Abstract
Cell-based drug delivery systems have generated an increasing interest in recent years. We previously demonstrated that systemically administered macrophages deliver therapeutics to CNS, including glial cell line-derived neurotrophic factor (GDNF), and produce potent effects in Parkinson’s disease (PD) mouse models. Herein, we report fundamental changes in biodistribution and brain bioavailability of macrophage-based formulations upon different routes of administration: intravenous, intraperitoneal, or intrathecal injections. The brain accumulation of adoptively transferred macrophages was evaluated by various imaging methods in transgenic Parkin Q311(X)A mice and compared with those in healthy wild type littermates. Neuroinflammation manifested in PD mice warranted targeting macrophages to the brain for each route of administration. The maximum amount of cell-carriers in the brain, up to 8.1% ID/g, was recorded followed a single intrathecal injection. GDNF-transfected macrophages administered through intrathecal route provided significant increases of GDNF levels in different brain sub-regions, including midbrain, cerebellum, frontal cortex, and pons. No significant offsite toxicity of the cell-based formulations in mouse brain and peripheral organs was observed. Overall, intrathecal injection appeared to be the optimal administration route for genetically modified macrophages, which accomplished targeted gene delivery, and significant expression of reporter and therapeutic genes in the brain.
Subject terms: Biotechnology, Medical research
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder in people over 65 years old, and one of the leading sources of disability affecting one million people in the US1. Furthermore, it is projected to reach 1.24 million in 2030. PD is characterized by the lack of the neurotransmitter, dopamine, due to a loss of dopaminergic neurons within the Substantia Nigra parts compacta (SNpc). Regrettably, there are no effective treatments or cures known. Currently available treatments provide only short-term benefits that is followed by the increased neurodegeneration and resistance to the existing therapies. Moreover, offsite toxicity leads to multiple complications, including dyskinesia, anxiety, depression, fatigue and pain2. In particular, the most common treatment of PD with L-DOPA that aims to increase the levels of dopamine in striatum may result in some improvements in motor functions at the beginning followed by fast decreases in dopaminergic activity and progression of the disease3. Clearly, the new treatment paradigms for nervous system disorders, and specifically, for PD to stop and reverse neurodegeneration must be developed.
PD is associated with brain inflammation4, microglia activation, and oxidative stress, all of which affect neuronal, astrocyte, and microglia survival by activating apoptotic processes5–8, and leading to neuronal demise9. Several lines of evidence suggest that mitochondrial dysfunction and oxidative stress have a central role in the dopaminergic neurodegeneration of PD10. In fact, the majority of CNS disorders have in common an inflammatory component11. In this respect, specialized cells of the immune system, including monocytes, macrophages, stem cells, and T cells, exhibit an intrinsic homing property of migrating toward the sites of injury, inflammation and tumor via the processes known as diapedesis and chemotaxis12. Thus, immunocytes are reported to cause blood–brain barrier (BBB) breakdown following brain inflammation13–16 trafficking primarily between adjacent endothelial cells, i.e. paracellulary through the junctional complexes17,18. Even in the healthy brain, perivascular macrophages, which reside on the parenchymal side of endothelial cells, originate from circulating phagocytes, monocytes and macrophages. It is well documented that under pathological conditions, the rate of transport of immunocytes to the inflamed brain tissues is further elevated14–16,19–22. In particular, these cells have shown a remarkable capability to cross an intact BBB with 80% turnover in 3 months23,24. Such frequent migration offers a unique opportunity to design novel cell-based drug formulations.
Two approaches are developed in the field of cell-mediated therapeutics. First approach utilizes the host cells that are loaded with a drug, usually incorporated into a protective container, and then carry the drug to the disease side25–29. Importantly, immunocytes have a high rate of endocytosis that allows efficient accumulation of micro- and nanoparticles with incorporated drugs within intracytoplasmic endosomes. These drugs are subsequently released from the cell-carriers through the recycling processes that include exocytosis. However, one of the main obstacles of this approach is efficient disintegration of the entrapped foreign particles by monocytes. Furthermore, loading of cytotoxic anticancer compounds requires the exceptionally strong protection of cell-carriers against their toxic cargo.
Second approach is based on the genetic modification of living cells to overexpress therapeutic molecules30–32. In particular, neural stem cells (NSC) were transfected to express vascular endothelial growth factor (VGEF), or glial cell-line neurotrophic factor (GDNF), or neurturin (NTN), and examined for the brain delivery of these therapeutics to treat PD33–35. Furthermore, bone marrow-derived macrophages (BMM) and microglia were also used in PD mouse models36,37. Specifically, BMM were transduced ex vivo with lentivirus to express GDNF, and adaptively transferred to recipient mice. GDNF-BMM decreased neuroinflammation and neurodegeneration that leaded to regeneration of axons in SNpc and improved motor functions in PD mice36. Likewise, genetically modified NSC were investigated for the delivery of neurotrophic factors38–42 to treat Alzheimer’s disease. Clearly, drug delivery systems based on living cells could serve as a new therapeutic approach.
Overall, drug delivery systems based on living cells can act as “Trojan horses” carrying concealed drug cargoes across impermeable barriers, such as the BBB or blood-tumor barrier, to the disease sites. These features make immunocytes attractive candidates for the CNS drug delivery. The neuroinflammation developed in the course of disease serves as a cue for recruitment of immune cells from periphery to the brain, providing the necessary spatial, temporal, and dosage control at targeted tissues.
Based on this exciting and promising therapeutic concept, we developed a novel platform technology for brain delivery of therapeutic agents, in which monocytes/macrophages are loaded ex vivo with therapeutics, and adoptively transferred to a disease-affected animal. Following adoptive transfer, drug-loaded macrophages accumulate at the disease tissues in the brain and exert therapeutic effect. We demonstrated the potential of this approach for the treatment of PD using diverse therapeutics (proteins and recombinant DNA) delivered within macrophages in toxin-induced PD mouse models43–48, and transgenic Parkin Q311X(A) mice49. In these prior studies, autologous bone marrow-derived macrophages (BMM) were administered into the blood stream by intravenous (i.v.) injections. The present study evaluates whether the route of systemic administration can alter the biodistribution and brain bioavailability of the cell-carriers in transgenic Parkin Q311X(A) mouse model. To assess brain and peripheral organ distribution of BMM in living mice, we used different imaging techniques, including In vivo Imaging System (IVIS), Positron Emission Tomography (PET), and Magnetic Resonance Imaging (MRI). We convincingly demonstrated significant brain accumulation of BMM injected through different administration routes in Parkin Q311X(A) mice. Importantly, the intrathecal (i.t.) route resulted in the superior BMM brain accumulation when compared to intravenous (i.v.) or intraperitoneal (i.p.) administrations. We hypothesized that the mild brain inflammation manifested in the transgenic PD mouse model warranted targeted gene delivery to the brain. We further evaluated the delivery of reporter and therapeutic genes to the brain in Parkin Q311X(A) mice by genetically-modified BMM. For the first time, we report significant increases in the expression of GDNF brain levels after a single i.t. injection of GDNF-transfected macrophages, providing additional evidence for clinical relevance of this cell-based delivery system for PD treatment.
Results
Biodistribution of autologous macrophages in Parkin Q311(X)A mice by bioluminescence imaging
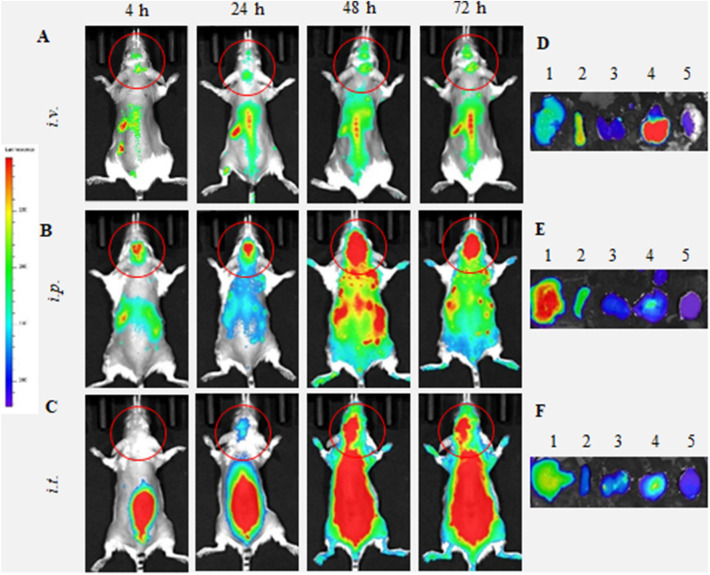
We evaluated i.v., i.p., and i.t. administration routes of BMM in Parkin Q311(X)A mice by IVIS (Fig. 1). To visualize the cell-carriers, their lipid membranes were labeled with a hydrophobic dye, DIR (DiIC18(7); 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide). In this experiment, we used maximal dose and volume of cell suspension allowed for each route of administration (specifically, 4 × 106 cells/200 µL/mouse for i.v. and i.p. injections, and 1 × 106 cells/50 µL/mouse for i.t. injections). These experimental conditions were chosen to replicate following therapeutic efficacy investigations. Significant levels of DIR-BMM were recorded in PD mouse brain at 24–72 h time frame for all three routes of administration (Figs. 1A–C). Lower fluorescence signals during the first hours in dorsal images were likely due to the fact that most DIR-BMM circulated in the bloodstream and were accumulated in main excretion organs, liver, spleen, and kidney, as seen in Supplementary Figure S1.
Figure 1.
Biodistribution of DIR-labeled BMM in Parkin Q311(X)A mice by IVIS. DIR-labeled BMM were administered in PD mice (12 mo. of age) (A, D) i.v. (4 × 106 cells/200 µL), (B, E) i.p. (4 × 106 cells/200 µL), or (C, F) i.t. (1 × 106 cells/50 µL). Images were recorded (A–C) in live animals at different time points, and (D–F) in main organs, liver (1), spleen (2), kidney (3), lungs (4), and brain (5), collected at the endpoint (72 h). Prone representative images show DIR signal accumulation in the brain for all administration routes examined, especially at 24–72 h. Accumulation of labeled macrophages was also observed in the main peripheral organs.
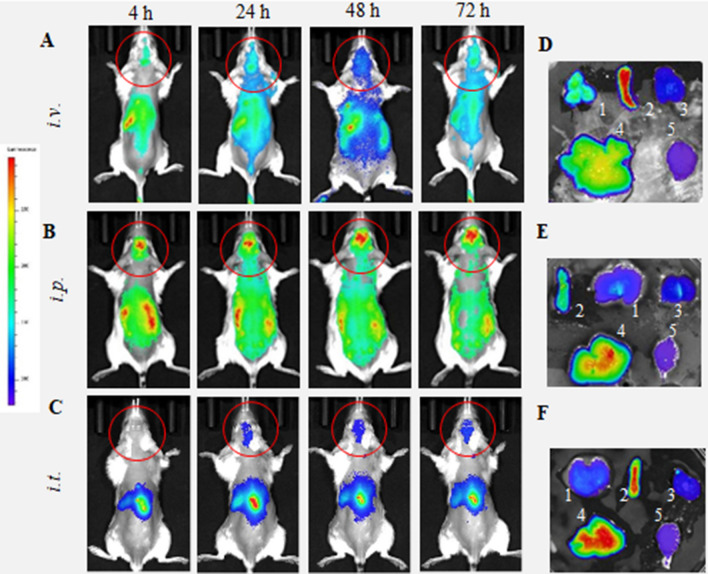
We also administered same doses of DIR-BMM in the age-matched wild type (WT) healthy mice (Fig. 2). DIR-BMM fluorescence was recorded in the brain of WT mice at 24–72 h after i.v. and i.p. injections (Fig. 2A–C). Very little if any brain fluorescence was recorded in the brain in healthy animals after i.t. administration of DIR-BMM. Additional supine images of WT littermates injected with DIR-BMM are also shown on Supplementary Figure S2.
Figure 2.
Biodistribution of DIR-labeled BMM in healthy mice by IVIS. DIR-labeled BMM were administered in healthy mice (12 mo. of age) (A, D) i.v. (4 × 106 cells/200 µL), (B, E) i.p. (4 × 106 cells/200 µL), or (C, F) i.t. (1 × 106 cells/50 µL). Images were recorded (A–C) in live animals at different time points, and (D–F) in main organs, liver (1), spleen (2), kidney (3), lungs (4), and brain (5), collected at the endpoint (72 h). Prone representative images of animals i.v., and i.p. suggest that DIR-BMM accumulate in the brain, although to a much lesser extent than same treatments in Parkin Q311(X)A mice (Fig. 1). Little if any DIR signal was observed in live animals after i.t. administration in healthy animals. Accumulation of labeled macrophages was also observed in the main peripheral organs.
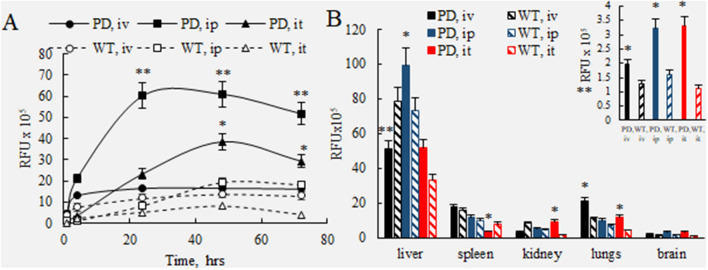
The live imaging data in PD and WT mice was quantified by IVIS Aura software (Fig. 3). For all routes of administration, the signals of DIR-BMM in the brain in living PD mice (Fig. 3A, filled symbols) were significantly greater than those in healthy WT littermates (Fig. 3A, empty symbols) throughout the entire observation period. Individual values of the DIR-BMM fluorescence for each animal are presented in Supplementary Tables S1 and S2. Remarkably, signals of DIR-BMM in the PD mouse brain at later time points (48–72 h) were significantly greater (p < 0.05) after i.p. and i.t. injections (Fig. 3A, filled squares and triangles, respectively), when compared to the signal observed after the i.v. injections (Fig. 3A, filled circles). Keeping in mind that the i.t. injected dose of DIR-BMM was four times less than those injected via i.p. and i.v. routes, the i.t. administration appears to be preferred for macrophage-based CNS delivery.
Figure 3.
Quantification of BMM accumulation in the main organs of PD and WT mice. Parkin Q311(X)A mice (12 mo. of age) were injected with DIR-BMM using different routes of administration and imaged by IVIS. Wild type mice were used as healthy controls. (A) Quantification of fluorescence levels in the brain area of PD mice (filled symbols) or WT animals (empty symbols) injected with DIR-BMM through i.v. (circles), i.p. (squares), or i.t. (triangles). The highest levels were detected in PD mice with i.p. and i.t. administrations at 24–72 h time frame. Accumulation levels of BMM in PD mouse brain were significantly greater than those in WT littermates. (B) At the endpoint (72 h), mice were sacrificed, perfused, and fluorescent levels of main organs (i.e. liver, spleen, kidney, lungs, and brain) were assessed by IVIS Aura software. The highest BMM accumulation was recorded in liver, spleen, and lungs. The amount of cell-carriers was significantly greater in the brain area of PD mice compared with WT littermates (insert). Values are the means ± SEM (N = 4), *p < 0.05; **p < 0.005 compared with WT healthy mice; #p < 0.05 compared to PD mice injected through i.v. route. Individual data points shown in Supplementary Tables S1 and S2.
The mice were sacrificed at the endpoint (72 h), perfused to eliminate the blood, and the main organs were imaged by IVIS for PD (Fig. 1D–F) and WT groups (Fig. 2D–F). The quantification of fluorescence levels at necropsy suggested that the fluorescence signal of DIR-BMM decreased in the row: liver > lungs ≅ spleen > kidney > brain (Fig. 3B). For all routes of administration the greatest DIR-BMM signal was detected in the liver, although, considerable signal was also seen in the brain (Figs. 1B, 3A), where DIR-BMM could possibly transport via the lymphatic system50. Significantly greater accumulation of DIR-BMM in the PD mouse brain were detected compared with their WT littermates, especially in case of i.t. and i.p. injections (Fig. 3B, insert). These results suggest that systemically administered macrophages migrate to the sites of inflammation and accumulate in the brain of transgenic Parkin Q311(X)A mice that is in good agreement with our earlier observations made in PD mice with acute toxin-induced brain inflammation43–48. One should keep in mind that quantification IVIS images of isolated organs reflects total fluorescence of whole organ, therefore high fluorescence count in liver may be not only due to the greater accumulation of DIR-BMM in this organ, but also to the overall larger liver mass compared to other organs. In fact, when fluorescent accumulation levels were normalized to the mass of the excited organ (RFU/g), the highest BMM uptake was observed in the spleen and lungs (Supplementary Table S3).
Biodistribution of 64Cu-labeled macrophages in Parkin Q311(X)A mice by PET
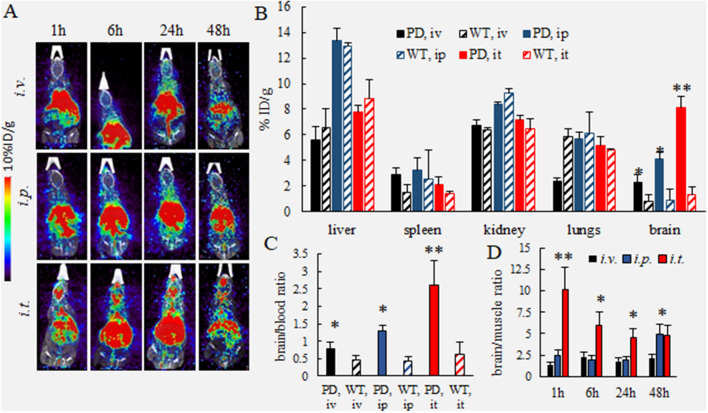
Next, biodistribution of adoptively transferred cell-carriers in PD and WT mice was studied by PET imaging, a highly sensitive technique that detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide. For this purpose, autologous BMM were labeled with 64Cu, and PD or WT littermates received a single injection of 64Cu-BMM (48.0 ± 2.8 µCi/1 × 106 cells/50 µL/mouse, N = 4) through i.v., i.p., or i.t. routes. Unlike the IVIS experiments described above, the comparable dose of 64Cu-labeled macrophages was used for all three administration routes in this experimental setup. The representative images of PD mice injected with 64Cu-BMM through i.v., i.p., and i.t. routes at various time points (up to 48 h) are shown on Fig. 4A. 64Cu-BMM brain levels for each administration route are shown in Supplementary Table S4. To quantify 64Cu-BMM levels, PD and WT mice with a single injection of radioactively-labeled macrophages were sacrificed at the endpoint (48 h), perfused, main organs and blood were harvested, and radioactivity levels were assessed using gamma counter (Fig. 4B). Individual values of the injected dose (%ID/g) for each animal are presented in Supplementary Tables S5–S7. Consistent with the results of IVIS experiments, the levels of 64Cu-BMM in the brain of PD mice with i.t. injections were significantly greater than those in i.v. and i.p. injected mice (Fig. 4A, B). Furthermore, the amount of BMM accumulated in PD mouse brain increased in a row: i.v. < i.p. < i.t. and for all injection routes was significantly greater than in WT littermates (Fig. 4B). The superior brain bioavailability for adoptively transferred macrophages, up to 8.1% of injected dose/g, was confirmed for PD mice at 48 h after a single i.t. injection. Of note, the brain/blood ratio in mice with i.t. injected macrophages also was significantly greater than in those treated i.v. and i.p. (Fig. 4C). Moreover, for all three administration routes, the brain/blood ratios of 64Cu-BMM in PD mice were significantly greater than those in WT mice (Fig. 4C), suggesting preferential transport and accumulation of BMM in the inflamed brain in PD animals. The same pattern was observed for the brain/muscle ratio at 48 h time point (Fig. 4D). Finally, as expected, considerable amounts of macrophages deposited in the main peripheral organs; although, i.t. route resulted in decreased levels in peripheral organs and increased amount of BMM in the brain compared to i.v. and i.p. routes (Fig. 4B).
Figure 4.
Biodistribution kinetics of 64Cu-labeled macrophages in Parkin Q311(X)A and WT mice by PET. Radioactively-labeled BMM were injected into PD or WT mice (12 mo. of age) though i.v., i.p., or i.t. routes (2.5 × 106 cells/50 µL). The animals were imaged by PET over 48 h-time period after the injection (A). At the endpoint (48 h), mice were sacrificed, perfused, and the radioactivity in the blood and main organs was measured. Panels present (A) representative PET images, (B) percentage of the injected dose (%ID/g), (C) brain/blood ratio, and (D) brain/muscle ratio. The greatest brain accumulation of 64Cu-BMM was detected in PD mice with i.t. injections. The amount of 64Cu-BMM accumulated in the PD mouse brain was significantly greater than those in WT animals. Values are the means ± SEM (N = 4), *p < 0.05; **p < 0.005 compared with WT healthy mice (B, C), or i.v. injections (D). Individual data points shown in Supplementary Tables S4–S6.
Biodistribution of 19F-labeled BMM in Parkin Q311(X)A mice by MRI
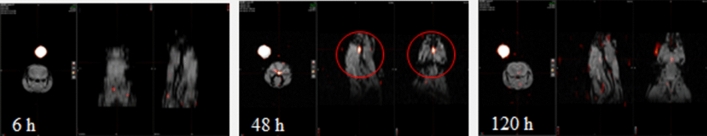
Fluorine 19 (19F) imaging is a technique utilizing non-radioactive version of fluorine in MRI scanners. This technique takes advantage of the minimal background signal in the body; there is very little endogenous fluorine. Furthermore, the perfluorocarbon agents are relatively inert and nontoxic, and have been proposed for applications such as blood substitutes, treatment of retinal detachment, ultrasound contrast agents, etc. Thus, the lack of ionizing radiation and no reduction in signal due to radioactive decay confers advantages to perfluorocarbon MR techniques as a method to perform cell tracking for clinical purposes. Therein, we validated the 19F tracking of BMM into the brain in Parkin Q311(X)A mice, and laid the foundation for translating this drug treatment technique in humans. For this purpose, BMM were labeled with 19F (CS-ATM DM Red, Celsense, Inc., Pittsburgh, PA), and the accumulation of 19F-BMM in PD mouse brain after i.t. injection was studied by MRI (Fig. 5). Dual mode optical and 19F MRI images confirmed that significant amount of i.t. administered BMM can reach the brain, especially at 48 h time point.
Figure 5.
Biodistribution of 19F-labeled macrophages in Parkin Q311(X)A mice over time by MRI. 19F- labeled BMM were injected i.t. into PD mice (12 mo. of age, 1 × 106 cells/50 µL) and 1H/19F fusion image was obtained. Anatomic image is represented in gray scale with cell loading represented by the color. A phantom standard is external to the animal. Representative dual mode brain MR images show significant accumulation of i.t. administered BMM in the brain, especially at 48 h time point.
Delivery of reporter and therapeutic genes via pre-transfected macrophages to the brain in Parkin Q311(X)A mice
Based on the results of IVIS, PET and MRI experiments, we selected the i.t. injection as the preferred route of administration of pre-transfected BMM for the delivery of reporter and therapeutic genes to the brain in Parkin Q311(X)A mice. For the reporter gene delivery study, the BMM cells transfected by electroporation with plasmid encoding a reporter gene, luciferase (Luc), Luc-pDNA were injected into PD mice, or their WT littermates through i.t. route. The luciferase expression levels in the brain regions (frontal cortex, midbrain, cerebellum, and pons) were examined 48 h after the single injection. To account for auto-luminescence, the relative luminescence values obtained for control animals injected with saline were subtracted from those injected with Luc-BMM for each brain region. Significant luminescence in Luc-pDNA/BBM treated animals was recorded in all brain sub-regions of both PD and WT mice (Fig. 6A). Individual values of the luciferase expression levels for each animal are presented in Supplementary Tables S8 and S9. Consistent with BMM brain biodistribution results obtained by IVIS (Figs. 1, 2, 3) and PET (Fig. 4) studies, the Luc expression in the brain of PD mice was significantly greater than those in WT littermates (Fig. 6A). Specifically, luminescence levels in the midbrain, cerebellum and pons of PD mice were up to 40 times higher than those in WT animals. No differences in Luc levels were found in the blood of PD and WT animals. We suggest that Luc-BMM targeted inflamed brain tissues in PD animals, resulting in overexpression of the encoded gene (Luc). Thus, pre-transfected adoptively transferred macrophages provided a sustained expression of the encoded protein in the inflamed brain. Luc brain/blood ratio in PD mice was also significantly (p < 0.005) greater than those in WT healthy mice (Fig. 6B).
Figure 6.
Reporter and therapeutic gene expression in brain of Parkin Q311(X)A mice after single i.t. injection of pre-transfected BMM. (A) Luc-transfected BMM (A, B) were injected through i.t. route (2.5 × 106 cells/50 µL) to PD mice (black bars) or WT mice (striped bars). Mice were sacrifices 48 h after injection, perfused, brains were harvested and luciferase activity was measured in different brain regions [i.e. frontal cortex (1), midbrain (2), cerebellum (3), and pons (4)), and blood (5)]. Luciferase activity in each brain region was significantly greater in PD mouse brain regions than those in WT counterparts (A). Total brain/blood ratio of luciferase activity in PD mice was 25 times greater than in WT mice (B), indicating that BMM target inflamed brain tissues. (C) PD mice were injected with the same amount of GDNF-transfected BMM through i.t. route (black bars), or saline (white bars). Mice were sacrifices 48 h after injection, perfused, brains were harvested and GDNF expression was measured in different brain regions by ELISA. GDNF levels in each brain region were greater in PD mouse brain regions injected with GDNF-BMM than controls injected with saline. Negligible differences in GDNF levels in the blood were observed. Values are means ± SEM (N = 6). *p < 0.05 compared to WT healthy mice (A, B), or saline injected control mice (C). Individual data points shown in Supplementary Tables S8–S10.
For the therapeutic gene delivery studies, Parkin Q311(X)A mice were injected i.t. with GDNF-transfected BMM, and GDNF-expression levels were examined in different brain regions 48 h after the administration (Fig. 6C). Individual values of the GDNF levels for each animal are presented in Supplementary Tables S10. The obtained data clearly demonstrate that a single injection of GDNF-BMM resulted in significant increases of GDNF expression in all brain regions examined. Similar to Luc expression, the highest levels of the GDNF was detected in pons. Consistently to the Luc-BMM studies, no significant increases in the blood GDNF levels were detected in the GDNF-BMM treated animals compared to saline-injected control group (Fig. 6C). Together, the studies demonstrated that cell-carriers efficiently target affected brain sub-regions in PD mice and deliver their therapeutic cargo.
Absence of cytotoxic effects in the mouse brain upon treatment with GDNF-transfected macrophages
To evaluate possible offsite toxicity of the transfected macrophages, healthy WT mice were injected i.t. with GDNF-BMM, or the same amount of sham BMM. Control group of mice was injected with saline. The animals were sacrificed 48 h after the injection, and the levels of pro-inflammatory cytokines and chemokines, including RANTES, MCP-1, TNF-a, and IL-6 were assessed in the brain, as well as in main organs; brain, kidney, spleen and liver by membrane-based antibody arrays and confirmed by colorimetric sandwich ELISA (Fig. 7). Individual values of the inflammatory signals levels for each animal are presented in Supplementary Tables S11–S14.
Figure 7.
Effect of BMM administration on the levels of inflammatory signals in mice. Healthy WT mice were injected with saline (white bars), sham BMM (stripped bars), or GDNF-transfected BMM (black bars), and the inflammation signals in the main organs was assessed by the levels of RANTES (A), TNF-a (B), MCP-1 (C), IL-6 (D). No significant increases of inflammatory signals were found in the brain. Some slight increases in RANTES, and MCP-1 were registered in spleen and liver (A, C). TNF-a levels were significantly decreased in spleen upon sham BMM injection. Values are means ± SEM (N = 10). *p < 0.05 compared to saline-injected control mice. Individual data points shown in Supplementary Tables S11–S14.
No signs of increased inflammation in the brain were detected. Moreover, administration of GDNF-transfected macrophages slightly reduced expression of all pro-inflammatory signals in the brain, compared to control saline-injected animals (Fig. 7A–D). Of note, this phenomenon may be due to a particular subset of M2 cell-carriers differentiated with MCSF that show mild neuroprotective effects themselves as reported earlier47. Next, the effect of GDNF-BMM and sham BMM administration on the expression of inflammatory molecules, 3-nitrotyrosine (3-NT), NF-kb, and P62, were evaluated in healthy mice, and expressed as a ratio of expression of these molecules and that of housekeeping protein, β-actin (Supplementary Figure S3). In consistence with the expression levels of chemokines and cytokines, adoptive transfer of cell-based formulations did not significantly affect the levels of 3-NT, NF-kb, and P62 in the brain. The administration of sham BMM increased NF-kb and p62 levels in kidney, and spleen, however, injection of GDNF-BMM eliminated this effect (Supplementary Figure S3). Finally, no differences in post-synaptic density (PSD-95) were detected in the brain of GDNF-BMM or sham BMM injected mice (Supplementary Figure S4A, B), indicating absence of toxic effects of macrophage-based drug delivery system on the synaptic transmission. Of note, some increases of glutamate receptor levels, NMDR2A and NMDR2B, were recorded upon administration of sham BMM and GDNF-BMM, respectively (Supplementary Figure S4B, C). Overall, no significant offsite toxicity was found in the brain upon GDNF-BMM, or sham BMM injections. The slight cytotoxic effects in peripheral organs, kidney, and spleen, observed in animals injected with sham BMM were eliminated, when the GDNF-transfected macrophages were administered.
Discussion
Cell-based drug delivery systems have a potential as a treatment modality for CNS disorders. However, this field is not well developed, and relatively little research has been done to advance these novel cell-based therapeutic agents. It was long time demonstrated that macrophages have extraordinary ability to cross body barriers, including the BBB, and reach the CNS, especially during a neurodegenerative process51. Therefore, we posit that the chronic inflammation in the brain at pathological conditions provides an opportunity for site-specific delivery using inflammatory response cells as vehicles for different potent therapeutics, including genes, proteins and low molecular compounds. In particular, we have developed cell-based drug delivery systems, in which immune cells are non-virally transfected, ex vivo, with pDNA encoding therapeutic proteins, and then systemically administered into mice with induced brain neurodegeneration, where they migrate to the sites of inflammation resulting in sustained expression of these therapeutics and prolonged neuroprotective effects44,47.
The critical information about biodistribution of cell-carriers upon different routes of administration would allow to optimize decisions on dosing, treatment frequency, and other clinical and regulatory parameters. Herein, we report that i.t. administration is the most efficient way to deliver macrophage-based drug formulations to the inflamed brain in the transgenic mouse model of PD, Parkin Q311(X)A mice. First, IVIS studies demonstrated that all examined routes of administration, namely i.v., i.p., or i.t. injections, provided considerable amount of fluorescently labeled macrophages in the PD mouse brain. A prolonged sustained accumulation of cell-carriers in the mouse brain with maximal amount at 48–72 h after single injection was recorded. In addition to the brain accumulation, a substantial amount of adoptively transferred macrophages was detected in the spinal column in PD mice, especially after i.t. administration. This may have additional therapeutic benefits, as a widespread alpha-synucleinopathy was shown not only in the brain, but also in the spinal cord of PD patients52. Thus, the delivery of therapeutics to the spinal cord may improve some nonmotor symptoms including urinary, sexual, and gastrointestinal, as well as of some motor symptoms. Next, we confirmed BMM accumulation in the brain in PD mice by PET with 64Cu-labeled macrophages. To have quantitative comparison for each administration route, the same dose of radioactively labeled macrophages was utilized in these settings. The results of PET experiments clearly demonstrated that i.t. administration lead to the highest accumulation of macrophages in the brain of Parkin Q311(X)A mice, with up to 8.1% of the injected dose was detected. The MRI studies with 19F-labeled BMM confirmed significant amount of i.t. administered BMM can reach the brain, especially at 48 h time point. We hypothesized that i.t. route allows to avoid entrapment of significant portion of BMM in peripheral organs, specifically, liver, lungs, and spleen, which was observed in case of i.v. and i.p. injections.
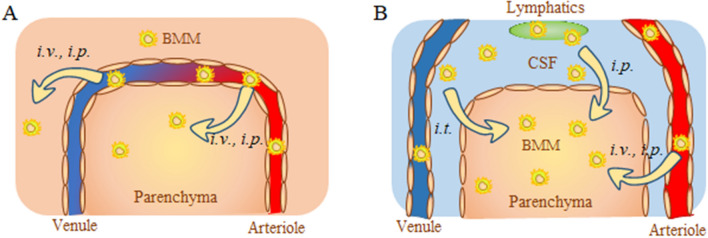
Surprisingly, i.p. administration resulted in significantly greater BMM levels in the brain compared to i.v. injection. We speculated that in addition to BMM penetration across the BBB brain from the bloodstream (Fig. 8A), a portion of i.p. injected macrophages may also reach the brain through lymphatic system (Fig. 8B). In fact, the meningeal lymphatics50 have been recently recognized as an important player in the complex circulation and exchange of molecular and cellular contents between the cerebrospinal fluid (CSF) and the interstitial fluid (ISF)53. Indeed, the perfusion procedure, which was carried out at the endpoint of IVIS and PET experiments, allowed to clear out the luminal space from circulating in the blood stream cell-carriers provided more accurate information about the amount of macrophages entered the brain tissues. However, it does not distinguish between the cells associated with the luminal wall and the brain parenchyma. The more comprehensive understanding of how adoptively transferred macrophages reach the inflamed brain upon different administration routes warrant further investigations.
Figure 8.
Schematic representation of different pathways for adoptively transferred macrophages to the brain. Autologous macrophages transfected with reporter or therapeutic—encoding pDNA were injected via i.v., i.p., or i.t. routes. Different ways of brain entrance across (A) the BBB, or (B): CSF barrier are shown. (A) Macrophages injected through i.v. or i.p. routes cross the BBB and deliver the encoded proteins to the parenchyma. (B) In addition, macrophages injected i.p. may enter the brain through lymphatic system. Macrophages administered through i.t. route mainly enter through the CSF barrier.
Equally important, in consistence with our previous reports44–46,54,55, we demonstrated here the superior accumulation levels of autologous macrophages in the PD mouse brain compared to their WT littermates. This effect was obtained for all routs of administration. We suggest that brain inflammation developed during the disease progression, and release of various cytokines and reactive oxygen species (ROS) by activated microglia attracted macrophages providing targeted transport of cell-carriers to the brain.
The most significant observation was made for PD mice that were i.t. injected with autologous macrophages pre-transfected ex vivo with reporter Luc and therapeutic GDNF genes. Genetically modified cell-carriers accomplished a site-specific delivery of the encoded gene to local areas of the disease, where the inflammatory processes are active. Indeed, Luc activity in the PD mouse brain was considerably greater (up to 40 times) than in those in healthy WT littermates. Moreover, a single i.t. injection of GDNF-BMM resulted in significant increases of GDNF levels in all examined brain regions, including pons, midbrain, frontal cortex, and cerebellum. Essential, no increases in GDNF blood levels were recorded. We hypothesized that transfected ex vivo and adoptively transferred macrophages can accomplish horizontal gene transfer to the neighboring brain cells resulting in transfection of brain tissues. Thus, pre-transfected macrophages serve as “donors” that pass or “horizontally transfer” the gene to acceptor cells. In this respect, we demonstrated recently that transfected macrophages can pass pDNA to muscle cells55, and siRNA to cancer cells56, causing significant antitumor effects. In addition, GDNF-BMM can also deliver the overexpressed neurotrophic factor. Our earlier in vitro experiments indicate that genetically modified macrophages express GDNF up to a week after transfection (~ 1.8 ng/mg protein on day one) and release it to the conditioned media (~ 16.1 ng/mL) over 7 days after transfection. Finally, absence offsite effects in the brain upon adoptive transfer GDNF-transfected macrophages or sham BMM that manifested in the lack of increases of pro-inflammatory cytokines and chemokines levels, as well as unchanged synaptic activity in the brain was demonstrated. Noteworthy, some signs of increased inflammation were recorded in peripheral organs, kidney, and spleen, in case of sham BMM injection that were alleviated when GDNF-macrophages were administered. In this respect, using human umbilical cord blood monocytes (HUCBM) in future clinical trials may be more beneficial, as it was reported that these cells have protective activities by releasing neurotrophic and anti-inflammatory factors, and even can rescue brain cells from hypoxic-ischemic injury57. Using these cells for gene delivery could have supplementary therapeutic benefits. Altogether, macrophages may pose an advantageous and efficient delivery carrier for a spectrum of various degenerative, cancerous, and infectious disorders.
Methods
Plasmids and reagents
The plasmid encoding the reporter gene luciferase (Luc) (gWILuc) and Human GDNF cDNA (NM_199234) were purchased from Gene Therapy Systems, (San Diego, CA), and OriGene (Rockville, MD), respectively. The sequence and condition for plasmids expansion can be found in our earlier publication49. A lipophilic fluorescent dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine iodide (DIR), was purchased from Thermo Fisher Scientific (Waltham, MA). Murine macrophage colony-stimulating factor (MCSF) was purchased from Peprotech Inc (Rocky Hill, NJ).
Cells and animals
Primary bone marrow-derived cells extracted from murine femurs (wild type littermates of Parkin Q311X(A) female mice, 2 months old) as described in20.
Two breeding pairs of Parkin Q311X(A) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) 12 weeks of age, and were treated in accordance to the Principles of Animal Care outlined by National Institutes of Health and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Several cohorts of transgenic mice as well as their wild type controls were bred in house. The genotyping of pups was carried by PCR analysis of Parkin Q311X(A) gene was performed for parents, a negative control wild mouse, and several mice generations according to manufacturers protocol. Mice were housed in a temperature and humidity-controlled facility on a 12 h light/dark cycle and food and water were provided ad libitum. According to NIH policy on Sex as a Biological Variable, both male and female mice were used in each group in 1:1 proportion. Significant sex differences were not detected in the analyses.
Transfection of macrophages
BMM were transfected by electroporation according to manufacturer’s protocol with some modifications. Briefly, BMM (50 × 106 cells) were spanned down at 1,000 RPM for 5 min, re-suspended in 700 µL electroporation buffer (Neon Transfection system, Thermo Fisher Scientific) and supplemented with 30 µg Luc-pDNA (300 µL), or GDNF-pDNA (300 µL) . The aliquots of cell suspension with Luc-pDNA (100 µL) were placed into electroporation cell, and electroporated at following setup: voltage 1,400; width 20; Pulses 2. Then, the cells were supplemented with 1 mL PBS, and centrifuged at 125G for 5 min. The supernatant was removed, and pellet was re-suspended in saline buffer and injected in PD or WT mice. To account for the expression levels of luciferase, a portion of the cells was cultured in CCM media for 24 h, then washed 3X with PBS, and lysed with luciferase lysis buffer (Promega, Madison, WI). The luciferase activity levels in BMM were assessed by luminescence (N = 4) and expressed in relative fluorescent units (RFU). Total luciferase activity in Luc-BMM was 693.560 × 106 RFU/106 cells. The GDNF levels in the cells were assessed by ELISA (Human GDNF ELISA kit, #EHGDNF, ThermoFisher Scientific, Waltham, MA). In addition, GDNF-BMM can also deliver the overexpressed neurotrophic factor. GDNF-transfected macrophages showed about 1.8 ng/mg protein on day one after transfection”.
Administration of cell-based formulations via different routes
Three different routes of administration were utilized: i.v., i.p., and i.t. injections. For i.v. administration route, PD or WT mice were injected with a BMM-based formulation (50–200 µL) intra-tail vein with the use of a restraining devise. For i.p. administration route, mice were injected into the left lower quadrant of the abdomen (50–200 µL) without restraining devise. For i.t. administration route, mice were anesthetized with 3% isoflurane, and the fur at the posterior end was shaved. The BMM-based formulation (50 µL) was slowly injected between the groove of L5 and L6 vertebrate.
Kinetics of biodistribution of autologous macrophages in Parkin Q311(X)A mice and WT healthy littermates by IVIS
To track systemically injected cell-carriers in PD and WT mice, BMM were labeled with DIR lipophilic dye (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocol. Briefly, BMM (1 × 106 cell/mL) were incubated with 7 µM DIR in PBS for 20 min at 37C. Followed incubation, the cells were spin down and washed 3 × with ice-cold PBS and re-suspended in saline. To establish background fluorescence levels, animals were imaged before the BMM administration. Then, Parkin Q311X(A) mice (12 mo. of age) and their WT littermates were injected with DIR-labeled BMM, via i.v., or i.p., or i.t. routes. In this experiment we used maximal dose and volume of cell suspension for each route of administration (specifically, 4 × 106 cells/200 µL/mouse for i.v. and i.p. injections, and 1 × 106 cells/50 µL/mouse for i.t. injections), and the animals were imaged by Xenogen IVIS Optical Imaging System. The conditions of this experiment were set up for a maximal volume of the cell suspension injected that is allowed for each route of administration. At the endpoint (72 h), animals were sacrificed and perfused as described earlier48, main organs were removed, washed, post-fixed in 10% phosphate-buffered paraformaldehyde, and evaluated by IVIS Aura software (Spectral Instrument Imaging, Tucson, AZ, USA). The background fluorescent level (approximately 0.2–0.3 × 105 RFU) were subtracted from the levels at each time point for each mouse and plotted versus time.
Labeling BMM with celsense for MRI studies
BMM were labeled with 19F (CS-ATM DM Red, Celsense Inc., Pittsburg, PA). CS-ATM is an aqueous colloidal nanoemulsion of a perfluorocarbon polymer with a red fluorescent label. This compound enables dual mode optical and 19F MRI imaging in small animals. For this purpose, BMM (4 × 106 cell/T25 flask) were supplemented with 0.32 mL Celsense solution (3 mg/mL) and incubated at 37 °C for 4 h. Then, labeled 19F-BMM were collected, washed 3X with ice-cold PBS, and re-suspended in saline. The labeling efficiency was evaluated by NMR with a fluorine probe with serial dilutions. A global calibration curve was determined based on BMM concentration versus NMR 19F signal. Serial dilutions of 19F-BMM were imaged utilizing the 9.4 T small animal MRI and 400 Hz NMR to calibrate concentration curves between the modalities. Next, ParkinQ311(X)A mice were i.t. injected with 19F-BMM (2 × 106 cell/50 µL/mouse) and brain images were obtained over 120 h after the injection on the 9.4 T small animal scanner with a custom dual mode 1H/19F volume coil using conventional sequences for anatomic (1H T2 RARE,1H T1 FLASH) and functional localization (19F T1 RARE). Animals with a sham injection served as a control.
Labeling BMM with 64Cu for PET imaging and biodistribution studies
To track the brain targeting efficiency of BMM cells administered via different routes, BMM were labeled with 64Cu as described earlier58. Briefly, BMM were incubated with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) (64Cu-PTSM) in 1 mL serum free medium at 37 °C for 1.5 h, and then washed with ice-cold PBS (pH 7.4). Parkin Q311X (A) mice (12 mo. of age) and their WT littermates were injected with 64Cu-BMM (48.0 ± 2.8 µCi/1 × 106 cells/50 µL/mouse) via i.v., i.p., or i.t. administrations, and imaged using SuperArgus PET/CT system at 1 h, 24 h and 48 h post-injection. After 48 h post-injection PET scan, the animals were euthanized. The brain and blood were collected and the accumulation of 64Cu-BMM was measured using gamma counter (2,470 Wizard, Perkin Elmer). The results were presented at percentage of injected dose per g of tissue (%ID/g).
Luciferase activity and GDNF levels in the brain and main peripheral organs
Parkin Q311(X)A mice (12 mo. old, N = 6) and their healthy WT littermates were injected with Luc-BMM (2.5 × 106 cells/50 µL) through i.t. route of administration. Followed 48 h mice were anesthetized with ketamine/xylazine cocktail and subjected to transcardial perfusion with ice-cold PBS for 5 min following 5 min of perfusion with 4% ice-cold paraformaldehyde (PFA) in PBS. Blood samples (100 μL) at the endpoint were taken into heparin-coated microhematocrit tubes (Braintree ScientificBraintee, MA), centrifuged for 5 min at 400×g, and luminescence levels were recorded in plasma. Brain was removed, washed in ice-cold saline, different brain regions (frontal cortex, midbrain, cerebellum, and pons) were dissected, blotted and weighed. The brain samples were supplemented with 0.5 mL of tissue solubilizer and then homogenized in a glass tissue homogenizer (Tissue-Tearor, BioSpec Products, Inc., Bartlesville, OK). The luciferase activity in 10 μL tissue homogenates was quantified using a TD20/20 or Glomax 20/20 luminometer (Promega, Fitchburg, WI) for an integration period of 20 s and 10 s respectively and normalized per mg of tissue as described before55.
To examine GDNF levels in the brain tissues in PD mice by ELISA, Parkin Q311(X)A mice (12 mo. old, N = 6) were injected with GDNF-BMM (2.5 × 106 cells/50 µL) through i.t. route. PD mice injected with saline were used as a control group. Followed 48 h, mice were sacrifices, perfused with PFA, brain was removed, dissected to different regions (frontal cortex, midbrain, cerebellum, and pons), and homogenized as described above. Blood samples were also collected. The lysates were spun 13,000 G for 10 min, supernatant was collected and added to the 96-well plate (100 µL/well) pre-coated with anti-human GDNF (Human GDNF ELISA kit, #EHGDNF, ThermoFisher Scientific, Waltham, MA). The plate was covered and incubated at RT for 2.5 h, then washed 4X with PBS, biotinylated antibody was added (100 µL/well) and incubated at RT for 1 h. Then, the plate was washed 4X, the streptavidin-HRP reagent (100 µL/well) was added, and incubated at RT for another 45 min. The plate was washed 4 × with PBS and TMB substrate (100 µL/well) was added. The color was developed at RT in the dark for 30 min and Stop solution (50 µL/well) was added. Absorbance was measured at 450 nm. The amount of GDNF was assessed based on the provided by manufacturer calibration curve and expressed in pg GDNF/mg protein.
Expression of cytokines and chemokines in the brain and main peripheral organs by ELISA and Western blotting
Organs were homogenized in cell lysis buffer (Thermo Scientific, Waltham, MA, USA) and the levels of interleukin (IL)-6, monocyte chemotactic protein-1 (MCP-1), regulated on activation, normal T cell expressed and secreted (RANTES) and tumor necrosis factor alpha (TNF-α) were measured by ELISA (R&D Systems, Minneapolis, MN, USA) as described in49.
Protein was extracted using RIPA buffer (Thermo Scientific) supplemented with a mixture of protease and phosphatase inhibitors followed by SDS-PAGE protein separation. Immunoblots were labeled with primary antibodies against 3-Nitrotyrosine (NT)—1:200 (Abcam, ab110282), PSD-95—1:200 (Abcam, ab76115), NMDAR2B—1:200 (Abcam, ab65783), NF-kB—1:200 (Santa Cruz Biotechnology, sc8008), P62/SQSTM1—1:500 (Novus Biologicals, NBP1—48,320) and β actin—1:200 (Santa Cruz Biotechnology, sc-47778) was used as internal control. Immunoblots were subsequently incubated with secondary antibodies conjugated to horseradish peroxidase (Millipore, Billerica, MA, USA), exposed to SuperSignal West Femto Substrate (Thermo Scientific) and visualized using a ChemiDoc imaging system (Bio-Rad,). Densitometric analysis was quantitatively measured using image J (NIH.gov).
Statistical analysis strategy
Previous studies guided our decisions regarding distributional assumptions, transformations of scales, and choices of estimators (mean vs. median, etc.). For each outcome measure in each experiment involving groups of N = 4, 6 or 10 mice, sample means were tabulated along with their corresponding standard errors (SEM): the estimated mean ± one SEM is an approximate 66% confidence interval. Hypothesis tests regarding treatment effects and differences between types (PD vs. WT) were performed using sets of pairwise two-sided t tests. The reported p values have not been adjusted for multiple comparisons. In the main analyses, rarely occurring missing data values were assumed to be caused by ignorable mechanisms. After the main analyses were completed, sensitivity analyses were performed to guide our level of trust in the main results by examining the their robustness/fragility; examples of these auxiliary computations include analysis of residuals, and examination of the impacts of influential observations, questionable data values, and alternative transformations of measurement scales (e.g., log10). Data values for the individual mice were tabulated for presentation in the Supporting Information section. All statistical computations were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and Microsoft Excel (Microsoft Corp., 2016).
Supplementary information
Acknowledgements
This study was supported in part by the National Institutes of Health Grant 1RO1 NS102412 (EVB), the North Carolina Biotechnology Center UNC TEG15-4849 (EVB), and the Eshelman Institute for Innovation Grant UNC EII29-201 (AVK). We are very grateful to Mr. T. Greenwood for financial support as well as various valuable comments and suggestions. We are also thankful to the Senior Director of Development at UNC Kelly Collins for her assistance with communication the strategy and facilitating the support of this project. We would like to express our gratitude to the Professor at the Department of Biostatistics UNC Dr. Paul Stewart for valuable comments regarding Statistical analysis.
Author contributions
Conceptualization: E.V.B., A.V.K. Investigation and validation: M.J.H., Y.Z., J.F., H.D., M.W., H.W., M.K.K. Writing—original draft: Z.L., Y.Z.L., N.E.H. Writing—review & editing: E.V.B.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-025-27452-5
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/25/2025
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-025-27452-5
Supplementary information
is available for this paper at 10.1038/s41598-020-68874-7.
References
- 1.Malek, N., Swallow, D. M. A., Grosset, K. A., Lawton, M. A., Marrinan, S. L., Lehn, A. C., Bresner, C., Baiai, N., Baker, R. A., Ben-Shlomo, Y., Burn, D. J., Foltynie, T., Hardy, J., Morris, H. R., Williams, N. M., Wood, N., & Grosset, D. G. Tracking Parkinson’s: study design and baseline patient data. J. Parkinsons Dis.5, 947–959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrer, M. J. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet.7, 306–318 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Gurme, S. T., Surwase, S. N., Patil, S. A., Jadhav, S. B. & Jadhav, J. P. Optimization of biotransformation of l-tyrosine to l-DOPA by Yarrowia lipolytica-NCIM 3472 using response surface methodology. Indian J. Microbiol.53, 194–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, Q., Liu, Y. & Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener.4, 19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology38, 1285–1291 (1988). [DOI] [PubMed] [Google Scholar]
- 6.Busciglio, J. & Yankner, B. A. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature378, 776–779 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Ebadi, M., Srinivasan, S. K. & Baxi, M. D. Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog. Neurobiol.48, 1–19 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Wu, D. C. et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA100, 6145–6150 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, P. H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab.21, 2–14 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Jin, H. et al. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim. Biophys. Acta2014, 1282–1294 (1842). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry, V. H., Bell, M. D., Brown, H. C. & Matyszak, M. K. Inflammation in the nervous system. Curr. Opin. Neurobiol.5, 636–641 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Kuby, J. Immunology (Freeman, WH. and Co., New York, 1994). [Google Scholar]
- 13.Anthony, D. C., Bolton, S. J., Fearn, S. & Perry, V. H. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain120(Pt 3), 435–444 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Anthony, D. C., Blond, D., Dempster, R. & Perry, V. H. Chemokine targets in acute brain injury and disease. Prog. Brain Res.132, 507–524 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Blamire, A. M. et al. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J. Neurosci.20, 8153–8159 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persidsky, Y. et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am. J. Pathol.155, 1599–1611 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlowski, N. A., Kaplan, G., Abraham, E. & Cohn, Z. A. The selective binding and transmigration of monocytes through the junctional complexes of human endothelium. J. Exp. Med.168, 1865–1882 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lossinsky, A. S. & Shivers, R. R. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol. Histopathol.19, 535–564 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Mahmood, A., Lu, D., Lu, M. & Chopp, M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery53, 697–702 (2003) (discussion 702–693). [DOI] [PubMed] [Google Scholar]
- 20.Dou, H. et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood108, 2827–2835 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron57, 178–201 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Alvarez, J. I., Cayrol, R. & Prat, A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta2011, 252–264 (1812). [DOI] [PubMed] [Google Scholar]
- 23.Lassmann, H., Schmied, M., Vass, K. & Hickey, W. F. Bone marrow derived elements and resident microglia in brain inflammation. Glia7, 19–24 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Vass, K., Hickey, W. F., Schmidt, R. E. & Lassmann, H. Bone marrow-derived elements in the peripheral nervous system. An immunohistochemical and ultrastructural investigation in chimeric rats. Lab. Investig. J. Tech. Methods Pathol.69, 275–282 (1993). [PubMed] [Google Scholar]
- 25.Visser, J. G., Van Staden, A. D. P. & Smith, C. Harnessing macrophages for controlled-release drug delivery: lessons from microbes. Front. Pharmacol.10, 22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans, M. A. et al. Macrophage-mediated delivery of light activated nitric oxide prodrugs with spatial, temporal and concentration control. Chem. Sci.9, 3729–3741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang, L. et al. A novel macrophage-mediated biomimetic delivery system with NIR-triggered release for prostate cancer therapy. J. Nanobiotechnol.17, 83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu, J. et al. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J. Control. Release204, 11–19 (2015). [DOI] [PubMed] [Google Scholar]
- 29.An, L. et al. Macrophages-mediated delivery of small gold nanorods for tumor hypoxia photoacoustic imaging and enhanced photothermal therapy. ACS Appl. Mater. Interfaces11, 15251–15261 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Haber, T., Baruch, L. & Machluf, M. Ultrasound-mediated mesenchymal stem cells transfection as a targeted cancer therapy platform. Sci. Rep.7, 42046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei, X. et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol. Sin.34, 747–754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiser, J. et al. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin. Biol. Ther.5, 1571–1584 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akerud, P., Canals, J. M., Snyder, E. Y. & Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci.21, 8108–8118 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casper, D. et al. Enhanced vascularization and survival of neural transplants with ex vivo angiogenic gene transfer. Cell Transplant.11, 331–349 (2002). [PubMed] [Google Scholar]
- 35.Yasuhara, T. et al. Neurorescue effects of VEGF on a rat model of Parkinson’s disease. Brain Res.1053, 10–18 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Biju, K. et al. Macrophage-mediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson’s disease. Mol. Ther.18, 1536–1544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biju, K. C. et al. Bone marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci. Lett.535, 24–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Serrano, A. & Bjorklund, A. Ex vivo nerve growth factor gene transfer to the basal forebrain in presymptomatic middle-aged rats prevents the development of cholinergic neuron atrophy and cognitive impairment during aging. Proc. Natl. Acad. Sci. USA95, 1858–1863 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Serrano, A., Hantzopoulos, P. A. & Bjorklund, A. Ex vivo gene transfer of brain-derived neurotrophic factor to the intact rat forebrain: neurotrophic effects on cholinergic neurons. Eur. J. Neurosci.8, 727–735 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Garcia, P. et al. Ciliary neurotrophic factor cell-based delivery prevents synaptic impairment and improves memory in mouse models of Alzheimer’s disease. J. Neurosci.30, 7516–7527 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Low, W. C. et al. Function recovery following neural transplantation of embryonic septal nuclei in adult rats with septohippocampal lesions. Nature300, 260–262 (1982). [DOI] [PubMed] [Google Scholar]
- 42.Pizzo, D. P., Coufal, N. G., Lortie, M. J., Gage, F. H. & Thal, L. J. Regulatable acetylcholine-producing fibroblasts enhance cognitive performance. Mol. Ther.13, 175–182 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Haney, M. J. et al. Blood-borne macrophage-neural cell interactions hitchhike on endosome networks for cell-based nanozyme brain delivery. Nanomedicine (Lond)7, 815–833 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haney, M. J. et al. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS ONE8, e61852 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Haney, M. J. et al. Cell-mediated transfer of catalase nanoparticles from macrophages to brain endothelial, glial and neuronal cells. Nanomedicine (Lond)6, 1215–1230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klyachko, N. L. et al. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine (Lond)9, 1403–1422 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, Y. et al. GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson’s disease mouse model. PLoS ONE9, e106867 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Zhao, Y. et al. Active targeted macrophage-mediated delivery of catalase to affected brain regions in models of Parkinson’s disease. J. Nanomed. Nanotechnol.S4, 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao, Y. et al. GDNF-expressing macrophages restore motor functions in transgenic mice at a severe late-stage of Parkinson’s disease. J. Control. Release10.1016/j.jconrel.2019.10.027 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature523, 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitch, M. T. & Silver, J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol.148, 587–603 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Raudino, F. & Leva, S. Involvement of the spinal cord in Parkinson’s disease. Int. J. Neurosci.122, 1–8 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Da Mesquita, S., Fu, Z. & Kipnis, J. The meningeal lymphatic system: a new player in neurophysiology. Neuron100, 375–388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brynskikh, A. M. et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine (Lond)5, 379–396 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahajan, V. et al. Horizontal gene transfer from macrophages to ischemic muscles upon delivery of naked DNA with Pluronic block copolymers. Biomaterials75, 58–70 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wayne, E. C. et al. Targeted targeted delivery of siRNA lipoplexes to cancer cells using macrophage transient horizontal gene transfer. Adv. Sci.6(21), 1900582. 10.1002/advs.201900582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saha, A. et al. Human umbilical cord blood monocytes, but not adult blood monocytes, rescue brain cells from hypoxic-ischemic injury: Mechanistic and therapeutic implications. PLoS ONE14, e0218906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, W. et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv. Mater.30, e1805557 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.