Abstract
Ticks carry pathogens that can cause disease in both animals and humans, and there is a need to monitor the distribution and abundance of ticks and the pathogens they carry to pinpoint potential high risk areas for tick-borne disease transmission. In a joint Scandinavian study, we measured Ixodes ricinus instar abundance at 159 sites in southern Scandinavia in August-September, 2016, and collected 29,440 tick nymphs at 50 of these sites. We additionally measured abundance at 30 sites in August-September, 2017. We tested the 29,440 tick nymphs in pools of 10 in a Fluidigm real-time PCR chip to screen for 17 different tick-associated pathogens, 2 pathogen groups and 3 tick species. We present data on the geolocation, habitat type and instar abundance of the surveyed sites, as well as presence/absence of each pathogen in all analysed pools from the 50 collection sites and individual prevalence for each site. These data can be used alone or in combination with other data for predictive modelling and mapping of high-risk areas.
Subject terms: Entomology, Bacterial infection, Ecological epidemiology
| Measurement(s) | pathogen • Ixodida • Abundance • tick instar abundance |
| Technology Type(s) | real-time PCR • Manual Count |
| Factor Type(s) | geographic location • time of data collection |
| Sample Characteristic - Organism | Ixodes ricinus |
| Sample Characteristic - Environment | forested area • meadow ecosystem |
| Kingdom of Denmark • Kingdom of Norway • Sweden |
Machine-accessible metadata file describing the reported data: 10.6084/m9.figshare.12523133
Background & Summary
Tick-borne pathogens have become more prevalent over the last decades1–7 causing disease in humans and animals world-wide. Incidences of tick-borne diseases such as Lyme borreliosis have increased in many regions – including Scandinavia8,9. Left untreated, Lyme borreliosis can pose serious health threats to humans. Other tick-borne diseases, affecting humans and animals such as anaplasmosis (caused by Anaplasma phagocytophilumn), relapsing fever (caused by Borrelia miyamotoi), babesiosis (caused by Babesia spp.), rickettsiosis (caused by Rickettsia spp.) and infections by Neoehrlichia mikurensis, are also on the rise10–13. As climate has changed over the past decades, there is great concern that a warmer climate has an impact on increased incidence of vector-borne diseases as vectors might expand their distribution to more northern climates in Scandinavia8,14. Human behaviour evidently affects the risk of exposure to ticks and their pathogens, but multiple studies have shown how tick abundance affect not only human tick-exposure but also the prevalence of pathogens harboured in ticks15–21. More effective control and prevention of these diseases requires a better understanding of factors affecting vectors and their associated pathogens. Hence, there is a great need for data-based analyses and models that can identify the main drivers of vector abundance and distribution as well as drivers of disease transmission. Developing predictive models for larger areas requires underlying data, which can oftentimes be economically and logistically unfeasible. Free access to data from multiple studies would not only support any large-scale modelling attempt but also act as a reference to future repeated surveys, and thus sharing of data would be of great value to the scientific community.
In Europe there are numerous studies on ticks and their pathogens that have been reported in scientific journals3–5,9,11,20,22–30, and some of these studies have also made data accessible through a free repository20,31–33. However, this is only a fraction of the published data, and finding data on tick abundance and tick-borne pathogen prevalence from a large number of sites for use in risk assessments and modelling, requires a thorough review of the immense amount of papers reporting these data, as for example was done by Estrada-Peña et al. both for European and South American tick data26,33–35. Common and free access to data on tick abundance and tick-borne pathogen prevalence would greatly benefit epidemiological research and sharing of data should always be encouraged. Therefore, we have created a public database for tick abundance and tick-borne pathogen prevalence in the figshare repository and describe the collected data in this manuscript.
The data is part of a multi-national study on ticks in southern Scandinavia. The tick abundance and prevalence data described here, was obtained using a standardised stratified design, comparable between the participating countries. The data consist of tick instar abundance measured from a total of 159 sites in 2016 and 30 sites in 2017 in Denmark, Norway and Sweden and constitute 75.6 km’s of flagged transects. We furthermore collected tick nymphs at 50 of the 159 surveyed sites, counting a total of 24,667 ticks. We used a Fluidigm real-time PCR chip to screen for 17 different pathogens and 2 pathogen groups expected to be in the region (Table 1) and furthermore screened for 3 different tick-species that have been observed in various parts of Scandinavia; Ixodes ricinus, I. persulcatus, and Dermacentor reticulatus. The dataset of 50 georeferenced sites of approximately 60 pools of ten nymphs each tested for 17 pathogens, 2 pathogen groups and 3 tick species thus consist of approximately 66,000 individual PCR tests. The field study was conducted within a short time-frame, from August to September in both 2016 and 2017, thus any differences between measures of abundance and pathogen prevalence within the same year is likely due to geographical differences within the region.
Table 1.
Pathogens and tick species included in the real-time PCR assay.
| Species |
|---|
|
Borrelia miyamotoi, B. burgdorferi sensu lato B. afzelii, B. burgdorferi sensu strictu, B. garinii, B. lusitaniae, B. spielmanii, B. valaisiana |
| Anaplasma phagocytophilum |
| Neoehrlichia mikurensis |
|
The spotted fever group (SFG) Rickettsiae Rickettsia helvetica |
| Francisella tularensis |
| Coxiella burnetii |
| Babesia canis, Babesia divergens, Babesia microti, Babesia venatorum |
| Bartonella henselae |
| Ixodes ricinus, Ixodes persulcatus, Dermacentor reticulatus |
The abundance data has previously been used to model the geographical distribution of I. ricinus nymphs36 and abundance of I. ricinus larva and nymphs37 in Scandinavia. This data along with the aggregated data on pathogen prevalence can be helpful in assessing tick distribution and tick-borne pathogen prevalence in southern Scandinavia and thereby assess human and animal disease risk. The data alone, or in combination with other data, can also be used in R0 models, disease mapping, and predictive modelling.
Methods
Study region
The stratification of the study region has been described in Kjær et al.36 and Kjær et al.37, but a short description follows. The study region encompassed all of Denmark, southern Norway and south-eastern Sweden. After dividing each national study region into a northern and a southern part of equal sizes, we used 1 km2 resolution land cover data from Corine38, to divide each 1 × 1 km pixel into forest, meadow and “other” habitats. Only forests and meadow habitats were selected as potential tick habitats. For each forest and meadow pixel, we used Fourier processed satellite imagery of the maximum normalized difference vegetation index39 (NDVI, 1 km2 resolution), to further divide these habitat pixels into high NDVI (NDVI values above the median value) and low NDVI (equal to or below the median NDVI value). An overview of the stratification scheme can be found in Fig. 1.
Fig. 1.
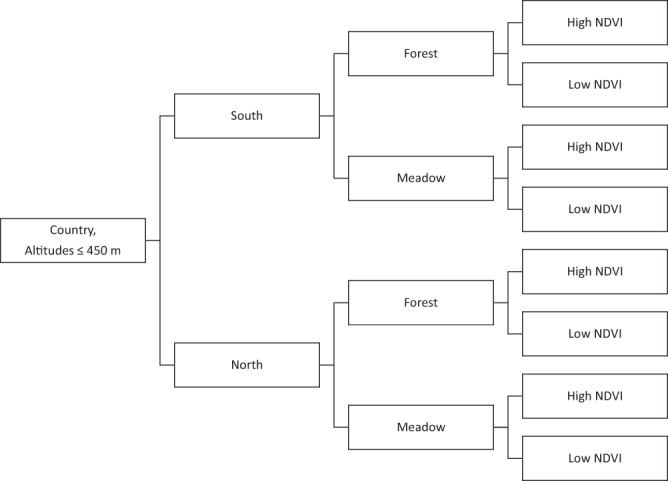
Stratification scheme Each country was divided into equally sized north and south strata. NDVI depicts the maximum normalized difference vegetation index. Forest and meadow classes are based on Corine Land Cover class definitions38. Forest includes the cover types: broad-leaved forest, coniferous forest and mixed forest. Meadow includes: land principally occupied by agriculture with significant areas of natural vegetation, natural grasslands, moors and heathland, and transitional woodland-shrub. All other land cover classes and altitudes >450 m (due to expected low tick abundance or complete absence) were excluded from the stratification and were not sampled for ticks.
Site selection
We randomly selected sites in Denmark, Norway and Sweden using R 3.4.240 (sampleStratified in the raster41 package). Our original aim was to both measure instar abundance and collect 600 tick nymphs from 30 1st priority sites consisting of 80% forest and 20% meadow in each country36. To account for potential difficulties collecting the required number of nymphs at some of the sites, we created 10 alternative sites with the same stratification score for each of the 30 first priority sites in each country, where these 10 alternative sites were ranked after smallest distance to the original site. To account for difficulties collecting enough tick nymphs in meadow sites, we also created 10 alternative forest sites for each meadow site with the same NDVI score. We also created 20 random sites (with 10 alternatives as above) along the Oslo Fjord in Norway, as we were interested in investigating ticks in that region.
For logistical reasons, we needed to ascertain whether it was possible to collect 600 tick nymphs within a reasonable time frame at a given site. Thus, we defined a measure of effort as the amount of nymphs collected by one person during 30 minutes. We determined that a site should allow collection of 600 nymphs in 10 man hours, and thus one person should be able to collect 30 nymphs in 30 minutes. If the number of nymphs collected was below 30, no attempt was made to collect 600 tick nymphs for pathogen testing and an alternative site was explored to obtain the 600 nymphs. Tick instar abundance was, however, measured at all sites, which could result in abundance measures from more than 30 sites per country.
Field study
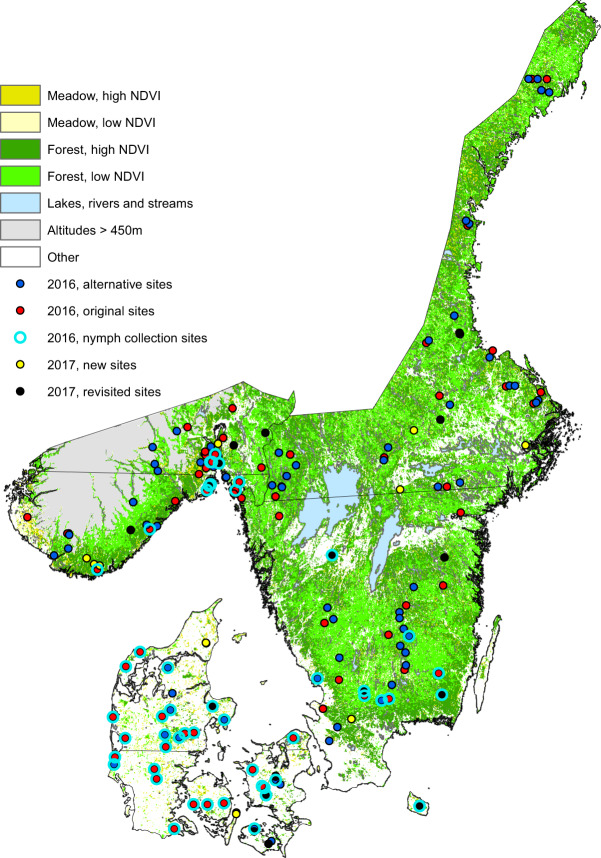
To measure tick abundance, we used two 100 m transects, one facing north and one facing east. We then “dragged”42 a white flannel cloth (1.05 × 1.15 m with lead weights in one end) along each transect in both directions (a total of 400 m’s) and counted and removed tick instar every 50 meters. We measured all tick instar abundance between 15 August and 30 September, 2016 within the hours of 11–16. After measuring abundance at a site, we used our preciously described effort measure to gauge whether it was feasible to collect 600 tick nymphs at the site. If the site was deemed feasible, we collected the tick nymphs, placed them in tubes and stored them on dry ice. When returning from the field, we stored the ticks at −80 °C until use. Within the allocated collection time period, we obtained abundance measures for 37 sites in Denmark, 47 sites in Norway and 75 sites in Sweden. Out of these sites, we collected approximately 600 tick nymphs from 30 sites in Denmark, 11 sites in Norway and 9 sites in Sweden (Fig. 2). Due to counting- and handling errors, we had less than 600 tick nymphs from 6 sites in Denmark and from 5 sites in Sweden, resulting in 29,440 collected tick nymphs in total. In 2017, we additionally measured abundance at 10 sites in each country, using the exact same procedure as in 2016 (August-September, between 11–16 hours). Six of these sites were previously sampled in 2016, and we chose the three sites with the lowest and the highest 2016 nymph abundance per country (excluding zeroes). We selected nymph abundance to choose the six sites in each country as nymphs were more evenly distributed than other instars. The remaining four 2017 sites were subjectively chosen by researchers in each country (Fig. 2).
Fig. 2.
Study region. The study region with the 159 sites from 2016 divided into original sites (red), alternative sites (blue), nymph collection sites (open cyan circles) as well as the revisited sites in 2017 (black) and the new sites (yellow) from 2017. Each country study region is divided into equally sized north and south strata, depicted by the black line. Habitat definitions are found on Fig. 1. NDVI = Normalized difference vegetation index. Similar maps without nymph collection sites depicted have been published elsewhere36,37.
Laboratory methods
DNA extraction
We aggregated the tick nymphs into pools of 10, and washed them for 5 min in 70% ethanol and then for 2 × 5 min in sterile water. We homogenized the ticks with three 3-mm Tungsten beads (Qiagen) using a TissueLyser II (Qiagen, Hilden, Germany) for 2 × 2.5 min at 25 Hz in a mixture of 75 µl Incubation buffer (D920, Promega, Madison, Wisconsin, USA) and 75 µl Lysis buffer (MC501, Promega, Madison, Wisconsin, USA). We briefly centrifuged the samples at 10,000 × g for 60 s and added 30 µl Proteinase K. After an overnight incubation of the samples at 56 °C, we added 300 µl Lysis buffer (MC501, Promega, Madison, Wisconsin, USA) and vortexed the samples for a short time. We then extracted Genomic DNA, using the Maxwell 16 LEV Blood DNA kit (Promega, Madison, Wisconsin, USA) on a Maxwell®16 Instrument.
Screening of tick-borne pathogens by real-time PCR
We performed high-throughput microfluidic real-time PCR, using the BioMark real-time PCR system (Fluidigm, San Francisco, California, USA). We applied the 192.24 dynamic arrays with 22 pathogen primers, as well as one positive E. coli control5 and one negative water control. We used primers for bacterial and parasitic tick-borne pathogens previously identified in the most common tick species, I. ricinus, from Scandinavia, and also included common tick-borne pathogens found throughout Europe5. To screen for different tick species, we used primers for I. ricinus, I. persulcatus, and D. reticulatus; tick species observed in various regions of Scandinavia43,44. All pathogens targeted in the PCR assays are listed in Table 1. We pre-amplified the DNA with 2.5 μL TaqMan PreAmp Master Mix (2X), 1.2 μL pooled primer mix (0.2X) and 1.3 μL of tick DNA (total volume of 5 μL). The PCR conditions for pre-amplification were as follows: one cycle at 95 °C for 10 min, 14 cycles at 95 °C for 15 s and 4 min at 60 °C. We watered down the pre-amplified DNA 5 times and then performed real-time PCRs, using FAM- and black hole quencher (BHQ1)-labeled TaqMan probes with TaqMan Gene Expression Master Mix (according to manufacturer instructions, Applied Biosystems, Foster City, California, USA). We ran thermal cycling in the following sequence: 50 °C for 2 min, 95 °C for 10 min, 40 cycles at 95 °C for 15 s, and 60 °C for 15 s. We used the BioMark real-time PCR system for data acquisition and used the Fluidigm real-time PCR Analysis software to analyse the data and obtain crossing point (CP) values.
We considered a PCR run to be valid (Pass/Fail) when all water controls were negative, all E. coli controls were positive, amplification curves were accepted by Fluidigm’s algorithm for ideal curves and CP values were ≤28. Sensitivity and specificity of the test have been discussed previously5,11,45.
Pathogen prevalence
We estimated the individual-level pathogen prevalence at each site based on the number of positive pools and number of examined pools of ten nymphs each using method 3 from Cowling et al.46, assuming 100% test sensitivity and specificity. Exact confidence limits (CIs) were calculated based on binomial theory46.
All of our pools were positive for I. ricinus and negative for I. persulcatus and D. reticulatus47. We did not detect F. tularensis, C. burnetii, B. canis or B. henselae in any of the pools, and these data are omitted from the published database.
Data Records
The database consists of 3 csv files48. The 3 files can be linked with each other through the SiteID variable, which is a unique identifier for each site.
-
A.
The tick instar abundance table has 12 columns: (1) the unique site ID, (2) the country where the site is situated, (3) the date of measurement, (4) the site selection criteria, (5) the longitude of the site, (6) the latitude of the site, (7) the habitat type of the site, (8) the total number of larva counted from both transects, (9) the total number of nymphs counted from both transects, (10) the total number of adult females counted from both transects, (11) the total number of adult males counted from both transects, (12) the total number of tick nymphs collected and used for pathogen screening.
-
B.
The pathogen pool results table has 19 columns: (1) the country where the site is situated, (2) the unique site ID, (3) The pool number for the particular site, (4) the Pass/Fail test result for B. miyamotoi, (5) the Pass/Fail test result for B. burgdorferi sensu lato, (6) the Pass/Fail test result for B. afzelii, (7) the Pass/Fail test result for B. burgdorferi sensu strictu, (8) the Pass/Fail test result for B. garinii, (9) the Pass/Fail test result for B. lusitaniae, (10) the Pass/Fail test result for B. spielmanii, (11) the Pass/Fail test result for B. valaisiana, (12) the Pass/Fail test result for A. phagocytophilum, (13) the Pass/Fail test result for B. divergens, (14) the Pass/Fail test result for B. microti, (15) the Pass/Fail test result for B. venatorum, (16) the Pass/Fail test result for N. mikurensis, (17) the Pass/Fail test result for R. helvetica, (18) the Pass/Fail test result for SFG Rickettsiae, (19) the tick species found within the pool.
-
C.
The pathogen individual nymph prevalence table has 17 columns: (1) the country where the site is situated, (2) the unique site ID, (3) the individual prevalence in percent along with exact confidence limits for B. miyamotoi, (4) the individual prevalence in percent along with exact confidence limits for B. burgdorferi sensu lato, (5) the individual prevalence in percent along with exact confidence limits for B. afzelii, (6) the individual prevalence in percent along with exact confidence limits for B. burgdorferi sensu strictu, (7) the individual prevalence in percent along with exact confidence limits for B. garinii, (8) the individual prevalence in percent along with exact confidence limits for B. lusitaniae, (9) the individual prevalence in percent along with exact confidence limits for B. spielmanii, (10) the individual prevalence in percent along with exact confidence limits for B. valaisiana, (11) the individual prevalence in percent along with exact confidence limits or A. phagocytophilum, (12) the individual prevalence in percent along with exact confidence limits for B. divergens, (13) the individual prevalence in percent along with exact confidence limits for B. microti, (14) the individual prevalence in percent along with exact confidence limits for B. venatorum, (15) the individual prevalence in percent along with exact confidence limits for N. mikurensis, (16) the individual prevalence in percent along with exact confidence limits for R. helvetica, (17) the individual prevalence in percent along with exact confidence limits for SFG Rickettsiae.
Technical Validation
Field study
We held several meetings to discuss the standardisation of measuring tick abundance, and a video demonstrating the “dragging” method was shared with each researcher in the group to ensure that the collections were standardised as much as possible. We furthermore ensured that each country used the same fabric and same fabric size of the cloth used for “dragging”, by having these cloths produced and made by one partner for all 3 countries.
Laboratory methods
The Fluidigm real-time PCR method has been validated through numerous studies, particularly studies pertaining to tick pathogens5,11,22,23,43,45.
Acknowledgements
We thank Simon Friis-Wandall, Mette Frimodt Hansen, Caroline Greisen, Ana Carolina Cuellar, Najmul Haider, Leif Kristian Sortedal, Philip Thomassen Neset, Preben Ottesen, Alaka Lamsal, Ruchika Shakya, Martin Strnad, Hanne Quarsten, Sølvi Noraas, Åslaug Rudjord Lorentzen, Chiara Bertacco, Kevin Hohwald, Catharina Schmidt, Coco de Koning, and Wenche Okstad for assistance in the field. We would also like to thank the Danish Ministry of the Environment, The Forest and Nature Agency as well as many private landowners for allowing us access to their properties to conduct our sampling. A special thank goes to the late Anastasia Isbrand who handled a large proportion of the DNA pools. This study was funded by the Interreg V Program (the ScandTick Innovation project, grant number 20200422) and the Danish Veterinary and Food Administration.
Author contributions
L.J.K. planned and managed the field work set-up, performed field work in Denmark and contributed to field work in Sweden, analysed the data and drafted the manuscript. K.K. performed DNA preparation, the PCR assays and contributed to drafting the manuscript. R.B. planned the original study, contributed to analysis and drafting the manuscript. A.S., K.S.E., H.E.H.L., K.M.P., A.K.A., L.K., V.K., A.S. and S.S. contributed to field work in Norway and drafting the manuscript. P.K., M.C. and M.T. contributed to field work in Sweden and drafting the manuscript. A.B. contributed to analysis and drafting the manuscript. L.M.J. contributed to fieldwork, DNA preparation, and drafting the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Estrada-Peña A, De J, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 2014;108:104–128. doi: 10.1016/j.antiviral.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Vu Hai V, et al. Monitoring human tick-borne disease risk and tick bite exposure in Europe: Available tools and promising future methods. Ticks Tick. Borne. Dis. 2014;5:607–619. doi: 10.1016/j.ttbdis.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors. 2012;5:8. doi: 10.1186/1756-3305-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skarphédinsson S, Jensen PM, Kristiansen K. Survey of tickborne infections in Denmark. Emerg. Infect. Dis. 2005;11:1055–1061. doi: 10.3201/eid1107.041265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelet L, et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyman P, et al. A clear and present danger: tick-borne diseases in Europe. Expert Rev. Anti. Infect. Ther. 2010;8:33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- 7.Medlock JM, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors. 2013;6:1–11. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jore S, et al. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasit. Vectors. 2011;4:1–11. doi: 10.1186/1756-3305-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjelland V, et al. Tick-borne encephalitis virus, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks collected from recreational islands in southern Norway. Ticks Tick. Borne. Dis. 2018;9:1098–1102. doi: 10.1016/j.ttbdis.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Rizzoli A, et al. Ixodes ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Heal. 2014;2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klitgaard K, Kjær LJ, Isbrand A, Hansen MF, Bødker R. Multiple infections in questing nymphs and adult female Ixodes ricinus ticks collected in a recreational forest in Denmark. Ticks Tick. Borne. Dis. 2019;10:1060–1065. doi: 10.1016/j.ttbdis.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen, B. N. et al. Distribution of Neoehrlichia mikurensis in Ixodes ricinus ticks along the coast of Norway: The western seaboard is a low‐prevalence region. Zoonoses Public Health zph. 12662, 10.1111/zph.12662 (2019). [DOI] [PubMed]
- 13.Jenkins A, et al. Detection of Candidatus Neoehrlichia mikurensis in Norway up to the northern limit of Ixodes ricinus distribution using a novel real time PCR test targeting the groEL gene. BMC Microbiol. 2019;19:199. doi: 10.1186/s12866-019-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindgren E, Gustafson R. Tick-borne encephalitis in Sweden and climate change. Lancet (London, England) 2001;358:16–18. doi: 10.1016/S0140-6736(00)05250-8. [DOI] [PubMed] [Google Scholar]
- 15.Del Fabbro S, Gollino S, Zuliani M, Nazzi F. Investigating the relationship between environmental factors and tick abundance in a small, highly heterogeneous region. J. Vector Ecol. 2015;40:107–116. doi: 10.1111/jvec.12138. [DOI] [PubMed] [Google Scholar]
- 16.Nazzi F, et al. Ticks and Lyme borreliosis in an alpine area in northeast Italy. Med. Vet. Entomol. 2010;24:220–6. doi: 10.1111/j.1365-2915.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- 17.Jaenson TGT, et al. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Med. Vet. Entomol. 2009;23:226–237. doi: 10.1111/j.1365-2915.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 18.Hudson PJ, et al. Tick-borne encephalitis virus in northern Italy: molecular analysis, relationships with density and seasonal dynamics of Ixodes ricinus. Med. Vet. Entomol. 2001;15:304–313. doi: 10.1046/j.0269-283x.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 19.Hubalek Z, Halouzka J, Juricova Z. Longitudinal surveillance of the tick Ixodes ricinusfor borreliae. Med. Vet. Entomol. 2003;17:46–51. doi: 10.1046/j.1365-2915.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- 20.Mysterud A, et al. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasit. Vectors. 2018;11:309. doi: 10.1186/s13071-018-2890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen PM, Hansen H. Spatial Risk Assessment for Lyme Borreliosis in Denmark. Scand. J. Infect. Dis. 2000;32:545–550. doi: 10.1080/003655400458857. [DOI] [PubMed] [Google Scholar]
- 22.Moutailler S, et al. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl. Trop. Dis. 2016;10:e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reye AL, et al. Prevalence of Tick-Borne Pathogens in Ixodes ricinus and Dermacentor reticulatus Ticks from Different Geographical Locations in Belarus. PLoS One. 2013;8:e54476. doi: 10.1371/journal.pone.0054476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada-Peña A. Distribution, Abundance, and Habitat Preferences of Ixodes ricinus (Acari: Ixodidae) in Northern Spain. J. Med. Entomol. 2001;38:361–370. doi: 10.1603/0022-2585-38.3.361. [DOI] [PubMed] [Google Scholar]
- 25.Estrada-Pena A, De La Fuente J. Species interactions in occurrence data for a community of tick-transmitted pathogens. Sci. Data. 2016;3:2–4. doi: 10.1038/sdata.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estrada-Peña A, et al. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int. J. Health Geogr. 2018;17:41. doi: 10.1186/s12942-018-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soleng A, Kjelland V. Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in Ixodes ricinus ticks in Brønnøysund in northern Norway. Ticks Tick. Borne. Dis. 2013;4:218–221. doi: 10.1016/j.ttbdis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Øines Ø, Radzijevskaja J, Paulauskas A, Rosef O. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasit. Vectors. 2012;5:156. doi: 10.1186/1756-3305-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strnad, M., Hönig, V., Růžek, D., Grubhoffer, L. & Rego, R. O. M. Europe-Wide Meta-Analysis of Borrelia burgdorferi Sensu Lato Prevalence in Questing Ixodes ricinus Ticks. Appl. Environ. Microbiol. 83 (2017). [DOI] [PMC free article] [PubMed]
- 30.Hornok S, et al. Occurrence of ticks and prevalence of Anaplasma phagocytophilum and Borrelia burgdorferi s.l. in three types of urban biotopes: Forests, parks and cemeteries. Ticks Tick. Borne. Dis. 2014;5:785–789. doi: 10.1016/j.ttbdis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Moutailler S, et al. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl Trop Dis. 2016;10(3):e0004539. doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reye AL, 2013. Pathogen prevalence in questing and feeding ticks. figshare. https://plos.figshare.com/articles/_Pathogen_prevalence_in_questing_and_feeding_ticks_/174458
- 33.Estrada-Peña A, De La Fuente J. 2016. Data from: Species interactions in occurrence data for a community of tick-transmitted pathogens. Dryad. [DOI] [PMC free article] [PubMed]
- 34.Estrada-Peña A, et al. Correlation of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the western palearctic. Appl. Environ. Microbiol. 2011;77:3838–45. doi: 10.1128/AEM.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estrada-Peña A. 2019. Data from: The dataset of ticks in South America. Dryad. [DOI]
- 36.Kjær LJ, et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Eurosurveillance. 2019;24:1800101. doi: 10.2807/1560-7917.ES.2019.24.9.1800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjær LJ, et al. Predicting the spatial abundance of Ixodes ricinus ticks in southern Scandinavia using environmental and climatic data. Sci. Rep. 2019;9:18144. doi: 10.1038/s41598-019-54496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corine Land Cover 2006 raster data. European Environment Agency, https://www.eea.europa.eu/data-and-maps/data/clc-2006-raster (2010).
- 39.Scharlemann JPW, et al. Global Data for Ecology and Epidemiology: A Novel Algorithm for Temporal Fourier Processing MODIS Data. PLoS One. 2008;3:e1408. doi: 10.1371/journal.pone.0001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, http://www.r-project.org (2018).
- 41.Hijmans RJ. raster: Geographic Data Analysis and Modeling. R package version. 2017;2:6–7. [Google Scholar]
- 42.Gray JS, Lohan G. The development of a sampling method for the tick Ixodes ricinus and its use in a redwater fever area. Ann. Appl. Biol. 1982;101:421–427. doi: 10.1111/j.1744-7348.1982.tb00842.x. [DOI] [Google Scholar]
- 43.Klitgaard K, Chriél M, Isbrand A, Jensen TK, Bødker R. Identification of Dermacentor reticulatus Ticks Carrying Rickettsia raoultii on Migrating Jackal, Denmark. Emerg. Infect. Dis. 2017;23:2072–2074. doi: 10.3201/eid2312.170919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaenson TGT, et al. First evidence of established populations of the taiga tick Ixodes persulcatus (Acari: Ixodidae) in Sweden. Parasit. Vectors. 2016;9:377. doi: 10.1186/s13071-016-1658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klitgaard K, et al. Screening for multiple tick-borne pathogens in Ixodes ricinus ticks from birds in Denmark during spring and autumn migration seasons. Ticks Tick. Borne. Dis. 2019;10:546–552. doi: 10.1016/j.ttbdis.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Cowling DW, Gardner IA, Johnson WO. Comparison of methods for estimation of individual-level prevalence based on pooled samples. Prev. Vet. Med. 1999;39:211–25. doi: 10.1016/S0167-5877(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 47.Kjær LJ, et al. A large-scale screening for the taiga tick, Ixodes persulcatus, and the meadow tick, Dermacentor reticulatus, in southern Scandinavia, 2016. Parasit. Vectors. 2019;12:338. doi: 10.1186/s13071-019-3596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kjær LJ, 2020. Spatial data of Ixodes ricinus instar abundance and nymph pathogen prevalence, Scandinavia, 2016–2017. figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Reye AL, 2013. Pathogen prevalence in questing and feeding ticks. figshare. https://plos.figshare.com/articles/_Pathogen_prevalence_in_questing_and_feeding_ticks_/174458
- Estrada-Peña A, De La Fuente J. 2016. Data from: Species interactions in occurrence data for a community of tick-transmitted pathogens. Dryad. [DOI] [PMC free article] [PubMed]
- Estrada-Peña A. 2019. Data from: The dataset of ticks in South America. Dryad. [DOI]
- Kjær LJ, 2020. Spatial data of Ixodes ricinus instar abundance and nymph pathogen prevalence, Scandinavia, 2016–2017. figshare. [DOI] [PMC free article] [PubMed]