it difficult to find criteria that adequately define what is “urban” at a global scale (United Nations 2018). Due to this heterogeneity, we relied on the categorisation of the study sites by the authors of the publications when determining their eligibility for our systematic review
Non-English studies, as well as grey literature, reviews, books, book chapters, conference papers and poster abstracts, were excluded. Furthermore, we did not consider studies investigating other species than R. rattus or R. norvegicus, studies conducted in rural ecosystems or unspecified locations, experimental studies and studies conducted on captive animals.
Data extraction and synthesis
For each study, two co-authors (ADL and DG) extracted the relevant data organized in 22 categories (Table 1) and compiled them into a database. When necessary, appendices and supplementary materials were also inspected. For each helminth species, we recorded the Latin binomial (i.e. the scientific name) and its synonyms as quoted in the article. Maps were built using QGIS 3.4.5 (QGIS Development Team 2018). The R package ggplot2 (Wickham 2016) was used for data visualisation.
Table 1.
Data extracted from the selected publications and used to address the review questions
| Category | Definition |
|---|---|
| Year | Year of publication |
| Reference | Authors. Year. Title. Journal. Volume. Issue. Pages |
| Research area | Journal scope |
| Scale of the study | Spatial scale: a city block, one city, multiple cities, a country |
| Location | Location(s) of the study |
| Trapping site(s) | Description of the trapping site(s) |
| Method of capture | Type(s) of trap |
| Host species | Rattus species investigated: R. rattus and/or R. norvegicus |
| Aim of the study | Main objective(s) of the research |
| Global relevance | Main scientific field covered by the study |
| Method(s) used for detection | Method(s) used to retrieve the helminths or evaluate intensity of infection |
| Morphological identification keys | Reference paper(s) cited for the identification of the helminth species |
| Sample size | Number of rats investigated |
| Helminth species | Latin binomial as quoted in the article |
| Organ | Organ where the helminth species was retrieved |
| Number of positive | Number of positive rats for each helminth species |
| Prevalence | Prevalence of infection for each helminth species (number of positive / sample size) |
| Helminth species richness | Number of helminth species per host (co-infection) |
| Parasite burden | Defined using different metrics: mean intensity (total number of helminths of a particular species found in a sample divided by the number of hosts infected with that helminth); abundance (number of individuals of a particular helminth species in a single host); mean abundance (total number of individuals of a particular helminth species in a sample of a particular host species divided by the total number of hosts of that species examined (including both infected and uninfected hosts), i.e. the average abundance of a helminth species among all members of a particular host population) (Bush et al. 1997) |
| Pathological findings | Gross and histological changes induced by the presence of an helminth species |
| Interspecies interaction | When the occurrence of one helminth species has an impact on the presence of another species |
| Risk factor(s) of infection | Risk factor(s) of infection statistically identified |
Results
PRISMA-guided study selection
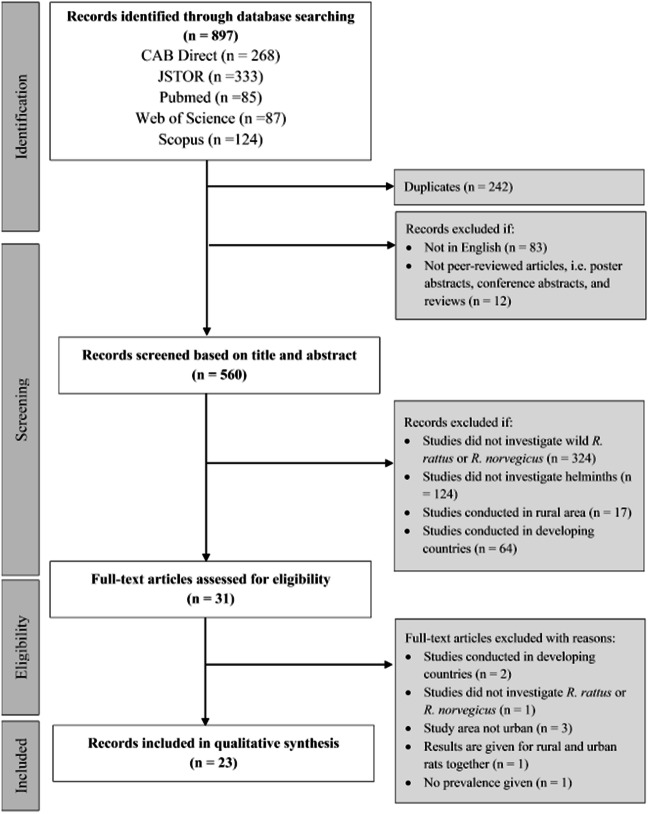
The flow diagram of the search strategy steps is presented in Fig. 1. Our first search yielded 897 results (268 from CAB Direct, 333 from JSTOR, 85 from Pubmed, 87 from Web of Science, and 124 from Scopus). After exclusion of duplicates (n = 242), reviews, poster presentations, proceeding papers (n = 12), and non-English publications (n = 83), 560 records were retained for the screening of titles and abstracts. Articles were excluded if the studies did not investigate wild R. rattus or R. norvegicus (n = 324), did not investigate helminths (n = 124), was conducted in rural area (n = 17) and/or in developing countries (n = 64). Thirty-one full-text articles met the eligibility criteria. After full examination, two of them were removed because they were not conducted in developed countries, one was excluded because it did not investigate R. rattus or R. norvegicus, three were excluded because they were not conducted in urban settings and two were excluded because prevalence could not be extracted from the results. Many studies had to be excluded on the basis of more than one criterion; numbers given in Fig. 1 indicate the first criterion of exclusion that has been identified. A total of 23 studies were ultimately included in our review. The details of the included studies are shown in the Supplementary Material 1.
Fig. 1.
PRISMA flow diagram of the methodology and selection process
Types of studies
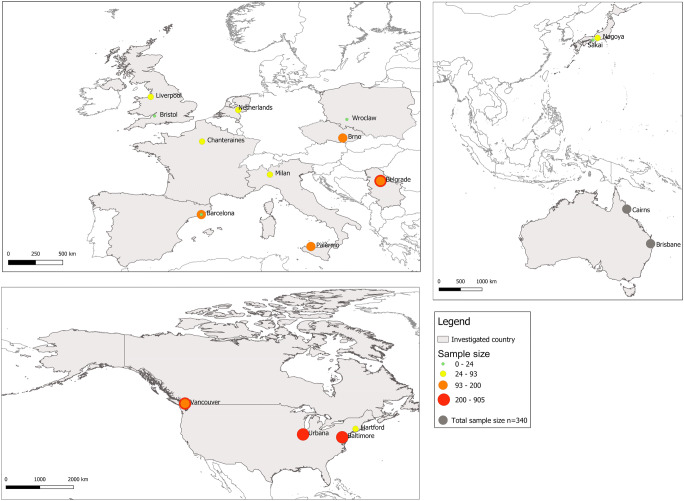
Year of publication of the 23 identified articles ranged between 1946 and 2019, with most of them (n = 13, 56.5%) published in the 2010s. Studies were conducted in 12 different developed countries (Fig. 2) on four continents, with the majority of them undertaken in Europe (n = 12 papers, 52.2%) and North America (8 papers, 37.8%). Ten papers (43.5%) were published in journals with parasitology as a main scope while five (21.7%) were published in journals showing veterinary medicine as a main scope. The scale of the studies varied greatly: the whole country in one study, two cities in one study, a city in 12 studies, a city and its zoo in two studies, the periurban area of a city in one study, an inner city urban neighbourhood and a port in three studies (those studies belonged to the same project, Vancouver Rat Project: http://www.vancouverratproject.com/vancouver_rat_project/home, and referred to the same sample), a city park in one study and one single animal in two studies. Eleven studies (47.8%) aimed at investigating a single helminth species, two studies aimed at investigating two specific helminth species and nine studies intended to conduct an exhaustive survey of the helminth fauna of urban rats. All studies reported data on urban R. norvegicus, while five (21.7%) reported also data on urban R. rattus. The median sample size (number of urban rats investigated) was 143 (min. = 1, max. = 905) (Fig. 2).
Fig. 2.
Geographic distribution of the 23 reviewed publications. The size of each circle is proportional to the sample size, i.e. the total number of urban rats (R. rattus and R. norvegicus) investigated per study
Research questions
Helminths of urban rats
Helminth diversity
Helminth taxa from three phyla were reported in urban rats from developed countries, i.e. Acanthocephala, Nematoda and Platyhelminthes, with 25 taxa attributed to the species level (Table 2) and eight to the genus level. Calodium hepaticum (syn. Capillaria hepatica) was the most commonly reported helminth species in urban rats (14 out of 23 studies), followed by Heterakis spumosa (8/23), Nippostrongylus brasilensis (7/23), Hymenolepis diminuta (the rat tapeworm) (6/23), Trichosomoides crassicauda (5/23), Taenia taeniaformis (syn. Strobilocercus fasciolaris, Cysticercus fasciolaris) larvae (4/23), Syphacia muris (the rat pinworm) (4/23) and Rodentolepis (syn. Hymenolepis) nana (3/23). The following taxa were recorded in two studies: Eucoleus gastricus, Mastophorus muris, Orientostrongylus ezoensis, Rodentolepis fraterna, Strongyloides ratti, Trichuris muris and Brachylaima sp. The following taxa were each recorded in one study: Angiostrongylus cantonensis (the rat lungworm), Aonchotheca annulosa, Aonchotheca murissylvatici, Ganguleterakis spumosa, Gongylonema neoplasticum, Moniliformis moniliformis, Notocotylus imbricatus, Plagiorchis proximus, Rodentolepis microstoma, Rodentolepis straminea and Strongyloides venezuelensis. All helminths were retrieved from R. norvegicus, among them four were described in R. rattus: A. cantonensis, A. murissylvatici, C. hepaticum and Eucoleus sp. (Table 2). Strongyloides venezuelensis and O. ezoensis were reported from Japan only (Yokota et al. 1991; Shintoku et al. 2005).
Table 2.
Summary of the helminths reported in urban rats (Rattus norvegicus and Rattus rattus) in cities from developed countries. Those zoonotic are indicated with an asterisk
| Helminth | Species authority reported in the reviewed papers | Synonyms found in the reviewed papers | Localisation | Host investigated | Prevalence (%) reported in the reviewed papers (number of studies) |
|---|---|---|---|---|---|
| Acanthocephala | |||||
| Acanthocephala sp. | Not indicated | na | Large intestine | R. norvegicus | 0.7a (1) |
| Moniliformis moniliformis* | Not indicated | Thorny-headed worm | Not indicated | R. norvegicus | 6 (1) |
| Cestoda | |||||
| Hymenolepis sp.* | Not indicated | na | Not indicated | R. norvegicus | na (1) |
| Hymenolepis diminuta* | Rudolphi, 1819 | Rat tapeworm | Small intestine | R. norvegicus | Min.: 1.2–Max. = 36.3 (6) |
| Rodentolepis microstoma* | Not indicated | na | Small intestine | R. norvegicus | 8.7 (1) |
| Rodentolepis nana* | Von Siebold, 1852 | Dwarf tapeworm, Hymenolepis nana | Small intestine | R. norvegicus | Min.: 13.3–Max. = 17.0 (3) |
| Rodentolepis fraterna* | Stilles, 1906 | Hymenolepis nana var. fraterna | Small intestine | R. norvegicus | Min.: 5.3– Max. = 17.8 (2) |
| Rodentolepis straminea* | Goeze, 1782 | na | Not indicated | R. norvegicus | 40.7 (1) |
| Taenia taeniaeformis larvae | Batsch, 1786 | Cysticercus fasciolaris, Strobilocercus fasciolaris | Liver | R. norvegicus | Min.: 3.7–Max. = 29.3 (4) |
| Nematoda | |||||
| Angiostrongylus cantonensis* | Not indicated | Rat lungworm | Lungs and pulmonary vasculature | R. norvegicus | 16 (1) |
| R. rattus | 27 (1) | ||||
| Aonchotheca annulosa | Not indicated | na | Not indicated | R. norvegicus | 12 (1) |
| Aonchotheca murissylvatici | Not indicated | na | Not indicated | R. norvegicus | 34.2 (1) |
| Not indicated | R. rattus | 50a (1) | |||
| Capillaria sp. | na | na | Not indicated | R. norvegicus | na (2) |
| Calodium hepaticum * | Bancroft, 1893; Moravec, 1982 | Capillaria hepatica | Liver | R. norvegicus | Min.: 10.9–Max. = 94.3b (14) |
| R. rattus | Min.: 0.0–Max. = 23.7 (2) | ||||
| Eucoleus sp. | na | na | Stomach (non-glandular part) and oesophagus | R. norvegicus | na (1) |
| R. rattus | na (1) | ||||
| Eucoleus gastricus | Not indicated | na | Stomach | R. norvegicus | Min.: 28.0–Max. = 30.1 (2) |
| Ganguleterakis spumosa | Not indicated | na | Not indicated | R. norvegicus | 1.8 (1) |
| Gongylonema sp. | Not indicated | na | Stomach | R. norvegicus | na (1) |
| Gongylonema neoplasticum* | Not indicated | na | Oesophagus | R. norvegicus | 20 (1) |
| Heterakis spumosa | Schneider, 1866 | na | Stomach, lower small intestine, large intestine, caecum, colon | R. norvegicus | Min.: 33.3–Max. = 82.5 (8) |
| Mastophorus muris | Gmelin, 1790 | na | Stomach | R. norvegicus | Min.: 2.4–Max. = 30.6 (2) |
| Nippostrongylus brasiliensis | Travassos, 1914 | na | Stomach, upper small intestine, lower small intestine, caecum, colon | R. norvegicus | Min.: 6.2–Max. = 100 (7) |
| Orientostrongylus ezoensis | Tada, 1975 | na | Stomach, upper small intestine, lower small intestine, large intestine, caecum, colon | R. norvegicus | Min.: 88.9–Max. = 94.1 (2) |
| Strongyloides sp. | na | na | Not indicated | R. norvegicus | na (1) |
| Strongyloides ratti | Sandground, 1925 | na | Stomach, upper small intestine, lower small intestine, large intestine, caecum, colon | R. norvegicus | Min.: 11.1–Max. = 97.1 (2) |
| Strongyloides venezuelensis | Not indicated | na | Stomach, upper small intestine, lower small intestine | R. norvegicus | 75.0 (1) |
| Syphacia muris | Yamaguti, 1935; Yamaguti, 1941 | Rat pinworm | Large intestine, caecum | R. norvegicus | Min.: 7.0–Max. = 55.0 (4) |
| Trichosomoides crassicauda | Schrank, 1788; Bellingham | na | Urinary bladder, ureters, and kidneys | R. norvegicus | Min.: 7.0–Max. = 65.4 (5) |
| Trichuris muris* | Schrank, 1788 | na | Not indicated | R. norvegicus | Min.: 2.6–Max. = 8.3 (2) |
| Trematoda | |||||
| Brachylaima sp. | na | na | Small intestine | R. norvegicus | Min.: 1.2–Max. = 8.4 (2) |
| Echinostoma sp.c | na | na | Not indicated | R. norvegicus | 5.3 (1) |
| Notocotylus imbricatus | Not indicated | na | Not indicated | R. norvegicus | 10.5 (1) |
| Plagiorchis proximus | Not indicated | na | Not indicated | R. norvegicus | 7.9 (1) |
na not applicable. Prevalence is not given when helminths are identified at the genus level only and this genus is reported elsewhere
aSample size = 1
bPrevalence calculated on a sample size of one individual excluded
cMorphological characteristics were consistent with that of a group of seven Echinostoma spp. (E. chloropodis, E. corvi, E. hystricosum, E. necopinum, E. rousseloti, E. sarcinum and E. travassosi) (Franssen et al. 2016)
Species richness
The helminth species richness per host ranged from one to six (Dyk et al. 1975; Ceruti et al. 2001; Shintoku et al. 2005; Kataranovski et al. 2010; Milazzo et al. 2010; Kataranovski et al. 2011; McGarry et al. 2015; Franssen et al. 2016; Desvars-Larrive et al. 2017; Galan-Puchades et al. 2018).
Species association
Within the rat host, a positive association was described between S. ratti and H. spumosa (Shintoku et al. 2005), S. muris and H. spumosa (Desvars-Larrive et al. 2017), and G. neoplasticum and M. moniliformis (Galan-Puchades et al. 2018). The study of Rothenburger et al. (2014b) on urban black and brown rats revealed that, in the host, the presence of C. hepaticum in the liver did not impact the presence of Eucoleus sp. in the stomach.
Prevalence
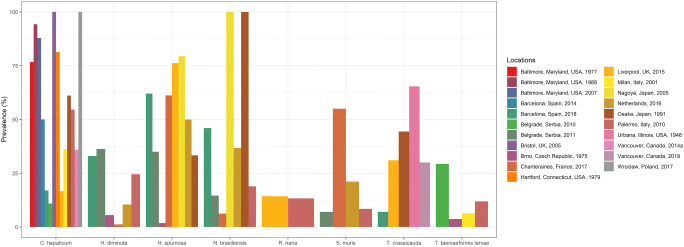
Prevalence of each helminth species varied greatly among studies (Table 2) although these prevalences are hardly comparable due to large variations in sample sizes and methods. Figure 3 shows the prevalence of the eight most commonly reported helminth species of the urban brown rat in developed countries.
Fig. 3.
Prevalence of the most common helminth species of the urban brown rat (R. norvegicus) in developed countries, as reported in the reviewed papers
Parasite burden
Parasite burden was reported for the liver larval stage of T. taeniformis (Kataranovski et al. 2010), A. cantonensis in the lungs (Aghazadeh et al. 2015b), several gastrointestinal helminth species (Yokota et al. 1991; Shintoku et al. 2005; Milazzo et al. 2010; McGarry et al. 2015; Franssen et al. 2016; Desvars-Larrive et al. 2017; Galan-Puchades et al. 2018) and T. crassicauda in the urinary tract (Smith 1946). Metrics used to quantify the parasite burden were variable (Supplementary Material 1).
Methods to investigate the helminths of urban rats
Identification of the helminths
Morphological identification
References to morphological identification keys for the genus or species were given in twelve (52.2%) papers (Yokota et al. 1991; Kataranovski et al. 2010; Milazzo et al. 2010; Kataranovski et al. 2011; Rothenburger et al. 2014a; Rothenburger et al. 2014b; Aghazadeh et al. 2015b; McGarry et al. 2015; Franssen et al. 2016; Bunkowska-Gawlik et al. 2017; Desvars-Larrive et al. 2017; Rothenburger et al. 2019). Drawings and photographs showing typical morphological features of the worms were provided in one (4.3%) (Yokota et al. 1991) and four (17.4%) (Kataranovski et al. 2010; Rothenburger et al. 2014b; Aghazadeh et al. 2015b; Franssen et al. 2016) papers, respectively. We did not consider histological photographs as suitable for morphological identification as they do not show the typical structures to allow species recognition.
Staining procedures for morphological characterisation of the helminths included (i) the use of lactophenol as a clearing solution for the nematodes (Yokota et al. 1991; Galan-Puchades et al. 2018); (ii) the use of Semichon acetocarmine followed by dehydration in alcohol, clearing in xylene and mounting in Canada balsam for the trematodes and cestodes and (iii) the use of Amann lactophenol for temporary mounting of the nematodes and acanthocephalans (Milazzo et al. 2010).
Molecular tools
Bunkowska-Gawlik et al. (2017) used a polymerase chain reaction (PCR) to identify C. hepaticum eggs retrieved from the liver. A molecular approach was also chosen by Franssen et al. (2016) to distinguish between H. diminuta, R. nana and H. fraterna, but the protocol was not detailed.
Helminths of the liver
Diagnosis of C. hepaticum and T. taeniaformis larvae was performed through macroscopic examination of the liver to search for typical lesions and confirmed through histological observation of adults and eggs (C. hepaticum) or larvae (T. taeniaformis) in liver tissue sections. Calodium hepaticum eggs cluster unevenly throughout the liver parenchyma, random sectioning is therefore not optimal for the diagnosis (McGarry et al. 2015). Calodium hepaticum eggs could also be recovered from liver press preparation (Conlogue et al. 1979; Childs et al. 1988). Quantitative assessment of C. hepaticum burden was performed through homogenization of the whole liver, followed by artificial digestion, filtration and count of the total number of eggs under a stereo microscope (McGarry et al. 2015).
Helminths of the digestive tract
The different segments of the gastrointestinal tract (stomach, upper small intestine, lower small intestine, caecum and colon) were processed separately. Helminths were collected under a stereo microscope after homogenization and filtration of the gastrointestinal content (Franssen et al. 2016) or incubation of the gastrointestinal content in a Baermann apparatus (Shintoku et al. 2005).
Coproscopic investigation was reported in three out of the 23 studies. Samples included fresh faeces collected on the ground (Dyk et al. 1975) and faeco-caecal content (Easterbrook et al. 2007; McGarry et al. 2015). The flotation technique was performed to concentrate eggs for identification of the helminth taxa (Dyk et al. 1975; Easterbrook et al. 2007). A modified McMaster concentration technique was used by McGarry et al. (2015) for egg quantification.
Helminths of the urinary tract
Presence of T. crassicauda was investigated through dissection and observation of the urinary tract under a stereo microscope followed by worm identification using light microscopy (Smith 1946; Yokota et al. 1991; Galan-Puchades et al. 2018) and through histological sections of the urinary tract (McGarry et al. 2015; Rothenburger et al. 2019).
Helminths of the lungs
Angiostrongylus cantonensis was retrieved through the examination of lungs and pulmonary vasculature under a stereo microscope. Identification was confirmed through the observation of male worms under a light microscope (Aghazadeh et al. 2015b).
Helminths of the muscles
Larvae of Trichinella spiralis in the muscle were searched through muscle examination using the compression method (Dyk et al. 1975) or artificial digestion of diaphragm and hind leg muscles followed by sequential filtering (Franssen et al. 2016). Both papers that investigated this parasite reported negative results.
Risk factors of infection
Seventeen papers (73.9%) investigated potential risk factors of helminth infection in urban brown rats. No risk factors were described for black rats.
Season
Impact of the season on helminth occurrence varied depending on the geographical location and helminth species. Concerning C. hepaticum, the prevalence was stable through the year in Baltimore, USA (Farhang-Azad 1977; Childs et al. 1988; Easterbrook et al. 2007), whereas autumn was at lowest risk of infection in Belgrade, Serbia (Kataranovski et al. 2011), but at highest risk in Vancouver, Canada (Rothenburger et al. 2014a). If the highest prevalences of H. spumosa, N. brasiliensis, O. ezoensis, S. ratti and S. venezuelensis were reported in spring (March–May) in Japan (Shintoku et al. 2005), the prevalence of N. brasiliensis in Europe (Kataranovski et al. 2011) and H. diminuta in the USA (Easterbrook et al. 2007) was significantly lower in spring. The prevalence of Trichuris muris and S. muris was higher in autumn compared to winter in Belgrade, Serbia (Kataranovski et al. 2011). The season did not influence the prevalence of Eucoleus sp. in Vancouver, Canada (Rothenburger et al. 2014b). Similarly, the overall prevalence of helminth infection did not show any seasonal variation in Belgrade, Serbia (Kataranovski et al. 2011).
Host sex
Most of the studies reported no impact of the host sex on the prevalence and parasite burden of helminth infection in urban brown rats (Smith 1946; Farhang-Azad 1977; Childs et al. 1988; Shintoku et al. 2005; Easterbrook et al. 2007; Kataranovski et al. 2010; Milazzo et al. 2010; Rothenburger et al. 2014a; Rothenburger et al. 2014b; McGarry et al. 2015; Desvars-Larrive et al. 2017; Galan-Puchades et al. 2018). However, Kataranovski et al. (2011) reported a higher global prevalence of intestinal helminth infection in male urban brown rats compared to females. Similarly, the prevalence of C. hepaticum (Kataranovski et al. 2011), the prevalence of Hymenolepis sp. (Easterbrook et al. 2007), the risk of S. muris occurrence (Desvars-Larrive et al. 2017), the mean abundance of M. moniliformis (Galan-Puchades et al. 2018) and the intensity of infection by S. ratti and S. venezuelensis (Shintoku et al. 2005) were significantly greater in male urban brown rats compared to females. Rothenburger et al. (2019) evidenced an increased odd of T. crassicauda infection in female R. norvegicus compared to males.
Host weight, age and sexual maturity
A positive association was evidenced between host weight and C. hepaticum prevalence (Farhang-Azad 1977; Childs et al. 1988; Easterbrook et al. 2007), Eucoleus sp. prevalence (Rothenburger et al. 2014b) and H. diminuta burden (Galan-Puchades et al. 2018). Smith (1946) and Rothenburger et al. (2019) reported an increased prevalence of T. crassicauda in adults and heavier rats (> 145 g), respectively. On the contrary, Brachylaima sp. prevalence was reported significantly higher in juveniles than adults (Milazzo et al. 2010) and risk of S. muris occurrence was reported significantly lower in heavier rats (Desvars-Larrive et al. 2017). In other studies, no association was evidenced between host weight (or age) and helminth prevalence (Easterbrook et al. 2007; Milazzo et al. 2010; Rothenburger et al. 2014a; Desvars-Larrive et al. 2017; Galan-Puchades et al. 2018). A positive association was found between sexual maturity and odd of infection by C. hepaticum (Rothenburger et al. 2014a) and Eucoleus sp. (Rothenburger et al. 2014b), independently of the host weight. Pregnancy had no effect on the prevalence of C. hepaticum and Hymenolepis sp. (Easterbrook et al. 2007).
Host site of capture
Fine-scale geographic variations in the prevalence of C. hepaticum (Farhang-Azad 1977; Childs et al. 1988; Rothenburger et al. 2014a) and S. muris (Desvars-Larrive et al. 2017) were reported.
Pathological findings in urban rats (R. norvegicus)
Fourteen (60.8%) of the 23 selected publications reported pathological changes related to the presence of helminths in R. norvegicus, i.e. gross lesions and/or histological changes.
Liver
Macroscopic findings associated to C. hepaticum consisted in focal, multifocal and sometimes coalescing greyish- or yellowish-white lesions ranging from 1 to 5 mm in diameter on the surface of the organ. The presence of tortuous tracts that resulted from migrating adult C. hepaticum was also characteristic (Farhang-Azad 1977; Conlogue et al. 1979; Childs et al. 1988; Ceruti et al. 2001; Easterbrook et al. 2007; Kataranovski et al. 2010; Millán et al. 2014; Rothenburger et al. 2014a; McGarry et al. 2015). Liver lesions were described as slight (i.e. involving a single hepatic lobe) or moderate (i.e. involving less than half of the liver) in the majority of the cases, and, less frequently, as severe (i.e. involving more than half of the liver) (Farhang-Azad 1977; Conlogue et al. 1979; Childs et al. 1988; Ceruti et al. 2001; Kataranovski et al. 2010; Millán et al. 2014). Early stage of infection was characterised by a minor inflammatory process that subsequently evolved into multifocal to coalescent pyogranulomatous hepatitis, progressing to well-defined granulomas surrounding parasitic and necrotic debris. Fibroblastic septa were described as connecting the portal tracts, sometimes also bridging with the terminal hepatic vein. Minor histopathological findings included increase in the diameter of portal and centrolobular veins, hepatic sinusoids containing erythrocytes and a few inflammatory cells, perilobular fibrosis of the liver, hepatocellular necrosis and haemorrhages in the periphery of the lesions and variable stages of biliary hyperplasia.
The presence of T. taeniaeformis larvae in the liver was associated with an inflammatory reaction characterised by the presence of periportal eosinophil infiltrates, micro-abscesses, sinusoid enlargement and prominent Kupffer cells (Kataranovski et al. 2010).
Urinary tract
Trichosomoides crassicauda was principally observed in the lumen and superficial mucosa of the urinary bladder (Smith 1946; McGarry et al. 2015; Rothenburger et al. 2019), but also in the lumen and superficial mucosa of the renal pelvis in 30% of the infected rats (Rothenburger et al. 2019) and in the ureters (Smith 1946). Histopathological changes associated with the presence of T. crassicauda in the urinary tract consisted in the formation of acellular and mucoid calculi of white to yellowish colour and different shapes in the urinary bladder, the presence of multinucleated cells in the epithelium of the urinary bladder together with a mild epithelial hyperplasia of the urothelium and mild lymphoplasmacytic inflammation of the urinary bladder submucosa (Smith 1946; McGarry et al. 2015; Rothenburger et al. 2019).
Digestive tract
Eucoleus sp. was observed in the non-glandular stomach of R. norvegicus. Lesions associated with the presence of this nematode or its eggs included hyperkeratosis, mucosal hyperplasia, keratin pustules and submucosal inflammation (Rothenburger et al. 2014b). A slight hyperaemia of the small intestine was associated with the presence of R. nana in (Dyk et al. 1975). On the contrary, McGarry et al. (2015) did not report any pathological changes associated with the presence of R. nana or M. muris.
Veterinary and public health importance of rat-borne helminthiases in cities
Sixteen (69.5%) studies highlighted the public health significance of rat-borne helminthiases. Nine potentially zoonotic helminths were mentioned (Table 2): C. hepaticum (Farhang-Azad 1977; Conlogue et al. 1979; Childs et al. 1988; Yokota et al. 1991; Ceruti et al. 2001; Easterbrook et al. 2007; Kataranovski et al. 2010; McGarry et al. 2015; Bunkowska-Gawlik et al. 2017; Galan-Puchades et al. 2018), A. cantonenis (Aghazadeh et al. 2015a), G. neoplasticum (Galan-Puchades et al. 2018), T. taeniaeformis (Kataranovski et al. 2010), R. microstoma (Desvars-Larrive et al. 2017), R. nana (Dyk et al. 1975; McGarry et al. 2015; Galan-Puchades et al. 2018), H. diminuta (Easterbrook et al. 2007; Kataranovski et al. 2011; Franssen et al. 2016; Desvars-Larrive et al. 2017; Galan-Puchades et al. 2018), Brachylaima sp. (Desvars-Larrive et al. 2017) and M. moniliformis (Galan-Puchades et al. 2018). A summary of the main characteristics of these rat-borne helminthiases is provided in Supplementary Material 2.
Seven (30.4%) papers mentioned the potential impact of rat-borne helminthiases on domestic and wild animal health (Conlogue et al. 1979; Childs et al. 1988; Ceruti et al. 2001; Kataranovski et al. 2010; Aghazadeh et al. 2015a; McGarry et al. 2015; Bunkowska-Gawlik et al. 2017). Ceruti et al. (2001) mentioned that only one case of hepatic capillariasis (out of 500 dogs submitted to necropsy over a 10-year period) has been described in domestic carnivores in the study area of Milan, Italy, although the prevalence in urban rats was 36% (unpublished data).
Discussion
The present systematic review provides an up-to-date overview of the helminths of urban rats in developed countries. Most of the studies were conducted in Europe and investigated brown rats. Twenty-five helminth species were identified at the species level, of which almost two-third belonged to the phylum Nematoda. Calodium hepaticum was the most commonly reported helminth in urban rats, mostly because it was the most searched parasite. Some species have a restricted distribution range and were therefore seldom reported. For example, the geographic distribution of A. cantonensis is limited to Southeast Asia, the Pacific Islands, South and Central America, and the Caribbean although the parasite is emerging in USA, Australia (Barratt et al. 2016) but also in Europe in the Canary Islands (Martin-Alonso et al. 2011). Similarly, S. venezuelensis and O. ezoensis are restricted to warmer climates (Viney 2017) and Asia (Fukumoto and Ohbayashi 1985), respectively.
This review underlined that helminth infection is common in wild urban rats and that co-occurrence of multiple helminth species within the same host is frequent. Prevalence, helminth species richness and association, and risk factors of helminth occurrence in urban rats varied greatly between studies. Observed differences may be related to the study design, statistical methods and helminth species investigated. Observed differences may also reflect a high impact of the microenvironment and local climate on the helminth eco-epidemiology.
Pathological findings related to the presence of helminths in their rat hosts were generally minor, except for C. hepaticum, which can induce severe hepatic lesions. Positive association between host weight (often, but not always, used as a proxy for age) and prevalence or intensity of helminth infection may suggest that the longer a rat lives, the more likely it is to be parasitized by helminths and the more intense is the infection. The absence of seasonal variation in the prevalence of most helminth species of urban rats probably reflects that transmission occurs year-round. Indeed, warmer temperature and reduced seasonality in cities could enhance the persistence of parasite transmission stages in the environment (Bradley and Altizer 2007). In the near future, climate change and other anthropogenic stressors may induce a loss of parasite biodiversity (Cizauskas et al. 2017) while some parasitic species may adapt and even expand geographically (Blum and Hotez 2018). The distribution range of some geographically restricted helminth species (e.g. A. cantonensis, S. venezuelensis, and O. ezoensis) may be modified due to the expansion of suitable habitat but also introduction events of their intermediate and definitive hosts (York et al. 2014). Therefore, monitoring helminths of urban rats and identifying changes in the parasite-host system may provide insights into the micro-environmental changes occurring within the urban ecosystem.
Urban rats can be definitive, intermediate and sometimes reservoir host of zoonotic helminths (Meerburg et al. 2009; Himsworth et al. 2013). However, the veterinary and public health importance of urban rat helminthiases remains under debate. Symptoms of rat-borne helminthiasis in humans are usually mild or absent, and go unnoticed. Consequently, prevalence of rat-borne helminthiases in the urban population from developed countries as well as environmental sources of contamination remain largely unknown. However, within some cities in the developed countries, poor urban infrastructure and sanitation affect positively rat abundance and increase rat-human contact rate (Byers et al. 2019). People living within impoverished urban areas are therefore more at risk of rat-borne infections, with drug users, immunocompromised and homeless people being the most vulnerable (Delobel et al. 2004; Himsworth et al. 2013; McVea et al. 2018).
This review stresses important limitations regarding research on urban rat helminths. The main limitation lied in the methods of investigation. Field work with urban rats encounters several physical and societal constraints (Desvars-Larrive et al. 2018) which could affect parasitological studies, i.e. place(s) investigated, method(s) of capture, sample size or season of capture. Moreover, helminthological methods are manual and time-consuming; they require a deep and long post-mortem examination of the animal and the collection of each worm individually (or the histological inspection of the liver parenchyma for hepatic helminths). For example, if collection, preservation and staining are straightforward techniques that require minimal equipment, taxonomic identification, especially to species, can be very tedious. Furthermore, helminthology suffers from the lack of consistency in the Latin binomials used to name the species. Discordant use of synonyms for certain helminth species renders literature search and study comparison difficult.
In more than two thirds of the studies, we noticed a dramatic lack of literature references for species identification and absence of morphological description of the reported species. This is particularly necessary for helminths of zoonotic importance, some of which are difficult to readily identify morphologically, i.e. Moliniformis spp. (Guerreiro Martins et al. 2017), Hymenolepididae (Casanova et al. 2001; Macnish et al. 2002; Cunningham and Olson 2010; Haukisalmi et al. 2010) and A. cantonensis (Aghazadeh et al. 2015a). Overall, the lack of references to the identification keys, the lack of drawings or photographs, the lack of consensus on the taxonomy and names of some helminth species, and the seldom use of molecular tools question the accuracy of the helminth species identification in urban rats.
Conclusion: future research needs
In order to fill the knowledge gap on the helminths of urban rats, and within the framework of an ethical research, an exhaustive collection and systematic storage of biological material and data related to wild rats, captured (and most of the time euthanized) for scientific purposes, are highly recommended. In this perspective, an extensive inventory of all helminth species from the different organs should be performed for each captured animal. Methods of collection should be standardized as proposed in Sepulveda and Kinsella (2013). Reference to the morphological identification keys of each helminth species is essential. To ensure reproducibility and verification of the study, but also to promote the re-use of the biological material and data for further research, scientists are encouraged, for each helminth species, to (i) deposit a morphological voucher of a male and a female, fixed in formalin (those are then not usable for DNA analysis), in museum collections (Sepulveda and Kinsella 2013); (ii) conserve a voucher specimen in 80–90% ethanol for further DNA analysis. Each voucher specimen should be linked to genetic data deposited in an open-source database (Astrin et al. 2013; Morand 2018), e.g. GenBank (Benson et al. 2012) or BOLD (Ratnasingham and Hebert 2007); (iii) preserve and share raw data (e.g. dataset of morphological data, environmental data, and helminthological data) via open-source repositories.
Helminth species characterisation through traditional DNA sequencing is common, e.g. H. diminuta (Julius et al. 2018), R. straminea, R. microstoma, R. fraterna (Macnish et al. 2002; Haukisalmi et al. 2010), H. spumosa (Zaleśny et al. 2010; Ribas et al. 2013; Šnábel et al. 2014), G. neoplasticum (Setsuda et al. 2018), M. muris (Julius et al. 2018), N. brasiliensis (Julius et al. 2018) and S. muris (Julius et al. 2018). However, facing the limitations of the morphological identification, helminthological studies would highly benefit in the development and use of universal primers for metabarcoding approaches that could screen for multiple parasites concurrently and therefore enable faster, cheaper, more accurate and standardized characterisation of the helminth community within a host species (Tanaka et al. 2014; Aivelo and Medlar 2018).
In addition, the estimation of the parasite burden for each helminth species through count of the individuals, although extremely time-consuming, is essential to the understanding of the ecology of the parasites, in particular to the assessment of potential interspecies interaction within the Rattus host. Research on the dynamics and socio-environmental risk factors of human-rat-helminth interaction are missing although they are essential to the understanding of the eco-epidemiology of rat-borne helminthiases in urban ecosystems and to the implementation of surveillance and prevention programmes. Furthermore, few of the reviewed publications stressed the significance of rat-borne helminth infections for other urban animal species. In particular, some helminths of urban rats can infect domestic (dogs and cats) and urban wild carnivores (e.g. foxes) (Štěrba and Baruš 1976; Butcher et al. 1998; Butcher and Grove 2001; Macnish et al. 2003; Jrijer et al. 2015; Mobedi et al. 2017). Finally, there is an important lack of research on the potential use of urban rat helminths as bioindicator for environmental pollution. For example, the host-parasite system rat-H. diminuta can be used as a sentinel system to monitor lead contamination in urban ecosystems (Sures et al. 2003). Similarly, M. moniliformis, H. diminuta and larval stage of T. taenaeiformis could be used as bioindicators for assessing environmental pollution by heavy metals (Teimoori et al. 2014).
Overall, we identified several research gaps regarding urban rat helminthology in developed countries, which can be classified into five main topics: (1) species identification through molecular tools, (2) impact of helminth infections on the dynamics of urban rat populations, (3) veterinary and public health importance of rat-borne helminthiases, (4) impact of urbanisation on the helminth populations and (5) potential use of helminths of urban rats as bioindicators for environmental pollution in urban ecosystems.
Electronic supplementary material
Characteristics of the 23 publications included in the systematic review and extracted data (XLSX 55.1 kb)
Overview of the rat-borne helminthiases mentioned in the 23 reviewed publications and associated reference list (DOCX 62.3 kb)
Acknowledgements
We would like to thank Annika Posautz for her scientific expertise on the pathological aspects.
Availability of data and material
All data extracted from the reviewed papers during this study are included in this published article and its supplementary information file.
Authors’ contributions
Conceived of or designed study: DSG, ADL; Performed research: DSG, ADL; Analysed data: DSG, BP, ADL; Wrote the paper: DSG, BP, CW, ADL
Funding Information
Open access funding provided by University of Veterinary Medicine Vienna. ADL was financially supported by a postdoctoral fellowship (FU-282-PDC) provided by the University of Veterinary Medicine Vienna, Austria.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aghazadeh M, Jones MK, Aland KV, Reid SA, Traub RJ, McCarthy JS, Lee R. Emergence of neural angiostrongyliasis in eastern Australia. Vector Borne Zoonotic Dis. 2015;15:184–190. doi: 10.1089/vbz.2014.1622. [DOI] [PubMed] [Google Scholar]
- Aghazadeh M, Reid SA, Aland KV, Restrepo AC, Traub RJ, McCarthy JS, Jones MK. A survey of Angiostrongylus species in definitive hosts in Queensland. Int J Parasitol Parasites Wildl. 2015;4:323–328. doi: 10.1016/j.ijppaw.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aivelo T, Medlar A. Opportunities and challenges in metabarcoding approaches for helminth community identification in wild mammals. Parasitology. 2018;145:608–621. doi: 10.1017/S0031182017000610. [DOI] [PubMed] [Google Scholar]
- Albon SD, Stien A, Irvine RJ, Langvatn R, Ropstad E, Halvorsen O. The role of parasites in the dynamics of a reindeer population. Proc Biol Sci. 2002;269:1625–1632. doi: 10.1098/rspb.2002.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angley LP, Combs M, Firth C, Frye MJ, Lipkin I, Richardson JL, Munshi-South J. Spatial variation in the parasite communities and genomic structure of urban rats in New York City. Zoonoses Public Health. 2018;65:e113–e123. doi: 10.1111/zph.12418. [DOI] [PubMed] [Google Scholar]
- Aronson MFJ, Nilon CH, Lepczyk CA, Parker TS, Warren PS, Cilliers SS, Goddard MA, Hahs AK, Herzog C, Katti M, La Sorte FA, Williams NSG, Zipperer W. Hierarchical filters determine community assembly of urban species pools. Ecology. 2016;97:2952–2963. doi: 10.1002/ecy.1535. [DOI] [PubMed] [Google Scholar]
- Astrin JJ, Zhou X, Misof B (2013) The importance of biobanking in molecular taxonomy, with proposed definitions for vouchers in a molecular context. Zookeys:67–70. 10.3897/zookeys.365.5875 [DOI] [PMC free article] [PubMed]
- Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Marriott D, Harkness J, Ellis J, Stark D. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology. 2016;143:1087–1118. doi: 10.1017/S0031182016000652. [DOI] [PubMed] [Google Scholar]
- Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2012;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AJ, Hotez PJ. Global “worming”: climate change and its projected general impact on human helminth infections. PLoS Negl Trop Dis. 2018;12:e0006370–e0006370. doi: 10.1371/journal.pntd.0006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunkowska-Gawlik K, Perec-Matysiak A, Burzynska K, Hildebrand J. The molecular identification of Calodium hepaticum in the wild brown rat (Rattus norvegicus) in Poland. Acta Parasitol. 2017;62:728–732. doi: 10.1515/ap-2017-0087. [DOI] [PubMed] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583 [PubMed]
- Butcher AR, Grove DI. Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst Parasitol. 2001;49:211–221. doi: 10.1023/A:1010616920412. [DOI] [PubMed] [Google Scholar]
- Butcher AR, Parasuramar P, Thompson CS, Grove DI. First report of the isolation of an adult worm of the genus Brachylaima (Digenea: Brachylaimidae), from the gastrointestinal tract of a human. Int J Parasitol. 1998;28:607–610. doi: 10.1016/S0020-7519(97)84372-X. [DOI] [PubMed] [Google Scholar]
- Byers KA, Cox SM, Lam R, Himsworth CG. “They’re always there”: resident experiences of living with rats in a disadvantaged urban neighbourhood. BMC Public Health. 2019;19:853–853. doi: 10.1186/s12889-019-7202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable J, Barber I, Boag B, Ellison AR, Morgan ER, Murray K, Pascoe EL, Sait SM, Wilson AJ, Booth M. Global change, parasite transmission and disease control: lessons from ecology. Philos Trans R Soc Lond Ser B Biol Sci. 2017;372:20160088. doi: 10.1098/rstb.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JC, Santalla F, Durand P, Vaucher C, Feliu C, Renaud F. Morphological and genetic differentiation of Rodentolepis straminea (Goeze, 1752) and Rodentolepis microstoma (Dujardin, 1845) (Hymenolepididae) Parasitol Res. 2001;87:439–444. doi: 10.1007/s004360100379. [DOI] [PubMed] [Google Scholar]
- Cavia R, Cueto GR, Suárez OV. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landsc Urban Plan. 2009;90:11–19. doi: 10.1016/j.landurbplan.2008.10.017. [DOI] [Google Scholar]
- Ceruti R, Sonzogni O, Origgi F, Vezzoli F, Cammarata S, Giusti AM, Scanziani E. Capillaria hepatica infection in wild brown rats (Rattus norvegicus) from the urban area of Milan, Italy. J Vet Med B Infect Dis Vet Public Health. 2001;48:235–240. doi: 10.1046/j.1439-0450.2001.00436.x. [DOI] [PubMed] [Google Scholar]
- Childs JE, Glass GE, Korch GW., Jr The comparative epizootiology of Capillaria hepatica (Nematoda) in urban rodents from different habitats of Baltimore, Maryland. Can J Zool. 1988;66:2769–2775. doi: 10.1139/z88-404. [DOI] [Google Scholar]
- Cizauskas CA, Carlson CJ, Burgio KR, Clements CF, Dougherty ER, Harris NC, Phillips AJ. Parasite vulnerability to climate change: an evidence-based functional trait approach. Royal Soc Open Sci. 2017;4:160535–160535. doi: 10.1098/rsos.160535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlogue G, Foreyt W, Adess M, Levine H. Capillaria hepatica (Bancroft) in select rat populations of Hartford, Connecticut, with possible public health implications. J Parasitol. 1979;65:105–108. doi: 10.2307/3280211. [DOI] [PubMed] [Google Scholar]
- Costantini D, Greives TJ, Hau M, Partecke J. Does urban life change blood oxidative status in birds? J Exp Biol. 2014;217:2994–2997. doi: 10.1242/jeb.106450. [DOI] [PubMed] [Google Scholar]
- Crompton DWT, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- Cunningham LJ, Olson PD. Description of Hymenolepis microstoma (Nottingham strain): a classical tapeworm model for research in the genomic era. Parasit Vectors. 2010;3:123–123. doi: 10.1186/1756-3305-3-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel P, Signate A, El Guedj M, Couppie P, Gueye M, Smadja D, Pradinaud R. Unusual form of neurocysticercosis associated with HIV infection. Eur J Neurol. 2004;11:55–58. doi: 10.1046/j.1351-5101.2003.00696.x. [DOI] [PubMed] [Google Scholar]
- Desvars-Larrive A, Baldi M, Walter T, Zink R, Walzer C. Brown rats (Rattus norvegicus) in urban ecosystems: are the constraints related to fieldwork a limit to their study? Urban Ecosyst. 2018;21:951–964. doi: 10.1007/s11252-018-0772-8. [DOI] [Google Scholar]
- Desvars-Larrive A, Pascal M, Gasqui P, Cosson J-F, Benoît E, Lattard V, Crespin L, Lorvelec O, Pisanu B, Teynié A, Vayssier-Taussat M, Bonnet S, Marianneau P, Lacôte S, Bourhy P, Berny P, Pavio N, Le Poder S, Gilot-Fromont E, Jourdain E, Hammed A, Fourel I, Chikh F, Vourc’h G. Population genetics, community of parasites, and resistance to rodenticides in an urban brown rat (Rattus norvegicus) population. PLoS One. 2017;12:e0184015. doi: 10.1371/journal.pone.0184015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyk V, Tilc K, Hanuskova Z. Endoparasites of the rat (Rattus norvegicus, Berkenhout, 1769) in old and modern city districts and in the zoological garden. Acta Vet Brno. 1975;44:245–251. [Google Scholar]
- Easterbrook J, Kaplan J, Vanasco N, Reeves W, Purcell R, Kosoy M, Glass G, Watson J, Klein S. A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiol Infect. 2007;135:1192–1199. doi: 10.1017/S0950268806007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang-Azad A. Ecology of Capillaria hepatica (Bancroft 1893) (Nematoda). 1. Dynamics of infection among Norway rat populations of the Baltimore zoo, Baltimore, Maryland. J Parasitol. 1977;63:117–122. doi: 10.2307/3280114. [DOI] [PubMed] [Google Scholar]
- Franssen F, Swart A, van Knapen F, van der Giessen J (2016) Helminth parasites in black rats (Rattus rattus) and brown rats (Rattus norvegicus) from different environments in the Netherlands. Infect Ecol Epidemiol 6. 10.3402/iee.v3406.31413 [DOI] [PMC free article] [PubMed]
- French SS, Fokidis HB, Moore MC. Variation in stress and innate immunity in the tree lizard (Urosaurus ornatus) across an urban-rural gradient. J Comp Physiol B. 2008;178:997–1005. doi: 10.1007/s00360-008-0290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeschke G, Matthee S. Landscape characteristics influence helminth infestations in a peri-domestic rodent - implications for possible zoonotic disease. Parasit Vectors. 2014;7:393. doi: 10.1186/1756-3305-7-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S-i, Ohbayashi M. Variations of synlophe of Orientostrongylus ezoensis Tada, 1975 (Nematoda: Heligmonellidae) among different populations in Japan. Jpn J Vet Res. 1985;33:27–43. [PubMed] [Google Scholar]
- Galan-Puchades MT, Sanxis-Furio J, Pascual J, Bueno-Mari R, Franco S, Peracho V, Montalvo T, Fuentes MV. First survey on zoonotic helminthosis in urban brown rats (Rattus norvegicus) in Spain and associated public health considerations. Vet Parasitol. 2018;259:49–52. doi: 10.1016/j.vetpar.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Gordon CA, McManus DP, Jones MK, Gray DJ, Gobert GN (2016) Chapter six—the increase of exotic zoonotic helminth infections: the impact of urbanization, climate change and globalization. In: Rollinson D, Stothard JR (eds) Advances in parasitology, vol 91. Academic Press, pp 311–397. 10.1016/bs.apar.2015.12.002 [DOI] [PubMed]
- Guerreiro Martins NB, del Rosario RM, Navone GT. A new species of Moniliformis from a Sigmodontinae rodent in Patagonia (Argentina) Parasitol Res. 2017;116:2091–2099. doi: 10.1007/s00436-017-5508-9. [DOI] [PubMed] [Google Scholar]
- Haukisalmi V, Hardman LM, Foronda P, Feliu C, Laakkonen J, Niemimaa J, Lehtonen JT, Henttonen H. Systematic relationships of hymenolepidid cestodes of rodents and shrews inferred from sequences of 28S ribosomal RNA. Zoll Scr. 2010;39:631–641. doi: 10.1111/j.1463-6409.2010.00444.x. [DOI] [Google Scholar]
- Himsworth CG, Parsons KL, Jardine C, Patrick DM. Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. 2013;13:349–359. doi: 10.1089/vbz.2012.1195. [DOI] [PubMed] [Google Scholar]
- Horsák M, Lososová Z, Čejka T, Juřičková L, Chytrý M. Diversity and biotic homogenization of urban land-snail faunas in relation to habitat types and macroclimate in 32 central European cities. PLoS One. 2013;8:e71783. doi: 10.1371/journal.pone.0071783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson PJ, Dobson AP, Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327. 10.1126/science.aam8327 [DOI] [PubMed]
- Jrijer J, Bordes F, Morand S, Neifar L. A survey of nematode parasites of small mammals in Tunisia, North Africa: diversity of species and zoonotic implications. Comp Parasitol. 2015;82(204–210):207–210. doi: 10.1654/4767.1. [DOI] [Google Scholar]
- Julius RS, Schwan EV, Chimimba CT. Molecular characterization of cosmopolitan and potentially co-invasive helminths of commensal, murid rodents in Gauteng Province, South Africa. Parasitol Res. 2018;117:1729–1736. doi: 10.1007/s00436-018-5852-4. [DOI] [PubMed] [Google Scholar]
- Kataranovski M, Mirkov I, Belij S, Popov A, Petrović Z, Gačić Z, Kataranovski D. Intestinal helminths infection of rats (Ratus norvegicus) in the Belgrade area (Serbia): the effect of sex, age and habitat. Parasite. 2011;18:189–196. doi: 10.1051/parasite/2011182189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataranovski M, Zolotarevski L, Belij-Rammerstorfer S, Mirkov I, Stosic J, Popov Aleksandrov A, Kataranovski D. First record of Calodium hepaticum and Taenia taeniaeformis liver infection in wild Norway rats (Rattus norvegicus) in Serbia. Arch Biol Sci. 2010;62:431–440. doi: 10.2298/ABS1002431K. [DOI] [Google Scholar]
- Macnish MG, Morgan-Ryan UM, Monis PT, Behnke JM, Thompson RC. A molecular phylogeny of nuclear and mitochondrial sequences in Hymenolepis nana (Cestoda) supports the existence of a cryptic species. Parasitology. 2002;125:567–575. doi: 10.1017/S0031182002002366. [DOI] [PubMed] [Google Scholar]
- Macnish MG, Ryan UM, Behnke JM, Thompson RCA. Detection of the rodent tapeworm Rodentolepis (=Hymenolepis) microstoma in humans. A new zoonosis? Int J Parasitol. 2003;33:1079–1085. doi: 10.1016/S0020-7519(03)00137-1. [DOI] [PubMed] [Google Scholar]
- Martin-Alonso A, Foronda P, Quispe-Ricalde MA, Feliu C, Valladares B. Seroprevalence of Angiostrongylus cantonensis in wild rodents from the Canary Islands. PLoS One. 2011;6:e27747–e27747. doi: 10.1371/journal.pone.0027747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JW, Higgins A, White NG, Pounder KC, Hetzel U. Zoonotic helminths of urban brown rats (Rattus norvegicus) in the UK: neglected public health considerations? Zoonoses Public Health. 2015;62:44–52. doi: 10.1111/zph.12116. [DOI] [PubMed] [Google Scholar]
- McIntyre NE, Knowles-Yánez K, Hope D. Urban ecology as an interdisciplinary field: differences in the use of “urban” between the social and natural sciences. Urban Ecosyst. 2000;4:5–24. doi: 10.1023/A:1009540018553. [DOI] [Google Scholar]
- McKinney ML. Urbanization as a major cause of biotic homogenization. Biol Conserv. 2006;127:247–260. doi: 10.1016/j.biocon.2005.09.005. [DOI] [Google Scholar]
- McKinney ML. Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 2008;11:161–176. doi: 10.1007/s11252-007-0045-4. [DOI] [Google Scholar]
- McVea DA, Himsworth CG, Patrick DM, Lindsay LR, Kosoy M, Kerr T. Exposure to rats and rat-associated Leptospira and Bartonella species among people who use drugs in an impoverished, inner-city neighborhood of Vancouver, Canada. Vector Borne Zoonotic Dis. 2018;18:82–88. doi: 10.1089/vbz.2017.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- Meillère A, Brischoux F, Parenteau C, Angelier F. Influence of urbanization on body size, condition, and physiology in an urban exploiter: a multi-component approach. PLoS One. 2015;10:e0135685. doi: 10.1371/journal.pone.0135685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo C, Ribas A, Casanova JC, Cagnin M, Geraci F, Di Bella C. Helminths of the brown rat (Rattus norvegicus) (Berkenhout, 1769) in the city of Palermo, Italy. Helminthologia. 2010;47:238–240. doi: 10.2478/s11687-010-0037-4. [DOI] [Google Scholar]
- Millán J, Chirife AD, Proboste T, Velarde R. Factors associated with the prevalence and pathology of Calodium hepaticum and C. splenaecum in periurban micromammals. Parasitol Res. 2014;113:3001–3006. doi: 10.1007/s00436-014-3962-1. [DOI] [PubMed] [Google Scholar]
- Mobedi I, Fakhar M, Irshadullah M, Rahimi-Esboei B, Gholami S, Fraija-Fernández N. New record of Brachylaima sp. (Digenea: Brachylaimidae) from a stray dog in North Iran. Iran J Parasitol. 2017;12:606–613. [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand S. Advances and challenges in barcoding of microbes, parasites, and their vectors and reservoirs. Parasitology. 2018;145:537–542. doi: 10.1017/S0031182018000884. [DOI] [PubMed] [Google Scholar]
- Mouritsen KN, Poulin R. Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology. 2002;124:101–117. doi: 10.1017/S0031182002001476. [DOI] [PubMed] [Google Scholar]
- Partecke J, Schwabl I, Gwinner E. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology. 2006;87:1945–1952. doi: 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Prange S, Gehrt SD, Wiggers EP. Demographic factors contributing to high raccoon densities in urban landscapes. J Wildl Manag. 2003;67:324–333. doi: 10.2307/3802774. [DOI] [Google Scholar]
- QGIS Development Team (2018) QGIS geographic information system. Open Source Geospatial Foundation Project. Version: 3.4.5. http://www.qgis.org/
- Ratnasingham S, Hebert PDN (2007) Bold: the barcode of life data system (http://www.barcodinglife.org). Mol Ecol Notes 7:355–364. 10.1111/j.1471-8286.2007.01678.x [DOI] [PMC free article] [PubMed]
- Ribas A, Bellocq J, Ros A, Ndiaye P, Miquel J. Morphometrical and genetic comparison of two nematode species: H. spumosa and H. dahomensis (Nematoda, Heterakidae) Acta Parasitol. 2013;58:389–398. doi: 10.2478/s11686-013-0156-4. [DOI] [PubMed] [Google Scholar]
- Rothenburger JL, Himsworth CG, Chang V, LeJeune M, Leighton FA. Capillaria hepatica in wild Norway rats (Rattus norvegicus) from Vancouver, Canada. J Wildl Dis. 2014;50:628–633. doi: 10.7589/2013-09-256. [DOI] [PubMed] [Google Scholar]
- Rothenburger JL, Himsworth CG, La Perle KMD, Leighton FA, Nemeth NM, Treuting PM, Jardine CM. Pathology of wild Norway rats in Vancouver, Canada. J Vet Diagn Investig. 2019;31:184–199. doi: 10.1177/1040638719833436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenburger JL, Himsworth CG, Lejeune M, Treuting PM, Leighton FA. Lesions associated with Eucoleus sp. in the non-glandular stomach of wild urban rats (Rattus norvegicus) International Journal for Parasitology Parasites and Wildlife. 2014;3:95–101. doi: 10.1016/j.ijppaw.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda MS, Kinsella JM (2013) Helminth collection and identification from wildlife. J Vis Exp:e51000–e51000. 10.3791/51000 [DOI] [PMC free article] [PubMed]
- Setsuda A, Ribas A, Chaisiri K, Morand S, Chou M, Malbas F, Yunus M, Sato H. Molecular genetic diversity of Gongylonema neoplasticum (Fibiger & Ditlevsen, 1914) (Spirurida: Gongylonematidae) from rodents in Southeast Asia. Syst Parasitol. 2018;95:235–247. doi: 10.1007/s11230-018-9778-0. [DOI] [PubMed] [Google Scholar]
- Shintoku Y, Kimura E, Kadosaka T, Hasegawa H, Kondo S, Itoh M, Islam MZ. Strongyloides ratti infection in the large intestine of wild rats, Rattus norvegicus. J Parasitol. 2005;91:1116–1121. doi: 10.1645/GE-3439.1. [DOI] [PubMed] [Google Scholar]
- Sitcharungsi R, Sirivichayakul C. Allergic diseases and helminth infections. Pathog Glob Health. 2013;107:110–115. doi: 10.1179/2047773213Y.0000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VS. Are vesical calculi associated with Trichosomoides crassicauda, the common bladder nematode of rats? J Parasitol. 1946;32:142–149. doi: 10.2307/3272587. [DOI] [PubMed] [Google Scholar]
- Šnábel V, Utsuki D, Kato T, Sunaga F, Ooi H-K, Gambetta B, Taira K. Molecular identification of Heterakis spumosa obtained from brown rats (Rattus norvegicus) in Japan and its infectivity in experimental mice. Parasitol Res. 2014;113:3449–3455. doi: 10.1007/s00436-014-4014-6. [DOI] [PubMed] [Google Scholar]
- Štěrba J, Baruš V. First record of Strobilocercus fasciolaris (Taeniidae - larvae) in man. Folia Parasitol. 1976;23:221–226. [PubMed] [Google Scholar]
- Strand TM, Lundkvist Å. Rat-borne diseases at the horizon. A systematic review on infectious agents carried by rats in Europe 1995-2016. Infect Ecol Epidemiol. 2019;9:1553461–1553461. doi: 10.1080/20008686.2018.1553461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sures B, Scheible T, Bashtar AR, Taraschewski H. Lead concentrations in Hymenolepis diminuta adults and Taenia taeniaeformis larvae compared to their rat hosts (Rattus norvegicus) sampled from the city of Cairo, Egypt. Parasitology. 2003;127:483–487. doi: 10.1017/S0031182003003901. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Hino A, Tsai IJ, Palomares-Rius JE, Yoshida A, Ogura Y, Hayashi T, Maruyama H, Kikuchi T. Assessment of helminth biodiversity in wild rats using 18S rDNA based metagenomics. PLoS One. 2014;9:e110769. doi: 10.1371/journal.pone.0110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teimoori S, Sabour Yaraghi A, Makki MS, Shahbazi F, Nazmara S, Rokni MB, Mesdaghinia A, Salahi Moghaddam A, Hosseini M, Rakhshanpour A, Mowlavi G. Heavy metal bioabsorption capacity of intestinal helminths in urban rats. Iran J Public Health. 2014;43:310–315. [PMC free article] [PubMed] [Google Scholar]
- Tompkins DM, Dunn AM, Smith MJ, Telfer S. Wildlife diseases: from individuals to ecosystems. J Anim Ecol. 2011;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- United Nations (2016) Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. United Nations. https://unstats.un.org/unsd/methods/m49/m49regin.htm#developed. Accessed 21/02/2017 2017
- United Nations . The 2018 Revision. New York: United Nations; 2018. World Urbanization Prospects. [Google Scholar]
- Viney M. Strongyloides. Parasitology. 2017;144:259–262. doi: 10.1017/S0031182016001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. New York DOI: Springer-Verlag; 2016. [Google Scholar]
- World Health Organization . Research priorities for Helminth infections. Italy: World Health Organization; 2012. [PubMed] [Google Scholar]
- Yokota M, Hashimoto M, Matsui T, Oku N, Kida M, Ishikawa K, Miyagawa H, Ohnaka T, Nakajima K. A nematode, Orientostrongylus ezoensis, from brown rats in Sakai, Osaka prefecture. J Vet Med Sci. 1991;53:159–160. doi: 10.1292/jvms.53.159. [DOI] [PubMed] [Google Scholar]
- York EM, Butler CJ, Lord WD. Global decline in suitable habitat for Angiostrongylus ( = Parastrongylus) cantonensis: the role of climate change. PLoS One. 2014;9:e103831–e103831. doi: 10.1371/journal.pone.0103831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleśny G, Hildebrand J, Popiołek M. Molecular identification of Heterakis spumosa Schneider, 1866 (Nematoda: Ascaridida: Heterakidae) with comparative analysis of its occurrence in two mice species. Annal Zool. 2010;60:647–655. doi: 10.3161/000345410X550517. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the 23 publications included in the systematic review and extracted data (XLSX 55.1 kb)
Overview of the rat-borne helminthiases mentioned in the 23 reviewed publications and associated reference list (DOCX 62.3 kb)
Data Availability Statement
All data extracted from the reviewed papers during this study are included in this published article and its supplementary information file.