Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2, has led to the death of hundreds of thousands of people worldwide. If infected, older individuals and those with diabetes, hypertension, cardiovascular disease, and compromised immune systems are at higher risk for unfavorable outcomes. These comorbidities are prevalent in patients with kidney disease, hence the significant burden of COVID-19 on kidney transplant programs. Multiple case series of kidney transplant recipients with COVID-19 have shown increased mortality compared to nontransplant patients. To date, we do not have high-level evidence to inform immunosuppression minimization strategies in infected transplant recipients. Most centers however have adopted early antimetabolite withdrawal in addition to other interventions. This review summarizes the published COVID-19 literature as it relates to outcomes and immunosuppression management in kidney transplant recipients. It also discusses challenges pertaining to pretransplant evaluation and wait-listed patients.
Key Words: SARS-CoV-2, Immunosuppression, Renal transplantation, Coronavirus disease
Clinical Summary.
-
•
COVID-19 is associated with significant mortality in kidney transplant recipients.
-
•
There is currently no evidence-based immunosuppression reduction strategy for kidney transplant recipients with COVID-19. Early anti-metabolite withdrawal would be reasonable.
-
•
Kidney transplant programs should establish internal protocols to guide their operations during the COVID-19 pandemic.
-
•
It is prudent to pay attention to nosocomial spread, physical distancing, proper PPE use, and to incorporate telemedicine wherever possible.
Coronavirus disease 2019 (COVID-19) is a respiratory and systemic illness that was first reported in Hubei province, China, in December 2019. Since declaring it a global pandemic by the World Health Organization on March 11, 2020, COVID-19 has quickly accelerated beyond its tipping point leading to the death of hundreds of thousands of people worldwide.1
COVID-19 is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an RNA beta-coronavirus that likely emerged from a wet animal and fish market in the city of Wuhan. In humans, COVID-19 is a highly contagious, air-borne disease with person-to-person viral transmission occurring via respiratory droplets. Since it is a novel virus, we lack effective therapies, and hence its increased morbidity and mortality rate of about 5% according to the Johns Hopkins Coronavirus Resource Center (http://coronavirus.jhu.edu). Our understanding of the clinical spectrum of COVID-19 continues to evolve rapidly. It has been consistent across all published experiences however that older patients and those with medical comorbidities or compromised immune system are at a higher risk for worse outcomes.2
This review discusses COVID-19 in kidney transplant recipients, and focuses on clinical characteristics and outcomes, immunosuppression management, and operational challenges facing transplant programs. Other aspects of the disease will be covered by separate articles in this issue of the journal.
Clinical Characteristics and Outcomes
SARS-CoV-2 incubation period is up to 14 days (mean of around 5 days), and its initial symptoms include fever, cough, dyspnea, and malaise that could progress to acute respiratory distress syndrome requiring intensive care and mechanical ventilation.1 More recently, multiple studies have elucidated that COVID-19 is a systemic disease that often manifests with gastrointestinal (GI) symptoms, liver injury, cardiac involvement, encephalitis, atypical stroke, and acute kidney injury (AKI) in addition to endothelial cell injury and coagulopathy, the likely mediators of multiorgan involvement.3, 4, 5, 6, 7, 8
Six retrospective studies from New York and Europe have reported on COVID-19 in kidney transplant recipients (Table 1 ).9, 10, 11, 12, 13, 14 In a 36-patient study, the median age was 60 years, 72% were male, 39% were African American, and 75% received deceased-donor kidney transplants (DDKTs).9 Comorbidities included diabetes (69%), hypertension (94%), smoking (36%), and cardiovascular disease (17%). Forty-two percent received lymphocyte depleting induction, and more than 85% were on mycophenolate/tacrolimus/prednisone maintenance immunosuppression. Upon presentation, less than a half had no fever, about a fifth presented with GI symptoms, and a third with myalgias. All but one of 28 hospitalized patients had radiologic evidence of a viral pneumonia. Out of those, eleven patients required mechanical ventilation and 6 developed AKI warranting renal replacement therapy (RRT). Ten patients died at a median of 21 days, among them 2 were being monitored at home. Both were recent transplant recipients who received antithymocyte globulin within 5 weeks of presentation.
Table 1.
Summary of the Published Literature on COVID-19 in Kidney Transplant Recipients
| Akalin et al.9 | Columbia University Experience10 | Nair et al.11 | Alberici et al.12 | Montagud-Marrahi et al.13 | Pereira et al.14 | |
|---|---|---|---|---|---|---|
| KTR (n) | 36 | 15 | 10 | 20 | 33 | 90 (46)∗ |
| Baseline characteristics | ||||||
| Median age (y) | 60 (Range: 32-77) | 51 (Range: 21-78) | 57 (IQR: 47-67) | 59 (IQR: 51-64) | 57 ± 17† | 57 (IQR: 46-68) |
| Male (%) | 72 | 65 | 60 | 80 | 58 | 59 |
| DDKT (%) | 75 | 80 | 40 | N/A | N/A | N/A |
| Median time since transplant | N/A | 49 mo (range: 0-232) | 2822 d (IQR: 1272-4592) | 13 y (IQR: 9-20) | 10.7 y (range: 4-14.7) | 6.64 y (IQR: 2.87-10.61) |
| Diabetes (%) | 69 | N/A | 60 | 15 | 46 | 46 |
| Hypertension (%) | 94 | N/A | 100 | 85 | 64 | 64 |
| Cardiovascular disease (%) | 17 | N/A | 20 | 15 | N/A | N/A |
| Lymphocyte depleting induction (%) | 42 | N/A | 30‡ | N/A | N/A | |
| Symptoms/signs | ||||||
| Fever (%) | 58 | 87 | 70 | 100 | N/A | 70 |
| Cough (%) | N/A | 60 | N/A | 50 | N/A | 59 |
| Dyspnea (%) | N/A | 27 | N/A | 5 | N/A | 43 |
| GI symptoms/diarrhea (%) | 22 | 20 | N/A | 15 | N/A | 31 |
| Myalgia (%) | 36 | 13 | N/A | 5 | N/A | 24 |
| Radiologic evidence of pneumonia (%) | 75 | 6 | 70 | 85§ | N/A | N/A |
| Disease course | ||||||
| Requiring hospitalization (%) | 78 | 100 | 90 | 100 | 79 | 76 |
| Requiring MV (%) | 31 | 27 | 30 | 50‖ | 6 | 35 |
| AKI (%) | N/A | 40 | 50 | 30 | N/A | N/A |
| Requiring RRT (%) | 17 | 13 | 10 | 5 | N/A | |
| Treatment | ||||||
| IS management | Antimetabolite withdrawal (67%), CnI withdrawal (17%) | Antimetabolite withdrawal (71%) | Antimetabolite withdrawal (100%), Tac trough target reduced to 3-5 ng/mL), prednisone continued. | All IS withdrawal, initiation of MP 16 mg daily. | MMF and m-TORi withdrawal in all patients, CnI withdrawal in case of lopinavir/ritonavir prescription, initiation of prednisone 12-20 mg daily. | Antimetabolite withdrawal (88%), CnI withdrawal or reduction (18%) |
| Hydroxychloroquine (%) | 67 | 87 | 90 | 95 | 14 | 91 |
| Azithromycin (%) | 36 | 60 | 90 | N/A | 43 | 66 |
| Antivirals (%) | N/A | N/A | N/A | 95 | 64 | 3 |
| Tocilizumab (%) | 6 | 7 | N/A | 30 | 36 | 21 |
| Mortality | ||||||
| Mortality (%) | 28 | 13 | 30 | 25 | 6 | 18 |
| Medan time to mortality (d) | 21 (Range: 14-28) | N/A | N/A | 15 (IQR: 15-19) | N/A | N/A |
Abbreviations: AKI, acute kidney injury; CnI, calcineurin-inhibitor; DDKT, deceased-donor kidney transplant; GI, gastrointestinal; IQR, interquartile range; IS, immunosuppression; KTR, kidney transplant recipients; n, number of kidney transplant recipients; MMF, mycophenolate mofetil; MP, methylprednisolone; m-TORi, mammalian target of rapamycin inhibitor; MV, mechanical ventilation; N/A, data not available; RRT, renal replacement therapy; Tac, tacrolimus.
Ninety solid organ transplants, 46 of which were kidney transplants.
Unit not specified.
Unknown induction in 4 patients.
Fifty percent had bilateral infiltrates and 35 unilateral infiltrates.
Mostly noninvasive ventilation.
In a study of 15 hospitalized kidney transplant recipients at Columbia University, the median age was 51 years, 65% were male, and 80% received DDKT.10 Maintenance immunosuppression consisted mostly of an antimetabolite/tacrolimus/prednisone regimen. Notably, the Columbia transplant program adopts an early steroid-withdrawal strategy; however, their sample was enriched with patients on prednisone maintenance (67%), which confirms the plausible role of enhanced immunosuppression as a susceptibility factor. It is also worth mentioning that 2 of their patients were maintained on a belatacept-based regimen. Upon presentation, about 90% of kidney transplant recipients had fever, two-thirds, one-third, and one-fifth had cough, dyspnea, and GI symptoms, respectively, whereas a third had no radiologic finding of pneumonia. Four and 2 patients required mechanical ventilation and RRT, respectively. Among those who were intubated, 2 were in their first year after transplant. One developed symptoms and deteriorated while on plasmapheresis and glucocorticoids treatment for antibody-mediated rejection. The other developed symptoms just after completing antithymocyte globulin induction. One patient had delayed graft function in the setting of COVID-19 infection, and 2 died. Nair and colleagues reported a case series of 10 kidney transplant recipients with COVID-19, median age of 57 years, 60% male, and 40% DDKT recipients.11 Ninety percent of those patients required hospitalization, 50% developed AKI, and 30% required mechanical ventilation and eventually died.
An article from Northern Italy reported on 20 patients with a median age of 59 years and 13 years since transplant.12 Eighty percent were male, 15% diabetic, 85% hypertensive, 15% had cardiovascular disease, and the majority were on a mycophenolate/calcineurin inhibitor/prednisone regimen. All patients presented with fever, half with cough, and about 15% with GI symptoms. Almost half of the patients required ventilation, and a third developed AKI, with 1 patient requiring RRT. Mortality was 25% at a median follow-up of 15 days from the onset of symptoms. In a 33-patient report from Spain, mortality was comparable to the general population at 6%; however, several patients where still hospitalized when the study was completed.13
The largest published experience to date comes from New York.14 It included 90 solid organ transplant recipients out of which 46 were kidney transplant patients. Median age was 57 years, 59% were male, 46% were diabetic, 64% hypertensive, and 63% had baseline chronic kidney disease. Atypical GI and constitutional symptoms were common upon presentation. Immunosuppression modification included antimetabolite withdrawal in 88% and calcineurin inhibitor cessation or reduction in 18% of the cohort. Hydroxychloroquine and azithromycin were used in the majority, whereas tocilizumab and glucocorticoids were used in about a fifth of the cases. Patients with a more severe disease tended to be older, and overall mortality was 18%. Also, important to note is that 8% who initially tested negative for COVID-19 before repeat testing—based on high clinical suspicion—turned positive.
Retrospective, unpublished data from the University of Washington on about 300 solid organ transplant patients with COVID-19, more than two-thirds of which are kidney transplant recipients, show that the demographics are consistent with the published reports: median age of 57 years and 35% African American. Interestingly, nearly half of these patients had no fever, 15% had absent respiratory symptoms, 25% had normal chest imaging, and more than 50% were lymphopenic upon presentation. Upward of 70% were hospitalized early in their course, 30% managed in ICU, and 22% required intubation. Most of them were also treated with some off-label or experimental therapy.
In summary, COVID-19 could present in an atypical fashion in kidney transplant recipients, with no fever, respiratory symptoms, or radiologic findings of pneumonia. This raises the question of the validity of using these symptoms and signs to rule out SARS-CoV-2 infection with good enough sensitivity. We should be also cognizant of GI symptoms, which could be confounded or augmented by immunosuppressive medications, mainly mycophenolate.4 , 5 The majority of patients were male, which is consistent with earlier studies from China, and in their sixth decade of life. This is slightly older than the general COVID-19 population and likely represents the relatively increased age of kidney transplant recipients.2 , 15 , 16 The AKI incidence was elevated. This reflects diminished kidney reserve in setting of a solitary kidney, calcineurin inhibitor use, and suboptimal baseline allograft function.17 The mortality rate was between 13% and 30%, which is higher than that of the general population (∼5%). This could be attributed to the increased prevalence of diabetes, hypertension, and cardiovascular disease in addition to the immunosuppressed state—all of which have been associated with severe COVID-19. Moreover, these studies are retrospective and focus on cohorts of patients who mostly required hospitalization. This prevents us from assessing the true incidence of infection in kidney transplant recipients and could have possibly skewed the AKI and mortality rates.
Immunosuppression Management
It remains unclear how to best manage immunosuppression in transplant recipients with COVID-19. Liang and colleagues have shown that cancer patients with neutropenia have unfavorable outcomes.18 This underscores the role of the immune system in fighting SARS-CoV-2. It is then plausible that there is room for immunosuppression reduction to help alleviate the progression of SARS-CoV-2 infection. The flip side, however, maybe losing the potential beneficial effect of immunosuppressive drugs in mitigating the systemic inflammatory response mediated by cytokine storm.19 Actually, in addition to antivirals, many of the treatment strategies currently being investigated for COVID-19 are examining adjunctive medications that primarily target the inflammatory response.20 Some of these therapies such as glucocorticoids, interleukin-6 (IL-6) receptor antagonists, anticomplement-5 inhibitors, intravenous immunoglobulins, and mammalian target of rapamycin inhibitors have been used for primary prevention and treatment of allograft rejection.21, 22, 23, 24
By using human cDNA libraries, genome-wide coronavirus yeast-two-hybrid experiments demonstrated that cyclophilin and FK-binding protein interacted with the SARS-CoV-1 nonstructural protein 1.25 Subsequent experiments showed in vitro efficacy of cyclosporine A and FK506 in inhibiting the replication of SARS-CoV-1 and other human coronaviruses.25 These data might offer a justification for leaving patients on a lower dose of calcineurin inhibitor while de-escalating other medications. A reasonable first step is to reduce or hold the antimetabolite. Indeed, up to 100% of the patients in the aforementioned experiences have had mycophenolate withdrawn. Furthermore, in a national survey by Boyarsky and colleagues, responders discontinued antimetabolites and reduced calcineurin inhibitor dose in 92% and 27% of kidney transplant recipients, respectively.26 In the case series from Italy and Spain, all maintenance immunosuppression was held upon presentation and substituted by methylprednisolone or prednisone.12 , 13 Another consideration is the potential interaction between antivirals such as lopinavir/ritonavir and other investigational medications with immunosuppressives, mainly calcineurin and mammalian target of rapamycin inhibitors.27 Immunosuppression dose adjustment and more frequent trough monitoring would be prudent in this case.
The increased mortality seen in transplant recipients with COVID-19 corroborates the role of diminished T- and B-cell immunity as a predisposing factor for severe infection. To date though, we do not have a level 1 evidence-based strategy to inform immunosuppression management. In those with moderate disease, it would be reasonable to hold the antimetabolite and continue low-dose calcineurin inhibitor with or without glucocorticoids. In patients with severe disease and those who are critically ill, it may be justifiable to hold all maintenance immunosuppression and initiate remdesivir in addition to steroids.28 Other investigational drugs should be offered under institutional review board–approved prospective, controlled, research protocols.
Inflammatory biomarkers such as C-reactive protein, IL-6 levels, and ferritin have correlated with the severity of disease, nevertheless with substantial overlap and variability among subjects.29 Trending biomarkers in individual patients however might play a role in guiding an individualized immunosuppression adjustment plan. An example would be using tocilizumab, a monoclonal antibody against the IL-6 receptor, in patients with significantly elevated IL-6 levels.
Operational Challenges
The COVID-19 pandemic has taken its toll on kidney transplant programs and resulted in suspending or restricting their operations. This led to unfavorable consequences that have impacted wait-listed patients and complicated the care of kidney transplant recipients. Some of the concerns and challenges that relate to kidney transplantation practices are discussed in the following sections.
Donor to Recipient Viral Transmission
The consensus is that kidneys from deceased or living donors with a confirmed infection or high exposure risk to COVID-19 should not be used.30 To date, donor to recipient viral transmission lacks conclusive confirming data; however, it is certainly plausible. This is supported by the following: (1) Angiotensin-converting enzyme 2 is the receptor that binds the spike protein of SARS-CoV-2 and facilitates its entry into target cells.31 Among all organs, the kidneys show the highest level of expression of angiotensin-converting enzyme 2.32 (2) Indeed, SARS-CoV-2 can directly infect the kidneys. Ultrastructural tissue analysis by electron microscopy demonstrated viral particles in tubular epithelial and glomerular capillary endothelial cells.33 , 34 This could contribute to the AKI seen in patients with COVID-19; however, this discussion is beyond the scope of this article. (3) SARS-CoV-2 viremia and viruria have been reported in 15% and 7% of COVID-19 patients, respectively.15 , 35 This might even be an underestimation as data from the 2003 SARS outbreak show an RNAemia rate of 78% in the earlier weeks of the illness.36 All this will become obsolete if an efficacious antiviral drug is discovered. Then, we would be dealing with a situation similar to that of hepatitis C after the discovery of direct-acting antivirals.37
Transplant Team Members as Vectors
Sixteen percent of all COVID-19 patients are health-care workers, and 29% of those had hospital-acquired infections.38 , 39 Data from the 2003 SARS and Middle East respiratory syndrome outbreaks suggest that immunosuppressed patients could have more prolonged shedding while staying asymptomatic.40 Therefore, organ procurement team members as well as transplant physicians following transplant recipients could act as vectors for nosocomial spread, which underscores the importance of appropriate physical distancing, hygiene, handwashing, and personal protective equipment for all team members.
Practices by Transplant Centers in Response to the COVID-19 Pandemic
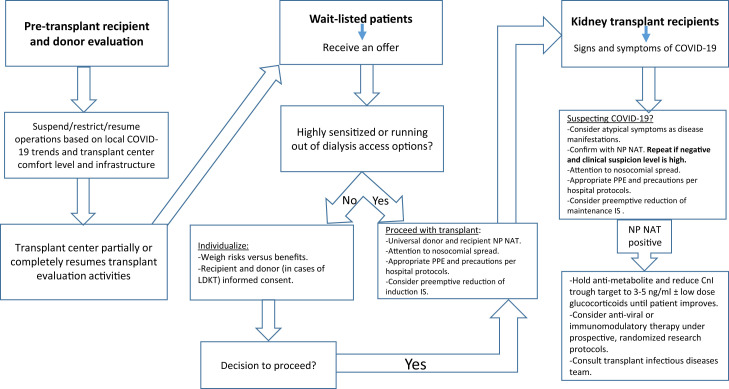
There has been significant heterogeneity among different kidney transplant program practices; however, the majority restricted their operations. In a survey of US centers by Boyarsky and colleagues, 72% and 24% of living donor transplant programs reported complete or partial suspension of activities, respectively.26 Only 20% of deceased donor programs continued with no restrictions.26 It is reasonable to defer DDKT for nonsensitized patients on the top of the list, as they will likely get more offers in the near future. We do not have this luxury however with the highly sensitized who might not get another opportunity soon enough. Therefore, an individualized approach to wait-listed patients would be inescapable (Fig 1 ).
Figure 1.
A proposed algorithm for approaching kidney transplant operations during the COVID-19 pandemic. Incorporate telemedicine wherever possible. CnI, calcineurin inhibitor; IS, immunosuppression; LDKT, living donor kidney transplantation; NP NAT, nasopharyngeal nucleic acid testing; PPE, personal protective equipment.
Most programs have adopted universal donor and recipient screening by nasopharyngeal (NP) nucleic acid testing (NAT) before transplantation. Only 17% had blood NAT available to rule out viremia.26 A study from Spain during the peak of the pandemic reported a moratorium on recipients and donors who had COVID-19 or a high exposure risk. However, they did consider infected donors 21 days after resolution of symptoms and only after viral clearance was confirmed.38 In another Chinese experience, all potential donors where screened with NP NAT in addition to chest CT. During their hospitalization, kidney transplant recipients received standard immunosuppression and wore surgical masks. If a patient was suspected with COVID-19, the patient would be isolated and only return to the regular transplant floor after CT chest and NAT NP came back negative.41
For posttransplant care of kidney transplant patients, 98% of centers limited in-person clinic visits, and 97% reported incorporating telemedicine instead.26
Impact on the Waiting List
Suspending kidney transplantation will prolong waiting times, so are we trading-off COVID-19–related mortality with mortality on the waiting list?38 , 42 It is still early to make this determination. Moreover, except for patients with end-stage kidney disease who are running out of dialysis access options, kidney transplantation remains a semi-elective procedure when we consider short-term outcomes. Between the magnitude of this pandemic, and its hopefully time-limited nature, it is reasonable to restrict and then gradually reinstate transplant activities based on the future COVID-19 trajectory. How this is executed will vary based on the geographic location of each transplant center.
Areas of Uncertainty
Several unanswered questions remain. When is it safe to accept kidneys from COVID-19 patients who have recovered?43 Is there a role for serologic testing? Does the presence of antibodies confer complete protection against reinfection? What is the optimal immunosuppression management strategy? What can we learn from allograft biopsies about the susceptibility of kidney tissue for direct infection by SARS-CoV-2? And finally, will COVID-19 be around until we have evidence-based answers to all these questions?
Conclusion
The COVID-19 pandemic has impacted transplantation and is associated with increased mortality among infected kidney transplant recipients. Without the availability of high-level evidence, most programs developed internal protocols to deal with new transplants and manage immunosuppression. Until effective therapies and a vaccine become available, we have to deal with this “new normal” while taking care of our susceptible patient population. It is imperative for transplant providers to have a low threshold for suspecting SARS-CoV-2 infection and promptly initiate appropriate evaluation and immunosuppression reduction (Fig 1).
Footnotes
Financial Disclosure: The author declares that he has no relevant financial interests.
References
- 1.Chavez S., Long B., Koyfman A., Liang S.Y. Coronavirus Disease (COVID-19): a primer for emergency physicians. The Am J Emerg Med. 2020;Mar 24 doi: 10.1016/j.ajem.2020.03.036. S0735-6757(20)30178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin X., Lian J.S., Hu J.H., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akalin E., Azzi Y., Bartash R., et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair V., Jandovitz N., Hirsch J.S., et al. COVID-19 in kidney transplant recipients. Am J Transpl. 2020;20(7):1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberici F., Delbarba E., Manenti C., et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagud-Marrahi E., Cofan F., Torregrosa J.V., et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transpl. 2020;May 5 doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira M.R., Mohan S., Cohen D.J., et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transpl. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United Network for Organ Sharing home page. http://www.unos.org Available from. Accessed May 12, 2020.
- 17.Abu Jawdeh B.G., Govil A. Acute kidney injury in transplant setting: differential diagnosis and impact on health and health care. Adv Chronic Kidney Dis. 2017;24(4):228–232. doi: 10.1053/j.ackd.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;Apr 13 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 21.Choi J., Aubert O., Vo A., et al. Assessment of tocilizumab (Anti-Interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transpl. 2017;17(9):2381–2389. doi: 10.1111/ajt.14228. [DOI] [PubMed] [Google Scholar]
- 22.Wan S.S., Ying T.D., Wyburn K., Roberts D.M., Wyld M., Chadban S.J. The treatment of antibody-mediated rejection in kidney transplantation: an updated systematic review and meta-analysis. Transplantation. 2018;102(4):557–568. doi: 10.1097/TP.0000000000002049. [DOI] [PubMed] [Google Scholar]
- 23.Moes D.J., Guchelaar H.J., de Fijter J.W. Sirolimus and everolimus in kidney transplantation. Drug Discov Today. 2015;20(10):1243–1249. doi: 10.1016/j.drudis.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Pascual J., Berger S.P., Witzke O., et al. Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol. 2018;29(7):1979–1991. doi: 10.1681/ASN.2018010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyarsky B.J., Chiang T.P., Werbel W.A., et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transpl. 2020;20(7):1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meziyerh S., Zwart T.C., van Etten R.W., et al. Severe COVID-19 in a renal transplant recipient: a focus on pharmacokinetics. Am J Transpl. 2020;20(7):1896–1901. doi: 10.1111/ajt.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishman J.A. The immunocompromised transplant recipient and SARS-CoV-2 infection. J Am Soc Nephrol. 2020;31(6):1147–1149. doi: 10.1681/ASN.2020040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah M.B., Lynch R.J., El-Haddad H., Doby B., Brockmeier D., Goldberg D.S. Utilization of deceased donors during a pandemic: an argument against using SARS-CoV-2 positive donors. Am J Transpl. 2020;20(7):1795–1799. doi: 10.1111/ajt.15969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du M., Cai G., Chen F., Christiani D.C., Zhang Z., Wang M. Multi-omics evaluation of gastrointestinal and other clinical characteristics of SARS-CoV-2 and COVID-19. Gastroenterology. 2020;158(8):2298–2301.e7. doi: 10.1053/j.gastro.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. 2020;31(8):1683–1687. doi: 10.1681/ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling Y., Xu S.B., Lin Y.X., et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34(2):75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durand C.M., Bowring M.G., Brown D.M., et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Ann Intern Med. 2018;168(8):533–540. doi: 10.7326/M17-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domínguez-Gil B., Coll E., Fernández-Ruiz M., et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transpl. 2020;May 2 doi: 10.1111/ajt.15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishman J.A., Grossi P.A. Novel Coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transpl. 2020;20(7):1765–1767. doi: 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Liu H., Buhler L.H., Deng S. Strategies to halt 2019 novel coronavirus (COVID-19) spread for organ transplantation programs at the sichuan academy of medical science and sichuan provincial people's hospital, China. Am J Transpl. 2020;20(7):1837–1839. doi: 10.1111/ajt.15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moeckli B., Peloso A., Oldani G., et al. The Swiss approach to the COVID-19 outbreak. Am J Transpl. 2020;20(7):1935–1936. doi: 10.1111/ajt.15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michaels M.G., La Hoz R.M., Danziger-Isakov L., et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transpl. 2020;20(7):1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]