Summary
The pathogenesis of psoriatic arthritis (PsA) involves several pathways, including the CD40/CD40L signaling which promotes the release of multiple cytokines. Transmembrane CD40L is also released in soluble form (sCD40L) and phosphodiesterase 4 (PDE4) seems to be involved in its cleavage. We aimed to investigate whether apremilast, a PDE4 inhibitor, could modify circulating levels of sCD40L in PsA patients, and the possible associations of these changes with clinical response. Consecutive PsA patients starting apremilast in routine clinical practice were prospectively observed. Disease Activity of Psoriatic Arthritis (DAPSA), Psoriasis Area Severity Index (PASI), Leeds Enthesitis Score (LEI) and serum samples were collected at baseline and at 6 months. Samples were run in a Bio‐Plex ProTM plate for sCD40L. To investigate the association of sCD40L level with DAPSA based minor response, low disease activity (LDA) and/or remission at 6 months of treatment, multivariate logistic regression models with backward selection (P < 0·05) were built. We studied 27 patients (16 of 27 women, 59·6%) with PsA and mean age [± standard deviation (s.d.)] of 58·4 ± 10 years. A significant reduction of the mean values of DAPSA, LEI and PASI was detected at 6 months. Mean serum levels of sCD40L decreased from baseline 5364 ± 2025 pg/ml to 4412 ± 2629 at 6 months (P = 0·01). Baseline DAPSA [odds ratio (OR) = 0·80, 95% confidence interval (CI) = 0·65–0·98] and sCD40L (OR = 1·001, 95% CI = 1·0001–1·0027) were independently associated with DAPSA LDA/remission at 6 months. In PsA patients, sCD40L levels decrease upon apremilast treatment and might predict short‐term clinical response to apremilast.
Keywords: apremilast, PDE4, psoriatic arthritis
Apremilast may decrease sCD40L level in PsA patients. Higher baseline serum sCD40L level may predict short‐term clinical response to apremilast.

Introduction
The pathogenesis of psoriatic arthritis (PsA) involves several pathways, including the CD40/CD40L interaction [1]. CD40 is a tumor necrosis factor receptor superfamily member expressed on several myeloid cells. Its ligand, CD40L, is induced in T cells early upon activation, and also expressed on B lymphocytes, monocytes, macrophages, endothelial cells and platelets. CD40L binds to CD40 on antigen‐presenting cells inducing the expression of important ligands for co‐stimulation, such as CD86 [2]. Of note, CD40–CD40L drive the interactions between dendritic cells and CD8+ T cells, which play a pivotal role in PsA synovitis [3] allowing a maximal T cell response in the absence of CD4+ T cells. [2]. Furthermore, it has been shown that CD40 expression was increased in synovial fluid B cells from PsA, but not in patients with rheumatoid arthritis [4]. CD40–CD40L linking can promote the release of proinflammatory cytokines and furthermore, soluble CD40L (sCD40L) may be shed upon CD40–CD40L dimerization acting as a cytokine itself, hence constituting an inflammatory biomarker [5].
Apremilast is a phosphodiesterase 4 (PDE4) inhibitor approved for the treatment of PsA and plaque psoriasis [6]. In‐vitro evidence suggests that it can decrease the expression of CD40 on macrophages, possibly reducing CD40L cleavage because of limited dimerization. Additionally, there is evidence that PDE4 blockade leads to a reduction of sCD40L through the inhibition of neutrophil–platelet interactions [7, 8]. Given these premises, here we investigate whether apremilast, a PDE4 inhibitor, could modify circulating level of sCD40L in PsA patients, and the possible association of these changes with clinical response.
Materials and methods
Patients
Consecutive patients with PsA according to Classification of Psoriatic Arthritis (CASPAR) criteria [9] starting apremilast for routine clinical practice between October 2018 and September 2019 in a single center were prospectively observed. Serum samples were collected at baseline and at the 6‐month follow‐up visit. Sera from healthy donors were also collected and stored at –80°C. Samples were run in a Bio‐Plex ProTM plate for sCD40L (cat. no. 17IBA014M, batch 64202666, exp. 2021‐11‐10) after successful calibration (cat. no. 17I203060, batch 64244495, exp. 2020‐10‐22) and validation (cat. no. 17I20301, batch 64239064, exp. 2020‐07‐06) of Bioplex® 200 instrument (Bio‐RAD, Hercules, CA, USA). For PsA patients at both observation times, demographic, laboratory and clinical data [sex, age at baseline, disease duration, presence/absence of comorbidity, oligoarticular/polyarticular phenotype, previous and concomitant therapies to apremilast, Disease Activity of Psoriatic Arthritis (DAPSA) [10], Psoriasis Area Severity Index (PASI), Leeds Enthesitis Score (LEI), Health Assessment Questionnaire‐Disability Index (HAQ), body mass index (BMI), erythrocyte sedimentation rate (ESR, mm/h) and C reactive protein (CRP, mg/l)] were collected. For healthy donors, demographic data were collected at sample collection.
Statistical analysis
Demographics and disease characteristics at baseline were analyzed using standard descriptive statistics. Categorical data are expressed as percentages, while normally distributed continuous variables are shown as mean ± 1 standard deviation (s.d.); t‐test or Wilcoxon’s signed‐rank test for paired samples were used when appropriate. To investigate the association of sCD40L level with DAPSA minor response (i.e. 40% difference from the baseline DAPSA value [11]) and DAPSA Low Disease Activity (LDA) and/or remission (i.e. DAPSA ≤ 14) at 6 months of treatment with apremilast, multivariate logistic regression models with backward selection (P < 0·05) were built, using sCD40L as an independent variable and adjusting for other variables associated with the outcomes of interest (P < 0·20) . For each model a receiver operating characteristic (ROC) curve was constructed, whereas the area under the curve (AUC) was validated with the 10‐fold cross‐validation. Statistical analysis was carried out with α = 0·05 unless otherwise indicated. GPower version 3.1 was used for power analysis (α = 0·05, 1β = 0·80) (Dusseldorf University, Dusseldorf, Germany). Data retrieved with Bio‐Plex ProTM Manager Software 6.0 (Bio‐RAD) were analyzed with STATA version 16.0 (StataCorp, College Station, TX, USA). The a priori power analysis indicated that 27 patients were necessary to obtain an effect size of 0·5 (medium) with α = 0·05 and 1β = 0·80 for matched observations. Furthermore, 21 healthy donors (HD) were needed to test a difference with PsA patients in term of sCD40L serum level at apremilast baseline (effect size = 0·8 (large) with α = 0·05 and 1β = 0·80).
No imputations for missing data were carried out. The study complies with Helsinki declaration and was approved by local Ethics Committee as part of the GISEA observational study (ClinicalTrial.gov NCT01543594, Local Approval no. DG‐624).
Ethics
This study complies with Helsinki declaration and had been approved by local Ethics Committee as part of the GISEA observational study (ClinicalTrial.gov NCT01543594, Local Approval no. DG‐624).
Results
We studied 27 patients (16 of 27 women, 59·6%) with PsA with mean age (± s.d.) of 58·4 ± 10·4 years. According to the Italian Medicine Agency, apremilast is indicated for PsA patients with inadequate response to conventional disease‐modifying anti‐rheumatic drugs (DMARDs) and not eligible for biological disease‐modifying anti‐rheumatic drugs (bDMARDs) and for those non‐responders to bDMARDs. Eleven out of 27 patients (40·7%) were naive for bDMARDS. In 29·6% (eight of 27) of patients apremilast was the second treatment after bDMARDs, whereas it was administered as the third or subsequent line for the remaining 29·6% (eight of 27). Further details on clinical, laboratory and demographic characteristics at baseline and 6 months are shown in Table 1. The HD group included 20 patients (16 of 20 females, 80%) with a mean age of 50 ± 10·7 years and BMI of 25·6 ± 2·6. Baseline DAPSA was 19·3 ± 9·3 and decreased significantly to 11·3 ± 9 after 6 months of treatment (P = 0·0005). In addition, 67·9% of patients (19 of 27) were in LDA remission. Similarly, LEI decreased from 0·7 ± 1·2 to 0·2 ± 0·8 (P = 0·002). Mean serum baseline sCD40L levels were significantly higher in patients with PsA than in healthy donors (P < 0·0001, Table 1) and after adjustment for sex, BMI and age. A significant reduction was observed for sCD40L level after 6 months of apremilast from 5364 ± 2025 to 4412 ± 2629 pg/ml (P = 0·01) (Fig. 1).
Table 1.
Demographic, clinical and laboratory characteristics of patients with psoriatic arthritis at baseline and 6 months. Demographic and soluble CD40L level of healthy donors are also shown
| PsA patients (27 patients) | 6‐months | P | HD (20 patients) | P (versus PsA patients) | |
|---|---|---|---|---|---|
| Baseline | |||||
| Female (n, %) | 16 (59·6) | 16 (80) | 0·01 | ||
| Age, years (mean ± s.d.) | 58·4 ± 10·4 | 50 ± 10·7 | 0·005 | ||
| BMI (mean ± s.d.) | 28·1 ± 5·3 | 27·2 ± 5 | 0·09 | 25·6 ± 2·6 | 0·02 |
| Comorbidities (n, %) | 23 (82·1) | ||||
| Disease duration, months (mean ± s.d.) | 127 ± 98·9 | ||||
| Polyarthritis (n, %) | 12 (44·4) | ||||
| Previous treatment (n, %) | |||||
| bDMARDs, naive | 11 (40·7) | ||||
| 1 bDMARD | 8 (29·6) | ||||
| ≥ 2 bDMARDs | 8 (29·6) | ||||
| MTX (n, %) | 9 (33·3) | 9 (33·3) | 0·9 | ||
| Glucocorticoids (n, %) | |||||
| No glucocorticoids | 17 (63) | 20 (74·1) | 0·005 | ||
| ≤ 5 mg/day prednisone | 9 (33·3) | 7 (25·9) | 0·005 | ||
| > 5 mg/day prednisone | 1 (3·7) | 0 | 0·005 | ||
| sCD40L, pg/ml (mean ± s.d.) | 5364 ± 2025 | 4412 ± 2629 | 0·01 | 2575 ± 843·1 | 0·0001 |
| PASI (mean ± s.d.) | 1·4 ± 2 | 0·8 ± 1·7 | 0·03 | ||
| HAQ median (IQR) | 1 (0·7) | 0·9 (0·7) | 0·54 | ||
| DAPSA (mean ± s.d.) | 19·3 ± 9·3 | 11·3 ± 9·4 | 0·0005 | ||
| DAPSA minor response (n, %) | 12(44·4) | ||||
| LDA or remission (n, %) | 18 (66·7) | ||||
| LEI (mean ± s.d.) | 0·7 ± 1·2 | 0·2 ± 0·8 | 0·002 | ||
| ESR, mm/1st h (mean ± s.d.) | 21·7 ± 20 | 22·1 ± 28·8 | 0·91 | ||
| CRP, mg/l (mean ± s.d.) | 9·1 ± 16·5 | 6·3 ± 9·9 | 0·35 |
bDMARDs = biological disease‐modifying anti‐rheumatic drugs; BMI = body mass index; CRP = C reactive protein; DAPSA = disease activity in psoriatic arthritis; ESR: erythrocyte sedimentation rate; HAQ = Health Assessment Questionnaire–disability index; HD = healthy donors; IQR = interquartile range; LDA = low disease activity; LEI = Leeds Enthesitis Index; MTX = methotrexate; PASI = Psoriasis Area Severity Index; PDN = prednisone; PsA = psoriatic arthritis; sCD40L = soluble CD40 ligand; s.d. = standard deviation.
Fig. 1.
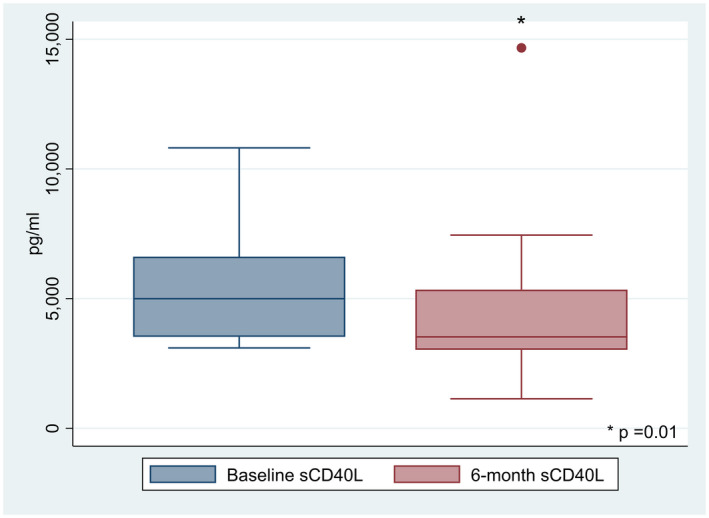
Box‐plot showing serum sCD40L levels at baseline and 6 months during apremilast treatment (P = 0·01).
At 6 months, apremilast was discontinued in three of 27 patients (11·1%) due to lack of efficacy. No adverse events occurred in the observation period.
Clinical correlations
No association was demonstrated between the baseline serum levels of sCD40L and the clinical features in PsA patients. Multivariate analysis, also including CRP level, showed that baseline serum sCD40L level was an independent predictor of DAPSA minor response at 6 months [odds ratio (OR) = 1·0006, 95% confidence interval (CI) = 1·0001–1·0012; AUC = 0·76, 95% CI = 0·55–0·97). Additionally, baseline DAPSA (OR = 0·80, 95% CI = 0·65–0·98) and baseline serum sCD40L level (OR = 1·001, 95% CI = 1·0001–1·0027) were independently associated with the achievement of DAPSA LDA/remission at 6 months in a multivariate model including baseline sCD40L, CRP, presence/absence of co‐morbidity, combination treatment with methotrexate (MTX), Health Assessment Questionnaire (HAQ), DAPSA and oligoarthritis/polyarthritis. The AUC for this model was 0·85 (95% CI = 0·69–0·98, Fig. 2).
Fig. 2.
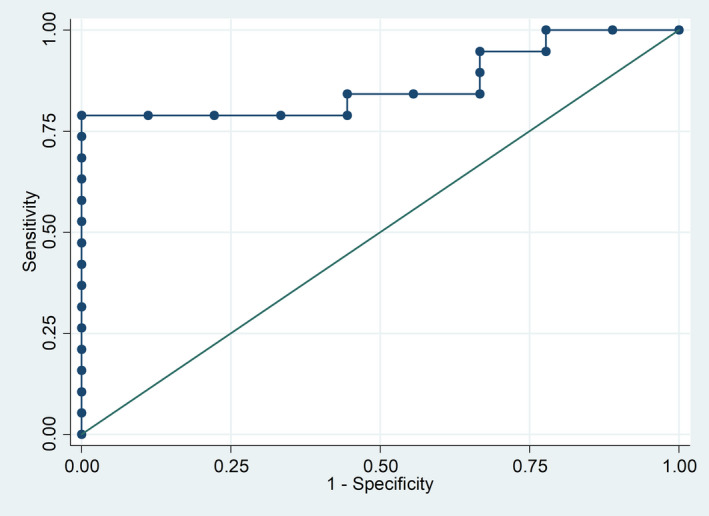
Receiver operating characteristic (ROC) curve for the model predicting LDA/remission at 6 months of apremilast treatment. Low baseline DAPSA and high sCD40L serum levels at the start of apremilast predict 6‐month clinical response. Variables left in the model: baseline DAPSA [odds ratio (OR) = 0·80 per single unit, 95% confidence interval (CI) = 0·65–0·98] and baseline soluble CD40L level (OR = 1·001 per single unit, 95% CI = 1·0001–1·0028). Area under the curve = 0·85 (95% CI = 0·69–0·98). DAPSA = disease activity in psoriatic arthritis; LDA = low disease activity.
Discussion
In the pathogenesis of psoriasis, CD40/CD40L interaction is an important pathway, being responsible for plaque maintenance [12]. This interaction may also play a role in the inflammatory process underlying PsA. In fact, evidence suggests that T lymphocytes of PsA patients over‐express CD40L compared to those from healthy subjects and with rheumatoid arthritis [1]. Despite this, there are no studies in the literature investigating CD40L as a prognostic biomarker in patients with PsA. The current availability of apremilast, a PDE4 inhibitor, for the treatment of PsA potentially capable of reducing the cleavage of sCD40L [13] had provided the rationale of this study.
Although we did not test sCD40L in other inflammatory arthritides, its strikingly high level found in PsA patients, compared with HD, may suggest a somewhat specific role of CD40 in PsA. In addition, serum sCD40L levels dropped along with the disease activity after 6 months of treatment with apremilast. The putative effect of apremilast to reduce sCD40L serum level opens interesting new perspectives. Of note, atherosclerosis‐related conditions such as obesity, metabolic syndrome and type II diabetes are the most prevalent comorbidities in PsA patients [14]. High levels of sCD40L may reflect an aberrant platelet activation and have been associated with increased cardiovascular risk [15]. Given that PDE4 blockade can down‐regulate the downstream pathway mediating polymorphonuclear leukocytes–platelets adhesion [8], we may speculate that a reduction of sCD40L by apremilast may also exert a modulating effect on platelet activation and thrombotic risk of patients with PsA, although this hypothesis needs to be corroborated by large‐scale prospective studies.
In addition, this study has highlighted an important association of sCD40L with clinical outcomes. The finding that high baseline sCD40L levels predict DAPSA response could make it a possible biomarker for profiling the patient suitable for apremilast therapy. The added value of sCD40L lies in the fact that, together with the DAPSA, allows identification of potential short‐term responders. Indeed, PsA patients with high sCD40L levels and low DAPSA value can benefit more effectively from apremilast and are more likely to reach the DAPSA LDA and/or remission. Of note, the latter association seems to be independent from CRP levels, suggesting that a dysregulation in CD40/CD40L pathway contributes to PsA disease activity, irrespective of the inflammatory burden. Furthermore, the clinical response to apremilast did not seem to be influenced by BMI, as reported for tumor necrosis factor inhibitors [16].
The main limitation of this study consists of having considered a large effect size for our analysis, using a small sample size. Additionally, a control group of patients with psoriasis to explore whether the excess CD40L‐shedding is a specific feature of PsA was lacking. Furthermore, we did not carry out in‐vitro studies to investigate whether the modulation of neutrophil–platelet interaction or the cleavage of sCD40L from T lymphocytes may be a specific effect of apremilast. However, this study has the strength of showing the potential of sCD40L as prognostic biomarker in patients with PsA on apremilast treatment, providing the rationale for larger assessments.
Disclosures
Authors declare no conflicts of interest for this work.
Acknowledgements
This study was partially funded be the University of Bari ‘Aldo Moro’ , Bari, Italy. We thank the ‘Consulta degli Specializzandi’ (‘Residents Conference’), Dr Francesca Vadacca and Dr Salvatore Fedele for logistic support.
References
- 1. Daoussis D, Antonopoulos I, Andonopoulos AP, Liossis SN. Increased expression of CD154 (CD40L) on stimulated T‐cells from patients with psoriatic arthritis. Rheumatology (Oxf) 2007; 46:227–31. [DOI] [PubMed] [Google Scholar]
- 2. Rizvi M, Pathak D, Freedman JE, Chakrabarti S. CD40‐CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med 2008; 14:530–8. [DOI] [PubMed] [Google Scholar]
- 3. Menon B, Gullick NJ, Walter GJ et al Interleukin‐17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014; 66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armas‐Gonzalez E, Diaz‐Martin A, Dominguez‐Luis MJ et al Differential antigen‐presenting B cell phenotypes from synovial microenvironment of patients with rheumatoid and psoriatic arthritis. J Rheumatol 2015; 42:1825–34. [DOI] [PubMed] [Google Scholar]
- 5. Davidson DC, Jackson JW, Maggirwar SB. Targeting platelet‐derived soluble CD40 ligand: a new treatment strategy for HIV‐associated neuroinflammation? J Neuroinflammation 2013; 10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar N, Goldminz AM, Kim N, Gottlieb AB. Phosphodiesterase 4‐targeted treatments for autoimmune diseases. BMC Med 2013; 11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanichakarn P, Blair P, Wu C, Freedman JE, Chakrabarti S. Neutrophil CD40 enhances platelet‐mediated inflammation. Thromb Res 2008; 122:346–58. [DOI] [PubMed] [Google Scholar]
- 8. Totani L, Amore C, Di Santo A et al Roflumilast inhibits leukocyte‐platelet interactions and prevents the prothrombotic functions of polymorphonuclear leukocytes and monocytes. J Thromb Haemost 2016; 14:191–204. [DOI] [PubMed] [Google Scholar]
- 9. Coates LC, Conaghan PG, Emery P et al Sensitivity and specificity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum 2012; 64:3150–5. [DOI] [PubMed] [Google Scholar]
- 10. Schoels MM, Aletaha D, Smolen JS. Defining remission and treatment success using the DAPSA score: response to letter by Helliwell and Coates. Ann Rheum Dis 2015; 74:e67. [DOI] [PubMed] [Google Scholar]
- 11. Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016; 75:811–8. [DOI] [PubMed] [Google Scholar]
- 12. Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol 2009; 21:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kishi Y, Ohta S, Kasuya N et al Ibudilast modulates platelet‐endothelium interaction mainly through cyclic GMP‐dependent mechanism. J Cardiovasc Pharmacol 2000; 36:65–70. [DOI] [PubMed] [Google Scholar]
- 14. Iannone F, Salaffi F, Fornaro M et al Influence of baseline modified Rheumatic Disease Comorbidity Index (mRDCI) on drug survival and effectiveness of biological treatment in patients affected with Rheumatoid arthritis, spondyloarthritis and psoriatic arthritis in real‐world settings. Eur J Clin Invest 2018; 48:e13013. [DOI] [PubMed] [Google Scholar]
- 15. Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation 2001; 104:2266–8. [DOI] [PubMed] [Google Scholar]
- 16. Iannone F, Lopalco G, Rigante D, Orlando I, Cantarini L, Lapadula G. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev 2016; 15:447–50. [DOI] [PubMed] [Google Scholar]


