By June 28, 2020, there were more than 10 million confirmed cases of coronavirus disease 2019 (COVID-19) and nearly 500,000 deaths in about 216 countries and regions.1 The number of cases is still increasing. In China, the spread of SARS-CoV-2 has been effectively controlled after strict management, with a total of 83,534 cases and 4634 deaths (by June 30).2 The integration of Traditional Chinese Medicine (TCM) with western interventions is a successful experience for the treatment of COVID-19.3 About 91.5% of the confirmed and suspected COVID-19 cases in China had been treated with TCM, alone or integrated with conventional western medicine (WM).4
There are hundreds of registered protocols and completed clinical studies. Evidence from published clinical trials suggested that TCM is helpful to treat COVID-19 and decrease mortality.5., 6. However, the voice of doubt about the efficacy of TCM for COVID-19 can still be heard. In opponents’ opinion, TCM was not approved to be effective for COVID-19, unless double-blind placebo-controlled randomized clinical trials (RCTs) were performed to provide evidence.7 The controversy is mainly focused on: what is reliable clinical evidence? We aimed to clarify the questions of clinical evidence of TCM for COVID-19 from the viewpoint of evidence-based medicine and real-world study.
Registered and published clinical trials of TCM for COVID-19
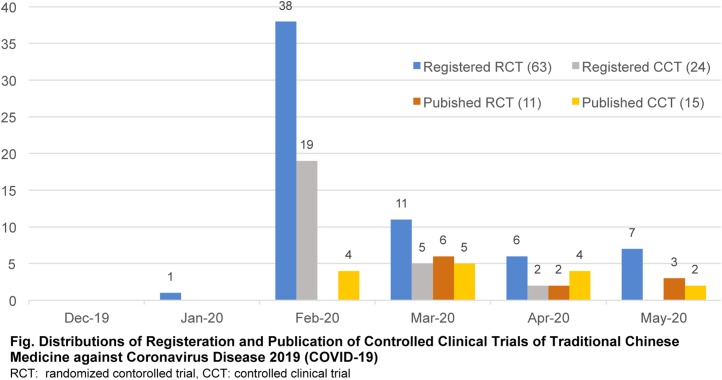
According to our review, there were 121 registered protocols of TCM for COVID-19 identified from Chinese Clinical Trial Registry (www.chictr.org.cn) and ClinicalTrials.gov (up to June 30), and 63 of them were RCTs (Fig. 1).
Fig. 1.
Distributions of registration and publication of clinical controlled trials of traditional Chinese medicine against coronavirus disease 2019 (COVID-19).
RCT: randomized contorolled trial, CCT: clinical controlled trial
After comprehensive literature search, we found 26 published clinical controlled trials of TCM for COVID-19, and 11 of them were RCTs with 1301 patients. However, none of them were placebo-controlled and double-blinded RCTs. The types of patients, interventions and outcomes in different RCTs varied significantly. In addition, most of the published trials have not registered their protocols before case recruitment.
Registered studies with protocols have not been completed, and published clinical trials were conducted in the absence of registered protocols, which reflected key problems in clinical trials of TCM for COVID-19. First, defects in methodology of clinical trials weakened the quality of evidence. Second, rigorously designed RCTs could not be completed due to the special environment dealing with COVID-19, which was a novel and highly infectious diseases.
Why was a rigorous RCT impractical in the early stage?
Knowledge of COVID-19 is limited for designing a good clinical trial protocol. COVID-19 is a novel infectious disease. Medical community knows only a little about the pathogenesis and evolution process of COVID-19, especially at the early stage of outbreak. As a consequence, it was difficult to design a scientific clinical trial protocol with clear patient inclusion criteria, appropriate treatment course and valuable outcomes. For example, the duration from onset to hospitalization of patients varied from several hours to more than one month. In addition, clinical symptoms and condition degree of patients were greatly heterogeneous and variable, which led to different treatment regime for individuals. Moreover, there was no consensus on clinical efficacy evaluation indicators at the beginning.8., 9. Therefore, registered protocols commonly deviated from the real clinical situation.
Most of the cases of COVID-19 were confirmed in Hubei province. Doctors, nurses and other medical staffs have overworked in the circumstance with high risk of infection. This situation was no reliable for doctors to serve as investigators in a clinical trial. In addition, different medical wards were managed by different medical teams, which increased the difficulty of implementing an unified research program.
The conditions for supporting a randomized double-blind placebo-controlled clinical trial on COVID-19 are not available as it is a novel infectious disease. There was no certain effective herbal drugs and therefore no prepared placebos. In addition, nearly no patient could agree to take any placebo for the fear of death. The willingness of patients is a critical factor for the feasibility in a RCT. Patients wished to receive potential effective treatments, especially herbal drugs which were helpful in their opinion. Therefore, in this case, it was virtually impossible to carry out patient informed, double-blind and placebo-controlled RCTs.
Suggestions for further clinical trials facing an epidemic outbreak
First, methodology and supporting condition for real-world studies should be strengthened. In response to the outbreak of novel diseases, observational studies and RCTs are both essential to provide evidence for diagnosis and treatment. Therefore, all the accessible data of patients need to be collected. A simple, efficient and intelligent data management system is essential, which is different from commonly used electronic data capture system (EDC). Diagnostic information such as data of tongue and pulse, imaging examination, laboratory tests and patient-reported outcomes can be obtained and analyzed simultaneously. Other functions including randomization, allocation concealment, drug management and follow-up reminding should be merged into the platform. Intelligent data analysis is an important function, which can shorten the time of evidence production for early diagnosis and treatment.
Second, an emergency medical team should include clinical investigators. For novel diseases, clinical treatment and clinical research are equally important. Clinical research can provide decision-making evidence and develop new effective treatments for clinical practice. In order to design and conduct a good clinical trial, clinical investigators should work in the first line with doctors. They have different and clear tasks and perform their own duties that are conducive to ensure the quality of clinical trials. Meanwhile, the normal training of clinical research methodology for doctors and nurses is also very important.
Third, the overall management of clinical research resources should be strengthened. Due to the lack of unified coordination and management of clinical studies, different research groups design different trials. Low-level duplicated studies cannot generate high-level evidence, which not only leads to the waste of research resources, but also the failure of high-quality research. Therefore, related administrative departments should pay more attention to manage clinical trials during a novel disease outbreak as it would be helpful to conduct unified high-quality studies to tackle the crucial problems in the condition of limited resources.10
Researchers always hope to carry out rigorously designed, large sample, double-blind and placebo-controlled RCTs to evaluate the efficacy of interventions. However, there is always a gap between the ideal and the reality. If divorced from the reality, the ideal is fantasy. For this reason, real-world studies have gained more and more attention in the field of clinical research.11., 12. The situation of clinical trials of TCM for COVID-19 is a typical example for the balance between scientificity and feasibility. Life first, treatment first. Clinical trials must be subject to clinical practice. For an emerging disease, we cannot fully recognize it in a short time, and we cannot produce all evidence for interventions in a short time. Although the current clinical evidence of TCM for COVID-19 is not at the highest level, it can still serve as necessary evidence for clinical decision-making. The follow-up clinical trials will continue to produce more evidence. It is not too late to mend. We have learned more based on the clinical trials of TCM for COVID-19, which are valuable reference for future studies especially on novel infectious diseases.
Author contributions
Conceptualization: JZ. Writing-Original Draft: BP. Writing-Review and Editing: JZ, ML and WZ.
Conflict of interest
The authors declared that there was no conflict of interest regarding the publication of this paper.
Funding
This work was funded by COVID-19 Prevention and Treatment Drug Development Program (2020-CMKYGG-03).
Ethical statement
Not applicable.
Data availability
Not applicable.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report-161. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200629-covid-19-sitrep-161.pdf?sfvrsn=74fde64e_2. Published June 29, 2020. Accessed June 30, 2020.
- 2.National Health Commission of the People’s Republic of China. Coronavirus disease 2019 (COVID-19) Situation Report - June 30, 2020. http://www.nhc.gov.cn/xcs/yqtb/202007/a98e49570be24eaf88de98e6e6217fc8.shtml. Published June 30, 2020. Accessed June 30,2020.
- 3.Gao S.M., Ma Y., Yang F.W., Zhang J.H., Yu C.Q. ZHANG Boli: traditional Chinese medicine plays a role in the prevention and treatment on novel coronavirus pneumonia. Tianjin J Trad Chin Med. 2020;37:121–124. [Google Scholar]
- 4.National Administration of Traditional Chinese Medicine. More than 90 percent received the treatment of traditional Chinese medicine. http://www.satcm.gov.cn/xinxifabu/meitibaodao/2020-03-24/14229.html. Published March 24, 2020. Accessed June 10, 2020.
- 5.Liu M., Gao Y., Yuan Y., Yang K.L., Shi S.Z., Zhang J.H. Efficacy and safety of integrated traditional chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9:1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyranoski D. China is promoting coronavirus treatments based on unproven traditional medicines[J] Nature. 2020 doi: 10.1038/d41586-020-01284-x. Available from: https://www-nature-com.rpa.skh.org.tw/articles/d41586-020-01284-x. Accessed June 9, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Jin X.Y., Pang B., Zhang J.H., Liu Q.Q., Yang Z.Q., Feng J.H. Core outcome set for clinical trials on coronavirus disease 2019 (COS-COVID) Engineering. 2020 doi: 10.1016/j.eng.2020.03.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin X.Y., Pang B., Wang H., Wang K.Y., Pang W.T., Zheng W.K. Outcomes and related issues of clinical trials for novel coronavirus pneumonia. Tianjin J Trad Chin Med. 2020:1–4. http://kns.cnki.net/kcms/detail/12.1349.R.20200312.1654.007.html [Epub ahead of print] [Google Scholar]
- 10.Yuan W.A., Hu Y.Y., Tang J.Y., Yu H., Zhang J.H., Yang Z.Q. Insight into current clinical research of Chinese medicine on COVID-19. SH J TCM. 2020;54:1–5. [Google Scholar]
- 11.Sherman R.E., Anderson S.A., Dal Pan G.J., Gray G.W., Gross T., Hunter N.L. Real-world evidence - what is it and what can it tell us? N Engl J Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J.H., Zheng W.K., Zhang B.L., Jin X.Y., Pang W.T., Pang B. Real world study. World Chin Med. 2019;14:3101–3105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.