Introduction
The eponymous syndrome first described by the Brugada brothers in the early 1990s consists of characteristic ST abnormalities and an increased risk of sudden cardiac death due to ventricular arrhythmias.1 Since that time, further genetic research has implicated hundreds of variants in 17 genes with mutations in SCN5A, coding for voltage-gated sodium channels, accounting for the majority of genotyped patients.2 Various triggers have been associated with the development of ventricular fibrillation (VF) in this patient population, including fever and alcohol intake.3
We present a case of electrical storm in the setting of acute febrile illness in a patient with Brugada syndrome and COVID-19 infection.
Case report
A 58-year-old woman with a history of hypertension, diabetes mellitus, and Brugada syndrome, for which she underwent implantable cardioverter-defibrillator (ICD) implantation in 2017, presented following a 3-day febrile illness and, on the day of presentation, multiple syncopal episodes. She had previously had a syncopal episode in 2019 that occurred in the setting of VF that was terminated by a single shock from her device.
Interrogation of her ICD in the emergency department revealed multiple episodes of VF, some of which were nonsustained, as well as 7 episodes that were terminated by appropriate ICD shocks. The fever persisted after admission despite the use of antipyretics, with a maximum temperature of 101.7°F in the first 24 hours. During these febrile periods, she continued to have additional episodes of VF requiring shock termination (Figure 1). Rapid nasal swab testing in the emergency department was positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and prompted admission to the intensive care unit. Isoproterenol infusion (2 μg bolus followed by 1 μg/min) was initiated, as well as aggressive treatment of the fever with standing acetaminophen and salsalate. Despite these measures, the fever persisted and additional temperature management with a cooling blanket was needed for fever control. She did not have any further ventricular arrhythmias after her temperature normalized (Figure 2).
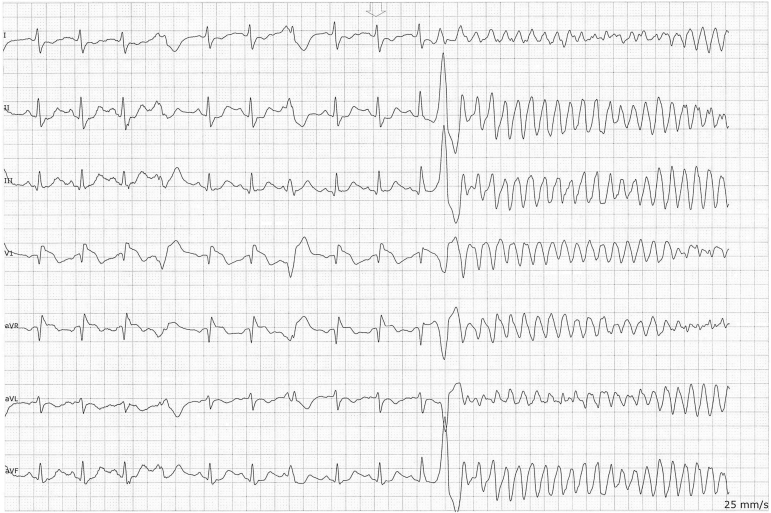
Figure 1.
One of multiple episodes of ventricular fibrillation requiring shock termination in the patient while febrile owing to COVID-19 infection. Of note is the coved-type ST pattern appreciated in V1.
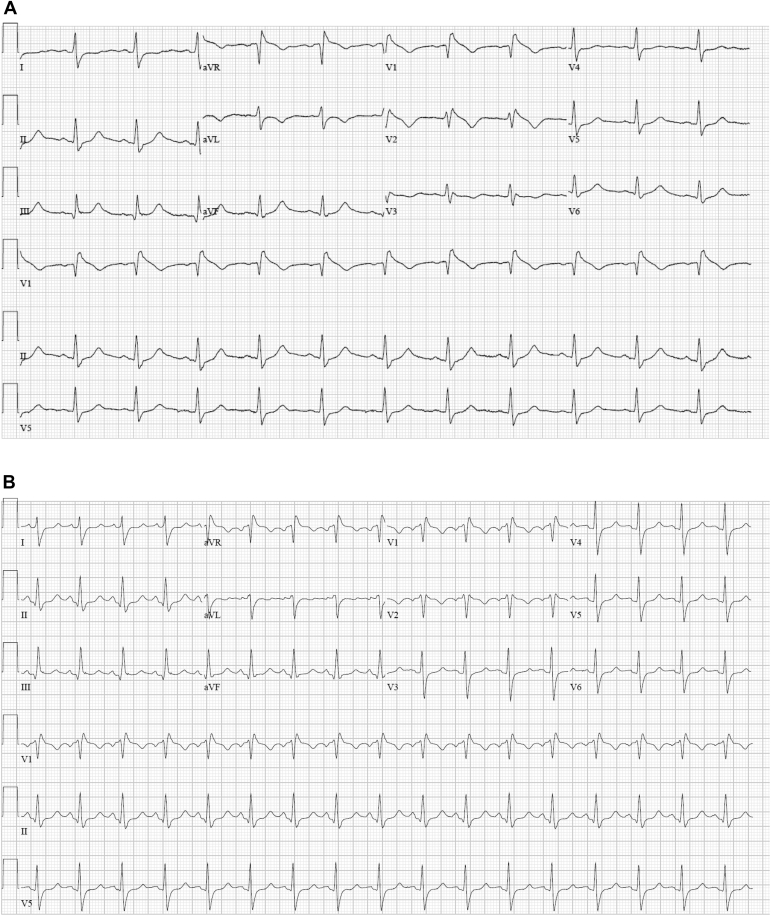
Figure 2.
Dynamic change in Brugada pattern with treatment. A: Twelve-lead electrocardiogram (ECG) obtained soon after presentation. B: ECG obtained following control of the patient’s fever and administration of isoproterenol.
She was started on hydroxychloroquine on hospital day 1; however, it was discontinued the following day due to QTc prolongation to 554 ms. Oxygenation proved difficult, and her respiratory status continued to decline despite the use of a non-rebreather mask and prone positioning. While her initial chest radiograph was unremarkable, serial radiographs revealed development of multifocal airspace and interstitial opacities consistent with acute respiratory distress syndrome from COVID-19-associated pneumonia. On hospital day 3, isoproterenol was discontinued owing to several sustained episodes of atrial tachycardia with rates of 140–150 beats/minute. Broad-spectrum antibiotics were started on hospital day 5 owing to concern for possible superimposed bacterial pneumonia. Remdesivir was initiated on hospital day 6; however, her respiratory function continued to decline, requiring intubation. During her hospital course, she developed laboratory evidence of severe disease with progressive lymphopenia (130/μL) and increased C-reactive protein (54.5 mg/dL), D-dimer (8474 ng/mL), fibrinogen (>1000 mg/dL), ferritin (1116 ng/mL), and procalcitonin (2.7 ng/mL). She was started on therapeutic anticoagulation with low-molecular-weight heparin owing to her hypercoagulable state.
She continued to decline, with development of septic shock requiring initiation of multiple vasopressors. Although she had infrequent episodes of atrial tachycardia, she had no further ventricular arrhythmias. During a sedation holiday on hospital day 18, she was noted to be unarousable. Computed tomography imaging revealed extensive intracranial hemorrhage with resultant mass effect. Given her severe and irreversible neurological injury, her family members decided to transition to comfort care.
Discussion
Brugada syndrome typically presents during adulthood, with a mean age of sudden cardiac death occurring at 41 ± 15 years.4 Abnormalities in SCN5A, a gene that encodes the α-subunit of cardiac sodium channels, remains the most common genotype. Mutation in this channel results in loss of sodium channel function with resultant delay in phase 0 action potential upstroke and slowing of conduction.3 The ventricular arrhythmias that develop in this patient population can be highly lethal. For this reason, ICDs are recommended for patients who are survivors of cardiac arrest and/or have documented spontaneous ventricular tachycardia/VF, as well as those with other high-risk features.5
Whereas Brugada syndrome has been well studied over the past several decades, COVID-19 is a relatively new entity. In early December 2019, the first cases of SARS-CoV-2 were identified in Wuhan, China.6 In the following months, the virus spread throughout the world, posing a significant global challenge to combat the pandemic. One of the most common symptoms in COVID-19 infection is fever, which is present in 83%–99% of patients.7 It is well known that a subset of COVID-19 patients will develop cytokine storm. This is a clinical entity characterized with high and unrelenting fevers, often with temperatures >39.4°C.8 We believe our patient to have developed secondary cytokine storm, which underscores the importance of temperature control in patients with Brugada syndrome and COVID-19 infection. In many patients it is not until fever is present that a type I electrocardiogram pattern becomes apparent, which itself carries an increased risk of fatal arrhythmias.9 It is believed that at increased temperatures sodium channel gating is augmented in patients with SCN5A mutations, resulting in altered net outward current during right ventricular depolarization.10 This relationship of fever unmasking electrocardiographic changes has been described in the COVID population.11
Electrical storm is the highly feared electrophysiological sequela of Brugada syndrome. As fever precipitates ventricular arrhythmias in Brugada syndrome, this concern is particularly amplified in patients with COVID-19 infection, a condition characterized by high fever curves.12 Acute management of electrical storm in Brugada syndrome with COVID infection includes treating any arrhythmic triggers, such as the utilization of acetaminophen for fever control.13 If antipyretics prove ineffective, our recommendation would be to use other nonpharmacologic measures, such as cooling blankets, packed ice, or even cooling catheters, normally reserved for therapeutic hypothermia protocols.
Other data have demonstrated benefit to isoproterenol infusion in the treatment of electrical storm (administered as a bolus injection of 1–2 μg followed by continuous infusion at a dose of 0.15–2.0 μg/min to maintain a 20% increase in heart rate). A nonspecific β-agonist, isoproterenol increases heart rate and has been shown to decrease J-point amplitude, change coved-type to saddleback-type ST-segment patterns, and even normalize ST-segment elevation, thereby suppressing VF.14 Quinidine is a class Ia antiarrhythmic agent that can be used in treatment of electrical storm in the Brugada population. It possesses anticholinergic effects and results in the inhibition of Ito in the ventricular epicardium to help suppress VF. Given the benefit of these medications, they are recommended by both American and European societies for the treatment of electrical storm.5,15 In our patient, the combination of antipyretic therapy and isoproterenol infusion was successful in suppressing ventricular arrhythmias.
Conclusion
This case describes the development of electrical storm in a patient with Brugada syndrome in the setting of an acute febrile illness due to COVID-19 infection. In addition to aggressively controlling her fever, isoproterenol was required for suppression of ventricular arrhythmias. While there is an established association between fever and ventricular arrhythmias in patients with Brugada syndrome,8 the presence of prolonged high-grade fever in those infected with COVID-19 may pose additional risks in these patients.
Key Teaching Points.
-
•
COVID-19 infection often presents with high fever. Among severely ill COVID-19 patients, some develop secondary cytokine storm syndromes characterized by high and unremitting fever.
-
•
Patients in this population with underlying Brugada syndrome are at particular risk of adverse outcomes due to the known association of fever and ventricular arrhythmias.
-
•
The prompt recognition and treatment of fevers and arrhythmias in patients with Brugada syndrome and COVID-19 infection is crucial for favorable outcomes.
Footnotes
No funding was required for this submission. There are no conflicts of interest for this submission with any authors.
References
- 1.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen M.W., Holst A.G., Olesen S.P., Olesen M.S. The genetic component of Brugada syndrome. Front Physiol. 2013;4:179. doi: 10.3389/fphys.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizusawa Y., Wilde A.A. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012;5:606–616. doi: 10.1161/CIRCEP.111.964577. [DOI] [PubMed] [Google Scholar]
- 4.Antzelevitch C., Brugada P., Borggrefe M. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 5.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Available at. Accessed May 15, 2020.
- 8.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin A.S., Meregalli P.G., Bardai A., Wilde A.A., Tan H.L. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149:216–218. doi: 10.7326/0003-4819-149-3-200808050-00020. [DOI] [PubMed] [Google Scholar]
- 10.Dumaine R., Towbin J.A., Brugada P. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 11.Chang D., Saleh M., Garcia-Bengo Y., Choi E., Epstein L., Willner J. COVID-19 infection unmasking Brugada syndrome [published online ahead of print, 2020 Mar 25] HeartRhythm Case Rep. 2020 doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: An update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020;48:737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 13.Wu C.I., Postema P.G., Arbelo E. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes [published online ahead of print, 2020 Mar 31] Heart Rhythm. 2020:S1547–S5271. doi: 10.1016/j.hrthm.2020.03.024. (20)30285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohgo T., Okamura H., Noda T. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4:695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Priori S.G., Blomström-Lundqvist C., Mazzanti A. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]