Abstract
Peripheral oxygen saturation (SpO2) measured by pulse oximetry is an unreliable surrogate marker for arterial oxygenation (SaO2) in critically ill patients. We hypothesized that a higher perfusion index (PFI) would be associated with better accuracy of SpO2 measurement. We retrospectively collected SaO2, SpO2, and PFI data for each arterial blood gas (ABG) analysis in a cohort of intensive care unit patients. PFI was categorised as low (PFI < 1.0), intermediate (1.0 ≤ PFI ≤ 2.5), or high (PFI > 2.5). The correlation between SpO2 and SaO2 was studied using Pearson’s correlation. The Bland–Altman plot was used to analyse the agreement between SpO2 and SaO2. Furthermore, the correlation between the (SpO2–SaO2) difference and PFI was assessed. The level of (dis)agreement was calculated for the three PFI categories separately. Overall, 281 patients and 1281 data points were analysed. There was a significant correlation between SaO2 and SpO2 (r = 0.69, p < 0.01). The Bland–Altman analysis revealed a mean difference between SaO2 and SpO2 of 0.2% with limits of agreement of ± 6% (SD ± 2%). The correlation between the PFI and the (SpO2–SaO2) difference was low; the (SpO2–SaO2) difference improved only marginally with higher PFI values. The accuracy of pulse oximetry for estimating arterial oxygenation was moderate and improved little with increasing PFI values. Thus, the additive value of PFI in clinical decision making is limited. Therefore, we advise performing an ABG before adjusting fraction of inspired oxygen (FiO2) settings.
Keywords: Critical illness, Intensive care unit, Oxygen saturation, Perfusion index, Pulse oximetry
Introduction
Pulse oximetry is routinely used to monitor peripheral oxygen saturation (SpO2) as a surrogate marker for arterial oxygen saturation (SaO2). Strict guidelines regarding an optimum target SpO2 are lacking. However, most clinicians will aim for SpO2 values between 88 and 96%, depending on the clinical situation, and might surmise that the real arterial oxygenation is above the measured values of the pulse oximeter [1]. However, one can erroneously accept lower arterial oxygenations than surmised. Although it is a useful method for monitoring intensive care unit (ICU) patients, it has limitations. In the critically ill, complex physiological disturbances such as altered blood flow, acid–base disturbances, and abnormalities in temperature regulation may occur; these affect the oxyhaemoglobin dissociation curve and might further complicate the interpretation of the SpO2 [2]. Moreover, the accuracy of a pulse oximeter is reduced in patients with low perfusion status, sepsis, and vasopressor use [3, 4]. Consequently, the new oximeters can calculate the perfusion index (PFI) from a pulsatile photo plethysmography signal and indirectly measure the perfusion variations. The PFI may function as a marker for peripheral perfusion and resembles vasomotor tone, with low and high PFI values indicating perfusion below and above average, respectively [5–9]. In routine ICU practice, the PFI could possibly be used as a surrogate marker for the accuracy of measured SpO2 [10].
The primary objective of our study was to assess whether, in a cohort of ICU patients, the use of the PFI in daily practice contributes to the accuracy of pulse oximetry. We hypothesized that higher PFI values would be associated with more accurate SpO2 measurement. Therefore, we studied the relationship between SaO2 and SpO2, and how this depended on the PFI. Furthermore, as a secondary objective, we tested whether multiple variables (temperature, MAP, pH, lactate, and inotropic drug use) influenced the (SpO2–SaO2) difference and PFI.
Materials and methods
We performed a retrospective study in a level 2 mixed ICU of a single-site teaching hospital. Patients were included in the study between May 2015 and September 2015. All admitted ICU patients on supplemental oxygen therapy, with at least one arterial blood gas (ABG) analysis and concomitantly measured SpO2, were eligible for inclusion.
Data collection
Data regarding patient characteristics, admission diagnosis, length of stay (LOS), days on non-invasive/invasive mechanical ventilation, SpO2, PFI, and variables that could potentially influence pulse oximetry measurements such as central body temperature, mean arterial pressure (MAP), pH, lactate, and inotropic drug use were retrieved from the electronic patient data management system (EPD). EPD data verification was performed by the research nurse. In patients treated with invasive ventilation, all ABG samples were included until extubation. In all other cases, one daily ABG was included until a maximum of 3 days after ICU admittance in order to limit the total amount of data, under the assumption that any further data would not strongly influence outcomes.
SaO2, PaO2, pH, and lactate were measured with a blood gas analyser (ABL 800 Flex, Radiometer, Copenhagen, Denmark) in the local clinical chemistry department; this method is considered the gold standard.
Pulse oximeters
SpO2 was measured continuously; however, for this study, SpO2 registered only at the time of blood withdrawal using the Philips M1191BL finger probe (Philips Healthcare, Eindhoven, The Netherlands) and the Philips M1194A ear probe (Philips Healthcare, Eindhoven, The Netherlands). According to the manufacturer, these pulse oximeters have an accuracy of ± 2.5% and ± 4% root mean square (RMS), respectively [11]. Both pulse oximeters were connected to the Philips MP70 monitor (Philips Healthcare, Eindhoven, The Netherlands) [10].
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) Continuous data are presented as a mean if normally distributed and as a median otherwise. Categorical data are presented as percentages.
Accuracy of pulse oximetry
We used the Pearson correlation coefficient (r) to express the relationship between SaO2 and SpO2. The Bland–Altman plot was used to graphically represent this relationship [12]. With this method, the difference between SpO2 and SaO2 (ΔSat) was plotted against the average of SpO2 and SaO2. A positive value indicated that the measured SaO2 was lower than SpO2. Furthermore, regression analysis was performed to determine whether there was positive, negative, or no correlation between the bias and the average [13]. The Pearson correlation coefficient was used to express the possible association of temperature, pH, lactate, and MAP with ΔSat. Furthermore, a Student’s t test was performed to test the association of sex with ΔSat. One-way ANOVA was used to test the association of inotropes with ΔSat.
The perfusion index
To evaluate the relationship between the (skewed) PFI and ΔSat, the Spearman correlation coefficient was calculated. Subsequently, we determined whether the PFI is a reliable surrogate for the accuracy of the pulse oximeter. We categorised PFI values as low (PFI < 1.0), intermediate (1.0 ≤ PFI ≤ 2.5), or high (PFI > 2.5) [5, 7, 10]. Accuracy was visualised by plotting ΔSat against the PFI. In addition, we calculated the percentages of measurements exceeding various limits of agreement for the three PFI categories.
The study protocol was approved by the local Ethics Review Board and only the primary investigator was able to link the study data with the patient data [14].
Results
A total of 320 patients were eligible for the study. The data from 39 patients were missing and the remaining 281 were included in the analysis, resulting in 1281 data points. Baseline patient characteristics are shown in Table 1.
Table 1.
Standard patient characteristics
| Patient characteristics (n = 281) |
n (%) |
|---|---|
| Sex | |
| Male | 177 (63) |
| Female | 104 (37) |
| Age in years (SD) | 65 (15) |
| Diagnosis at admittance* | |
| Pneumonia | 31 (11.0) |
| Cardiac arrest | 28 (10.0) |
| Trauma | 23 (8.2) |
| GI bleeding | 18 (6.4) |
| Sepsis | 16 (5.7) |
| COPD exacerbation | 15 (5.3) |
| Length of stay in days (range) | 2 (0–67) |
| Days intubated (range) | 3 (0–58) |
| Inotropic use | |
| Norepinephrine | 102 (36) |
| Dobutamine | 10 (4) |
| Combination | 19 (7) |
| APACHE IV* | 87 (35) |
| SaO2 in % (SD)* | 96.1 (3.8) |
| SpO2 in % (SD)* | 96.3 (3.9) |
| PFI in % (range)* | 1.4 (0.1–19.2) |
Continuous data are presented as means (SD) or medians (interquartile range). Categorical data are presented as percentages
*GI Bleeding gastrointestinal bleeding, COPD chronic obstructive pulmonary disease, APACHE IV acute health and chronic health evaluation IV, SaO2 arterial oxygen saturation, SpO2 peripheral oxygen saturation, PFI perfusion index
On average, SaO2 and SpO2 correlated moderately (r = 0.69, p < 0.01, n = 1281). The Bland–Altman plot revealed a mean ΔSat of 0.21%, with a standard deviation (SD) of 3.04% (Fig. 1). Limits of agreement within 2 SDs of the mean showed a ΔSat of − 5.75% and + 6.17%. Furthermore, a non-significant linear regression between the bias (ΔSat) and the magnitude of the measurements (mean of SaO2 and SpO2) was found (F (1,1279) = 1.44, p < 0.23), with a R2 of 0.001, indicating no correlation between the bias and the error and verifying reliable use of the Bland–Altman analysis.
Fig. 1.
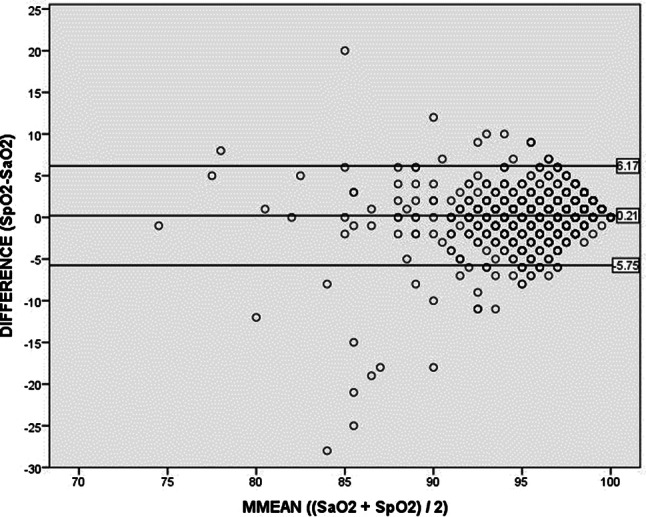
Bland–Altman plot, in which the difference between SpO2 and SaO2 is plotted against their average. The mean difference is 0.21% (middle line), with a SD (precision) of 3.04%. Limits of agreement were calculated using mean ± (1.96 * SD), resulting in an upper limit of 6.17% and a lower limit of − 5.75% (outer lines)
The mean ΔSat was not different between males and females (0.27 versus 0.13, p = 0.42) and was not associated with inotropic drugs use (F (3,1277) = 0.94, p = 0.42). In addition, other independent variables showed no or poor association with ΔSat (pH: r = − 0.18, p < 0.01; lactate: r = 0.02, p = 0.60; MAP: r = − 0.006, p = 0.82).
In the case of the largest measured ΔSat, we measured both methaemoglobin (MetHb) and carboxyhaemoglobin (COHb). All values of combined MetHb plus COHb were under 2% (data not shown).
The perfusion index
PFI and ΔSat correlated weakly (r = − 0.17, p < 0.01), indicating a slightly better agreement between SaO2 and SpO2 with increasing PFI (Fig. 2, Table 2). A large proportion of values exceeded the limits of agreement, which did not decrease drastically with increasing PFI. For example, even with a PFI > 2.5, which is generally considered adequate, 15.9% of all measurements still showed a difference between SpO2 and SaO2 of more than 2% (Table 2). Thus, the effect of PFI on ΔSat is limited.
Fig. 2.
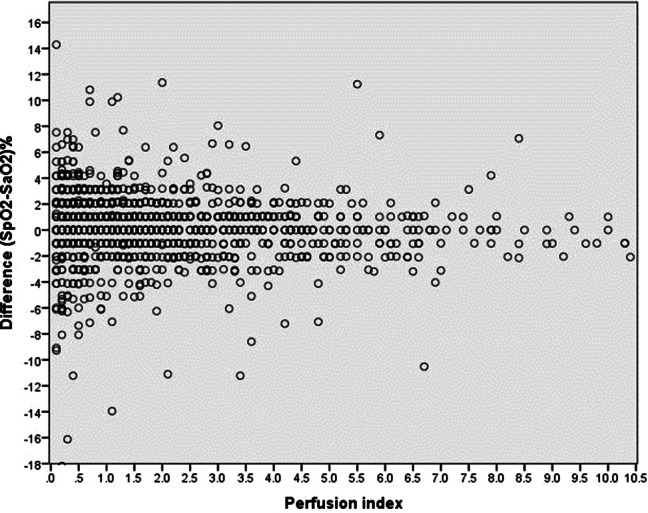
The ΔSat as percentages ((SpO2–SaO2/SaO2) × 100%) plotted against the PFI
Table 2.
Percentages and datapoints (n) of measurements exceeding varying limits of agreements (columns) for the various PFI categories (rows)
| ΔSat (SpO2–SaO2) PFI |
> 1% | > 2% | > 3% | > 4% |
|---|---|---|---|---|
| < 1.0 | 52.8% (246) | 30.7% (143) | 18.7% (87) | 10.5% (49) |
| 1.0–2.5 | 38.3% (153) | 18.3% (73) | 8.5% (34) | 4.8% (19) |
| > 2.5 | 36.2% (130) | 15.9% (57) | 6.9% (25) | 4.1% (15) |
Discussion
We performed a retrospective, single-centre study to demonstrate the contribution of the PFI to the accuracy of pulse oximetry in critically ill adult patients. The main finding of our study is that even with high PFI values, the accuracy of pulse oximetry in estimating arterial oxygenation remains moderate; therefore, the additive value of PFI is limited. In our study, pulse oximetry and SaO2 measurements correlated moderately (r = 0.69). Even when the outliers were removed, results remained identical. As the correlation coefficient found between SaO2 and SpO2 was 0.69, only 50% of the variability of SpO2 could be explained by the difference in SaO2. This is partly explained by a 3% variability of the pulse oximeter, compared to a variability of 0.2% of the blood gas analyser. This indicates that various other factors, such as acid–base disorders or factors contributing to macro- or microcirculation, might play a role in determining the absolute values of both SpO2 and SaO2. This comparison between SpO2 and SaO2 was also substantiated in the Bland–Altman plot, showing only moderate accuracy with varying clinical conditions.
Our findings regarding a discrepancy between SaO2 and SpO2 measurements are in line with the results of previous studies conducted with similar cohorts of critically ill patients. Perkins et al. demonstrated that changes in SpO2 do not reliably predict changes in SaO2, and neither anaemia nor acidosis altered the relationship between SpO2 and SaO2 [15]. We only found a small and weak negative correlation between the pH and ΔSat. In a cohort of ventilator-dependent patients, Seguin et al. showed that SpO2 overestimated SaO2, and a minimum SpO2 value of 96% to ensure SaO2 > 90% was incorporated into a nurse-driven protocol [16]. In a study by van de Louw et al., large differences between SpO2 and SaO2 were found with poor SpO2 reproducibly. Both studies suggested an SpO2 above 94% to ensure SaO2 > 90% [16, 17]. The accuracy of pulse oximetry was studied by Wilson et al. in a cohort of septic patients admitted to the emergency department. Their main finding was that pulse oximetry overestimated ABG-determined SaO2 by 2.75% [4]. When SaO2 needed to be determined, ABG analysis was recommended. A key problem in interpreting the findings reported in the literature on the use of pulse oximetry is that different kinds of pulse oximeters are used with different patented techniques for calculating SpO2, making comparisons difficult [18]. Corrections for the PFI have not been made in any of these studies.
Several possible explanations can be put forward to explain the disparity between the ΔSat and the PFI values. Vasodilation in skin and muscle is not equally distributed in acid–base disorders. Higher pH results in vasodilation in the muscle but vasoconstriction in skin arteries where the SpO2 measurements are taken [19]. This skin vasoconstriction may result in lower SpO2 values and therefore the ΔSat (SpO2–SaO2) may decrease, resulting in a negative correlation. Moreover, a lower pH results in a lower SaO2 due to the rightward shift of the oxygen dissociation curve (Bohr Effect), resulting in an increased ΔSat. Furthermore, in general and in isolated arteries, a lower pH results in vasodilation and therefore higher PFI values [19]. This association might result in a negative correlation. However, in our patient group a positive correlation was found. This might be explained by the fact that acidotic patients are frequently hemodynamically less stable, have a lower cardiac output, and are at risk for higher inotropic use. These factors might independently contribute to a lower PFI. In conclusion, many interacting factors may result in vasodilation or vasoconstriction. Our results have small correlation coefficients and may result in divergent values of blood oxygen levels.
To our knowledge, this is the first clinical study that correlated SpO2 accuracy with the PFI in adults. However, there are several limitations to our study. In our ICU, the ABG measurements were performed in our local central laboratory rather than with point-of-care equipment. A delay in the laboratory measurements could result in a lower arterial oxygen content and thus increased frequency of a positive SpO2–SaO2 difference. In our analyses this was not found, so we believe that this may not have influenced our results significantly. A second point of concern might be that we only used Philips pulse oximetry equipment, making head-to-head comparison with other manufacturers and extrapolation to different clinical settings difficult. However, a study by Louie et al. demonstrated that the Philips, Masimo, and Nellcor pulse oximeters were similarly effective in detecting hypoxemia [18]. Furthermore, besides the surmised but not proven value concerning the reliability of the linear relationship between SpO2 and SaO2, PFI values have also been used to predict vasopressor requirements or mortality [2, 20–23]. Clearly, differences in patient population may account for this, and our study was neither powered nor designed to assess inotropic drug use or mortality. Another possible drawback of our study is that it was conducted in a single centre; however, bias can also be introduced easily in a multi-centre trial as equipment and laboratory procedures may differ slightly.
Given that SpO2 does not reliably predict SaO2 values, despite in accordance to the manufacturer’s precision (both around 2–3%), the question arises as to how supplemental oxygen therapy can be adjusted without creating a hypoxic or hyperoxic state [11]. For example, in our ICU, we would rather be informed if the patient has an SaO2 of 89% instead of 93%, whereas the corresponding monitor indicates 91%. Moreover, our results imply that the PFI is not an accurate marker for SaO2 extrapolation and therefore will not be the primary determinant for adjusting the fraction of inspired oxygen (FiO2) values to improve supplemental oxygen delivery. Hence, we suggest performing an ABG analysis to measure SaO2.
Conclusions
Our results indicate a clinically relevant discrepancy between SaO2 and SpO2 and only a small decrease in their difference in the presence of high arterial perfusion. However, even at high PFI values, differences in saturation can still be clinically significant. These findings may influence daily practice on how to adjust oxygen supply therapy based on SpO2 measurements only. In critical situations, we advise collecting ABG measurements instead of adjusting oxygen supply based on SpO2 values, regardless of the PFI values.
Author contributions
MT selected the study design, acquired the data, drafted, and completed the manuscript for publication. LJ performed the statistical analysis, revised the manuscript, and approved it for publication. JN revised the manuscript and approved it for publication. NF selected the study design, revised the manuscript, gave the final approval for publication. All authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mark Thijssen, Email: m.thijssen@zuyderland.nl.
Loes Janssen, Email: loes.janssen@mmc.nl.
Jos le Noble, Email: jlenoble@maastrichtuniversity.nl.
Norbert Foudraine, Email: nfoudraine@viecuri.nl.
References
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Hasanin A, Mukhtar A, Nassar H. Perfusion indices revisited. J Intensive Care. 2017;5:24. doi: 10.1186/s40560-017-0220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen LA, Onyskiw JE, Prasad NG. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung. 1998;27:387–408. doi: 10.1016/S0147-9563(98)90086-3. [DOI] [PubMed] [Google Scholar]
- 4.Wilson BJ, Cowan HJ, Lord JA, Zuege DJ, Zygun DA. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective cohort study. BMC Emerg Med. 2010;10:9. doi: 10.1186/1471-227X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Genderen ME, Bartels SA, Lima A, Bezemer R, Ince C, Bakker J, van Bommel J. Peripheral perfusion index as an early predictor for central hypovolemia in awake healthy volunteers. Anesth Analg. 2013;116:351–356. doi: 10.1213/ANE.0b013e318274e151. [DOI] [PubMed] [Google Scholar]
- 6.Klijn E, Groeneveld AB, van Genderen ME, Betjes M, Bakker J, van Bommel J. Peripheral perfusion index predicts hypotension during fluid withdrawal by continuous Veno-venous hemofiltration in critically ill patients. Blood Purif. 2015;40:92–98. doi: 10.1159/000381939. [DOI] [PubMed] [Google Scholar]
- 7.Lima AP, Beelen P, Bakker J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med. 2002;30:1210–1213. doi: 10.1097/00003246-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 8.De Felice C, Latini G, Vacca P, Kopotic RJ. The pulse oximeter perfusion index as a predictor for high illness severity in neonates. Eur J Pediatr. 2002;161:561–562. doi: 10.1007/s00431-002-1042-5. [DOI] [PubMed] [Google Scholar]
- 9.Nitzan M, Romem A, Koppel R. Pulse oximetry: fundamentals and technology update. Med Devices (Auckl). 2014;7:231–239. doi: 10.2147/MDER.S47319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philips (2006) Intellivue Patiëntmonitor Mp70. M8000-9004g. Germany.
- 11.Philips . Reusable sensors that last durable and cost effective reusable SpO2 sensors. Eindhoven: Royal Philips Electronics; 2008. [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 13.Ho KM. Using linear regression to assess dose-dependent bias on a bland-altman plot. J Emerg Crit Care Med. 2018;2:68. doi: 10.21037/jeccm.2018.08.02. [DOI] [Google Scholar]
- 14.General Assembly of the World Medical A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–18. [PubMed] [Google Scholar]
- 15.Perkins GD, McAuley DF, Giles S, Routledge H, Gao F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care. 2003;7:R67. doi: 10.1186/cc2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seguin P, Le Rouzo A, Tanguy M, Guillou YM, Feuillu A, Malledant Y. Evidence for the need of bedside accuracy of pulse oximetry in an intensive care Unit. Crit Care Med. 2000;28:703–706. doi: 10.1097/00003246-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Van de Louw A, Cracco C, Cerf C, Harf A, Duvaldestin P, Lemaire F, Brochard L. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001;27:1606–1613. doi: 10.1007/s001340101064. [DOI] [PubMed] [Google Scholar]
- 18.Louie A, Feiner JR, Bickler PE, Rhodes L, Bernstein M, Lucero J. Four types of pulse oximeters accurately detect hypoxia during low perfusion and motion. Anesthesiology. 2018;128:520–530. doi: 10.1097/ALN.0000000000002002. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JH, Wildenthal K, Johnson RL., Jr The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1:375–389. doi: 10.1038/ki.1972.48. [DOI] [PubMed] [Google Scholar]
- 20.Rasmy I, Mohamed H, Nabil N, Abdalah S, Hasanin A, Eladawy A, Ahmed M, Mukhtar A. Evaluation of perfusion index as a predictor of vasopressor requirement in patients with severe sepsis. Shock. 2015;44:554–559. doi: 10.1097/SHK.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 21.Janak JC, Howard JT, Goei KA, Weber R, Muniz GW, Hinojosa-Laborde C, Convertino VA. Predictors of the onset of hemodynamic decompensation during progressive central hypovolemia: comparison of the peripheral perfusion index, pulse pressure variability, and compensatory reserve index. Shock. 2015;44:548–553. doi: 10.1097/SHK.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 22.He H, Long Y, Liu D, Wang X, Zhou X. Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care. 2015;19:330. doi: 10.1186/s13054-015-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He HW, Liu DW, Long Y, Wang XT. The peripheral perfusion index and transcutaneous oxygen challenge test are predictive of mortality in septic patients after resuscitation. Crit Care. 2013;17:R116. doi: 10.1186/cc12788. [DOI] [PMC free article] [PubMed] [Google Scholar]


