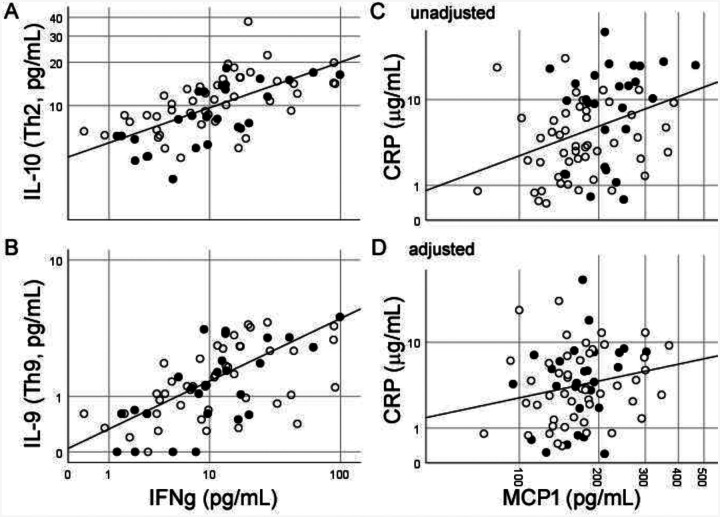
We read with interest Huang and colleagues’ description of early patients with 2019 novel coronavirus in China, their plasma inflammatory protein profiles, and the clinical and biochemical distinctions from SARS.1 We have routinely analyzed inflammatory protein levels in healthy aging, neurological disorders, and infectious diseases over 10 years,2–5 and we express the strongest caution in postulating therapeutic protocols based on uncontrolled plasma cytokine analysis. Plasma biomarkers (including those measured here) are gaining popularity due to ease of phlebotomy and availability of commercial assays, but their associations with disease are also notoriously difficult to reproduce across laboratories, cohorts, and assay platforms even in well-controlled settings.2 Reasons for this include non-standard protocols to generate plasma (from whole blood), unpublished half-lives for most proteins in blood (minutes to days), proprietary methods for quantitation (antigenic site for antibodies, peptide fragment for mass spectrometry), and absence of high quality reference values from well-characterized healthy subjects. Plasma cytokines independent of demographic factors are also highly correlated entities in health (Fig 1A, B) as well as disease, and false associations can in turn result from effects of age or race (Fig 1C, D). Thus, elevated Th2-related cytokines in 2019-nCoV better inform an intact Th1-Th2 axis than putative efficacy of immunomodulatory therapies.1 If blood cytokine profile (with or without immunophenotyping) are considered for biomarkers to complement drug development or clinical monitoring, we recommend with the greatest urgency the creation of an international reference laboratory to enhance rigor and translatability.
Figure 1.
Plasma protein levels from 75 healthy middle-aged Black (filled circles) and White (empty circle) Americans. Cytokines associated with different Th cells highly correlate with Th1-associated IFN-γ levels (A, B), and the relationship between proteins influenced by race and age (C) diminishes after adjusting for these factors (D). Axis values represent actual concentrations shown on log10 scale due to non-normal distributions.
Funding Sources:
This study was funded by NIH K01AG42498 (WW) and R01AG54046 (WTH). The funders have no role in the data analysis or manuscript preparation.
Footnotes
Conflict of interests: WTH has served as a consultant to ViveBio LLC, Biogen Inc., and AARP Inc.; received research support from Fujirebio USA; and has a patent on CSF-based diagnosis of FTLD-TDP (assigned to Emory University).
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu WT, Holtzman DM, Fagan AM, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology 2012; 79(9): 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu WT, Howell JC, Ozturk T, et al. CSF Cytokines in Aging, Multiple Sclerosis, and Dementia. Front Immunol 2019; 10: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu WT, Chen-Plotkin A, Arnold SE, et al. Biomarker discovery for Alzheimer’s disease, frontotemporal lobar degeneration, and Parkinson’s disease. Acta neuropathologica 2010; 120(3): 385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozturk T, Kollhoff A, Anderson AM, et al. Linked CSF reduction of phosphorylated tau and IL-8 in HIV associated neurocognitive disorder. Sci Rep 2019; 9(1): 8733. [DOI] [PMC free article] [PubMed] [Google Scholar]