Abstract
We investigated whether pre-treatment with neonatal sustained hypoxia (SH) prior to chronic intermittent hypoxia (SH+CIH) would modify in vitro carotid body (CB) chemoreceptor activity and the excitability of neurons in the caudal nucleus of the solitary tract (nTS). Sustained hypoxia followed by CIH exposure simulates an oxygen paradigm experienced by extremely premature infants who developed persistent apnea. Rat pups were treated with 5 days of SH (11% O2) from postnatal age 1 (P1) followed by 10 days of subsequent chronic intermittent hypoxia (CIH, 5% O2/5mins, 8hrs/day, between P6–15) as described previously (Mayer et al., Respir. Physiol. Neurobiol. 187(2): 167–75, 2013a). At the end of SH+CIH exposure (P16), basal firing frequency was reduced, and the hypoxic sensory response of single unit CB chemoafferents was attenuated. Further, basal firing frequency and the amplitude of evoked excitatory post-synaptic currents (ESPC’s) of nTS neurons was augmented compared to age-matched rats raised in normoxia. These effects were unique to SH+CIH exposure as neither SH or CIH alone elicited any comparable effect on chemoafferent activity or nTS function. These data indicated that pre-treatment with neonatal SH prior to CIH exposure uniquely modified mechanisms of peripheral (CB) and central (nTS) neural function in a way that would be expected to disturb the ventilatory response to acute hypoxia.
Keywords: Chronic intermittent hypoxia, Carotid body, nTS
2.0. Introduction:
Preterm infants experience frequent bouts of O2 de-saturation associated with apnea of prematurity (AOP; Di Fiore et al., 2010; Martin et al., 2011). Recent clinical trials aimed to target preterm infants to a lower level of O2 saturation (SaO2) for the first few months of life resulted in a sustained increase in the incidence of de-saturation events (Di Fiore et al., 2012) and an increase in infant mortality (Stenson et al., 2011; Carlo et al., 2010). Using a previously devised model aimed to closely mimic the O2 characteristics experienced by these preterm infants, we showed that neonatal sustained hypoxia exposure (SH, 11% O2 between postnatal (P) age P1-5 days) prior to chronic intermittent hypoxia (CIH, 5% O2/5min, 8hrs/day, between P6-15) attenuated the acute hypoxic ventilatory response in rats (Mayer et al., 2013a). In the current study, we investigated whether the same neonatal SH+CIH exposure modified in vitro carotid body (CB) O2 sensitivity and excitability of nucleus tractus solitarius (nTS) neurons where the vagus and chemoafferents terminate.
The CB is the primary peripheral O2 chemoreceptor located in the bifurcation of the carotid artery and initiates afferent transmission of blood oxygenation to first order neurons of the nTS (Mifflin, 1992; Andreson and Kunze et al., 1994; Finley and Katz et al., 1992). CB input is then relayed to other brainstem respiratory neural control regions to generate the appropriate ventilatory response and preserve SaO2 in defense against tissue O2 deprivation. A significant portion of the CB-nTS neural pathway develops during the first postnatal weeks in the rat (Bamford et al., 1999; Donnelly 2000; Liu et al., 2010a,b) but development can be modified by early life experiences. Numerous studies have shown neonatal SH, CIH or hyperoxia exposure, for example, exert various effects on chemoafferent mechanisms of respiratory control (Bavis and Mitchell, 2008; Eden and Hanson 1987; Hanson et al., 1989a,b; Sladek et al., 1993; Wyatt et al., 1995; Bisgard et al., 2005; Bavis et al., 2011; Pawar et al., 2008; 2009; Peng et al., 2004; Julien et al., 2011). However, virtually nothing is known about the effects of neonatal hypoxia (or hyperoxia) on nTS neuronal function and the majority of studies are performed on adults. Stimulation of the commissural solitary tract, the conduit for visceral vagal and CB afferent fibers, in the in vitro brainstem slice preparation from adult rats exposed to 10 days of CIH increased nTS neuron excitatory postsynaptic current (EPSC) firing (Kline et al., 2007). Further, the magnitude of acute hypoxia-induced increase in the KATP –dependent outward current in nTS neurons was reduced following 7 days of either SH or CIH exposure, which would be expected to translate into an enhanced chemoreflex response to acute hypoxia (Zhang et al., 2008). Despite the diverse effects of hypoxia exposure on CB and nTS neuron function, no studies have investigated the combined effects of neonatal SH and CIH on the function of CB/PG, and nTS neuron activity.
We showed previously that neonatal SH treatment prior to CIH (SH+CIH) attenuated the ventilatory response to acute hypoxia in neonatal rats, whereas, neither SH or CIH alone elicited any comparable effect on respiratory function (Mayer et al., 2013a). We developed this exposure paradigm to closely mimic some of the hypoxia profiles experienced by preterm infants in a recent clinical trial (Carlo et al., 2010). Specifically, preterm infants were maintained below (89% SaO2) a lower than normal (>91% SaO2) O2 saturation target starting within hours of birth. These infants developed a profound increase in the incidence of intermittent hypoxia events after the second week of life (presumably related to apnea), which remained elevated through to 2 months of age (Di Fiore et al., 2012) and there was an unexpected degree of infant mortality compared to infants maintained at a higher level of SaO2 (>91% SaO2; Carlo et al., 2010). The cause of the mortality remains unknown, although a prolonged period of low SaO2 followed by a predominance of CIH could indicate a possible disturbance in the respiratory neural control system sub-serving the reflex response to hypoxia. Therefore, in the current study, we used a neonatal SH+CIH exposure paradigm that: 1) aimed to closely replicate the infant scenario in the clinical trial; and 2) we previously showed attenuated the acute hypoxic ventilatory response of rats (Mayer et al., 2013a), to investigate whether it disturbs in vitro CB and nTS neuronal excitability.
3.0. Experimental procedures:
Time-pregnant Lewis rats were purchased from a commercial vendor (Charles River, colony PO6) and were later observed to give birth at CWRU; experiments were performed on 16 day old male rats following 15 days of hypoxia exposure (see below). Lewis rats were chosen as we showed previously that this inbred strain exhibits an attenuated ventilatory response to acute hypoxia following neonatal SH+CIH (Mayer et al., 2013a). All procedures were carried out in accordance with the National Institute of Health (NIH) guidelines for care and use of laboratory animals and were approved by the Animal Care and Use Committee at Case Western Reserve University.
3.1. Hypoxia exposures:
Following the day of birth (P0), the dam (and her pups) were assigned to one of 4 groups that received either of the following experimental protocols until the pups were 15 days of age: normoxic (NX) raised rat pups; CIH only treated rats: pups raised in normoxia (NX) for 5 days, followed by 10 days of CIH (5% O2/5 mins, 8 hrs/day); SH+CIH treated rats: pups raised in sustained hypoxia (SH, 11% O2) for 5 days, followed by 10 days of CIH; SH treated rats: pups raised in SH for 5 days, followed by 10 days of NX. SH was achieved by placing the mother and pups inside a plexiglas chamber (30 x 50 x 28 cm) connected to adjustable rotameters for mixing air and nitrogen (N2). Oxygen levels were monitored (TED 60T, Teledyne Analytical Instruments; CA, USA) and adjusted if necessary to maintain SH (~11% O2). Airflow through the chambers was maintained at ~3L/min and carbon dioxide (CO2) levels in the airflow exiting the chambers was measured to ensure flow was adequate to prevent CO2 accumulation. SH exposure lasted 24 hrs/day for 5 consecutive days. At the end of the 5th day of exposure, the rats were then raised for a further 10 days in either normoxia or CIH, according to the groups outlined above.
Chronic intermittent hypoxia was achieved by purging N2 inside custom-made chambers (53 x 58 x 23cm) until the O2 levels reached 5% O2 at which time air was purged into the chambers to return to room air (see Mayer et al., 2013a). Switching between air and N2 flow through the chamber was achieved using time-controlled solenoid valves so that each hypoxic nadir (i.e. 5% O2) occurred every 5 minutes, 8hrs/day for 10 days. CIH exposure occurred during the nocturnal period of their light cycle (between 9am-5pm). Cages, water and food were replaced every 3 days. Normoxic rats received room air for the same time period. At the end of the experiment, the pups were anaesthetized with isoflurane and decapitated for removal of the CB containing an intact PG or for sectioning of the brainstem containing the nTS and commissural solitary tract.
3.2. Carotid body recording:
At the end of the exposure period (P16), the left and right carotid artery bifurcation containing the intact CB and PG was removed for in vitro extracellular single unit recordings of PG cells containing functional connections with the CB as described previously (Donnelly and Rigual, 2000). The CB/PG complex and carotid artery bifurcation were located ventrally, the trachea was dissected and retracted rostrally, and the superior cervical ganglion was removed along with the entire carotid bifurcation containing the intact PG and CB. The tissue was transferred to a chamber filled with Ringers containing collagenase (0.1%, Roche Diagnostics type P) and protease (0.02% Sigma type XIV) at 37°C for 30 min and gently agitated in a hybridization incubator at 4 revolutions per minute. The bifurcation was then removed for further manual dissection to isolate the CB, carotid sinus nerve, glossopharyngeal nerve and petrosal/nodose ganglia. The CB-PG complex was then placed in a recording chamber perfused at a constant rate (7ml/min; MPII, Harvard Instruments, Holliston, MA) with Ringers Solution (in mM: 125 NaCl, 5 KCl, 2 CaCl2, 1 Na2HPO4, 1 MgSO4, 26 NaHCO3, and 5 dextrose) bubbled with 21% O2 and 5% CO2 (balanced N2) at 37°C (TC-324B, Warner Instruments, Hamden, CT). Chamber O2 levels were measured during several recordings using a fiber-optic electrode (Oxymicro, World Precision Instruments, Sarasota, FL.) positioned immediately proximal to the CB. Individual cell bodies in the PG were identified and drawn into a suction electrode to then determine the sensory response to graded hypoxia (8% and 0% O2; 5 min each) in 5% CO2. A stimulating electrode (pipette filled with 1M NaCl; 2μm tip diameter) placed in the CB to elicit an evoked response was used to first determine that the PG cell intended for recording was in fact connected to the CB (see Fig. 1A, inset). A constant–current stimulus (100–400μA; 0.1ms duration) was delivered at 1 Hz using a stimulus isolation unit (Isostim A320, World Precision Instruments, Sarasota, FL) and subsequent detection of an evoked orthodromic action potential was determined in the immediate post-stimulus period. An initial 1–2 minutes of initial recording was analyzed using the spike discrimination module in LabChart (ADInstruments) to confirm the activity was from an individual cell. If action potential clusters exhibiting distinctly different amplitude, shape, or firing frequencies (indicative of multiple cells) the recording was discontinued and a different cell was located. After a 10 minute baseline period of single unit activity was recorded, hypoxia (8% O2) was administered for 3 minutes by switching the upstream flow of perfusate; the solution was then changed to 0% O2 for a further 3 minutes.
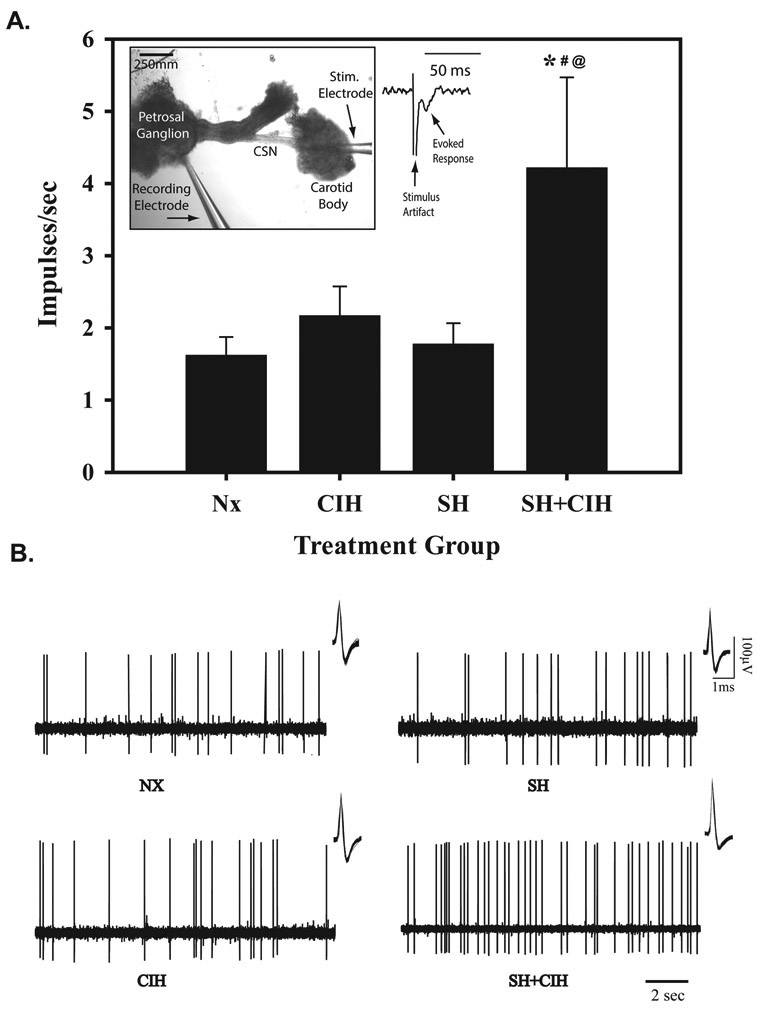
Figure 1.
Average firing frequency (A) and representative raw signals (B) of spontaneous single unit in vitro PG extracellular action potential recordings during baseline (21% O2) in NX, CIH, SH and SH+CIH treated rats. Note, spontaneous firing frequency was increased only in SH+CIH treated rats compared to other treatment groups. Values in (A) for each group are calculated from a 60sec recording of baseline activity prior to bath-applied acute hypoxia (see Fig. 2). Before each recording from an individual PG cell, the presence of an evoked response (inset) elicited by a stimulating electrode positioned into the CB was used to ensure the cell was connected to and received inputs from the CB. An overlay of all the individual action potentials analyzed for each group is also shown (insets, B). Values in (A) are means ± 1S.E.; *significantly different from Nx, #SH, and @CIH treated groups (P<0.05).
The response to graded hypoxia was detected using a suction electrode (A-M systems, Carlsborg, WA) filled with krebs solution and the signal was amplified, bandpass-filtered (0.2–3 kHz) using a P511 amplifier (Grass Technologies, Warwick, RI), digitized (10 kHz sample rate) and stored on computer for later analysis (LabChart, ADInstruments). Analysis of single unit activity was discriminated using the event detection program and the number of individual spikes per second was graphed as a function of time and a 3 s moving average was used for determining peak firing frequencies. On most occasions, a second cell was located after return to normoxia, but only 2 cells were recorded/preparation. The breakdown of CB recordings for each treatment group were as follows: NX: 7 rats from 5 litters, N=12 CB’s; SH: 6 rats from 4 litters, N=11 CB’s; CIH: 8 rats from 5 litters, N=13 CB’s; and SH+CIH: 6 rats from 5 litters, N=11 CB’s.
3.3. In vitro nTS recordings:
After deeply anesthetizing with isoflurane, the rats were decapitated and the brainstem was removed while the head was immersed in a 95% O2, 5% CO2 (carbogen)-gassed artificial cerebral spinal fluid (aCSF; in mM: 124 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 0.5 NaHPO4, 25 NaHCO3, 30 d-glucose). Horizontal medullary slices were made on a vibratome (Leica VT1000, Instruments, St. Louis, MO), while continuously perfused with chilled (3–6°C) carbogen gassed aCSF. 300 μm slices containing the commissural portion of the nTS and the solitary tract were cut for recording of individual nTS neurons. After sectioning, the slice was placed in a polycarbonate chamber (26GLP, Warner instruments, Hamden, CT) and held in place with a platinum ring overlain with nylon threads. The slice was then continuously perfused with aCSF bubbled with carbogen and gabazine (30μM) to block inhibitory transmission, while maintaining the preparation at 27°C.
Putative first order relay neurons of the commissural nTS were identified under an upright microscope (Leica DM/LFS) for whole-cell patch clamp recording of spontaneous EPSCs. Patch pipettes (Borosilicate glass BF150, Sutter Instruments, Novato, CA) were pulled (1.5–2μm tip diameter; Sutter Instruments P-97) and filled with the recording solution (in mM: 10 NaCl, 130 K+ gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 0.2 NaGTP, 2 MgATP). Pipette resistance was 6.5–7.5MΩ before obtaining a tight seal (>1GΩ) on the cell, and the membrane was then ruptured by applying a negative pressure to the electrode. Capacitance and series resistances were measured and compensated for using software (PatchMaster software suite, HEKA Elektronik) and transients were eliminated using automated and manual compensation (EPC10, HEKA Elektronik, Bellmore, NY). A bipolar concentric electrode (FHC, Bowdoin, ME) was used to stimulate the solitary tract to assess evoked responses. Monosynaptic connections were determined by the occurrence of a single evoked EPSC by the neuron in response to solitary tract stimulation. Cells that exhibited a standard deviation in the jitter <250μs between the artifact from the solitary tract stimulation and the EPSC latency were used to confirm it was a second order neuron monosynaptic and directly connected to solitary tract sensory fibers (Kline et al., 2002). The average jitter was not significantly different between the treatment groups (NX: 0.23±0.01 ms; CIH: 0.21±0.01 ms; SH: 0.21±0.01 ms; SH+CIH: 0.21±0.01ms). Two sweeps at each stimulus intensity were performed for each cell at 30 second intervals. Stimulation intensity for evoked EPSCs were set at 1.5X the minimum current needed to evoke an EPSC from a given neuron, and stimulation frequency set to 10 or 20Hz (all neurons were held at −65mV). Cells that exhibited leak currents in excess of 100 pA were not used for analysis. We analyzed spontaneous burst frequency, amplitude, rise time, decay time, and current area, using Mini Analysis software (Synaptisoft Inc., Decatur, GA.). The breakdown of nTS recordings for each treatment group are as follows: NX: 3 rats from 2 litters, N= 7 cells; SH: 3 rats from 2 litters, N=9 cells; CIH: 3 rats from 2 litters, N=11 cells; and SH+CIH: 4 rats from 3 litters, N= 10 cells.
3.4. Statistical analysis:
Statistical comparisons of baseline CB and nTS spontaneous activity between groups were performed using a One-way ANOVA. Comparisons between treatment groups for in vitro CB responses to graded acute hypoxia and nTS evoked responses were made using a Two-way, Repeated Measures ANOVA using a Student-Newman-Keuls post-hoc analysis. Differences were considered significant at p < 0.05. All values are expressed as mean ± SEM.
4.0. Results:
4.1. Petrosal ganglion sensory responses to in vitro acute hypoxia:
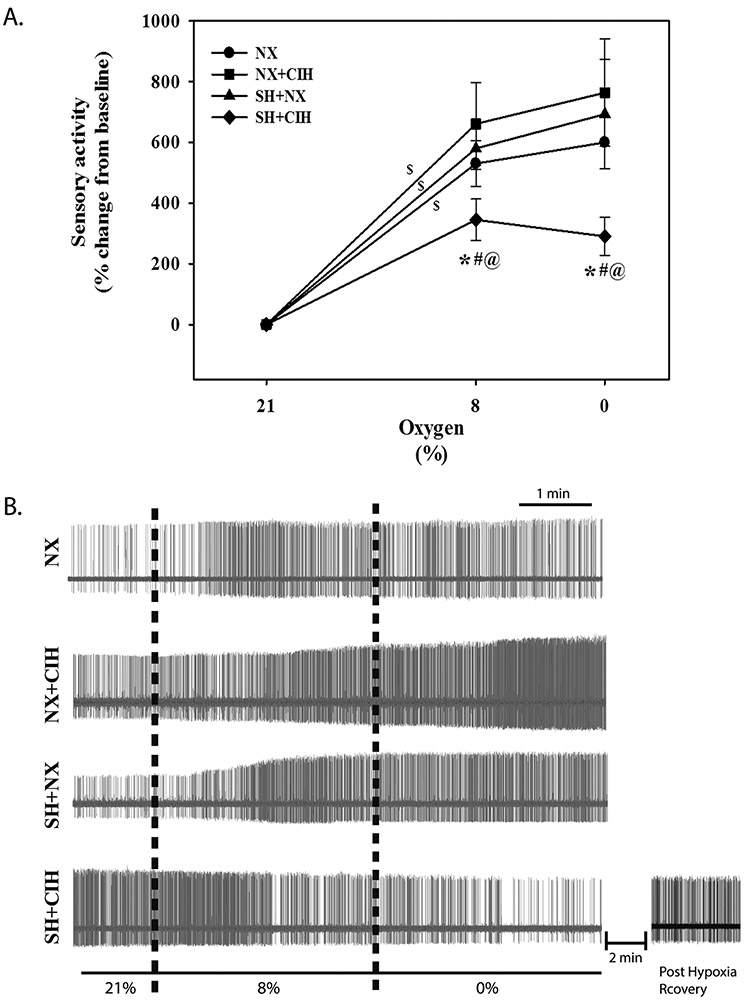
The basal (21% O2) spontaneous firing frequency of PG cells collected from neonatal rats exposed to SH+CIH was augmented (4.25 ± 1.3.4 imp/s) compared to PG cells from rats raised in NX (1.6 ± 0.2 imp/s) and exposed to either SH (1.8 ± 0.3 imp/s) or CIH (2.2 ± 0.4 imp/s) alone (Fig. 1A and B). Basal firing frequency of PG cells collected from rats exposed to either SH or CIH alone were similar to age-matched rats raised in NX. With the exception of the PG cells recorded from SH+CIH treated rats, all groups exhibit a significant response to graded hypoxia (slope) (Fig. 2A), although there was no significant difference in the average response between 8 and 0% O2 for any group. Indeed, firing frequency of PG cells from SH+CIH treated rats was significantly less during bath-applied acute hypoxia (Fig. 2A; 344.9 ± 68.2 % and 290.5 ± 62.2% change from baseline; 8 and 0% O2, respectively) compared to NX raised rats (530.2 ± 75.5 % and 599.8 ± 85.8 %; 8 and 0% O2, respectively). The hypoxic sensory response of PG cells from rats exposed to either SH or CIH alone, however, were quantitatively similar to NX rats and higher than SH+CIH animals regardless of the level of bath hypoxia (Fig. 2).
Figure 2.
Hypoxic sensory response of PG cells in NX, CIH, SH, and SH+CIH treated rats. A) average peak response to 8% and 0% O2 for each treatment group (as indicated) and B) representative recordings. Note the attenuated sensory response of SH+CIH treated rats at each level of acute hypoxia and the tendency for it to decrease during 0% as demonstrate in the representative recording in (B) bottom; also shown is the post-hypoxia recording in a SH+CIH cell demonstrating recovery of activity ~2 minutes after return to baseline O2. Values in A) are expressed as a % change from baseline; means ± 1S.E. Vertical dashed lines (B) designate switching to the hypoxic perfusate. *significant difference from NX, #SH, and @CIH treated groups for respective level of bath-applied hypoxia (P<0.05); $significant slope of the response to hypoxia.
4.2. nTS neuronal spontaneous and evoked responses:
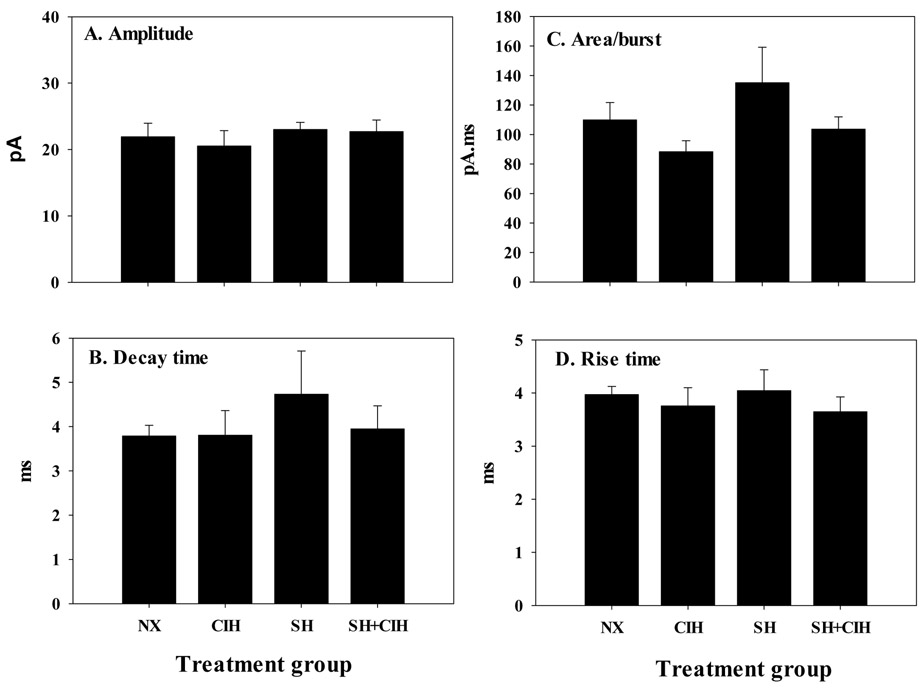
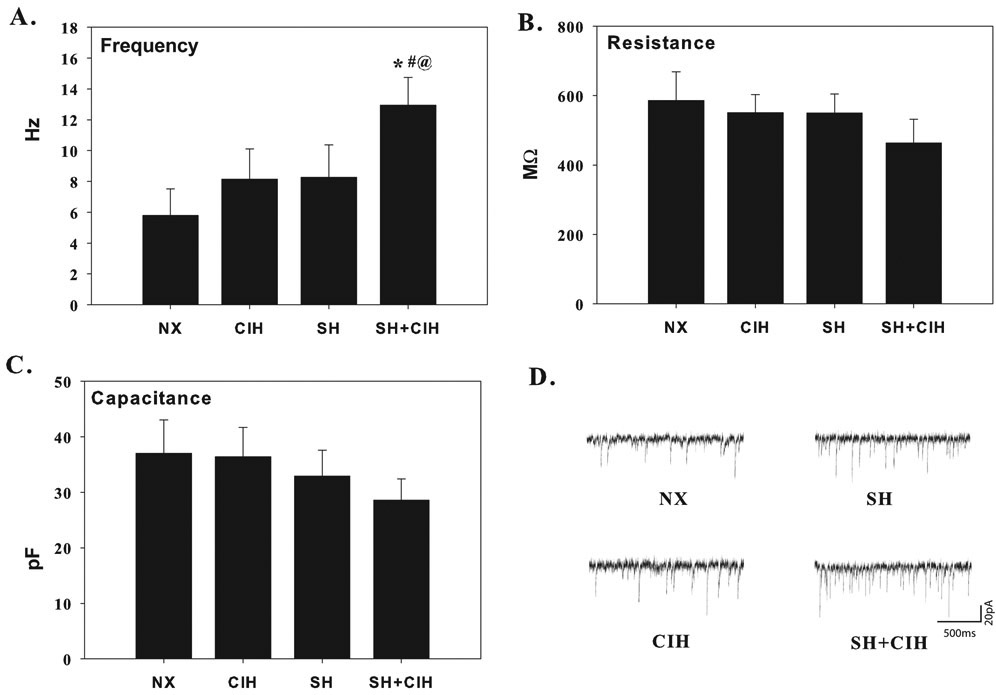
Spontaneous EPSC firing characteristics are indicated in figure 3 and 4. Average spontaneous ESPC amplitude, decay time, rise time and current area were not different between treatment groups (Fig. 3A-D). Spontaneous EPSC firing frequency, however, was increased in SH+CIH (12.9 ± 1.8 Hz) treated rats compared to either NX (5.8 ± 1.7 Hz), or SH (8.3 ± 2.1 Hz) and CIH (8.1 ± 2.0 Hz) treatment; the increased frequency was unique to the SH+CIH group as there was no difference between each of the other hypoxia groups (Fig. 4A). Membrane resistance and capacitance was also not affected by hypoxia (Fig. 4B and C).
Figure 3.
Average spontaneous excitatory postsynaptic current (EPSC’s) burst amplitude (A), decay time (B), current area (C), and rise time (D) of whole-cell patch clamped in vitro nTS neurons from NX, CIH, SH and SH+CIH treated rats. Note, there were no differences between treatment groups for any of the measured variables. Values for each group are calculated from a 2 sec recording of baseline activity. Values are means ± 1S.E.
Figure 4.
Average spontaneous excitatory postsynaptic current (EPSC) firing frequency (A), membrane resistance (B) and capacitance (C) of whole-cell patch clamped in vitro nTS neurons in NX, CIH, SH and SH+CIH treated rats. Note, spontaneous firing frequency was significant enhanced for SH+CIH treated rats compared to all other groups, whereas resistance and capacitance was no affected by either hypoxia exposure. Representative traces of a neuron from each treatment group are also provided (D). Values in for each group are calculated from a 2 min recording of baseline activity. Values are means ± 1S.E.; *significant difference from NX, #SH, and @CIH treated groups (P<0.05).
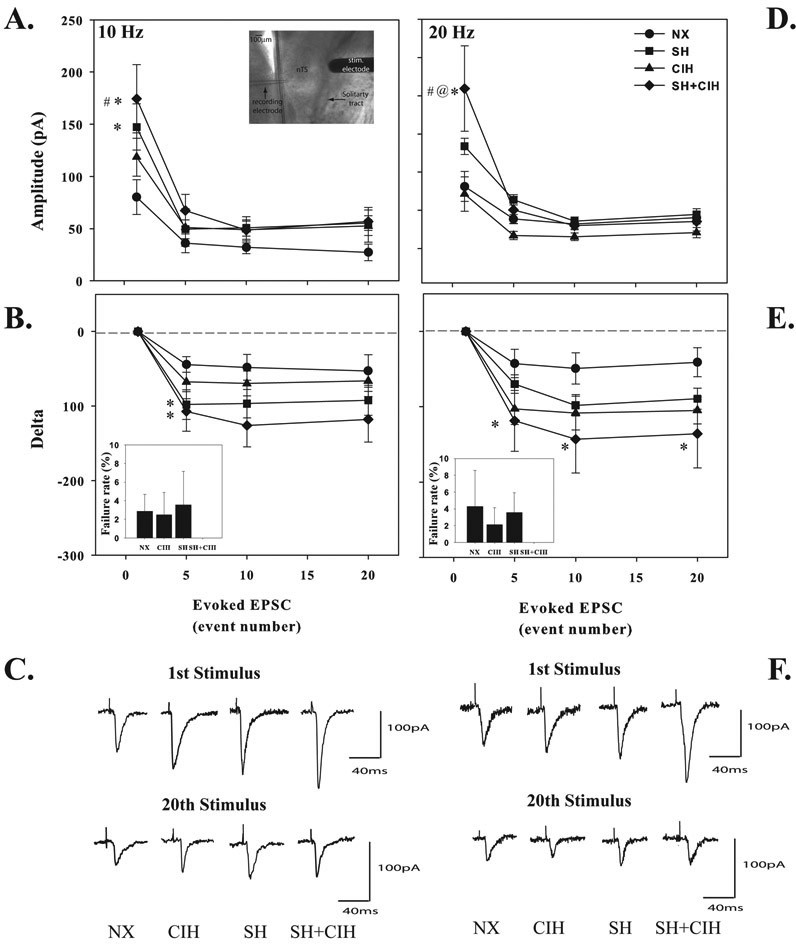
Although the amplitude of spontaneous nTS neuron EPSC’s were similar between groups, the amplitude of only the initial evoked response to 10Hz stimulation (20 stims/train) was augmented in neurons from SH (147.3 ± 22.1 pA) and SH+CIH (174.5 ± 32.7 pA) treated rats compared to NX controls (80.1 ± 16.7 pA; Fig. 5A), although there was no difference between SH and SH+CIH groups. There was no statistical difference between groups in the amplitude of evoked responses after the initial stimulus event of the train. Similarly, the initial evoked EPSC of the 20 Hz stimulus train was augmented (versus all other groups) in neurons following SH+CIH (209.3 ± 55.9 pA) treatment (Fig. 5D). When evoked EPSC’s were expressed as a delta from baseline, there was a greater initial (5th stimulation event number) depression in the SH and SH+CIH treated groups at 10Hz stimulation (Fig. 5B) compared to NX rats. However, there was also a greater initial (5th event number) and sustained (10th and 20th event numbers) depression of evoked EPSC’s that occurred exclusively in the SH+CIH treated rats at 20Hz stimulation compared to NX rats (Fig. 5E). Finally, there was no difference in the failure rate between any treatment groups for either 10Hz or 20Hz stimulation, although it was interesting to note that evoked EPSC’s of neurons from SH+CIH treated rats never failed in response to stimulation (Figure 5B and E, insets).
Figure 5.
Average 10Hz (A) and 20Hz (D) evoked EPSC’s burst amplitude of whole-cell patch clamped nTS neurons in NX, CIH, SH and SH+CIH treated rats. Values in B and E are 10Hz and 20Hz evoked EPSC’s expressed as a delta from the first stimulus event to highlight the magnitude of EPSC depression. Note the initial evoked burst amplitude was increased only in SH and SH+CIH treated groups at 10Hz (A), whereas only SH+CIH treated rats exhibited increased evoked responses at 20Hz (D). Neurons from SH+CIH treated rats exhibited the significant EPSC depression (20Hz) compared to cells from NX rats. Representative 10Hz (C) and 20Hz (F) evoked responses for the 1st and last (20th) stimulation event are shown below. Inset: Photo of the recording and stimulating electrode positioned into the nTS region (A) and failure rate for both 10Hz and 20Hz stimulus trains (B and D, inset) . Values are means ± 1S.E.; *significantly different from NX; #significantly different from CIH; @significantly different from SH treated rats within corresponding stimulus event number (P<0.05).
5.0. Discussion:
The primary results of the current study indicated that neonatal SH exposure prior to CIH (SH+CIH): 1) enhanced baseline PG and presumably CB spontaneous firing frequency, 2) attenuated the PG/CB response to in vitro acute hypoxia, but 3) enhanced evoked EPSC amplitude of neurons in the nTS where vagal and PG/CB afferents terminate. These data demonstrate the combination of SH and CIH exposure disturbs functional development of the peripheral (CB) and possibly central (nTS) mechanisms mediating the reflex response to acute hypoxia. We first provide a critique of our methods before discussing the significance of these findings.
5.1. Critique of methods:
We recorded the firing properties of EPSC from monosynaptic neurons located in the caudal nTS where vagal and PG/CB chemoafferents terminate. However, it isn’t possible to determine whether the changes in firing characteristics of these neurons following SH+CIH were unique to those that communicated directly with the CB without performing appropriate back-labeling experiments. Technical limitations and the need for invasive surgical procedures in such small animals make it difficult for such experiments that would allow us to determine possible differences in characteristics of CB-related nTS neurons from other vagal-related neurons. Therefore, caution should be expressed with interpretation of these data as others have shown excitability of CB-related nTS neurons may be differently affected by hypoxia exposure than other neurons that may not be directly involved in the chemoreflex pathway (Zhang et al., 2008).
5.2. CB responses:
Neonatal hypoxia exposure augmented in vitro single unit PG cell basal spontaneous firing frequency and attenuated the sensory response to bath-applied acute hypoxia although these effects were unique to the SH+CIH exposure group. Although these measurements were made from PG cells, these data suggest SH+CIH exposure most likely modified the O2-sensitivity of the CB chemoreceptor. Development of CB sensitivity to acute hypoxia occurs largely during the first postnatal weeks (Kholwadwala and Donnelly 1992; Donnelly, 2000; Carroll, 2003) so it is not surprising it may be vulnerable to prolonged O2-related challenges. SH alone delayed CB development and function (Hanson et al., 1989a,b; Sterni et al., 1999) whereas CIH enhanced CB basal activity in both neonatal and adult rats (Pawar et al., 2008). In the current study, however, CIH did not affect basal activity unless the rats were pre-treated with SH exposure (between P1–5). The discrepancies between these effects of SH or CIH on CB basal activity could be related to timing of CIH exposure which began at P6 suggesting we may have missed an earlier more vulnerable period. As little as 16 hours of IH exposure in 2-day old animals was sufficient to elicit changes in basal CB activity, suggesting an early period of augmented CB vulnerability to even a brief bout of intermittent hypoxia exposure (Peng et al., 2004). Finally, SH exposure during the first 5 postnatal days in our study did not affect basal firing frequency of the CB/PG, although the measurements were made 10 days after the rats were allowed to recover in room air. It is difficult to know if SH exhibited an early effect on CB function that, without subsequent CIH exposure, may have resolved on its own. On the other hand, differences in the strain of rats used in the various studies and how they are affected by hypoxia exposure might also explain discrepancies.
The consequences of augmented CB basal activity are unclear, although it could translate to increased resting ventilatory drive, ultimately leading to a potentially undesirable relative hyperventilation and destabilization of breathing (MacFarlane et al., 2013). Indeed, we noted previously that SH+CIH increased resting (during normoxia) minute ventilation compared to NX treated rats (Mayer et al., 2013a), although whether this was attributed to increased basal CB drive is difficult to determine since these rats also exhibited an elevated metabolic rate, which in itself could explain the increased ventilation.
Neonatal SH+CIH exposure also attenuated the sensory response to bath-applied acute hypoxia which, similarly to the effects on basal activity, wasn’t seen in rats exposed to either SH or CIH alone. In fact, SH+CIH treatment eliminated the slope of the response to acute hypoxia, indicating a reduction in the gain setting of CB O2 sensitivity (Fig. 2A). Further, in some SH+CIH PG cells (see Fig. 2B), we noted severe hypoxia (0% O2) tended to attenuate firing frequency (compared to 8% O2), suggesting a severe disturbance to CB sensory mechanisms even though activity was restored within minutes of return to recovery in baseline O2 levels (Fig. 2B). Prolonged periods of neonatal O2 exposure can have diverse effects on CB sensitivity to subsequent acute hypoxia. Specifically, neonatal SH (Sterni et al., 1999; Wyatt et al., 1995) or hyperoxia (Bavis et al., 2004; Bavis et al., 2011; Kim et al., 2013) suppressed CB hypoxic sensory responses, whereas CIH enhances it in both neonates (Pawar et al., 2008; Peng et al., 2004) and adults (Peng et al., 2003; Peng and Prabhakar 2004). Whereas neonatal SH exposure attenuated CB sensory responses, it can (but not always) have the opposite effect in the adult (Liu et al., 2013; Wilkinson et al., 2010; Vizek et al., 1987), suggesting the presence of a transitional stage of development during which the effects of SH on the neonatal respiratory system contrasts markedly from its effects on the adult (Mayer et al., 2013b).
The mechanisms mediating attenuated hypoxic sensory responses following neonatal SH+CIH are beyond the scope of this study, although the CIH-induced enhancement of basal CB activity (during 21% O2) and the augmented hypoxic sensory response was prevented by antioxidant treatment (Pawar et al., 2009). Similarly, SH or CIH may initiate a pro-inflammatory response that could modify CB sensory responses (Powell 2009; Del Rio et al., 2012; Lam et al., 2008, 2009; Liu et al., 2012), whereas SH altered glomus cell K+, Na+ and Ca2+ currents (Hatton et al., 1997; Hempleman 1996; Wyatt et al., 1995). Since nTS neurons integrate CB chemoafferent inputs and relay systemic hypoxia signals to increase respiratory drive during acute hypoxia, we investigated the firing properties of nTS neurons in vitro located in the vicinity of where CB afferents terminate.
5.3. nTS activity:
Spontaneous nTS neuron EPSC firing frequency was augmented following neonatal hypoxia exposure compared to NX rats and, similarly to the CB, these effects were exclusive to the SH+CIH treatment group. All other parameters including membrane resistance, capacitance, current amplitude, rise and decay time, and current area were unchanged by either hypoxia exposure paradigm. The majority of nTS studies have focused on adults and there is evidence for changes in the central neural integration of afferents following SH (Dwinell et al., 2000) and CIH (Kline et al., 2007). However, in one study on neonatal monkeys, basal nTS neuron excitability was enhanced following intermittent ozone exposure and attenuated the response to solitary tract stimulation (Chen et al., 2003). The cause for the augmented basal nTS neuron firing following SH+CIH treatment is unknown, but could involve modification of pre-synaptic neurotransmitter release mechanisms (Kline et al., 2007), including changes in ATP sensitive potassium channels, which were modified by either SH or CIH in adults (Zhang et al., 2008). Indeed, the magnitude of evoked EPSCs depression was greater in SH+CIH treated rats suggesting a disturbance in the pre-synaptic release mechanisms. Also of interest, was the presence of increased spontaneous currents (termed “asynchronous” activity) during and after a 20Hz stimulus train in nTS neurons of adult rats exposed to CIH (Kline et al., 2007). We did not observe asynchronous activity in any of the hypoxia exposures used in the current study, adding support to the concept that developmental influences may be important determinants to the vulnerability of neural function to prolonged hypoxia exposure. Finally, since the effects of hypoxia exposure (e.g acute intermittent hypoxia) on the respiratory neural control system can vary across species and also within rat strains (Baker-Herman et al., 2010), genetic determinants could also explain the discrepancies between our studies and others.
The amplitude of evoked (10 Hz stimulation) EPSC’s were augmented following neonatal hypoxia and, at this level of stimulus intensity, these effects were common to both SH and SH+CIH treated rats. These data are consistent with SH effects on evoked EPSC responses of adult rats (Zhang et al., 2009) whereas adult CIH exposure had the opposite effect (Kline et al., 2007). At higher stimulus intensity (20 Hz), the augmented evoked EPSC’s was unique to the SH+CIH treated animals, whereas neither SH or CIH alone had any comparable effect. Although the cause of augmented evoked ESPC amplitude or depression following neonatal SH+CIH was not a focus of the current study, the enhanced evoked response in adult rats following SH was associated with increased GluR2, but not GluR1 receptor expression (Zhang et al., 2009). Collectively, these data could indicate alterations in neurotransmitter release sites and/or release probability and warrant further investigation. Future studies should aim to investigate whether changes in glutamatergic receptor signaling are affected by neonatal hypoxia exposure since nTS neuron GluR2/3 AMPA receptor expression increases during the first 10 postnatal days in the rat (Whitney et al., 2000) and are important modulators of the hypoxic ventilatory response (Ohtake et al., 2000).
The reasons for changes in evoked EPSC firing properties following neonatal hypoxia exposure are difficult to determine, but could reflect either a direct effect of hypoxia on nTS neuron function, or an indirect effect in response to hypoxia-induced modification in the firing characteristics of afferent inputs (Kline et al., 2007; Zhang et al., 2008). The reduced amplitude of nTS evoked ESPC’s in adult rats following CIH, for example, could represent a homeostatic compensatory response to counterbalance augmented afferent excitatory inputs (Kline et al., 2007). Maintaining a stable balance between nTS neurotransmission and modified afferent inputs may be important for manifesting efficient hypoxic signaling (Zhang et al., 2008). This hypothesis of homeostatic regulation between integrative function and sensory inputs are in part consistent with the results of the current study, indicating the enhanced evoked response could be an attempt to compensate for attenuated afferent inputs. Alternatively, over-excitation of nTS neurons has been shown to limit efficient neurotransmission. For example, dampening of nTS neuron excitability could protect against over-excitation due to excessive glutamate release to optimize the subsequent reflex response to hypoxia (Zhang et al., 2008). Thus, although the enhanced nTS evoked response following SH+CIH is counter-intuitive to the attenuated HVR we observed previously (Mayer et al., 2013a), hyper-excitable nTS neurons may not be conducive to efficient neurotransmission of low intensity peripheral sensory inputs.
5.5. Summary and significance:
The results of the current study suggest early life experiences to specific patterns and/or sequences of hypoxia could cause major disturbances to CB function and excitability of nTS neurons that may be responsible for central integration of vagal afferent inputs. An augmented CB and nTS excitability during basal conditions could exacerbate normoxic respiratory pattern irregularities in a way that may precipitate apnea, while an attenuated CB hypoxic sensitivity could disturb the ability to terminate an apneic event (MacFarlane et al., 2012; Di Fiore et al., 2013). Although the design of the study does not allow us to confirm whether SH+CIH uniquely affected second order neurons directly involved in the CB chemoreceptor pathway, these data show a potentially deleterious effect on the excitability of neurons in a key relay station that receives afferent sensory input with likely projections to other respiratory related regions. These data emphasize the importance of maintaining adequate levels of oxygenation in the early neonatal period to protect against potentially deleterious effects of hypoxia on neural function, which may be particularly relevant to preterm infants who may be more susceptible and vulnerable to prolonged periods of continuous and intermittent hypoxemia.
6.0. Acknowledgements:
Funded by the Department of Pediatrics, Rainbow Babies & Children’s Hospital and Case Western Reserve University. Special thanks go to Ryan W. Bavis for temporary loan of the fiber optic O2 sensor.
7.0 References:
- Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. 2014. Am J Physiol Lung Cell Mol Physiol. July 15;307(2):L129–L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL.1994. Nucleus tractus solitarius gateway to neural circulatory control. Ann. Rev. Physiol 56, 93–116 [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkderson JER, Golder FJ, MacFarlane PM, Watters JJ, Behan M, Mitchell GS 2010. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir. Physio. & Neurobiol 170(3), 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. 1999. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 276(5 Pt 1), L875–L884. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS, 2008. Long-term effects of the perinatal environment on respiratory control. J. Appl. Physiol 104(4),1220–1229. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB Jr., Vidruk EH, Fuller DD, Mitchell GS, 2004. Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J. Physiol 557(2), 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Wenninger JM, Miller BM, Dmitrieff EF, Olson EBJ, Mitchell GS, Bisgard GE, 2008. Respiratory plasticity after perinatal hyperoxia is not prevented by antioxidant supplementation. Respir. Physiol. Neurobiol 160(3), 301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Kim I, Pradham N, Nawreen N, Dimitrieff ET, Carroll JL, Donnelly DF, 2011. Recovery of carotid body O2 sensitivity following chronic postnatal hyperoxia in rats. Respir. Physiol. Neurobiol 177(1), 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Olson EB Jr, Bavis RW, Wenninger J, Nordheim EV, Mitchell GS. 2005. Carotid chemoafferent plasticity in adult rats following developmental hyperoxia. Respir Physiol Neurobiol. 145(1), 3–11. [DOI] [PubMed] [Google Scholar]
- Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, Yoder BA, Faix RG, Das A, Poole WK, Schibler K, Newman NS, Ambalavanan N, Frantz ID III, Piazza AJ, Sánchez PJ, Morris BH, Laroia N, Phelps DL, Poindexter BB, Cotton CM, Van Meurs KP, Duara S, Narendran V, Sood BG, O'Shea TM, Bell EF, Ehrenkranz RA, Watterberg KL, Higgins RD, SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. 2010. Target ranges of oxygen saturation in extremely preterm infants. NEJM. 362(21), 1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL. 2003. Developmental plasticity in respiratory control. J. Appl. Physiol 94(1), 375–389. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Kim I, Dbouk H, Yang DJ, Bavis RW, Donnelly DF. 2009. Time-dependence of hyperoxia-induced impairment in peripheral chemoreceptor activity and glomus cell calcium response. Adv Exp Med Biol.648, 299–306. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bonham AC, Plopper CG, Joad JP, 2003. Neuroplasticity in nucleus tractus solitarius neurons after episodic ozone exposure in infant primates. J. Appl. Physiol 94(2). 819–27. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R. 2012. Contribution of inflammation on carotid body chemosensory potentiation induced by intermittent hypoxia. Adv Exp Med Biol. 758, 199–205. [DOI] [PubMed] [Google Scholar]
- Di Fiore JM, Walsh M, Wrage L, Rich W, Finer N, Carlo WA, Martin RJ, SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. 2012. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J. Pediatr 161(6), 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore JM, Orge F, Schutt A, Schluchter M, Cheruvu VK, Walsh M, Finer N, Martin RJ, 2010. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr 157, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore JM, Martin RJ, Gauda EB 2013. Apnea of prematurity – a perfect storm. Respir Physiol Neurobiol. 189(2), 213–222. [DOI] [PubMed] [Google Scholar]
- Donnelly DF. 2000. Developmental aspects of oxygen sensing by the carotid body. J Appl Physiol. 88(6), 2296–301. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Rigual R, 2000. Single unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. J. Appl. Phyisol, 88(4), 1489–1495. [DOI] [PubMed] [Google Scholar]
- Dwinnell MR, Powell FL 1999. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J. Appl. Physiol 87(2), 817–823. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA 1987. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J. Physiol 392, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JC, Katz DM. 1992. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 572(1-2), 108–16. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Kumar P, Williams BA 1989a. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J. Physiol 411, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Eden GJ, Nihuis JG, Moore PJ. 1989b. Peripheral chemoreceptors and other O2 sensors in the fetus and newborn In: Lahiri S, Forster RE, Davies RO, Pack AI (Eds). Chemoreceptors and Reflexes in Breathing. NY, Oxford University Press; Pp.113–120. [Google Scholar]
- Hatton CJ, Carpenter E, Pepper DR, Kumar P, and Peers C. Developmental changes in isolated rat type I carotid body cell K+ currents and their modulation by hypoxia. J Physiol 501: 49–58, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempleman SC. Increased calcium current in carotid body glomus cells following in vivo acclimatization to chronic hypoxia. J Neurophysiol 76: 1880–1886, 1996. [DOI] [PubMed] [Google Scholar]
- Julien CA, Joseph V, Bairam A, 2011. Alteration of carotid body chemoreflexes after neonatal intermittent hypoxia and caffeine treatment in rat pups. Respir. Physiol. Neurobiol 177(3), 301–12. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF. 1992. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol. 453, 461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Yang D, Carroll JL, Donnelly DF. 2013. Perinatal hyperoxia exposure impairs hypoxia-induced depolarization in rat carotid body glomus cells. Respir Physiol Neurobiol. 188(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Takacs K, Ficker E, Kunze D. 2002. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J. Neurophysiol 88, 2736–2744. [DOI] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. 2007. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci. 27(17), 4663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S-Y, Tipoe GL, Liong E, 2008. Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem. Cell. Biol 130, 549–559. [DOI] [PubMed] [Google Scholar]
- Lam S-Y, Liu Y, Ng KM, Lau CF, Liong EC, Tippoe GL, Fung ML, 2012. Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem. Cell Biol 137(3), 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, He L, Dinger B, Stensaas L, Fidone S. 2012. Effect of endothelin receptor antagonist bosentan on chronic hypoxia-induced inflammation and chemoafferent neuron adaptation in rat carotid body. High Alt Med Biol.13(3), 209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, He L, Dinger B, Stensaas L, Fidone S. 2013. Sustained exposure to cytokines and hypoxia enhances excitability of oxygen-sensitive type I cells in rat carotid body: correlation with the expression of HIF-1a protein and adrenomedullin. High Alt Med Biol. 14(1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Ribeiro AP, Martin RJ 2013. Carotid chemoreceptor development and neonatal apnea. Respir. Physiol. Neurobiol. 185(1), 170–176. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Wang K, Köroglu Ö, Di Fiore JM, Kc P, 2011. Intermittent Hypoxic episodes in preterm infants: Do they matter? Neonat. 100, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CA, Ao J, Di Fiore JM, Martin RJ, MacFarlane PM, 2013a. Impaired hypoxic ventilatory response following neonatal sustained and subsequent chronic intermittent hypoxia in rats. Respir. Physiol. Neurobiol 187(2), 167–175. [DOI] [PubMed] [Google Scholar]
- Mayer CA, Di Fiore JM, Martin RJ, MacFarlane PM, 2013b. Vulnerability of neonatal respiratory neural control to sustained hypoxia during a uniquely sensitive window of development. J. Appl. Physiol Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. 1992. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol. 263(2 Pt 2), R368–75. [DOI] [PubMed] [Google Scholar]
- Nuritova F, Frenguelli BG, Putative depolarisation-induced retrograde signalling accelerates the repeated hypoxic depression of excitatory synaptic transmission in area CA1 of rat hippocampus via group I metabotropic glutamate receptors. 2012. Neuroscience. October 11;222:159–72. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Simakajornboon N, Fehinger MD, Xue YD, Gozal D, 2000. N-methyl-D-aspartate receptor expression in the nucleus tractus solitarii and maturation of the hypoxic ventilatory response in the rat. Am. J. Respir. Crit. Care. Med 162, 1140–1147. [DOI] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR 2008. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J. Appl. Physiol 104(5), 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR, 2009. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 296(3), R735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR, 2004. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J. Appl. Physiol. 97, 2020–2025. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR, 2003. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc. Natl. Acad. Sci. USA. 100, 10073–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, 2009. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am. J. Physiol 296(2), L156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek M, Parker RT, Grogaard JB, Sundell HW 1993. Long-lasting effect of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr. Res. 34(6), 821–828. [DOI] [PubMed] [Google Scholar]
- Stenson B, Brocklehurst P, Tarnow-Mordi W, 2011. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. NEJM. 364(17), 1680–1682. [DOI] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL, 1999. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am. J. Physiol. 21, L645–L652. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV, 1987. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol. 63(6), 2403–10. [DOI] [PubMed] [Google Scholar]
- Whitney GM, Ohtake PJ, Simakajornboon N, Xue Y-D, Gozal D, 2000. AMPA glutamate receptors and respiratory control in the developing rat: anatomic and pharamacological aspects. Am. J. Physiol. Reg. Integ. Comp. Physiol 278, R520–R528. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Huey K, Dinger B, He L, Fidone S, Powell FL, 2010. Chronic hypoxia increases the gain of the hypoxic ventilatory response by a mechanism in the central nervous stem. J. Appl. Physiol 109(2), 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C, 1995. O2-sensitive K+ currents in carotid chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc. Natl. Acad. Sci. USA 92(1), 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Carreño FR, Cunningham JT, Mifflin SW, 2008. Chronic sustained hypoxia enhances both evoked EPSCs and norepinephrine inhibition of glutamatergic afferent inputs in the nucleus of the solitary tract. J Neurosci. 29(10), 3093–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]