Summary
Major histocompatibility complex class I (MHC‐I)‐dependent inhibitory receptors on natural killer (NK) cells have been found to contribute to NK cell dysfunction in hepatocellular carcinoma (HCC). However, the roles of MHC‐I‐independent inhibitory receptors on NK cells in HCC remain poorly defined. In this study, we analyzed the expression of the MHC‐I‐independent inhibitory receptors sialic acid‐binding immunoglobulin‐like lectin (Siglec)‐7 and Siglec‐9 on NK cells by analyzing the peripheral blood of 35 HCC patients and 63 healthy donors. We observed that HCC patients had lower frequencies and total numbers of NK cells in the peripheral blood. Importantly, both the expression levels of Siglec‐7 on NK cells and the frequencies of Siglec‐7+ NK cells were significantly reduced in HCC patients, which was accompanied by a decrease in activating receptor and an increase in inhibitory receptor expression on NK cells. Moreover, Siglec‐7+ NK cells expressed higher levels of activating receptors and displayed stronger effector functions, compared with Siglec‐7− NK cells. Our findings demonstrate for the first time that reduced Siglec‐7 expression predicts NK cell dysfunction in HCC patients, suggesting that Siglec‐7 may be a potential marker of functional NK cell subset in HCC patients.
Keywords: hepatocellular carcinoma, natural killer cells, Siglec‐7
The expression levels of Siglec‐7 on NK cells and the frequencies of Siglec‐7+ NK cells were significantly reduced in HCC patients. Siglec‐7+ NK cells expressed higher levels of activating receptors and displayed stronger effector functions, compared with Siglec‐7 NK cells. Reduced Siglec‐7 expression predicts NK cell dysfunction in HCC patients.
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most common cancer but the second leading cause of cancer‐related deaths worldwide [1]. Unfortunately, only a limited choice of effective therapeutic regimens for HCC exist to date. Increasing evidence indicates that immunotherapy may represent a promising strategy for HCC treatment [2]. The human liver is an organ enriched in diverse lymphocyte populations, of which natural killer (NK) cells account for 25–50% [3]. NK cells have potent anti‐tumor activity through direct cell lysis and/or cytokine production. Accumulating studies have shown that the decrease in NK cell numbers and their dysfunction (also called NK cell exhaustion) is associated with the progression and poor clinical outcome of HCC [4]. This suggests that NK cell‐based immunotherapies to improve NK cell effector function including, but not limited to, checkpoint blockade, cytokine therapy or NK cell adaptive transfer, have the potential to be applied to HCC treatment. However, the lack of detailed knowledge relating to the exact mechanisms underlying NK cell exhaustion has deterred the use of NK cell immunotherapy in HCC.
NK cells comprise ~15% of total lymphocytes in the human peripheral blood mononuclear cell (PBMC) population, and are divided into two subsets (CD56brightCD16− and CD56dimCD16+), according to the expression of CD56 (dim or bright) and CD16 (presence or absence). CD56dimCD16+ NK cells account for ~90–95% of the total NK cell population and exhibit higher cytotoxic activity than CD56brightCD16− NK cells [5]. NK cell function is regulated by (i) activating receptors, such as natural killer group (NKG)2D, NKp46, NKp44 and NKp30; and (ii) inhibitory receptors such as NKG2A, lymphocyte‐activation gene (LAG‐3), T cell immunoglobulin mucin‐3 (TIM‐3), T cell immunoglobulin and immunoreceptor tyrosine‐based inhibition motif (ITIM) domain (TIGIT) and CD96 [6]. Healthy cells protect themselves against NK cell‐mediated killing via the interaction between their self‐major histocompatibility complex class I (MHC‐I) molecules and the inhibitory receptors on NK cells [7]. This strategy is also utilized by cancer cells to escape NK cell‐mediated anti‐tumor immunity [8]. Recently, accumulating studies have demonstrated that MHC‐I‐independent inhibitory receptors also contribute to NK cell dysfunction during cancer progression [9, 10]. Deciphering the roles of these inhibitory receptors in cancer pathogenesis will therefore enhance our understanding of the mechanisms implicated in NK cell exhaustion and benefit the development of effective HCC therapies.
Sialic‐acid‐binding immunoglobulin‐like lectin‐7 (Siglec‐7) belongs to the CD33‐related Siglec family, predominantly expressed on human NK cells [11]. The cytoplasmic domain of Siglec‐7 has an ITIM, which defines its action as an inhibitory receptor on NK cells [12]. Although the ligation of Siglec‐7 with specific antibodies or ligands inhibits NK cell function [13], Siglec‐7 displays a similar expression pattern to that of NK cell‐activating receptors. The down‐regulation of Siglec‐7 on NK cells, which represents an early marker of NK cell dysfunction, has been observed during chronic viral infection [14, 15]. The expression of Siglec‐7 is also reduced on NK cells in obese humans, whose NK cell anti‐tumor activity is impaired [16, 17]. Thus, cell surface Siglec‐7 expression defines a highly functional NK cell phenotype, despite its role in suppressing NK cell activity. However, the changes in Siglec‐7 expression experienced by NK cells and their association with the clinical outcomes of HCC remain largely unknown.
In this study, we observed that both the level of Siglec‐7 expression on NK cells and the frequency of Siglec‐7+ NK cells were significantly reduced in HCC patients, which was accompanied by a decrease in activating receptor and an increase of inhibitory receptor expression on NK cells, as well as overall NK cell functional impairment. Therefore, reduced Siglec‐7 expression on NK cells predicts circulating NK cell dysfunction in HCC patients, suggesting that Siglec‐7 may be a potential marker of functional NK cell subset in HCC patients.
Materials and methods
Human samples
None of the HCC patients selected for this study were receiving chemotherapy or radiotherapy. Venous blood was collected from 35 untreated patients with primary HCC and 63 healthy donors (HDs) between 2017 and 2019. Patients were recruited from the First Affiliated Hospital of Anhui Medical University. This study was approved by the Medical Ethics Committee of the First Hospital of Anhui Medical University, and the protocols were carried out in accordance with the approved guidelines. All participants gave written informed consent to participate in the study.
Isolation and activation of PBMCs
PBMCs were isolated from the peripheral blood of HCC patients or HDs by gradient centrifugation with Ficoll‐Paque Plus (Solarbio, Beijing, China), according to the manufacturer’s instructions. To assess intracellular cytokine production, PBMCs were isolated and stimulated with phorbol 12‐myristate 13‐acetate (PMA), ionomycin and monensin for 4 h.
Flow cytometry
Single‐cell suspensions were stained with the fluorescently‐labeled antibodies, including fluorescein isothiocyanate (FITC)‐anti‐human CD3 (clone OKT3), Brilliant Violet 421 anti‐human CD56 (clone 5.1H11), phycoerythrin (PE) anti‐human CD366 (TIM‐3; clone F38‐2E2), peridinin chlorophyll/cyanine 5.5 (PerCP/Cy5.5) anti‐human CD337 (NKp30; clone P30‐15), PE/Cy7‐CD335 (NKp46; clone 9E2), PE anti‐human CD328 (Siglec‐9; clone K8), APC‐anti‐human CD328 (Siglec‐7; clone 6‐434), APC/Fire™ 750 anti‐human CD16 (clone 3G8), PE‐anti‐human tumor necrosis factor (TNF)‐α (clone MAb11), PerCP/Cy5.5 anti‐human CD107a [lysosomal‐associated membrane protein 1 (LAMP‐1); clone H4A3] and PE/Cy7 anti‐human interferon (IFN)‐γ (clone B27). Isotype controls were used to determine cut‐off levels for positive staining. All antibodies and isotypes were purchased from Biolegend (San Diego, CA, USA). Data were acquired using a flow cytometer (Beckman Coulter; Brea, CA, USA) and analyzed using FlowJo version 10 software (Tree Star; Ashland, OR, USA).
Statistical analysis
Data are shown as means ± standard deviation (s.d.). The difference between groups was analyzed using a paired‐ or unpaired‐samples t‐test. Any potential correlations between variables were analyzed using Pearson’s correlation test. Analyses yielding P‐values of less than 0·05 were considered statistically significant; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001. Statistical analyses were performed using prism software, version 6 (GraphPad).
Results
Abnormal frequencies and functions of NK cells observed in the peripheral blood of HCC patients
To analyze NK cells in the peripheral blood, CD3−CD56+ NK cells were first identified within the lymphocyte gate and then subdivided into CD56dimCD16+ and CD56brightCD16− NK subsets (Fig. 1a). Our results show that the frequencies of total NK cells in the peripheral blood of HCC patients were lower compared to HDs (Fig. 1b). Moreover, the proportion of the CD56dimCD16+ NK subset within the total NK cell population decreased, while the proportion of the CD56brightCD16− NK subset increased in HCC patients compared to HD controls, implying a shift in the NK cell composition of HCC patients (Fig. 1c,d). Accordingly, the absolute number of NK cells in the peripheral blood of HCC patients was markedly lower than in that of HDs (Fig. 1e). The same was true for the absolute numbers of both CD56dimCD16+ and CD56dimCD16− NK cell subsets (Fig. 1f,g). Moreover, the NK cells of HCC patients displayed impaired IFN‐γ production (Fig. 1h,i). Taken together, our data reveal a reduction in the frequency, number and function of NK cells in HCC patients.
Fig. 1.
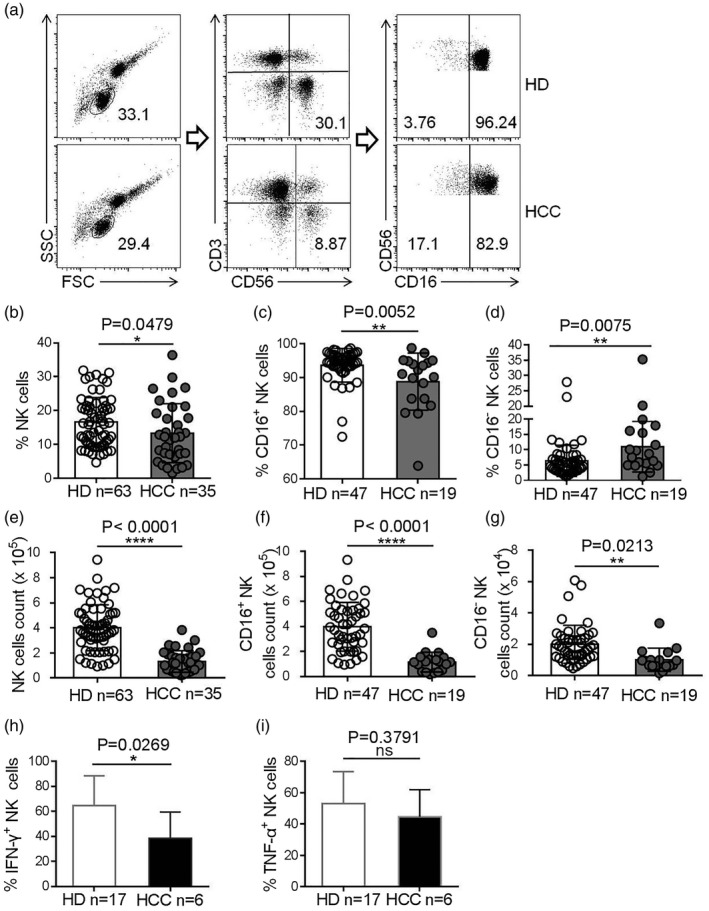
Lower natural killer (NK) cell numbers detected in the peripheral blood of hepatocellular carcinoma (HCC) patients. (a) The gating strategy used for the cytometric analysis of NK cell subsets in peripheral blood mononuclear cell (PBMC) samples. (b–d) Frequencies of NK cells within the lymphocyte population (b), CD16+ NK cell subset (c) and CD16− NK cells subset (d) in total NK cells of peripheral blood from patients with HCC patients and healthy donors (HDs). (e–g) The numbers of NK cells (e), CD16+ NK cells (f) or CD16− NK cells (g) in the peripheral blood of HCC patients and HDs. (h,i) The proportion of interferon (IFN)‐γ+ NK cells (h) or tumor necrosis factor (TNF)‐α+ NK cells (i) in HCC patients and HDs. Data are shown as means ± standard deviation (s.d.). Analyses yielding P‐values of less than 0·05 were considered statistically significant. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Abnormal expression of activating and inhibitory receptors on NK cells in HCC patients
NK cell anti‐tumor activity is regulated by activating and inhibitory receptors, which led us to investigate the expression characteristics of these receptors on NK cells in HCC patients compared to HDs. We found that the frequencies of NKp30+ and NKp46+ NK cells were lower in the peripheral blood of HCC patients compared to HDs (Fig. 2a–f). In contrast, the frequency of TIM‐3+ NK cells was higher in HCC patients (Fig. 2g,h). In addition, the frequency of programmed cell death 1 (PD‐1+) NK cells was slightly higher in HCC patients, although the frequency was very low in both HCC patients and HDs (Supporting information, Fig. S1a–c). The frequency of TIGIT+ NK cells in HCC patients was similar to that in HDs (Supporting information, Fig. S1d–f). No significant difference of frequencies of NKp44+ and NKG2D+ NK cells was observed in HCC patients and HDs (Supporting information, Fig. S1g–l). Taken together, our data reveal that the expression of activating and inhibitory receptors on NK cells was abnormal in HCC patients, indicating NK cell dysfunction.
Fig. 2.
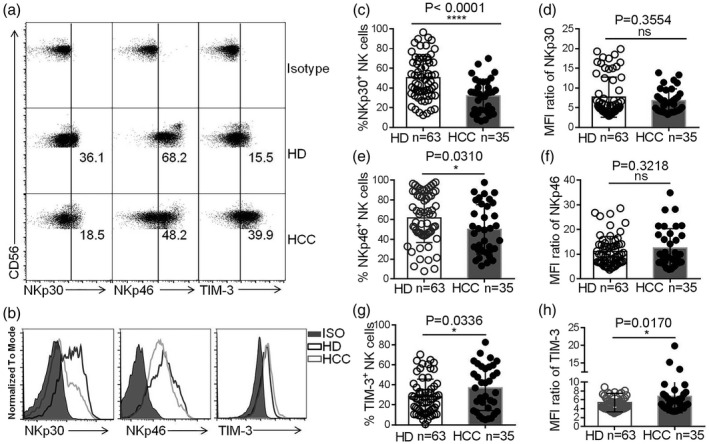
Abnormal expression of cell‐surface activating and inhibitory receptors on natural killer (NK) cells in hepatocellular carcinoma (HCC). (a,b) Dot‐plot (a) and histogram (b) representations of T cell immunoglobulin mucin‐3 (TIM‐3), NKp30 and NKp46 expression on NK cells in HCC patients and healthy donors (HDs). (c,e,g) The proportion of NKp30+ NK cells (c), NKp46+ NK cells (e) or TIM‐3+ NK cells (g) in HCC patients and HDs. (d,f,h) The median fluorescence intensity (MFI) ratios of NKp30 (d), NKp46 (f) or TIM‐3 (h) on NK cells. MFI ratio of X = X‐MFI/isotype‐MFI. Data are shown as means ± standard deviation (s.d.). Analyses yielding P‐values of less than 0·05 were considered statistically significant. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Reduced Siglec‐7 expression on NK cells in the peripheral blood of HCC patients
Siglec‐7 and Siglec‐9 are MHC‐I‐independent NK cell inhibitory receptors. We found that the frequency of Siglec‐9+ NK cells in HCC patients was similar to that in HDs (Fig. 3a) However, the median fluorescence intensity (MFI) level of Siglec‐7 expression on HCC patient NK cells was significantly lower compared with HDs (Fig. 3b,c). The frequency and number of Siglec‐7+ NK cells were also lower in the peripheral blood of HCC patients (Fig. 3d,e). Moreover, the number of Siglec‐7+ NK cells in HCC patients with alpha‐fetoprotein (AFP) (> 25) was significantly lower than that in HCC patients with AFP (≤ 25) (Table 1). It had been reported that the frequency of Siglec‐7+ NK cells is reduced in hepatitis B virus (HBV)‐infected individuals [15]. However, we found no difference in the frequency of Siglec‐7+ NK cells between HBV− and HBV+ HCC patients (Fig. 3f), suggesting that HBV infection was not the reason for Siglec‐7 down‐regulation in HCC patient NK cells. The expression of activating and inhibitory receptors on NK cells was further investigated on different NK cell subsets. The frequencies of CD56dimCD16+NKp30+, CD56dimCD16+NKp46+ and CD56dimCD16+Siglec‐7+ NK cells were all reduced in HCC patients compared to HDs (Fig. 4a–e). Taken together, our data demonstrate that the expression of Siglec‐7 was lower on NK cells in the peripheral blood of HCC patients.
Fig. 3.
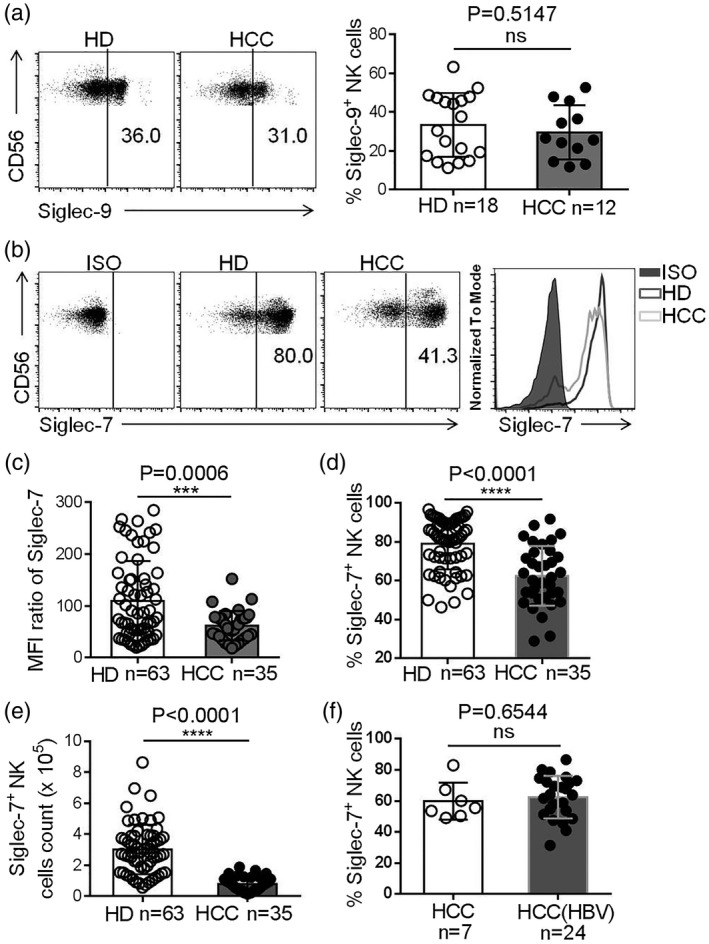
Decreased Siglec‐7 expression on natural killer (NK) cells in hepatocellular carcinoma (HCC). (a) A representative dot‐plot showing Siglec‐9+ expression on the NK cells of HCC patients and healthy donors (HDs), assessed by flow cytometry (left panel) and comparison between Sigleg‐9 expression on the NK cells of HCC patients and HDs (right panel). (b) Representative dot‐plot (left panel) and histogram (left panel) of surface Siglec‐7 expression on NK cells in HCC patients and HDs. (c) The Siglec‐7 median fluorescence intensity (MFI) ratio on the NK cells of HCC patients and HDs. (d) The proportion of Siglec‐7+ NK cells in HCC patients and HDs. (e) Siglec‐7+ NK cell counts in HCC patients and HDs. (f) The comparison of Siglec‐7+ NK cells in HCC patients with or without hepatitis B virus (HBV) infection. Data are shown as means ± standard deviation (s.d.). Analyses yielding P‐values less than 0·05 were considered as statistically significant. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Table 1.
Clinical characteristics of HCC patients
| Characteristics | Number | Mean with s.e.m. (Siglec‐7+ NK cell count) | P‐value (unpaired Student’s t‐test) |
|---|---|---|---|
| Age (years) | |||
| ≤ 56 | 19 | 81 864 ± 11 701 | 0·5996 |
| > 56 | 16 | 73 520 ± 10 039 | |
| Gender | |||
| Male | 31 | 79 218 ± 8505 | 0·6816 |
| Female | 4 | 68 998 ± 1819 | |
| AFP (ng/ml) | |||
| ≤ 25 | 15 | 99 116 ± 1241 | 0·0163 |
|
> 25 |
20 | 62 249 ± 8526 | |
| ALT, U/l | |||
| ≤ 40 | 18 | 93 122 ± 10 065 | 0·0438 |
| > 40 | 17 | 62 091 ± 10 880 | |
| Tumor size, cm | |||
| ≤ 5 | 16 | 77 624 ± 10 953 | 0·9608 |
| > 5 | 19 | 78 408 ± 11 189 | |
| Tumor multiplicity | |||
| Solitary | 22 | 76 363 ± 9099 | 0·7820 |
| Multiple | 13 | 80 904 ± 14 587 | |
| Vascular invasion | |||
| Absent | 19 | 81 197 ± 11 309 | 0·6651 |
| Present | 16 | 74 312 ± 10 697 | |
| Intrahepatic metastasis | |||
| No | 23 | 77 662 ± 8323 | 0·9462 |
| Yes | 12 | 78 791 ± 16 641 |
Analyses yielding P‐values of less than 0·05 were considered as statistically significant.
AFP = a‐fetoprotein; ALT = alanine aminotransferase; HCC = hepatocellular carcinoma; NK = natural killer; s.e.m. = standard error of the mean.
Fig. 4.
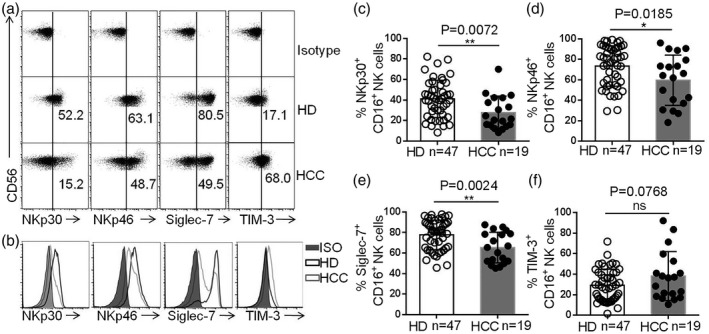
Decreased Siglec‐7 expression on the CD16+ natural killer (NK) cells subset in hepatocellular carcinoma (HCC). (a,b) Dot‐plot (a) and histogram (b) representation of NKp30, NKp46, Siglec‐7 and T cell immunoglobulin mucin‐3 (TIM‐3) expression on CD16+ NK cells. (c–f) The proportions of NKp30+CD16+ NK cells (c), NKp46+CD16+ NK cells (d), Siglec‐7+CD16+ NK cells (e) and TIM‐3+CD16+ NK cells (f) within the total NK cell population in the peripheral blood of HCC patients and healthy donors (HDs). Data are shown as means ± standard deviation (s.d.). Analyses yielding P‐values less than 0·05 were considered statistically significant. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Siglec‐7+ NK cells exhibit the hyperresponsiveness in HCC patients
Lower numbers of Siglec‐7+ NK cells were observed in the peripheral blood of HCC patients compared to HDs. In order to more clearly characterize Siglec‐7+ NK cells in HCC patients, we evaluated their activating and inhibitory receptor expression profiles and measured their effector functions. Our data suggest that Siglec‐7+ NK cells express higher levels of the activating receptors NKp30 and NKp46 compared with Siglec‐7− NK cells, both in HCC patients and HDs (Fig. 5a–f). Moreover, Siglec‐7+ NK cells produced more TNF‐α compared with Siglec‐7− NK cells after stimulation with by PMA and ionomycin (Fig. 6a,b). No significant difference was observed in the production of IFN‐γ and the expression of CD107a by the Siglec‐7+ NK cells and Siglec‐7− NK cells (Fig. 6c–f). Thus, our data demonstrate that Siglec‐7+ NK cells express higher levels of activating receptors and exhibit hyperresponsiveness in HCC patients.
Fig. 5.
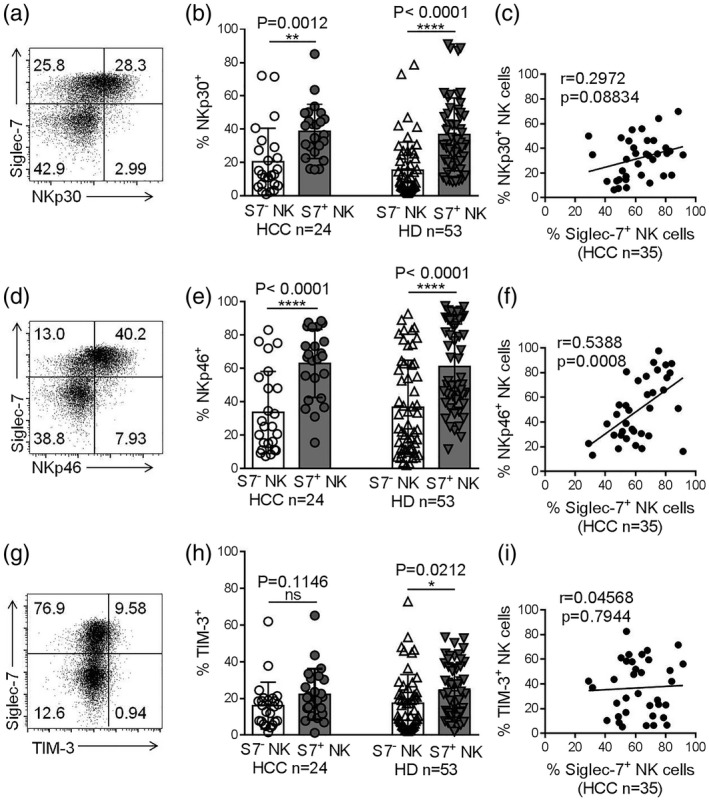
Siglec‐7+ natural killer (NK) cells express higher levels of activating receptors. (a) A representative dot‐plot showing NKp30 expression on Siglec‐7+ and Siglec‐7− NK cells, assessed by flow cytometry. (b) The proportions of NKp30+Siglec‐7+ NK cells and NKp30+Siglec‐7− NK cells in hepatocellular carcinoma (HCC) patients and healthy donors (HDs). (c) The correlation between Siglec‐7 and NKp30 expression on NK cells. (d) Dot‐plot representation of NKp46 expression on Siglec‐7+ and Siglec‐7− NK cells. (e) The proportions of NKp46+Siglec‐7+ and NKp46+Siglec‐7− NK cells in HCC patients and HDs. (f) The correlation between Siglec‐7 and NKp46 expression on NK cells. (g) A representative dot‐plot showing T cell immunoglobulin mucin‐3 (TIM‐3) expression on Siglec‐7+ and Siglec‐7− NK cells. (h) The proportions of TIM‐3+Siglec‐7+ and TIM‐3+Siglec‐7− NK cells in HCC patients and HDs. (i) The correlation between Siglec‐7 and TIM‐3 expression on NK cells. Data are shown as means ± standard deviation (s.d.). Analyses yielding P‐values less than 0·05 were considered statistically significant. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Fig. 6.
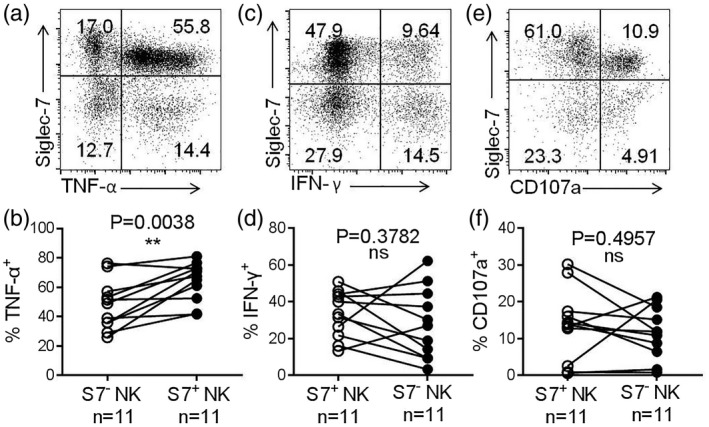
Siglec‐7+ natural killer (NK) cells exhibit stronger effector functions than Siglec‐7− NK cells. (a) A representative dot‐plot showing TNF‐α production by Siglec‐7+ and Siglec‐7− NK cells, assessed by flow cytometry. (b) Comparison of TNF‐α production by Siglec‐7+ NK cells and Siglec‐7− NK cells. (c) A representative dot‐plot showing the production of IFN‐γ by Siglec‐7+ and Siglec‐7− NK cells. (d) Comparison of IFN‐γ production by Siglec‐7+ and Siglec‐7− NK cells. (e) A representative dot‐plot showing CD107a expression by Siglec‐7+ and Siglec‐7− NK cells. (f) Comparison of CD107a expression by Siglec‐7+ and Siglec‐7− NK cells. Data are shown as means ± standard deviation (s.d.). Analyses yielding P‐values less than 0·05 were considered statistically significant. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Discussion
Siglec‐7 is an MHC‐I‐independent inhibitory receptor on human NK cells that recognizes sialic acid‐containing carbohydrates [18]. It has been reported that the expression of Siglec‐7 on NK cells is reduced in HBV‐ or hepatitis C virus (HCV)‐infected patients [15]. In the present study, we found that Siglec‐7 expression was also reduced on the NK cells of HCC patients. Siglec‐7+ NK cells displayed higher levels of activating receptor expression compared with Siglec‐7− NK cells. Therefore, reduced Siglec‐7 expression on NK cells appears to predict NK cell dysfunction in the context of primary HCC.
Like cytotoxic CD8+ T cells, NK cells play important roles in host immunity against cancer. NK immunotherapy may therefore provide a feasible, if not an improved, alternative to the more conventional type of cancer immunotherapy involving T cells [19, 20, 21]. A specific NK cell‐mediated immune response has been confirmed in diverse cancer types, including hematological malignancies, non‐small‐cell lung cancer, colorectal cancer and HCC [22, 23, 24, 25]. It has been widely reported that the peripheral and tumor‐infiltrating NK cells of HCC patients are associated with an abnormal frequency, phenotype and function. Our study has further confirmed these findings. We found that the frequency and absolute number of NK cells in the peripheral blood of HCC patients was lower than in that of HDs. Moreover, the proportion of the CD56dimCD16+ NK cell subset was reduced, while the proportion of the CD56dimCD16− NK cell subset was elevated in HCC patients. In addition, the IFN‐γ production capacity of NK cells of HCC patients was also impaired compared to HD controls. The activity and homeostasis of NK cells is regulated by activating, inhibitory and cytokine receptors, which are also utilized as a means of escape from the NK cell‐mediated immune response [26]. In HCC, NK dysfunction can be caused by a number of cell‐specific receptors and their molecular interactions, including: (i) myeloid‐derived suppressor cells (MDSC), via NKp30 [27]; (ii) monocytes/macrophages, via the CD48–2B4 interaction [28]; (iii) HCC‐associated fibroblasts, via prostaglandin E2 (PGE2) and indoleamine 2,3 dioxygenase (IDO) [29]; (iv) the soluble form of MHC‐I‐related chain A (sMICA); and (v) the MHC‐I‐dependent inhibitory receptor NKG2A [30]. Therefore, one of the central areas of current NK cell‐based immunotherapy research is to decipher the potential immune checkpoints modulating NK cell activity in HCC.
Siglecs are a family of sialic acid‐binding immunoglobulin‐like co‐receptors that recognize sialic acid‐containing ligands and regulate immune cell function [31, 32, 33]. The interaction of Siglecs with ligands helps cancer cells to escape the anti‐tumor immune attack [34]. Recently, Siglec‐15 became recognized as an immune suppressor and potential target for normalization cancer immunotherapy [35]. Furthermore, tumor‐expressed CD24 mediates immune evasion through its interaction with Siglec‐10 on macrophages [36]. Siglec‐10 is also reported to be associated with lower rates of survival among HCC patients, and Siglec‐10+ NK cells exhibit impaired effector functions [37]. Siglec‐9 has been found to regulate effector memory CD8+ T cells in the context of melanoma [38]. Human NK cells ubiquitously express Siglec‐7, whereas a smaller subset expresses Siglec‐9 [39]. Both Siglec‐7 and ‐9 contain ITIMs to recruit Src homology‐2 (SH2) phosphatases and suppress kinase phosphorylation [31]. Siglec‐9 defines a subset of mature cytotoxic NK cells with enhanced chemotactic potential, and CD56dimSiglec‐9+ NK cell numbers have been shown to be reduced in the peripheral blood of patients with colon adenocarcinoma or malignant melanoma [39]. The study authors have reported that Siglec‐7 is expressed at similar levels on the peripheral blood NK cells of these patients and HDs [39]. In our study, however, there was no significant difference in Siglec‐9 expression on the NK cells of HCC patients and HDs. Instead, both the frequency Siglec‐7+ peripheral NK cells and the levels of NK cell surface Siglec‐7 expression were significantly reduced in HCC patients compared to HDs. We also observed that Siglec‐7+ NK cells express higher levels of activating receptors and exhibit stronger effector functions. Moreover, the reduction in Siglec‐7 expression on peripheral blood NK cells in HCC patients was not associated with HBV infection, despite previous reports of Siglec‐7 being significantly reduced on the NK cells of HBV‐infected patients [15]. Therefore, reduced Siglec‐7 levels on NK cells appear to predict NK cell dysfunction in the context of HCC.
Siglec‐7 has a preference for α2, 6‐linked sialic acid and α2, 8‐linked sialic acid [18]. Cao et al. reported that α2,6‐linked sialic acid was positive on HCC tissues, while normal hepatocytes and preneoplastic foci of altered hepatocytes did not express α2,6‐linked sialic acid [40]. Gong et al. also reported that α2,6‐sialic acids increased significantly on the hepatocyte membrane after the carcinomatous change [41]. These findings demonstrate that Siglec‐7 ligands exist in HCC tissues. Accumulating studies report that Siglec‐7 ligands inhibit NK cell activation and cytotoxicity, which have been used by glycocalyx engineering to protect against NK cell killing [13]. Siglec‐7 ligands expressed on K562 cells significantly suppressed cytolysis of K562 cells by peripheral NK cells [39]. Disialosyl globopentaosylceramide (DSGb5) expressed on renal cell carcinoma (RCC) cells inhibited NK cell cytotoxicity in a DSGb5–Siglec‐7‐dependent manner [42]. Therefore, it is suggested that Siglec‐7 ligands in HCC tissues might inhibit NK cell function in the hepatic location. However, the precise mechanisms responsible for: (i) the changes in Siglec‐7 expression on NK cells in HCC patients; and (ii) the role of Siglec‐7 in modulating NK cell‐mediated anti‐tumor activity and ultimately HCC patient survival, still require further investigation.
In summary, our study has revealed for the first time, to our knowledge, that the extent and expression levels of Siglec‐7 on peripheral blood NK cells were significantly reduced in HCC patients, and that in HCC, Siglec‐7+ NK cells exhibit powerful anti‐tumor activity. These findings serve to deepen our understanding of Siglec‐7+ NK cell properties in the context of HCC and provide new avenues for NK‐based cancer immunotherapy.
Author contributions
J. L. and X. W. conceived the study, designed the experiments and wrote the paper. L. T. and S. W. performed the experiments, analyzed the data and wrote the paper. L. Y. and L. J. provided technical assistance in certain experiments.
Disclosures
The authors declare no competing interests.
Supporting information
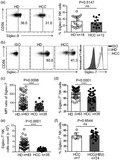
Fig. S1. The alteration of activating receptors and inhibitory receptors on peripheral NK cells in HCC patients.
Acknowledgements
This work was supported by the Anhui Provincial Natural Science Foundation (grant numbers: 1808085QC83 and 1708085QH183) and Young Top Talent Support Program of Anhui Medical University, and Research Improvement Program of Anhui Medical University.
Contributor Information
J. Li, Email: lijing1812@ahmu.edu.cn.
X. Wang, Email: wangxuefu@ahmu.edu.cn.
References
- 1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019; 16:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown ZJ, Greten TF, Heinrich B. Adjuvant treatment of hepatocellular carcinoma: prospect of immunotherapy. Hepatology 2019;70:1437–42. [DOI] [PubMed] [Google Scholar]
- 3. Mikulak J, Bruni E, Oriolo F, Di Vito C, Mavilio D. Hepatic natural killer cells: organ‐specific sentinels of liver immune homeostasis and physiopathology. Front Immunol 2019; 10:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell‐based immunotherapy. Acta Pharmacol Sin 2015; 36:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol 2016; 16:310–20. [DOI] [PubMed] [Google Scholar]
- 6. Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol 2019; 16:430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shifrin N, Raulet DH, Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol 2014; 26:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalan E, Charni S, Jaime P et al MHC‐I modulation due to changes in tumor cell metabolism regulates tumor sensitivity to CTL and NK cells. Oncoimmunology 2015; 4:e985924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Q, Bi J, Zheng X et al Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti‐tumor immunity. Nat Immunol 2018; 19:723–32. [DOI] [PubMed] [Google Scholar]
- 10. Sun H, Huang Q, Huang M et al Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology 2019; 70:168–83. [DOI] [PubMed] [Google Scholar]
- 11. Nicoll G, Ni J, Liu D et al Identification and characterization of a novel siglec, siglec‐7, expressed by human natural killer cells and monocytes. J Biol Chem 1999; 274:34089–95. [DOI] [PubMed] [Google Scholar]
- 12. Angata T, Varki A. Siglec‐7: a sialic acid‐binding lectin of the immunoglobulin superfamily. Glycobiology 2000; 10:431–8. [DOI] [PubMed] [Google Scholar]
- 13. Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec‐based mechanism for NK cell immunoevasion. Nat Chem Biol 2014; 10:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brunetta E, Fogli M, Varchetta S et al The decreased expression of Siglec‐7 represents an early marker of dysfunctional natural killer‐cell subsets associated with high levels of HIV‐1 viremia. Blood 2009; 114:3822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varchetta S, Mele D, Lombardi A et al Lack of Siglec‐7 expression identifies a dysfunctional natural killer cell subset associated with liver inflammation and fibrosis in chronic HCV infection. Gut 2016; 65:1998–2006. [DOI] [PubMed] [Google Scholar]
- 16. Rosenstock P, Horstkorte R, Gnanapragassam VS, Harth J, Kielstein H. Siglec‐7 expression is reduced on a natural killer (NK) cell subset of obese humans. Immunol Res 2017; 65:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michelet X, Dyck L, Hogan A et al Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 2018; 19:1330–40. [DOI] [PubMed] [Google Scholar]
- 18. Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y. A small region of the natural killer cell receptor, Siglec‐7, is responsible for its preferred binding to alpha 2,8‐disialyl and branched alpha 2,6‐sialyl residues. A comparison with Siglec‐9. J Biol Chem 2002; 277:6324–32. [DOI] [PubMed] [Google Scholar]
- 19. Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol 2011; 11:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol 2016; 17:1025–36. [DOI] [PubMed] [Google Scholar]
- 21. Fang F, Xiao W, Tian Z. NK cell‐based immunotherapy for cancer. Semin Immunol 2017; 31:37–54. [DOI] [PubMed] [Google Scholar]
- 22. Pittari G, Filippini P, Gentilcore G, Grivel JC, Rutella S. Revving up natural killer cells and cytokine‐induced killer cells against hematological malignancies. Front Immunol 2015; 6:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jobin G, Rodriguez‐Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high‐risk subjects undergoing colonoscopy. Gastroenterology 2017; 153:980–7. [DOI] [PubMed] [Google Scholar]
- 24. Cai L, Zhang Z, Zhou L et al Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129:428–37. [DOI] [PubMed] [Google Scholar]
- 25. Lin M, Liang SZ, Wang XH et al Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non‐small cell lung cancer. Immunol Res 2017; 65:880–7. [DOI] [PubMed] [Google Scholar]
- 26. Muntasell A, Ochoa MC, Cordeiro L et al Targeting NK‐cell checkpoints for cancer immunotherapy. Curr Opin Immunol 2017; 45:73–81. [DOI] [PubMed] [Google Scholar]
- 27. Hoechst B, Voigtlaender T, Ormandy L et al Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Y, Kuang DM, Pan WD et al Monocyte/macrophage‐elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 2013; 57:1107–16. [DOI] [PubMed] [Google Scholar]
- 29. Li T, Yang Y, Hua X et al Hepatocellular carcinoma‐associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 2012; 318:154–61. [DOI] [PubMed] [Google Scholar]
- 30. Sun C, Xu J, Huang Q et al High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017; 6:e1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macauley MS, Crocker PR, Paulson JC. Siglec‐mediated regulation of immune cell function in disease. Nat Rev Immunol 2014; 14:653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007; 7:255–66. [DOI] [PubMed] [Google Scholar]
- 33. Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol 2012; 30:357–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barenwaldt A, Laubli H. The sialoglycan–Siglec glyco‐immune checkpoint – a target for improving innate and adaptive anti‐cancer immunity. Expert Opin Ther Target 2019;1–15. [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Sun J, Liu LN et al Siglec‐15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 2019; 25:656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barkal AA, Brewer RE, Markovic M et al CD24 signalling through macrophage Siglec‐10 is a target for cancer immunotherapy. Nature 2019; 572:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang P, Lu X, Tao K et al Siglec‐10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J Surg Res 2015; 194:107–13. [DOI] [PubMed] [Google Scholar]
- 38. Haas Q, Boligan KF, Jandus C et al Siglec‐9 regulates an effector memory CD8(+) T‐cell subset that congregates in the melanoma tumor microenvironment. Cancer Immunol Res 2019; 7:707–18. [DOI] [PubMed] [Google Scholar]
- 39. Jandus C, Boligan KF, Chijioke O et al Interactions between Siglec‐7/9 receptors and ligands influence NK cell‐dependent tumor immunosurveillance. J Clin Invest 2014; 124:1810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cao Y, Karsten U, Otto G, Bannasch P. Expression of MUC1, Thomsen–Friedenreich antigen, Tn, sialosyl‐Tn, and alpha2,6‐linked sialic acid in hepatocellular carcinomas and preneoplastic hepatocellular lesions. Virchows Arch 1999; 434:503–9. [DOI] [PubMed] [Google Scholar]
- 41. Gong ZY, Liao CX, Wang Y, Liao XX, Qin AC, Huang YP, Liao H. Variations of the amount of sialic acids on hepatocellular carcinoma cell membrane. J South Med University 2010; 30:2323–6. [PubMed] [Google Scholar]
- 42. Kawasaki Y, Ito A, Withers DA et al Ganglioside DSGb5, preferred ligand for Siglec‐7, inhibits NK cell cytotoxicity against renal cell carcinoma cells. Glycobiology 2010; 20:1373–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The alteration of activating receptors and inhibitory receptors on peripheral NK cells in HCC patients.


