Abstract
Findings on the effects of probiotics on salivary cytokines and immunoglobulines have been conflicting. We aimed to perform a systematic review and meta-analysis on clinical trials that examined the effects of oral intake and local administration of probiotics on salivary cytokines and immunoglobulines in adults. We searched PubMed, MEDLINE, SCOPUS, EMBASE, and Google Scholar up to April 2020 for all relevant published papers assessing probiotic intakes and salivary cytokines and immunoglobulines. We included all randomized clinical trials that investigated the effect of oral probiotic supplementation or lozenges tablets on inflammatory biomarkers in adults. Studies that reported their effect sizes as mean ± SD or mean ± SEM were included. After excluding non-relevant papers, 8 studies remained in this review. Combining findings from 3 studies with 4 effect sizes, we found no significant reduction in salivary IgA concentrations after oral probiotic supplementation [weighted mean difference (WMD): −0.26; 95% CI: (−0.86, 0.35)]. A significant increase in salivary IL-1β concentrations reached after local probiotic supplementation (WMD: 28.21; 95% CI: 18.42, 38.01); however, no significant changes in salivary IL-6 concentrations after local probiotic supplementation was found (WMD: 0.36; 95% CI: −0.85, 1.56). We observed a significant increase in salivary IL-8 concentrations after local probiotic supplementation (WMD: 31.82; 95% CI: 27.56, 36.08). In case of salivary IL-10 concentrations after local probiotic administration, no significant reduction was seen (WMD: −0.02; 95% CI: −0.10, 0.06). we found that oral and local administrations of probiotics might influence some of salivary cytokines. However, additional clinical trials are required to examine these effects on further pro- and anti-inflammatory cytokines and immunoglobulines.
Subject terms: Interleukins, Risk factors
Introduction
Probiotics have been defined as livings micro-organisms that are selectively fermented1. They were reported to have beneficial effects on human health1,2. Regular intakes of probiotic supplements alter the gastrointestinal microbiota composition and activity and results in major changes in immune system responses3.
Probiotics may influence and enhance innate and adaptive immune response4. Several studies have reported the immune-modulatory effects of probiotics in humans5,6. Reduction in the production of inflammatory cytokines7,8 and elevation of intestinal secretory immunoglobulin A (sIgA) were also reported by probiotics administration9. Despite the overall anti-inflammatory effects of probiotics, the potential mechanisms of action are not clearly understood yet. It seems that the stimulatory and regulatory effects of probiotics in immune system confer their immunological protection by changings pro- and anti-inflammatory cytokines profile including TNF-α, IL-1β, IL-6, IL-8 and IL-1010,11. In case of their immune-modulatory effects, probiotics beneficially compete with pathogens, nutrients and antagonistic substances, through which they lead to healthy and diverse flora with regulated responses of immune system12. Probiotics have been reported to have local (direct) and systemic (indirect) effects on immune system4. For instance, they have been involved in maintaining of oral health through inhibiting the growth of pathogens13,14. Oral intake of probiotic drinks or supplements enhanced the secretory IgA in saliva2,6,15. In addition, local administration of probiotics in lozenges results in higher levels of salivary IgA and specific cytokines13,14. However, some other studies failed to find significant changes in salivary immunoglobulines or inflammatory cytokines by either oral intake or local administration of probiotics2–5,7,15–17. Despite earlier investigations, there is no comprehensive systematic review or meta-analysis summarizing earlier findings in this regard. We conducted this systematic review and meta-analysis to summarize the available data about the effects of oral intake and local administration of probiotics on salivary cytokines and immunoglobulines in adults.
Methods
Search strategy
This systematic review and meta-analysis of clinical trials was conducted based on Cochrane library checklist. All articles published earlier than April 2020 were searched through PubMed, MEDLINE, SCOPUS, EMBASE, and Google Scholar, by two independent investigators to identify relevant articles. To obtain suitable MESH and non-MESH text words, an initial search on Medline was undertaken. The systematic search strategies through each database were provided in the supplementary material file. We had no restrictions of language or time of publication. To avoid missing any publication, a manual search was conducted on reference lists of all included studies as well as review articles. We didn’t include unpublished data and grey literature, including dissertations, thesis, congress papers, and patentsin the current meta-analysis. In addition, duplicate citations were removed.
Inclusion criteria
We included all randomized clinical trials that investigated the effect of oral probiotic supplementation or lozenges tablets on inflammatory biomarkers in adults. Studies that reported their effect sizes as mean ± SD or mean ± SEM were included. Publications were independently assessed by two reviewers considering the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist. Any disagreements between the reviewers were resolved through discussion.In case of several publications with the same data set, we included only the most complete one13,16. If data for specific probiotics were reported separately, we considered them as a separate study in the analysis1.
Exclusion criteria
Studies were excluded if they were observational, editorial, letter to editor, comments, ecological or review papers. In addition, studies in which random allocation was not performed, had not control group or those conducted on animal models, pregnant or lactating women, children or elderlies were not included. Publications that examined the effect of another intervention along with probiotic supplementation, those that used symbiotics, examined only gene expression of inflammatory biomarkers or concentrations of inflammatory biomarkers in-vivo were not also considered eligible for the current study. Publications that examined gingival index, plaque index, bleeding, depth of pocket and etc. were excluded. The study by Garaiova et al. was excluded from systematic review and meta-analysis because its study population was children18. We also excluded the study of Dong et al. study form the meta-analysis due to not reporting any effect size3. In addition, the study of Jorgensen et al.16 was excluded because the data were repeatedly reported in the study of Braathen et al.13. After these exclusions, 8 papers remained for the primary systematic review. We didn’t consider two studies in the meta-analysis due not to reporting the data for control group6 and in the end of trial for both groups5. Figure 1 illustrates the study selection process for systematic review and meta-analysis.
Figure 1.
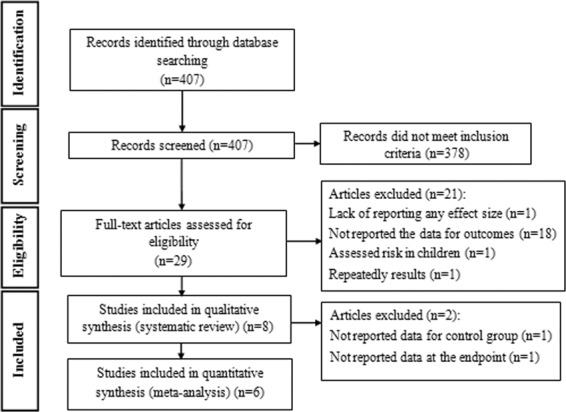
Flowchart of study selection process.
Data extraction
The data were extracted independently and cross-checked by two reviewers (SE and AM). Any disagreements between reviewers were consulted by principal investigator (AE). Quantitative data regarding effect-size measures such as mean and Standard Deviations (SDs) or mean and Standard Errors (SEs) or median and Interquartile Range (IQR) of inflammatory biomarkers before and after intervention in each groups; and mean (SD) changes in inflammatory markers after intervention in each group were extracted.In addition, information on first author’s last name, publication year, subjects’ heath condition, sample size, participants’ sex, number of subjects in each group, participants’ age, type of probiotics, study design (parallel/cross-over/other), type of control, duration of intervention and covariates were obtained. If data were reported as SEs or IQR, they were converted to SDs using appropriate formulas. When the concentration of an inflammatory biomarker was reported in different units, it was converted to the most frequently used one. Three studies had reported results in Figs. 1, 2, 6. We obtained the values from the figures by online “webplot digitizer” converting 2D Bar Plot to data. The values for SD changes were calculated using √S12 + S22 − 2 × r × S1 × S2 formula, in which r was computed for each individual study using SD12 + S22 - SD change2/2SD1SD2. The quality of studies and risk of bias of all eligible studies were assessed using the Cochrane Collaboration’s tool for quality assessment of randomized controlled trials19. The quality assessment tool encompasses the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other probable sources of biases.
Figure 2.
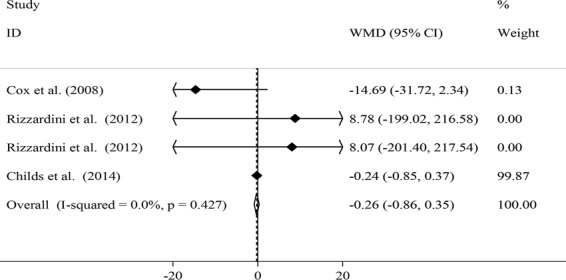
Effects of oral probiotic supplementation on salivary IgA concentrations.
Figure 6.
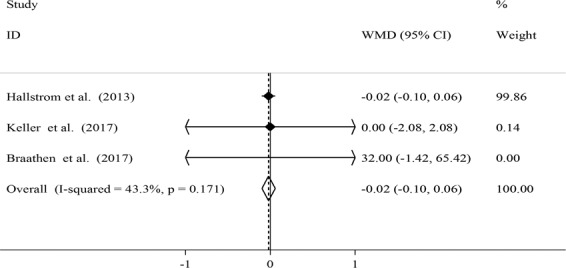
Effects of local probiotic supplementation on salivary IL-10 concentrations.
Statistical analysis
All effect sizes were calculated as mean ± SD of changes in the concentrations of inflammatory biomarkers between probiotic and control groups. The fixed-effects model was used to calculate the overall effect sizebecause random-effects model gives larger weights to small extreme studies20. We examined between-study heterogeneity by the Cochran’s Q test and I2 statistic. To find probable sources of between-study heterogeneity, subgroup analyses were conducted based on sex (Male/Female/Both genders), age (<40 year/>40 year), study design (Parallel/Cross-over), supplement dosage (=109/>109 CFU/day), duration of intervention (<3 /≥3 weeks) and probiotic type (Lactobacillus/Bifidobacter/Different types), using a fixed-effects model. The duration of 3 weeks and the dosage of 109 CFU/day were selected based on previous studies21,22. All statistical analyses were done using Stata software, version 11.2 (Stata Corp, College Station, TX). P < 0.05 was considered as statistically significant.
Results
Findings from the systematic review
The initial literature search yielded 407 unique studies. Based on titles and abstracts, 378 studies were excluded. Out of these, 21 studies were also excluded due to above-mentioned reasons. Finally, 8 articles that reported the effects oforal probiotic intake or probiotic containing lozenges tablets on salivary immunoglobulins or cytokines remained for the current study. Main characteristics of five studies that examined the effects of oral probiotic intake on salivary immunoglobulins are presented in Table 1. Five studies were done on healthy adults1,2,5,6,15. These studies were published between 2008 and 2016. Except for one study on men15, four other studies were performed on both genders. Total sample sizes in intervention and control groups were 231 and 129, respectively (54.92% female and 45.07% male). Participants in these studies were healthy people aged ≥18 years.Three studies were parallel1,5,6 and 2 studies were cross-over trials2,15. Participants consumed the probiotic supplements or placebos as capsules1,15 or milk- or fruit juice-based drinks2,5,6. Daily dose of supplementation ranged from 109 to 35 × 109. All studies had control group, except for the study of Harbige et al.6. Administered probiotics were lactobacillus1,5,6,15, bifidobacter1,2,5 and propionibacterium5. Three studies had used more than one type of probiotic1,2,16. Duration of trial ranged from 3 to 6 weeks. Measured outcomes were salivary IgA1,2,5, IgA16,12, IgA26, IgG1, IgM1 and INF-γ6. The method of assessment of outcome in all studies was enzyme-linked immunosorbent assay (ELISA). Three studies had reported mean ± SE of salivary immunoglobuline concentrations before and after intervention6 or their changes1,2. Table 2 presents the results of quality assessment of eligible studies on oral probiotic intake. Two studies had poor quality5,6, two had good quality1,2 and the remaining one study had fair quality15. The risk of bias was attributed to random sequence generation and blinding of outcome assessment in the included studies. Due to limited number of studies, we did not perform subgroup analysis by quality of primary studies.
Table 1.
Effects of oral probiotic intake on salivary immunoglobulins.
| Author (yaer) | Subjects and gender | Age range/ And mean (year) | Design | Intervention type | Bacteria type | Duration (wk/d) | Outcomes | Outcome assessment method | outcome | Any other intervention (from) | Notes about subjects | Adjustment or matching | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (name and composition) | Control (name and composition) | Intervention mean ± SD and number | Control mean ± SD and number | |||||||||||
| Harbige et al. (2016) | F: 10 M: 8 Both: 18 Probiotic: 14 Placebo: 4 | 18–49 | CT (clinical trial) | Daily drink with breakfast: two 65mlbottles equivalent intake of 1.3 × 1010 live Lactobacillus caseiShirota (LcS). | No treatment | Lactobacillus caseiShirota (LcS) | 4 week intervention, 6 week break, followed by 4 week intervention | Salivary IgA1, Salivary IgA2, Salivary INF-γ (For 10 probiotic subjects) | Salivary INF-γ: ELISA Salivary IgA1, 2: radial immunodiffusion assay | SIgA1(mg/mL): Before:0.04 ± 0.13 Week 4:0.04 ± 0.16 Week 10: 0.04 ± 0.15 Week 14: 0.05 ± 0.17 N = 10 SIgA2(mg/mL): Before:0.0 3 ± 0.09 Week 4:0.03 ± 0.11 Week 10: 0.03 ± 0.12 Week 14: 0.03 ± 0.13 N = 10 SINF- γ(mg/mL):NR | No | No | Salivary samples just obtained from 10 probiotic subjects. Subjects were healthy volunteers | No |
| Childs et al. (2014) | F: 22 M: 22 Probiotic:42 Placebo: 41 | 25–65 43 ± 12 | Cross-over | The volunteer were given 2 sachets of daily supplements which powders dissolved in water, milk or fruit juice: Prebiotic (xylo-oligosaccharide, XOS, 8 g/d), Probiotic (Bifidobacteriumanimalis subsp. lactis Bi-07, 109 colony-forming units (CFU)/d), Synbiotic (8 g XOS + 109 CFU Bi-07/d) | The volunteer were given 2 sachets of daily placebo which powders dissolved in water, milk or fruit juice: Placebo. maltodextrin (MDX; Syral) | Bifidobacterium animals subsp. Lactis Bi-07, 109 CFU | 21 days | Salivary IgA | enzyme-based colorimetry | SIgA(mg/mL): Change: −0.18 ± 0.50 N = 42 | SIgA(mg/mL): Change: 0.06 ± 1.92 N = 41 | No | BMI for all subjects: were 25 ± 5 kg/m2. Subjects were healthy volunteers Symbiotic: 41 Prebiotic: 42 | Sex, age, BMI |
| Rizzardini et al. (2012) | F: 118 M: 93 BB-12 cap: 53 (25/28) Placebo cap: 48 (27/21) L. casei 431: 56 (31/25) placebo drink: 54 (35/19) | 20–60 | Parallel | Intervention groups consumed a minimum of 109 colony-forming units of BB-12 (capsule) or L. casei 431 (dairy drink) once daily (110 ml). | Placebo groups consumed matched placebo capsule or placebo drink once daily (110 ml). | Bifidobacteriumanimalis ssp. lactis (BB-12) capsule and Lactobacillus paracasei ssp. paracasei (L. casei 431) drink | 6 weeks | Salivary IgA, IgG, IgM | salivary IgA were analysed using Human Secretory IgA SIgA ELISA Kit, total salivary IgGand IgM were analysed using the Quantitative Human IgG/IgM ELISA Kit | BB-12: Change, SIgA (mg/mL): Change: 57.88 ± 612.98 N = 53 Change, SIgG (U /mL): Change: 2.74 ± 153.02 N = 53 Change, SIgM (U/mL): Change: 1.38 ± 220.66 N = 53 L. casei 431: Change, SIgA(U/mL): Change: 59.33 ± 594.73 N = 56 Change, SIgG (U/mL): Change: −4.61 ± 176.15 N = 56 Change, SIgM (U/mL): Change: 4.29 ± 203.53 N = 56 | BB-12: Change, SIgA (U/mL): Change: 49.1 ± 446.36N = 48 Change, SIgG (U/mL): Change: -5.23 ± 173.94 N = 48 Change, SIgM(U/mL): Change: 6.77 ± 240.12 N = 48 L. casei 431: Change, SIgA (U/mL): Change: 51.26 ± 525.08 N = 54 Change, SIgG (U/mL): Change: -1.90 ± 133.99 N = 54 Change, SIgM (U/mL): Change: 0.88 ± 204.55 N = 54 | 2 weeks after intervention, a seasonal influenza vaccination was given to all subjects. | BMI for subjects: BB-12 cap: 22.8 ± 4.1 Placebo cap: 22.4 ± 3.8 L. casei 431: 24.6 ± 4.3 placebo drink: 22.8 ± 3.6 Subjects were healthy volunteers | No |
| Cox et al. (2008) | F: 0 M: 20 Both: 20 | 27.3 | Cross-over | Intervention group was given 3hard gelatin capsules twice daily with food (L fermentum VRI-003 (PCC), contained a minimum of two billion of Lactobacillus fermentum strain VRI-003) | Placebo group was given identical 3 placebo capsules twice daily with food. | Lactobacillus fermentum strain VRI-003 | 1 month (28 days) intervention 4 months (14 week) | Salivary IgA, IgA1 and albumin | SIgA and SIgA1: ELISA assay | SIgA (mg/mL): Before:56.0 ± 35.4%Change:29.0 ± 80.7 N = 20 SIgA1 (mg/mL): Before:94.5 ± 63.4%Change:21.3 ± 67.0 N = 20 | SIgA (mg/mL): Before:69.2 ± 44.7%Change:27.5 ± 58.9 N = 20 SIgA1 (mg/mL): Before:92.7 ± 34.4%Change: 23.6 ± 64.6 N = 20 | No | Subjects were healthy volunteers | No |
| Kekkonen et al. (2008) | F: 45 M: 17 Both: 62 Lactobacillus rhamnosus GG: 13 Bifidobacteriumanimalis ssp. LactisBb12: 16 Propioni-bacterium freudenreichii ssp. Shermanii JS: 17 Placebo: 16 | 44 23–58 | Parallel | The subjects were advised to consume a 250 mL milk-based fruit drink daily for 3 wk containing either: L. rhamnosus GG (ATCC 53103) (LGG) bacteria, on average 6.2 × 107 cfu/mL (daily dose of 1.6 × 1010 cfu); B. animalis ssp. lactis Bb12 (Bb12) bacteria, 1.4 × 108 cfu/mL (daily dose of 3.5 × 1010cfu); P. freudenreichii ssp. shermanii JS (DSM 7067) (PJS) bacteria, 1.3 × 108 cfu/mL (daily dose of 3.3 × 1010 cfu) | Control group received a placebo drink without any probiotic bacteria. | Lactobacillus rhamnosus GG (LGG), Bifidobacterium animalis ssp. lactis Bb12 (Bb12), or Propionibacteriumfreudenreichii ssp. shermanii JS (PJS) | 3 weeks | Salivary IgA | ELISA assay | LGG: SIgA(mg/mL): Before:270 ± 210 After: same before N = 13 BB-12: SIgA (mg/mL): Before:400 ± 450 After: same before N = 16 PJS: SIgA (mg/mL): Before:280 ± 240 After: same before N = 17 | Placebo:SIgA (mg/mL):Before:230 ± 140 After: same before N = 16 | No | BMI for subjects: 2418–30 Subjects were healthy volunteers | No |
Table 2.
Study quality and risk of bias assessment of included studies on oral probiotic intake according to the Cochrane Collaboration’s tool.
| Study (year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Overall quality* |
|---|---|---|---|---|---|---|---|---|
| Harbige et al. (2016) | U | U | U | U | L | L | H | Poor |
| Childs et al. (2014) | L | L | L | L | L | L | U | Good |
| Rizzardini et al. (2012) | L | L | L | U | L | L | L | Good |
| Cox et al. (2008) | U | L | L | U | L | L | L | Fair |
| Kekkonen et al. (2008) | U | U | U | U | L | L | L | Poor |
U; unclear risk of bias, L; low risk of bias, H; high risk of bias.
*Good quality: all criteria met; Fair quality: one criterion not met (i.e. high risk of bias for one domain or two criteria unclear); Poor quality: two or more criteria listed as high or unclear risk of bias.
Table 3 provides characteristics of three studies4,13,14 that examined the effects oflocal administration of probiotic tablets as lozengeson salivary cytokines and immunoglobulins. These studies were published between 2007 and 2017 and were conducted on both genders except for one study on females14. Total sample sizes in intervention and control groups were 93 and 66, respectively (68.79% female and 31.21% male). Participants were healthy people aged ≥18 years. Two studies were cross-over13,14 and one study was parallel trial4. In these publications, participants were healthy participants13,14 or periodontal patients4. Daily dose of supplementation ranged from 0.1 × 109 to 3 × 109. The administered probiotics in these papers were various strains of lactobacillus. Duration of trials ranged from 3 weeks to 12 weeks. Measured outcomes were salivary IgA13, IL-1β4,13,14, IL-64,13,14, IL-84,13,14, IL-104,13,14, IL-1814 and TNF-α4,13,14. The method of assessment of all these variables was enzyme-linked immunosorbent assay (ELISA). All studies had reported mean ± SD of salivary cytokines and immunoglobuline concentrations before and after intervention. The quality assessment of included studies on local administration of probiotic tablets as lozenges revealed that two studies had fair quality4,14 and the remaining one study13 had good quality (Table 4). Allocation concealment and blinding of outcome assessment were the major sources for risk of bias. Again, due to limited number of studies, we were not able to do subgroup analysis.
Table 3.
Effects of local administration of probiotic tablets as lozenges on salivary cytokines and immunoglobulins.
| Author (yaer) | Subjects and gender | Age range/ mean (year) | Design | Intervention type | Bacteria type | Duration (week) | Outcomes | Outcome assessment method | outcome | Any other intervention (from) | Notes about subjects | Adjustment or matching | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (name and composition) | Control (name and composition) | Intervention mean ± SD and number | Control mean ± SD and number | |||||||||||
| Keller et al. (2017) | F: 34 M: 13 Both: 47 Probiotic: 23 Placebo: 24 | Probiotic: 26.9 Placebo: 25.7 | Parallel | The participants were instructed to take one tablet of in the morning and one in the evening30 min after tooth brushing. The probiotic tablets contained an equal mix of Lactobacillus rhamnosus PB01 DSM14869 and Lactobacillus curvatus EB10 DSM32307 at a total dose of ≤108 cfu/tablet | The placebo tablets were identical in size and composition but without the addition of the probiotic strains. | Lactobacillus rhamnosus PB01 DSM14869 and Lactobacillus curvatus EB10 DSM32307 | 4 weeks intervention | IL-1β, IL-10, IL-8, IL-6, TNF-α | xMAP technology multiplex immunoassay | IL-1β (pg/mL): Before: 50 ± 125 4 weeks:71 ± 155 IL-6 (pg/mL): Before:6.3 ± 9.8 4 weeks:5.6 ± 14.1 IL-8 (pg/mL): Before: 100 ± 113 4 weeks:74 ± 119 IL-10 (pg/mL): Before:9.2 ± 14.3 4 weeks: 9.9 ± 9.2 TNF-α (pg/mL): Before:2.8 ± 3.3 4 weeks:3.1 ± 6.6 | IL-1β (pg/mL): Before:25 ± 41 4 weeks:21 ± 35 IL-6 (pg/mL): Before:4.0 ± 5.4 4 weeks:3.1 ± 4.2 IL-8 (pg/mL): Before:94 ± 88 4 weeks:87 ± 79 IL-10 (pg/mL): Before:7.0 ± 8.7 4 weeks:6.3 ± 8.6 TNF-α (pg/mL): Before:3.1 ± 2.9 4 weeks:3.1 ± 3.7 | All participants used fluoride toothpaste (1,100-1,450 mg/kg) on a daily basis | There were no significant differences in the baseline characteristics (age, sex, flow rate, oral hygiene routines) between the two study groups. Subjects were patients | No |
| Braathen et al. (2017) | Both: 47 F: 36 M: 11 Prob: 23 Placebo: 24 | 18–32 23.9 ± 3.3 | Cross-over | The active intervention was twice daily intake of one lozenge containing two strains of the probiotic bacterium L. reuteri Prodentis (DSM 17938 1 × 109 cfu/lozenge and 12 5289 2 × 109 cfu/lozenge). The participants were instructed to ingest either probiotic or placebo lozenges twice daily (morning and evening) for three weeks followed by a three-week wash-out period. Hereafter, the participants crossed-over and received the opposite lozenges twice daily for three weeks. The intervention period terminated with a three-week wash-out period | The placebo lozenges were identical in taste, colour, texture and size but without active bacteria | Lactobacillus reuteri | 12 weeks | Salivary IgA, IL-1β, IL-10, IL-8, IL-6, TNF-α | Salivary IgA: ELISA Cytokines: xMAP technology multiplex immunoassay | Salivary IgA (mg/100 mL): Baseline:7.7 ± 4.1 Follow-up:9.3 ± 4.6 IL-1β (pg/mL): Baseline:149 ± 365 Follow-up:166 ± 400 IL-6(pg/mL): Baseline:21 ± 22 Follow-up:100 ± 293 IL-8(pg/mL): Baseline:211 ± 187 Follow-up:262 ± 434 IL-10(pg/mL): Baseline:26 ± 25 Follow-up:38 ± 87 NB = 11 NF = 17 | Salivary IgA (mg/100 mL): Baseline: 8.6 ± 5.9 Follow-up:5.5 ± 2.3 IL-1β (pg/mL): Baseline:114 ± 161 Follow-up:90 ± 132 IL-6(pg/mL): Baseline:40 ± 71 Follow-up:25 ± 30 IL-8(pg/mL): Baseline:207 ± 218 Follow-up:198 ± 171 IL-10(pg/mL): Baseline:43 ± 75 Follow-up:23 ± 29 NB = 30 NF = 24 | No | Subjects were healthy volunteers | No |
| Hallstrom et al. (2013) | F: 18 Total: 18 | 38 | Cross-over | Lozenges containing two strains of L. reuteri (ATCC55730 and ATCC PTA5289; 1 × 108 CFU of each strain) were taken twice a day during the experimental periods | Lozenges containing placebo were taken twice a day during the experimental periods. | L. reuteri (ATCC55730 and ATCC PTA5289 | 3 weeks | IL-1β, IL-6, IL-8, IL-10, IL-18, TNF-α | Cytokines determined using the commercial Bio-Plex Cytokine Assay (Bio-Rad Laboratories, Hercules, CA) | TNF-α (pg/mL): Baseline:0.72 ± 0.81 Follow-up:1.45 ± 4.14 IL-1β (pg/mL): Baseline:27.6 ± 22.4 Follow-up:76.6 ± 70.2 IL-6(pg/mL): Baseline:3.77 ± 8.56 Follow-up:5.15 ± 16.2 IL-8(pg/mL): Baseline:80.9 ± 57.7 Follow-up:36.8 ± 34.0 IL-10(pg/mL): Baseline:0.36 ± 0.30 Follow-up:0.43 ± 0.46 IL-18(pg/mL): Baseline:42.3 ± 59.8 Follow-up:98.6 ± 105.7 N = 18 | TNF-α (pg/mL): Baseline:0.47 ± 0.30 Follow-up:0.66 ± 1.03 IL-1β (pg/mL): Baseline:31.2 ± 27.7 Follow-up:60.5 ± 65.4 IL-6(pg/mL): Baseline:1.69 ± 1.67 Follow-up:1.58 ± 2.45 IL-8(pg/mL): Baseline:81.9 ± 65.3 Follow-up:33.4 ± 27.5 IL-10(pg/mL): Baseline:0.29 ± 0.20 Follow-up:0.38 ± 0.26 IL-18(pg/mL): Baseline:34.0 ± 47.9 Follow-up:116.2 ± 112.1 N = 18 | No | Subjects were healthy volunteers | No |
Table 4.
Study quality and risk of bias assessment of included studies on local administration of probiotic tablets as lozenges according to the Cochrane Collaboration’s tool.
| Study (year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Overall quality* |
|---|---|---|---|---|---|---|---|---|
| Keller et al. (2017) | U | U | L | L | L | L | L | Fair |
| Braathen et al. (2017) | L | L | L | U | L | L | L | Good |
| Hallstrom et al. (2013) | L | U | L | U | L | L | L | Fair |
U; unclear risk of bias, L; low risk of bias, H; high risk of bias.
*Good quality: all criteria met; Fair quality: one criterion not met (i.e. high risk of bias for one domain or two criteria unclear); Poor quality: two or more criteria listed as high or unclear risk of bias.
Findings from meta-analysis
Combining findings from 3 studies1,2,15 with 4 effect sizes, we found no significant reduction in salivary IgA concentrations after oral probiotic supplementation [weighted mean difference (WMD): −0.26; 95% CI: (−0.86, 0.35)] (Fig. 2). There were no significant between-study heterogeneity (I2 = 0.0%, P = 0.427). No particular study had a significant influence on the summary effect in our sensitivity analysis. There was no proof of significant publication bias (Egger’s test: 0.494).
There were 3 clinical trials examining local administration of probiotic tablets as lozenges on salivary IL-1β, IL-6, IL-8 and IL-104,13,14. Combining three effect sizes from clinical trials, we found a significant increase in salivary IL-1β concentration after local probiotic supplementation (WMD: 28.21; 95% CI: 18.42, 38.01) (Fig. 3). There were no significant between-study heterogeneity (I2 = 11.9%, P = 0.32). No particular study had a significant influence on the summary effect in our sensitivity analysis. There was no proof of significant publication bias (Egger’s test: 0.89).
Figure 3.
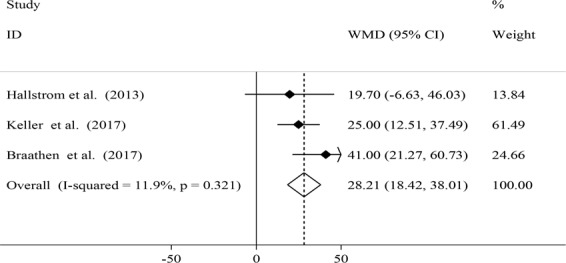
Effects of local probiotic supplementation on salivary IL-1β concentrations.
When we combined three effect sizes, we found no significant change in salivary IL-6 concentrations after local probiotic supplementation (WMD: 0.36; 95% CI: −0.85, 1.56) (Fig. 4). There were no significant between-study heterogeneity (I2 = 28.2%, P = 0.248) and evidence of significant publication bias (Egger’s test: 0.085).
Figure 4.
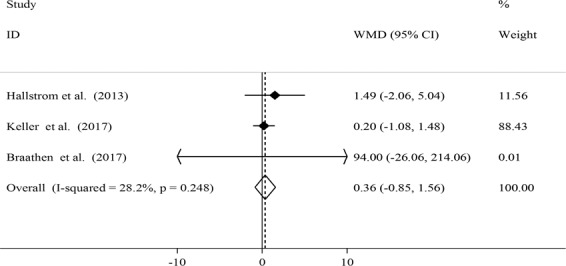
Effects of local probiotic supplementation on salivary IL-6 concentrations.
A significant increase in salivary IL-8 concentrations was observed after local probiotic supplementation (WMD: 31.82; 95% CI: 27.56, 36.08) (Fig. 5). However, a significant between-study heterogeneity was found (I2 = 72.7%, P = 0.026). Due to limited number of studies we did not perform subgroup analysis to find possible source of this heterogeneity.
Figure 5.
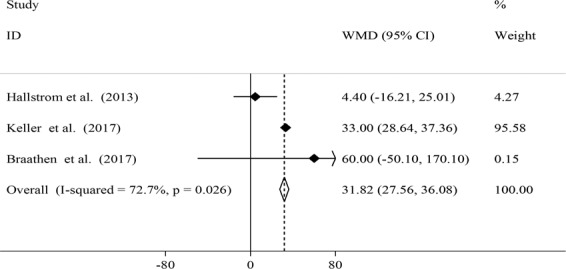
Effects of local probiotic supplementation on salivary IL-8 concentrations.
In case of salivary IL-10 concentrations after local probiotic administration, no significant reduction was seen (WMD: −0.02; 95% CI: −0.10, 0.06) (Fig. 6). No evidence of between-study heterogeneity (I2 = 43.3%, P = 0.171) and publication bias (Egger’s test: 0.482) was seen.
Disscusion
In the current meta-analysis, we found a significant increase in salivary IL-1β and IL-8 concentrations after local probiotic supplementation. However, no significant effects of oral probiotic supplementation on salivary IgA levels and also, no significant effects of local probiotic supplementation on salivary IL-6 and IL-10 concentrations were found in our meta-analysis. To the best of our knowledge, this is the first systematic review and meta-analysis summarizing the effects of oral and local probiotic supplementation on salivary immunoglobulines and cytokines.
Our findings from the current meta-analysis were in line with previous clinical trials that showed no significant increase in salivary IgA levels after oral probiotic treatments compared to placebo5,15. In contrast, some studies indicated a significant increase in serum IgA concentrations by probiotic consumption1,6. Whereas Childs et al. reported a significant decrease in salivary IgA concentrations after probiotic intake2. Although some earlier studies have shown the effect of probiotic supplementation on systemic IgA antibody releasing and B cell stimulatory activity23,24, the salivary concentrations of IgA, as a marker of mucosal immunity, did not influence by probiotic supplementation. This might be explained by the age of participants. Most studies have enrolled elderly people, whom antibody responses might be different from healthy middle-age adults. Moreover, saliva volume and its contents might be affected by several environmental and neural factors. Therefore, salivary levels of IgA could also be influenced by psychological and physical stress24. Due to limited number of publications, we were unable to do subgroup analysis by sex, age group, design and duration of trials, dose and type of probiotics. These factors may also affect our findings. It must also be taken into account that exposure to probiotics in early life through diet might also contribute to immune responses and secretion of immune-globulins in body liquids25.
We found a significant increase in some salivary inflammatory cytokines including IL-1β and IL-8 concentrations by local probiotic administration. However, no significant changes in IL-6 and IL-10 were observed following probiotic supplementation. These findings were in agreement with several other reports from randomized clinical trials that showed a significant increase in salivary cytokines including IL-1β4,14. Against to this finding, some investigators failed to find any significant effects on salivary cytokines4,13,17. One should keep in mind that local administration of probiotics is different from oral supplementation. The effects of local ingestion of probiotics on immune system function basically depend on individual oral biofilm environment and oral hygiene and gingival inflammation26. Individual oral biofilm and inflamed gums or healthy gums can differently respond to probiotic treatments. In addition, in case of gingivitis, in which we face with acute inflammation, local administration of probiotics for short-term cannot cool down inflammation due to elevated levels of inflammatory cytokines in these patients27. Moreover, in spite of immune-modulatory effects of local administration of probiotics and secretion to saliva, regular intake of probiotic products does not seem to be enough to initiate major alterations in oral biofilm4. It should also be kept in mind that the quality of primary studies can strongly influence the overall effect size. We assessed study quality in the current investigation and excluded studies with poor quality from the current analysis because of not reporting reliable effect sizes5,6. However, we could not perform subgroup-analysis based on quality of studies due to the limited number of publication in each area.
The possible mechanisms through which probiotic administration might affect salivary cytokines and immunoglobulines are not clearly understood. Among the possible suggested mechanisms are the effects of probiotics on increasing Treg function, through which they can induce the anti-inflammatory cytokine production, such as TGF-β, which can consequently lead to increased levels of IgA28–31. In addition, secretions of anti-inflammatory cytokines are up-regulated by probiotics through encouraging the anti-inflammatory M2 macrophages32,33.
Despite being the first meta-analysis on salivary cytokines and immunoglobulines, some limitations need to be considered. Due to limited number of publications, we were unable to do the meta-analysis on some other cytokines and immunoglobulines. The effects of probiotics are strongly dependent to age and primary exposure of host. This should be considered in the interpretation of the findings. We confined our meta-analysis to adult population and did not include studies that investigated children or adolescences. Moreover, despite the effects of salivary flow rate on the levels of salivary cytokines and immunoglobulins on one hand34,35 and the effect of probiotic supplementation on salivary flow rate on the other hand12, none of the studies had considered normalized levels of cytokines for salivary flow rate. In addition, we did not register the protocol of the current study on PROSPERO registry system due to the delay in processing the submitted protocols for studies outside the UK. This lack of registration might be a source of bias for this review. However, this review and meta-analysis was designed and performed according to the Cochrane guidelines.
In conclusion, we found that oral and local administrations of probiotics were significantly associated with increased levels of IL-1β and IL-8 in adult population. However, additional clinical trials are required to examine these effects on further pro- and anti-inflammatory cytokines and immunoglobulines.
Supplementary information
Author contributions
S.E.K., A.E. and B.L. designed research; S.E.K. and A.M. conducted research and analyzed data; S.E.K. and A.E. wrote the paper; A.E. had primary responsibility for final content. All authors read and approved the final manuscript and all authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67037-y.
References
- 1.Rizzardini G, et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12 and Lactobacillus paracasei ssp. paracasei, L. casei 431 in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012;107:876–84. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 2.Childs CE, et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animal is subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br. J. Nutr. 2014;111:1945–56. doi: 10.1017/S0007114513004261. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Rowland I, Thomas LV, Yaqoob P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013;52:1853–63. doi: 10.1007/s00394-012-0487-1. [DOI] [PubMed] [Google Scholar]
- 4.Keller MK, Brandsborg E, Holmstrøm K, Twetman S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: a randomised controlled trial. Benef. Microbes. 2018;9:487–494. doi: 10.3920/BM2017.0104. [DOI] [PubMed] [Google Scholar]
- 5.Kekkonen RA, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World. J. Gastroenterol. 2008;14:2029–36. doi: 10.3748/wjg.14.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbige LS, Pinto E, Allgrove J, Thomas LV. Immune Response of Healthy Adults to the Ingested Probiotic Lactobacillus casei Shirota. Scand. J. Immunol. 2016;84:353–364. doi: 10.1111/sji.12495. [DOI] [PubMed] [Google Scholar]
- 7.O’Mahony L, et al. lactobacillus and bifidobacterium irritable bowel syndrome symptom responses a relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Borruel N, et al. Effects of nonpathogenicbacteria on cytokine secretion by human intestinal mucosa. Am. J. Gastroenterol. 2003;98:865–70. doi: 10.1111/j.1572-0241.2003.07384.x. [DOI] [PubMed] [Google Scholar]
- 9.Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 2004;70:1176–81. doi: 10.1128/AEM.70.2.1176-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019;38:522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Azad AK, Sarker M, Wan D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed. Research. International. 2018;8063647:1–10. doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ericson D, Hamberg K, Bratthall G, Sinkiewicz-Enggren G, Ljunggren L. salivary IgA response to probiotic bacteria and mutans streptococci after the use of chewing gum containing Lactobacillus reuteri. Pathog. Dis. 2013;68:82–7. doi: 10.1111/2049-632X.12048. [DOI] [PubMed] [Google Scholar]
- 13.Braathen G, Ingildsen V, Twetman S, Ericson D, Jørgensen MR. Presence of Lactobacillus reuteri in saliva coincide with higher salivary IgA in young adults after intake of probiotic lozenges. Benef. Microbes. 2017;8:17–22. doi: 10.3920/BM2016.0081. [DOI] [PubMed] [Google Scholar]
- 14.Hallström H, et al. Effect of probiotic lozenges on inflammatory reactions and oral biofilm during experimental gingivitis. Acta. Odontol. Scand. 2013;71:828–33. doi: 10.3109/00016357.2012.734406. [DOI] [PubMed] [Google Scholar]
- 15.Cox AJ, Pyne DB, Saunders PU, Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports. Med. 2010;44:222–6. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen MR, et al. Lactobacillus reuteri supplements do not affect salivary IgA or cytokine levels in healthy subjects: A randomized, double-blind, placebo-controlled, cross-over trial. Acta. Odontol. Scand. 2016;74:399–404. doi: 10.3109/00016357.2016. [DOI] [PubMed] [Google Scholar]
- 17.Riccia DN, et al. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral. Dis. 2007;13:376–85. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 18.Garaiova I, et al. Probiotic and vitamin C for the prevention of respiratory tract infections in children attending preschool: a randomised controlled pilot study. Eur. J. Clin. Nutr. 2015;69:373–9. doi: 10.1038/ejcn.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N-Linked Glycoprotein Side-Chain Biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: An Analysis From the JUPITER Trial. J. Am. Heart Assoc. 2016;5:1–12. doi: 10.1161/JAHA.116.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivamaruthi BS, Kesika P, Chaiyasut C. Effect of Probiotics Supplementations on Health Status of Athletes. Int. J. Environ. Res. Public Health. 2019;16:4469. doi: 10.3390/ijerph16224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minelli EB, Benini A. Relationship between number of bacteria and their probiotic effects. Microbial Ecology in Health and Disease. 2008;20:180–183. doi: 10.1080/08910600802408095. [DOI] [Google Scholar]
- 23.O’Connel E, Allgrove J, Pollard L, Xiang M, Harbige LS. A pilot study investigating the effects of yakult fermented milk drink (L. casei Shirota) on salivary IFN-γ, sIgA, IgA1 and IgA2 in healthy volunteers. Proceedings of the Nutrition Society. 2010;69:E267. doi: 10.1017/S002966511000056X. [DOI] [Google Scholar]
- 24.Boge T, et al. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomized controlled trials. Vaccine. 2009;27:5677–84. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Björkstén B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J. Pediatr. Gastroenterol. Nutr. 2009;49:349–54. doi: 10.1097/MPG.0b013e31818f091b. [DOI] [PubMed] [Google Scholar]
- 26.Offenbacher S, et al. Changes in gingival crevicular fluid inflammatory mediator levels during the induction and resolution of experimental gingivitis in humans. J. Clin. Periodontol. 2010;37:324–33. doi: 10.1111/j.1600-051X.2010.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J. Leukoc. Biol. 2003;73:213–24. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 28.Donkor ON, et al. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin. Exp. Immunol. 2012;167:282–95. doi: 10.1111/j.1365-2249.2011.04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter CA, Bermudez L, Beernink H, Waegell W, Remington JS. Transforming growth factor-beta inhibits interleukin-12-induced production of interferon-gamma by natural killer cells: a role for transforming growth factor-beta in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur. J. Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 30.Strober W, et al. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol. Today. 1997;18:61–4. doi: 10.1016/S0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 31.Starovoĭtova SO, Tymoshok NO, Horchakov VI, Spivak MIA. Immunomodulation characteristics of Lactobacillus genus bacteria. Mikrobiol. Z. 2009;71:41–7. [PubMed] [Google Scholar]
- 32.Mendonça FH, et al. Effects of probiotic bacteria on Candida presence and IgA anti-Candida in the oral cavity of elderly. Braz. Dent. J. 2012;23:534–8. doi: 10.1590/S0103-64402012000500011. [DOI] [PubMed] [Google Scholar]
- 33.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu. Rev. Med. 1997;48:341–51. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 34.Streckfus C, Bigler L, O’Bryan T. Aging and salivary cytokine concentrations as predictors of whole saliva flow rates among women: a preliminary study. Gerontology. 2002;48:282–8. doi: 10.1159/000065250. [DOI] [PubMed] [Google Scholar]
- 35.Cregger RA, Langworthy KL, Salako NO, Streckfus C. Relationship between Salivary Cytokines, and Caries Experience in Children with Different Body Mass Indices. J. Dent. Oral. Health. 2017;5:1–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


