Abstract
In China, the prevalence of Clonorchis sinensis (C. sinensis) infections is only evaluated at the provincial level by national sampling surveys, and data from villages and counties are still lacking. In this study, we conducted a cross-sectional survey in 10 villages located along the Lalin River in northeast China. Clonorchiasis was diagnosed using a modified Kato–Katz method that detects the C. sinensis egg in stools. A total of 3,068 persons were screened and 2,911 were recruited for the study. Overall, the prevalence of C. sinensis infection was 29.3%. Among 175 participants who were cured after antiparasitic treatment, 54 (30.86%) were re-infected in this survey. After calibration of potential confounders, male gender, occupation as a farmer, smoking, and occasionally or frequently eating raw fish were independent risk factors for C. sinensis infection. The results of laboratory examinations in the C. sinensis/hepatitis B or C virus co-infection group were similar to those in the hepatitis B or C virus mono-infection groups. In conclusion, C. sinensis is highly endemic in villages along the Lalin River, and the primary route of infection is the consumption of raw freshwater fish. Co-infection with C. sinensis did't aggravate the clinical manifestations of viral hepatitis in this cross-sectional study.
Subject terms: Gastroenterology, Health care
Introduction
Clonorchis sinensis (C. sinensis) is an important food-borne zoonotic parasite that has infected approximately 15 million people worldwide; countries in eastern and Southeast Asia, including China, Japan, Korea, and Vietnam, account for a large proportion of infections1-4. There are 13 million people infected with Clonorchis sinensis in China, which is the country with the largest number of infections in the world5,6. Three large-scale clonorchiasis investigations have been conducted in mainland China. The first national parasite investigation, which covered 30 provinces/municipalities/autonomous regions (P/M/As) from 1988 to 1992 (hereinafter referred to as 1992), showed that the prevalence of clonorchiasis was 0.37%7. The prevalence increased to 0.58% in the second national parasite investigation, which included 31 P/M/As from 2001 to 20048. Another special clonorchiasis investigation in 27 endemic P/M/As was conducted during the years 2001–2004 and showed that the prevalence was 2.40%, with 12.49 million people infected8,9. Jilin Province is one of the major endemic regions in China8. The prevalence of Clonorchis sinensis infections is only evaluated at the provincial level by national sampling surveys in China, and data from villages and counties are still lacking. The current study results supplement C. sinensis infection data in rural areas along rivers in Jilin Province.
Persistent and chronic infections of C. sinensis often cause the development and progression of hepatobiliary diseases, such as cholangitis, cholelithiasis, cholecystitis, pancreatitis, hepatic fibrosis, liver cancer, and cholangiocarcinoma (CCA)10. Cholangiocarcinoma is the most severe complication of C. sinensis infection11,12. Although great progress has been made in the treatment of cholangiocarcinoma in recent years, 5-year survival rates remains very low13. A Korean study reported that C. sinensis infection causes 25% of CCA cases in endemic areas; approximately one-tenth of CCA cases are estimated to be caused by C. sinensis infection, and the risk of CCA in areas hyper-endemic for C. sinensis is higher than in other areas14. However, the cause of most cholangiocarcinomas is still not identified. Recently, researchers have reported that CCA may be caused by viral hepatitis B and C or hepatolithiasis cirrhosis15,16. Similar to C. sinensis infection, the prevalence rates of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are also considerably high in China. HBV, HCV and C. sinensis target the same organ, but little is known about whether co-infection can aggravate symptoms, and current data regarding whether there is any association between co-infection and hepatocellular carcinoma or cholangiocarcinomas are limited.
This cross-sectional study obtained baseline data for our long-term follow-up observation in areas highly endemic for C. sinensis. It is expected that the follow-up data of C. sinensis infection and cholangiocarcinoma cases will aid in the evaluation of the disease burden of C. sinensis infection and ultimately promote C. sinensis prevention. We also focused on whether C. sinensis co-infection affected the disease state of HBV and HCV infections. We look forward to clarifying the impact of hepatitis virus on the occurrence and development of cholangiocarcinoma in a long-term follow-up study.
Results
Demographic characteristics and prevalence of C. sinensis infection
Of the 3,068 participants who participated in the survey, 2,911 (male, 1,496 [51.4%]; female, 1,415 [48.6%]) participants were enrolled in the study. 157 participants were excluded due to incomplete or unreliable information. Out of 2,911 study participants, 2,056 (70.6%) were aged between 31 and 60 years. There were 1,496 (51.4%) males and 1,415 (48.6%) females. In all, the prevalence of subjects infected with C. sinensis was 29.3% (95% CI: 27.6%-31.0%). In Table 1, the positive rates of C. sinensis infection varied markedly in the different age groups. The highest C. sinensis positivity rate(30.7%) was in group aged 31–60 years, whereas subjects under 30 years had the lowest prevalence rate (16.8%) of C. sinensis-positive results. Furthermore, gender differences in the C. sinensis positivity rates were observed, with higher rates found in males (39.4%) than in females (18.7%). Among 2,911 individuals, 2,759 tests included assays for detecting serum HCV antibodies and HBV serological markers. After 152 cases without HBV and HCV tests were excluded, 165 (5.99%) of the remaining 2,759 participants were hepatitis B surface antigen (HBsAg) positive, and 850 (30.8%) of 2,759 participants were HCV antibody positive.
Table 1.
Univariate and multivariate analysis of socio-demographic characteristics by Clonorchis sinensis infection among residents in riverside villages.
| Variable | Total N | Clonorchiasis + N (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | OR | 95% CI for OR | P value | |||
| Total | 2,911 | 854 (29.3) | – | – | – | – | – |
| Sex | |||||||
| Female | 1,415 | 265 (18.7) | – | – | Reference | Reference | Reference |
| Male | 1,496 | 589 (39.4) | 2.818 (2.380–3.337) | < 0.001 | 1.95 | 1.58–2.40 | < 0.001 |
| Age | |||||||
| ≤ 30 years old | 95 | 16 (16.8) | – | 0.005 | – | – | – |
| 31–60 | 2056 | 631 (30.7) | 2.186 (1.267–3.772) | 0.004 | – | – | – |
| ˃ 60 years old | 760 | 207 (27.2) | 1.848 (1.055–3.237) | 0.03 | – | – | – |
| Education | |||||||
| College | 167 | 29 (17.4) | – | < 0.001 | – | – | – |
| Middle school | 1,015 | 339 (33.4) | 2.386 (1.566–3.636) | < 0.001 | – | – | – |
| Primary school | 1729 | 486 (28.1) | 1.861 (1.230–2.814) | 0.003 | – | – | – |
| Occupation | |||||||
| Non-farmer | 278 | 49 (17.6) | – | – | Reference | Reference | Reference |
| Farmer | 2,633 | 805 (30.6) | 2.058 (1.495–2.833) | 0.000 | 2.31 | 1.66–3.22 | < 0.001 |
| Cigarette smoking | |||||||
| No | 1675 | 432 (25.8) | – | 0.000 | Reference | Reference | Reference |
| Yes | 1,092 | 384 (35.2) | 1.561 (1.322–1.842) | < 0.001 | 1.1 | 0.92–1.32 | 0.302 |
| Quit smoking | 144 | 38 (26.4) | 1.031 (0.701–1.518) | 0.875 | 0.68 | 0.45–1.03 | 0.069 |
| Alcohol consumption | |||||||
| No | 1985 | 463 (23.3) | – | 0.000 | Reference | Reference | Reference |
| Yes | 816 | 352 (43.1) | 2.494 (2.097–2.965) | 0.000 | 1.31 | 1.06–1.63 | 0.013 |
| quit drinking | 110 | 39 (35.5) | 1.806 (1.205–2.705) | 0.004 | 1.11 | 0.71–1.73 | 0.649 |
| Eating raw fish | |||||||
| Never | 1,049 | 154 (14.7) | – | 0.000 | Reference | Reference | Reference |
| Occasionally | 1787 | 660 (36.9) | 3.403 (2.797–4.141) | 0.000 | 2.58 | 2.10–3.18 | < 0.001 |
| Frequently | 75 | 40 (53.3) | 6.642 (4.090–10.785) | 0.000 | 3.93 | 2.38–6.51 | < 0.001 |
| Reared cats and dogs as pets or guardians | |||||||
| No | 1928 | 544 (28.2) | – | – | – | – | – |
| Yes | 983 | 310 (31.5) | 1.172 (0.992–1.385) | 0.063 | – | – | – |
| HBV infection | |||||||
| No | 2,594 | 753 (29.0) | – | 0.039 | – | – | – |
| Yes | 165 | 62 (37.6) | 1.471 (1.062–2.039) | 0.02 | – | – | – |
| Unclear | 152 | 39 (25.7) | 0.844 (0.581–1.226) | 0.373 | – | – | – |
| HCV infection | |||||||
| No | 1909 | 578 (30.3) | – | 0.263 | |||
| Yes | 850 | 237 (27.9) | 0.890 (0.745,1.06) | 0.203 | |||
| Unclear | 152 | 39 (25.7) | 0.795 (0.545,1.159) | 0.231 | |||
Univariate analysis of variables associated with C. sinensis infection
The results of the univariate comparisons are presented in Table 1. The results revealed that 1,862 (64.0%) participants reported eating raw fish. Most of them ate raw fish less than 2 times per week, and only 75 (4.0%) of them ate raw fish frequently (more than 2 times per week). Participants with a habit of eating raw fish were more likely to have C. sinensis infection than those who did not eat raw fish; the higher the frequency was, the greater the risk. Male gender (OR = 2.818, 95% CI: 2.380–3.337), older age (OR = 2.186, 95% CI: 1.267–3.772; OR = 1.848, 95% CI: 1.055–3.237), a low literacy level (OR = 2.386, 95% CI: 1.566–3.636; OR = 1.861, 95% CI: 1.230–2.814), cigarette smoking (OR = 1.561, 95% CI: 1.322–1.842), alcohol consumption (OR = 2.494, 95% CI: 2.097–2.965; OR = 1.806, 95% CI: 1.205–2.705) and occupation as a farmer (OR = 2.058, 95% CI: 1.495–2.833) were significantly associated with an increased risk of C. sinensis infection.
Multivariate analysis of variables associated with C. sinensis infection
Age, gender, occupation, education, cigarette smoking, alcohol consumption, HBV infection and eating raw fish, which were associated with C. sinensis infection in univariate analysis, were included in the multiple logistic regression model. After multivariate correction, male gender (OR = 1.95, P < 0.001), occupation as a farmer (OR = 2.31, P < 0.001), alcohol consumption (OR = 1.31, P = 0.013), and eating raw fish occasionally (OR = 2.58, P < 0.001) and frequently (OR = 3.93, P < 0.001) were independently associated with C. sinensis infection in the multivariate analysis (Table 1). Eating raw fish had the most significant associations with C. sinensis infection, and the odds ratios indicated that eating raw fish frequently (OR = 6.642, 95% CI:4.090–10.785) were at highest risk for C. sinensis infection. Age, cigarette smoking and HBV infection were not found to be significantly associated with C. sinensis infection in this study.
C. sinensis/HBV or C. sinensis/HCV co-infection
Among 854 participants with C. sinensis infection (C. sinensis eggs in stools), 46 were co-infected with HBV (HBsAg positive), 60 were co-infected with HCV (HCV-RNA positive) and 1 was co-infected with HBV and HCV (HBsAg positive and HCV-RNA positive). Age and gender were used to match co-infected and mono-infected individuals. Compared with the C. sinensis mono-infection group, the AST to platelet ratio index(APRI) levels in the HBV co-infection and HBV mono-infection group were significantly higher (P = 0.049). The HBV/C. sinensis co-infection group had a higher eosinophilic granulocyte level than the HBV mono-infection group. There is no significant difference of other indicators in the three groups (Table 2). The baseline characteristics of the HCV/C. sinensis co-infection group are presented in Table 3. The HCV/C. sinensis co-infection group had similar alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), albumin (ALB), glucose (GLU) and total platelet (PLT) levels and AST to PLT ratio index (APRI) and fibrosis-4 scores (FIB-4) compared to those of the HCV mono-infected group. ALT, AST, total protein (TP), total bilirubin (TBIL), indirect bilirubin (IBIL), GGT, ALP, APRI and FIB-4 scores were significantly higher in the co-infection group than in the C. sinensis mono-infected group. The differences between the two groups were statistically significant. The albumin to globulin (A/G), triglyceride (TG), cholesterol, low-density lipoprotein (LDL), white blood cell (WBC), and PLT levels were higher in the mono-infection group than in the co-infection group. The differences were statistically significant.
Table 2.
Baseline characteristics of HCV/C. sinensis co-infection group and HCV or C. sinensis mono-infection groups.
| Hepatitis B (n = 46) | HBV/C. sinensis coinfection (n = 46) | C. sinensis (n = 46) | P1 | P2 | |
|---|---|---|---|---|---|
| Age (years, mean ± SD) | 50.59 ± 10.01 | 50.65 ± 9.81 | 50.63 ± 9.76 | 0.999 | 0.999 |
| Sex (Male, %) | 31 (67.4) | 31 (67.4) | 31 (67.4) | 1.000 | 1.000 |
| ALT (IU/mL) | 41.0 (34.3, 49.5) | 38.5 (32.8, 51.0) | 39.5 (30.5, 49.3) | 0.56 | 0.912 |
| AST (IU/mL) | 33.0 (26.0, 42.5) | 32.5 (27.8, 39.0) | 29.5 (25.0, 40.3) | 0.922 | 0.137 |
| Total protein (g/L) | 78.0 (72.8, 84.3) | 77.0 (72.0, 83.3) | 78.5 (72.0, 81.3) | 0.46 | 0.587 |
| ALB (g/L) | 44.5 (41.0, 48.3) | 44.0 (41.0, 47.0) | 44.5 (42.0, 46.0) | 0.45 | 0.543 |
| ALB/GLB | 1.40 (1.08, 1.52) | 1.36 (1.19, 1.54) | 1.34 (1.17, 1.59) | 0.934 | 0.936 |
| TBIL (µmol/L) | 18.0 (13.0, 23.0) | 18.0 (14.0, 22.0) | 17.0 (13.8, 21.0) | 0.745 | 0.766 |
| IBIL (µmol/L) | 11.0 (6.75, 16.0) | 9.0 (7.0, 14.0) | 10.0 (6.0, 13.0) | 0.484 | 0.829 |
| GGT (U/L) | 28.5 (18.8, 59.5) | 31.5 (21.8, 74.3) | 33.5 (24.8, 65.8) | 0.292 | 0.614 |
| ALP (U/L) | 69.0 (50.0, 83.0) | 61.5 (45.5, 72.3) | 67.0 (53.8, 79.5) | 0.184 | 0.183 |
| Creatinine (μmol/L) | 62.0 (54.5, 73.0) | 64.5 (58.0, 70.5) | 63.5 (55.5, 75.3) | 0.763 | 0.699 |
| BUN (mmol/L) | 5.1 (4.4, 5.9) | 5.25 (4.50, 6.03) | 5.5 (4.6, 6.2) | 0.716 | 0.39 |
| Uric acid (µmol/L) | 354 (304, 435) | 362.0 (308.3, 422.8) | 360.0 (311.5, 435.3) | 0.679 | 0.885 |
| TG (mmol/L) | 1.34 (0.95, 1.83) | 1.52 (1.11, 2.20) | 1.79 (1.19, 2.91) | 0.226 | 0.328 |
| Cholesterol (mmol/L) | 3.45 (2.80, 3.88) | 3.60 (3.10, 4.30) | 3.90 (3.30, 4.50) | 0.088 | 0.148 |
| LDL (mmol/L) | 1.23 (0.91, 1.56) | 1.40 (0.88, 2.02) | 1.45 (1.12, 2.08) | 0.517 | 0.293 |
| GLU (mmol/L) | 3.40 (3.10, 3.55) | 3.80 (3.30, 4.90) | 3.50 (3.25, 4.15) | 0.388 | 0.873 |
| WBC (*109/L) | 6.96 (6.22, 8.27) | 6.78 (5.75, 7.90) | 6.73 (5.81, 7.63) | 0.571 | 0.932 |
| RBC (*1,012/L) | 4.64 (4.23, 5.15) | 4.64 (4.16, 5.00) | 4.67 (4.40, 4.97) | 0.363 | 0.679 |
| PLT (*109/L) | 159.5 (133.8, 190.0) | 161.0 (129.8, 198.0) | 178.5 (145.8, 216.5) | 0.767 | 0.135 |
| Lymphocyte (*109/L) | 2.27 (1.89, 3.04) | 2.41 (1.95, 3.04) | 2.38 (1.96, 2.71) | 0.87 | 0.582 |
| Neutrophil (*109/L) | 3.75 (3.27, 4.22) | 3.51 (2.80, 4.30) | 3.29 (2.90, 4.26) | 0.461 | 0.746 |
| Eosinophil count (*109/L) | 0.15 (0.11, 0.21) | 0.18 (0.12, 0.34) | 0.19 (0.12, 0.42) | 0.509 | 0.673 |
| Haemoglobin (g/L) | 153.5 (139.0, 169.3) | 155.5 (134.5, 167.0) | 152.5 (144, 160) | 0.54 | 0.749 |
| lg10 (HBV-DNA) | 3.39 (2.30, 4.63) | 2.91 (2.36, 5.60) | – | 1 | – |
| APRI | 0.45 (0.31, 0.62) | 0.47 (0.33, 0.65) | 0.38 (0.28, 0.47) | 0.904 | 0.049 |
| FIB-4 | 1.70 (1.12, 2.44) | 1.65 (1.08, 2.69) | 1.28 (1.02, 1.80) | 0.907 | 0.082 |
ALT alanine aminotransferase, AST aspartate aminotransferase, ALB albumin, GLB globulin, ALB/GLB albumin to globulin, TBIL total bilirubin, IBIL indirect bilirubin, GGT gamma-glutamyl transpeptidase, ALP alkaline phosphatase, BUN blood urea nitrogen, TG triglyceride, LDL low-density lipoprotein, GLU glucose, WBC white blood cell, RBC red blood cell, PLT platelet, APRI, levels and AST to PLT ratio index, FIB-4 fibrosis-4 scores.
Table 3.
Baseline characteristics of HBV/C. sinensis co-infection group and HBV or C. sinensis mono-infection groups.
| Hepatitis C (n = 59) | HCV/C. sinensis coinfection (n = 59) | C. sinensis (n = 59) | P1 | P2 | |
|---|---|---|---|---|---|
| Age (years, mean ± SD) | 57.78 ± 7.91 | 57.54 ± 8.47 | 57.46 ± 8.33 | 0.876 | 0.956 |
| Sex (Male, %) | 46 (78.0) | 46 (78.0) | 46 (78.0) | 1.000 | 1.000 |
| ALT (IU/mL) | 54.0 (41.0, 81.0) | 62.0 (42.0, 103.0) | 38.0 (31.0, 48.0) | 0.236 | < 0.001 |
| AST (IU/mL) | 45.0 (32.0, 67.0) | 54.0 (38.0, 87.0) | 28.0 (24.0, 35.0) | 0.052 | < 0.001 |
| Total protein (g/L) | 78.0 (72.0, 85.0) | 79.0 (75.0, 85.0) | 77.0 (72.0, 82.0) | 0.292 | 0.031 |
| ALB (g/L) | 43.0 (41.0, 45.0) | 43.0 (42.0, 45.0) | 44.0 (42.0, 46.0) | 0.184 | 0.539 |
| ALB/GLB | 1.22 (1.07, 1.43) | 1.18 (1.09, 1.35) | 1.36 (1.23, 1.53) | 0.51 | 0.001 |
| TBIL (µmol/L) | 17.0 (12.0, 22.0) | 19.0 (14.0, 24.0) | 16.0 (13.0, 20.0) | 0.164 | 0.036 |
| IBIL (µmol/L) | 10.0 (6.0, 13.0) | 11.0 (7.0, 15.0) | 9.0 (6.0, 12.0) | 0.111 | 0.025 |
| GGT (U/L) | 53.0 (29.0, 104.0) | 69.0 (37.0, 143.0) | 36.0 (25.0, 69.0) | 0.114 | 0.001 |
| ALP (U/L) | 73.0 (61.0, 93.0) | 78.0 (61.0, 93.0) | 70.0 (56.0, 81.0) | 0.577 | 0.035 |
| Creatinine (μmol/L) | 65.0 (58.0, 73.0) | 65.0 (56.0, 75.0) | 66.0 (58.0, 74.0) | 0.732 | 0.724 |
| BUN (mmol/L) | 5.5 (4.5, 6.6) | 5.80 (4.60, 6.60) | 5.5 (4.8, 6.2) | 0.628 | 0.554 |
| Uric acid (µmol/L) | 363.0 (330.0, 404.0) | 377.0 (315.0, 424.0) | 365.0 (305.0, 438.0) | 0.317 | 0.844 |
| TG (mmol/L) | 1.38 (0.85, 1.73) | 1.28 (0.89, 2.04) | 1.54 (1.19, 2.54) | 0.861 | 0.020 |
| Cholesterol (mmol/L) | 3.30 (2.80, 3.70) | 3.30 (2.90, 4.00) | 3.80 (3.20, 4.40) | 0.425 | 0.003 |
| LDL (mmol/L) | 1.22 (0.91, 1.68) | 1.27 (0.87, 1.77) | 1.66 (1.16, 2.23) | 0.998 | 0.011 |
| GLU (mmol/L) | 3.85 (3.53, 4.05) | 4.10 (3.60, 4.40) | 3.70 (3.35, 6.48) | 0.377 | 0.535 |
| WBC (*109/L) | 6.28 (4.82, 7.73) | 6.02 (5.12, 7.30) | 6.81 (5.83, 8.09) | 0.72 | 0.012 |
| RBC (*1,012/L) | 4.49 (4.28, 4.97) | 4.57 (4.18, 4.93) | 4.64 (4.34, 4.85) | 0.901 | 0.825 |
| PLT (*109/L) | 144.0 (102.0, 179.0) | 136.0 (106.0, 176.0) | 180.0 (147.0, 215.0) | 0.677 | < 0.001 |
| Lymphocyte (*109/L) | 2.12 (1.73, 2.68) | 2.00 (1.73, 2.49) | 2.30 (1.97, 2.53) | 0.62 | 0.029 |
| Neutrophil (*109/L) | 3.28 (2.29, 4.66) | 3.30 (2.63, 4.03) | 3.62 (2.88, 4.40) | 0.733 | 0.039 |
| Eosinophil count (*109/L) | 0.15 (0.09, 0.20) | 0.17 (0.11, 0.28) | 0.25 (0.16, 0.47) | 0.177 | 0.004 |
| Haemoglobin (g/L) | 152.0 (144.0, 161.0) | 153.0 (142.0, 164.0) | 153.0 (145.0, 161.0) | 0.784 | 0.981 |
| lg (HCV-RNA) | 6.43 (5.71, 6.92) | 6.27 (5.49, 6.82) | – | 0.587 | – |
| HCV-Genotype (1b, %) | 32 (54.2) | 27 (45.8) | – | 0.361 | – |
| APRI | 0.74 (0.49, 1.47) | 0.88 (0.55, 1.88) | 0.35 (0.28, 0.52) | 0.156 | < 0.001 |
| FIB-4 | 2.54 (1.65, 4.22) | 3.01 (1.93, 5.39) | 1.52 (1.18, 2.08) | 0.188 | < 0.001 |
ALT alanine aminotransferase, AST aspartate aminotransferase, ALB albumin, GLB globulin, ALB/GLB albumin to globulin, TBIL total bilirubin, IBIL indirect bilirubin, GGT gamma-glutamyl transpeptidase, ALP alkaline phosphatase, BUN blood urea nitrogen, TG triglyceride, LDL low-density lipoprotein, GLU glucose, WBC white blood cell, RBC red blood cell, PLT platelet, APRI, levels and AST to PLT ratio index, FIB-4 fibrosis-4 scores.
Repeated infection
Praziquantel is the recommended drug for the treatment of clonorchiasis17. Since local disease control often distributes local therapeutic drugs, among our participants, 766 people received quinolone treatment (25 mg/kg praziquantel thrice daily for 1 or 2 consecutive days); 175 were cured after treatment, 561 were not reviewed, and 30 were not cured. Among the 175 people who were cured after the first treatment, this study found that 54 people were re-infected, accounting for 30.86% (Fig. 1).
Figure 1.
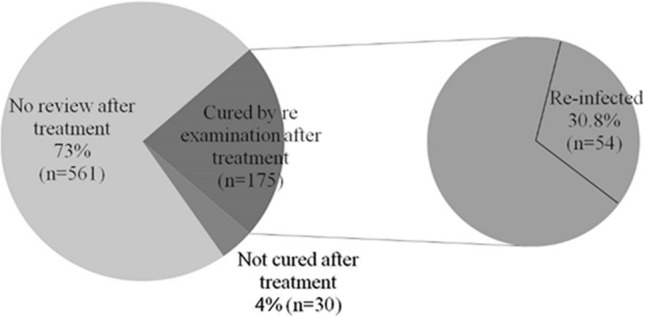
Clonorchis sinensis re-infection rate in this survey. Among 766 people who received quinolone treatment, 175 were cured after treatment, 561 were not reviewed, and 30 were not cured at one-month follow-up. Among the 175 people who were cured after the first treatment, 54 people were re-infected, accounting for 30.86%.
Discussion
In this study, the prevalence of C. sinensis infection in riverside villages in Fuyu City was 29.3%, which is tenfold higher than the prevalence of C. sinensis infection in the known endemic areas of China (2.4%)8. Our results showed that the prevalence of C. sinensis infection was higher in males than in females. This is because males are more likely to eat raw fish and shrimp than are females. A similar difference in the prevalence of C. sinensis infection between men and women has been shown in previous C. sinensis infection investigations in China from 2002 to 20048. Infection was identified in all age groups, with the highest prevalence in the age group of 31–60 years (30.7%). These results are similar to others, which indicated that adults had high infection rates in certain provinces in which the main infection method was raw fish consumption. Regarding occupation, there was a higher prevalence among farmers (30.6%, 805/2,633) than among non-farmers (17.6%, 49/278). This may be related to a relatively low literacy level and lack of personal hygiene knowledge in farmers. For example, many farmers use water directly from the river as domestic or drinking water, which may increase the chance of C. sinensis infection. Further, the farmers in our study were likely to have unhealthy dietary habits, which has been reported in other studies18. Our previous study reported that the prevalence of HCV infection was high among residents in Fuyu19. There are many HCV and C. sinensis co-infections in Fuyu, and the potential interaction between the two diseases has not received wide attention. Trepo C et al. reported that hepatitis B infection is highly prevalent in many clonorchiasis-endemic areas20. In the present study, we also found that C. sinensis infection was highly prevalent in HBsAg-positive participants (37.6% vs. 29.0%), which confirms the above results.
In the multivariate model, independent risk factors associated with C. sinensis infection were male gender, occupation as a farmer, drinking alcohol and eating raw fish. The risk factor most strongly associated with C. sinensis infection was eating raw fish, which supports the hypothesis that eating raw fish was an important predisposing factor for the establishment of a large reservoir of C. sinensis infection in Fuyu City. People who habitually eat raw or undercooked fish and eat outside frequently have a higher infection risk ratio than those who do not. In our findings, a greater proportion of men have this unhealthy eating habit than women, as do farmers compared to people in other occupations, which is similar to other studies21,22. In the present study, alcohol consumption was significantly associated with an increased risk of C. sinensis infection in the multivariate analysis, and this result was consistent with another report23 since the local residents drank alcohol while eating raw fish and shrimp. Additionally, more males (50.3%) drank while eating raw fish in comparison with females (5%, P = 0.000); thus, this outcome could have resulted from the influence of gender. This finding reflects Chinese culture, in which it is more common for men than for women to drink alcohol. In addition, many people mistakenly think that alcoholic drink can kill bacteria and parasites in the food, and so they are not afraid of being infected by pathogens from potentially contaminated food. This also explains why drinking alcohol is one of the risk factors for liver fluke infection.
Our previous study found that Fuyu City is highly endemic for hepatitis C19. This study also confirms that result, and among 854 C. sinensis-infected participants, 237 (27.8%) were anti-HCV positive and 59 (6.9%) were HCV-RNA positive. We compared the results of laboratory examinations between the co-infection group and mono-infection group and found that the results of laboratory examinations in the C. sinensis/HBV group were similar to those in the C. sinensis and HBV mono-infection groups. Xu's study indicated that the C. sinensis/HBV co-infection group presented decreased liver function and increased HBV DNA copies24. However, these results were not observed in our research. Moreover, there was no significant difference in liver function between the C. sinensis/HBV co-infection group and the C. sinensis mono-infection group in this study. The reason may be that the HBV viral load of HBV-infected people in the general population is low (approximately 70% of HBV DNA < 1,000 IU/mL), most of them are in the immune tolerance stage, and liver function is at normal levels.
Among the C. sinensis/HCV co-infection, C. sinensis mono-infection and HCV mono-infection groups, the results of laboratory examinations were significantly different between the C. sinensis/HCV co-infection and C. sinensis mono-infection groups, but there was no significant difference between the C. sinensis/HCV co-infection and HCV mono-infection groups. This finding suggests that clonorchiasis did not aggravate the clinical manifestations of viral hepatitis. This may simply be a cross-sectional result, and it is not clear whether the long-term outcome will be the same. According to the association between eosinophil count and intensity of C. sinensis infection18, the C. sinensis mono-infection group had a heavier burden of C. sinensis than the C. sinensis/HCV co-infection group, but this result should be further confirmed. There was no difference in liver function indicators between the C. sinensis/HBV co-infection group and the C. sinensis mono-infection group, while the liver function indicators in the C. sinensis/HCV co-infection group were significantly higher than those in the C. sinensis mono-infection group. The reason for this difference may be that liver fibrosis was mild in the C. sinensis/HBV co-infection group (middleAPRI = 0.45, middleFIB4 = 1.65), while liver fibrosis in the C. sinensis/HCV co-infection group was severe in this study (middleAPRI = 0.88, middleFIB4 = 3.01).
The C. sinensis prevention and control strategies usually involve a combination of two or more measures, including health and diet safety education, environmental hygiene improvement and promotion of good personal hygiene practices, etc.25. Avoiding eating raw or undercooked fish and shrimp foods is the most effective way to avoid C. sinensis infections26. However, most residents in the epidemic areas find it difficult to change their habit of eating raw fish/shrimp. In our study, the population in Fuyu showed high reinfection rates after effective treatment. Therefore, people should pay more attention on the safety of freshwater fish. The distribution of freshwater fish and snails infected with metacercaria should be investigated in endemic regions, and infected water sources need to be treated in a timely manner. Finally, infected fish cannot be sold on the market27. The high reinfection rate suggests that exposure prevention and treatment of the second intermediate host of clonorchiasis are urgently needed. Human clonorchiasis infection rates are positively related to animal (e.g., dog, cat, etc.) infection. Animals that defecate freely lead to faster transfer of eggs into rivers and ponds, shortening the parasite’s life cycle, and finally resulting in the aggravation of the epidemic28. In rural areas, removing toilets and swine enclosures from fishpond areas are helpful measures for environmental improvement27.
In addition to focusing on the prevention of C. sinensis, the interaction between C. sinensis and its hosts and the mechanism involved in cholangiocarcinoma remain unclear. We need to further clarify the molecular mechanisms and high-risk population of C. sinensis-related cholangiocarcinoma, identify feasible advanced technologies29,30 that can screen for molecular markers for early diagnosis and disease progression monitoring, and identify drug targets.
The use of a non-random convenience sampling and self-reported risk behaviours are the key limitations of our study. The study sample may not be representative of the general population in riverside villages in Jilin Province. At the same time, the self-reporting by participants may lead to subjects' recall bias. To minimise recall bias, questions related to risk behaviour (such as "have you consumed raw fish" and "how often you consume raw fish") were included in the questionnaire. Despite these limitations, the survey demonstrated a high prevalence of C. sinensis infections in riverside villages in Jilin Province, where eating raw fish was the most important risk factor for C. sinensis transmission.
In conclusion, ten villages along the Lalin River in Desheng County are endemic areas for clonorchiasis, and the main route of infection is consuming raw freshwater fish. Co-infection with C. sinensis did not aggravate the clinical manifestations of viral hepatitis in this cross-sectional study, but the long-term outcomes require a follow-up survey. The high reinfection rate in our study suggests that changing dietary habits is necessary to prevent C. sinensis infection.
Methods
Study population and recruitment
In August 2017, we conducted a cross-sectional survey in 10 villages along the Lalin River in Fuyu City, Jilin Province, China31. The detailed survey method is similar to the HCV infection investigation we conducted in 201219. First, we selected 10 villages (Beijiang, Desheng, Hejiang, Niuyingzi, Qianyang, Xiaojia, Xiaoweizi, Huandong, Linhe, and Zaixing) along the Lalin River. In the second stage, we contacted and obtained demographic information from the village chiefs. In the third stage, more than 100 village committee members and rural doctors in all 10 villages were recruited and trained for 2 days on effective publicity for the survey. They then publicised the study in their villages using recruitment cards and flyers, which was necessary for the investigators to perform cluster sampling. In the fourth stage, our research team went to each household to count the residents and distribute faecal bags. The survey was conducted for 2–3 days at each survey location. The enrolled participants were encouraged to inform their peers about the study. After screening for eligibility and obtaining informed consent, each participant completed a questionnaire. Participants who did not complete the questionnaire were excluded from the study.
Data collection
Each participant was asked to complete a questionnaire that included information on demographic variables (age, gender and race), risk factors for C. sinensis infection, and history of other diseases (e.g., presence or absence of hypertension, thyroid disease, diabetes, previous history of surgery, etc.). Behavioural data regarding dietary habit use (especially eat raw fish or not and frequency of use), alcohol consumption, smoking and infection history of C. sinensis were also collected. People who had been confirmed to be infected with C. sinensis and treated with praziquantel before this cross-sectional investigation were asked to provide stool samples for testing of C. sinensis eggs one month after receiving treatment. Those people who were negative for 3 consecutive days one month after treatment were considered to be cured. In this investigation, people who were cured and were subsequently diagnosed with C. sinensis infection were considered to have a repeated infection.
C. sinensis and virus detection
A modified Kato–Katz method was used to detect the Clonorchis sinensis eggs32. HBsAg, antibodies against HBsAg (anti-HBsAg), hepatitis B core antigen (anti-HBc) and hepatitis C antibody (anti-HCV) were tested in blood samples using an Abbott ARCHITECT i2000SR Immunoassay System (Abbott Laboratories; Abbott Park, IL, USA). Serum HBV DNA and HCV RNA were tested using an HBV DNA assay (Haoyuan Co., Ltd., Shanghai, China) and HCV RNA assay (Haoyuan Co., Ltd., Shanghai, China), respectively. The lower detection limits of these two kits are 50 IU/mL and 100 IU/mL, respectively. The above virological tests were completed at the clinical laboratory of the First Hospital of Jilin University.
Statistical analysis
We conducted statistical analysis using SPSS software package 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as medians and interquartile ranges or means and standard deviations, as appropriate, and were compared using Student’s t-test or the Mann–Whitney U-test. Categorical variables were compared using the chi-square test or Fisher’s exact test. Variables with statistical significance (P ≤ 0.05) in the univariate analysis were considered for inclusion in the multivariable logistic regression model. Then, we performed backward stepwise logistic regression to screen independent risk factors retained in the final model. A P-value ≤ 0.05 was considered statistically significant; odds ratios (ORs) with 95% confidence intervals (95% CIs) are presented to demonstrate the strength and direction of these associations.
Ethical considerations
The study was approved by the Ethics Committee at the First Hospital of Jilin University (2017-023), Changchun, China. Each participant signed an informed consent form before participating in the survey. During the investigation, we protected participants from potential ethical violations and complied with data confidentiality agreements. Data collection was conducted at the township health centre. During the questionnaire process, the interviewee and interviewer were required to be present at the same time, and only the two of them were present to ensure the maintenance of confidentiality. Those agreeing to take part in the study were asked to have their blood collected for further evaluation of anti-HCV and anti-HBV activities, always in the presence of the researcher. The collection of tissues and inclusion of patients were approved by the Ethics Committee at the First Hospital of Jilin University (2017-023), Changchun, China. Moreover, this study was in accordance with the regulations set by Chinese law for the use of human material for research33.
Acknowledgements
We are grateful to the Center for Disease Control in Fuyu City for supporting this study. This work was sponsored by the National Key Research Plan "precision medicine research" key project (2017YFC0908103), the National Natural Science Foundation of China (Grants No. 81972265), the National Natural Science Foundation of Jilin Province (20200201324JC, 20200201532JC), the National Science and Technology Major Project (2017ZX10202202), the JLU Norman Bethune Research Plan (2018B32), the Program for JLU Science and Technology Innovative Research Team (2017TD-08) and the Fundamental Research Funds for the Central Universities.
Author contributions
Y.H.G., Y.Q.L., X.W.L., T.Z., G.Y., Y.W., Y.S., Y.X., J.M., X.L., C.J., H.M.M., L.T.L., Q.Y., X.Y., F.P., Y.X.Z., W.G.Q., X.Z., T.Q.R., L.L.L., J.M.Z., F.YZ., A.Y., Y.P., H.Q.X., and J.Q.N. conducted the cross-sectional survey in this study. X.M.C., X.M.W., X.Z.G., R.H.W., Y.Y.Z., L.H., S.J.S., Y.Z.G. and Y.X. performed the laboratory testing. H.Q.X. performed statistical analyses and drafted the manuscript. J.Q.N. and Y.H.G. participated in the design of the study and applied for the funding. All authors have read and approved the final manuscript.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hongqin Xu and Junqi Niu.
Contributor Information
Hongqin Xu, Email: hongqinxu86@jlu.edu.cn.
Junqi Niu, Email: junqiniu@aliyun.com.
References
- 1.Furst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 2.Qian MB, Chen YD, Liang S, Yang GJ, Zhou XN. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect. Dis. Poverty. 2012;1:4. doi: 10.1186/2049-9957-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian MB, Chen YD, Yan F. Time to tackle clonorchiasis in China. Infect Dis. Poverty. 2013;2:4. doi: 10.1186/2049-9957-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Control of Foodborne Trematode Infections. WHO Technical Report Series849 (1995). [PubMed]
- 5.Ma YX. Epidemiological evolvement of Clonorchiasis sinensis in China for the past 60 years. Int. J. Med. Parasit. Dis. 2009;36:6. [Google Scholar]
- 6.Qian MB, Utzinger J, Keiser J, Zhou XN. Clonorchiasis. Lancet. 2016;387:800–810. doi: 10.1016/S0140-6736(15)60313-0. [DOI] [PubMed] [Google Scholar]
- 7.Yu SH, et al. Nationwide survey of human parasite in China. Southeast Asian J. Trop. Med. Public Health. 1994;25:4–10. [PubMed] [Google Scholar]
- 8.Fang, Y. Y. et al. [Current prevalence of Clonorchis sinensis infection in endemic areas of China]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi26, 99–103, 109 (2008) (in Chinese). [PubMed]
- 9.Li T, He S, Zhao H, Zhao G, Zhu XQ. Major trends in human parasitic diseases in China. Trends Parasitol. 2010;26:264–270. doi: 10.1016/j.pt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg. Infect Dis. 2005;11:1507–1514. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouvard V, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: the carcinogenic human liver fluke. World J. Gastroenterol. 2008;14:666–674. doi: 10.3748/wjg.14.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma evolving concepts and therapeutic strategies. Nature reviews. Clin. Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590–597. doi: 10.5483/bmbrep.2016.49.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J. Hepatol. 2010;2(12):419–427. doi: 10.4254/wjh.v2.i12.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang MH, et al. Relation of hepatolithiasis to helminthic infestation. J. Gastroenterol. Hepatol. 2005;20:141–146. doi: 10.1111/j.1440-1746.2004.03523.x. [DOI] [PubMed] [Google Scholar]
- 17.WHO, Sustaining the drive to overcome the global impact of neglected tropical diseases: Second WHO report on neglected tropical diseases. World Health Organization Geneva (2013).
- 18.Xie W, Deng Y, Chen S, Yang Q. Association between eosinophil count and cholelithiasis among a population with Clonorchis sinensis infection in Foshan City, China. J. Helminthol. 2019;94:e107. doi: 10.1017/S0022149X19001019. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, et al. Use of parenteral caffeinum natrio-benzoicum: an underestimated risk factor for HCV transmission in China. BMC Public Health. 2015;15:928. doi: 10.1186/s12889-015-2299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 21.D. Coordinating Office of the National Survey on the Important Human Parasitic [A national survey on current status of the important parasitic diseases in human population]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi23, 332–340 (2005). [PubMed]
- 22.Yu, S. et al. [Report on the first nationwide survey of the distribution of human parasites in China. 1. Regional distribution of parasite species]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi12, 241–247 (1994) (in Chinese) [PubMed]
- 23.Vinh HQ, et al. Risk factors for Clonorchis sinensis infection transmission in humans in northern Vietnam: a descriptive and social network analysis study. Parasitol. Int. 2017;66:74–82. doi: 10.1016/j.parint.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, et al. Clonorchis sinensis co-infection could affect the disease state and treatment response of HBV patients. PLoS Negl. Trop. Dis. 2016;10(6):e0004806. doi: 10.1371/journal.pntd.0004806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu W, Qian X, Huang Y, Hong Q. A review of the control of clonorchiasis sinensis and Taenia solium taeniasis/cysticercosis in China. Parasitol. Res. 2012;111:1879–1884. doi: 10.1007/s00436-012-3152-y. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, et al. Development and evaluation of loop-mediated isothermal amplification (LAMP) for rapid detection of Clonorchis sinensis from its first intermediate hosts, freshwater snails. Parasitology. 2013;140:1377–1383. doi: 10.1017/S0031182013000498. [DOI] [PubMed] [Google Scholar]
- 27.Val F, et al. Respiratory complications of plasmodium vivax malaria: systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2017;97:733–743. doi: 10.4269/ajtmh.17-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang JD, L. R., Zhu XQ. Animals infected with clonorchis sinensis in China. Chin J Zoonoses23, 177–179 (in Chinese) (2007).
- 29.Wang X, et al. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. 2011;12:R107. doi: 10.1186/gb-2011-12-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, et al. The carcinogenic liver fluke, Clonorchis sinensis: new assembly, reannotation and analysis of the genome and characterization of tissue transcriptomes. PLoS ONE. 2013;8:e54732. doi: 10.1371/journal.pone.0054732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao, Y. et al. Epidemiology, risk factors of clonorchis sinensis infections in northeast in China. Hepatology70, 317, 10.1002/hep.30941 (2019) (Abstract).
- 32.Hong ST, Choi MH, Kim CH, Chung BS, Ji Z. The Kato–Katz method is reliable for diagnosis of Clonorchis sinensis infection. Diagn. Microbiol. Infect Dis. 2003;47:345–347. doi: 10.1016/s0732-8893(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 33.Ministry of Science and Technology of the People's Republic of China. Regulations of the People's Republic of China on the Management of Human Genetic Resources. https://www.most.gov.cn/mostinfo/xinxifenlei/fgzc/xzfg/201906/t20190612_147044.htm (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon request.


