Abstract
The coronavirus infection (COVID-19) has turned into a global catastrophe and there is an intense search for effective drug therapy. Of all the potential therapies, chloroquine and hydroxychloroquine have been the focus of tremendous public attention. Both drugs have been used in the treatment and prophylaxis of malaria. Long-term use of hydroxychloroquine is the cornerstone in the treatment of several auto-immune disorders. There is convincing evidence that hydroxychloroquine has strong in vitro antiviral activity against SARS-CoV-2. A few small uncontrolled trials and several anecdotal reports have shown conflicting results of such drug therapy in COVID-19. However, the results of preliminary large-scale randomized controlled trials have failed to show any survival benefit of such drug therapy in COVID-19. Despite the lack of such evidence, hydroxychloroquine has been used as a desperate attempt for prophylaxis and treatment of COVID-19. The drug has wide-ranging drug interactions and potential cardiotoxicity. Indiscriminate unsupervised use can expose the public to serious adverse drug effects.
Keywords: Chloroquine, Hydroxychloroquine, COVID-19, SARS-CoV-2, Coronavirus, Pandemic
1. Introduction
The coronavirus infection, which originated from Wuhan, China in December 2019, has turned into a global catastrophe [1]. The virus has been designated as severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and the disease caused by the agent as coronavirus disease 2019 (COVID-19) [2]. The World Health Organization (WHO) pronounced the disease as a pandemic on 11 March 2020 [3]. The world community has responded to the challenge with resilience and determination [4]. There was a major understanding of the disease and the pathogen in a matter of days and weeks rather than years and decades, which is an unprecedented occurrence in the history of medicine. This has led to the sharing of knowledge, preparedness measures to be implemented, containment measures to mitigate against the virus morbidity and mortality, and collaborative research to quickly address critical gaps in knowledge [5].
2. Drugs for COVID-19
There have been intensive attempts to explore drug therapy for the prophylaxis and treatment of SARS-CoV-2 infection during this COVID-19 pandemic [6], [7], [8], [9]. Several drugs have been identified based on their differing modes of action on the virus and various pathways it traverses, including: several antivirals (lopinavir/ritonavir combination, remdesivir and favipiravir); two antimalarials (chloroquine and hydroxychloroquine); ACE2 inhibitor (losartan); immunosuppressive agents (tocilizumab, leronlimab and corticosteroids); TMPRSS2 inhibitor (camostat mesylate); anti-parasitic drugs (ivermectin and nitazoxanide); a gold-containing drug, auranofin, an immunomodulator used in sepsis and leprosy (Sepsivac, mycobacterium w heat-killed injections); allogeneic PLacental eXpanded (PLX) cells; and convalescent plasma [6], [7], [8], [9], [10] (Table 1 ). Apart from Dexamethasone, there is currently no other medication or vaccine proven to be effective for the treatment or prevention of COVID-19 [11], [12], [13], [99].
Table 1.
List of potential drugs explored for the treatment of COVID-19.
| Class/Drug | Dose | Rationale | Trials |
|---|---|---|---|
| Antivirals | |||
| Lopinavir/ritonavir (LPV/RTV) | i. LPV 400 mg/RTV 100 mg BID PO x 14 d ii. LPV 400 mg/RTV 100 mg PO BID x 21 d iii. LPV 400 mg/RTV 100 mg PO x 14 d ± ribavirin (loading dose 4 g, 1.2 g x 8 hourly PO) |
HIV protease inhibitor In vitro activity vis-à-vis SARS-CoV and NERS-CoV No data vis-à-vis SARS-CoV-2 |
Randomized trial: not effective A cohort study and anecdotal experience: results inconsistent |
| Remdesivir | i. 200 mg IV x d1; 100 mg IV x d2–5 ii. 200 mg IV x d1; 100 mg IV x d2–10 iii. 200 mg IV x d1; 100 mg IV x daily up to 10 days |
Nucleoside analogue Broad-spectrum antiviral against coronaviruses |
Shortens the time to recovery in adults with no effect on mortality |
| Favipiravir (Avigan) | 200 mg tablets (1200 mg PO first dose; 400 mg PO x d1; 400 mg BID PO xd2–5) | Activity against RNA viruses and indicated in influenza resistant to Tamiflu It has a teratogenic effect |
Chinese non-randomized trial-effective |
| Antimalarials | |||
| Chloroquine (CQ) | 500 mg BID PO x 10 d | Immunomodulatory effect and reduce the production of cytokines. In vitro antiviral activity vis-à-vis SARS-CoV-2; HCQ is more potent and less toxic |
Chinese and French trials; non-randomized; results inconclusive Anecdotal reports Included for treatment and prophylaxis in protocols. Preliminary report from large scale randomized trial did not show any significant reduction in 28-day mortality. HCQ also show no beneficial effect in post-exposure prophylaxis against COVID-19 |
| Hydroxychloroquine (HCQ) | i. 400 mg BID PO x d1; 200 mg BID PO x d2–5 ii. 200 mg TID PO x 10 days (French trial) iii. 400 mg BID PO x d1; 400 mg PO once weekly x 3–7 wk (ICMR, prophylaxis) |
||
| Antihypertensive drug | |||
| Losartan | 50 mg QID POi | Hypothetical: may block ACE2 receptors and inhibit virus binding Can also upregulate ACE2, which may harm host |
Clinical trial underway |
| Immunosuppressive drugs | |||
| Tocilizumab | IV infusion: 4-8 mg/kg x 60 min; if needed repeat at 12 hr (max dose 800 mg) | Recombinant humanized monoclonal antibody against IL-6 receptor To treat cytokine storm syndrome |
Case study and series, rapid improvement in cytokine-related symptoms |
| Corticosteroids | Parenteral | Anti-inflammatory to treat extended cytokine response; treatment for ARDS and sepsis | Dexamethasone 6 mg once daily lowered 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support. Treat shock and/or ARDS |
| Antibiotic | |||
| Azithromycin | i. 500 mg QID PO x d1; 250 mg QID PO x d2–5 ii. 500 mg PO QID x 7 days iii. 500 mg PO x QID 5 days |
Macrolide and antibacterial immunomodulators downregulate inflammatory response; reduce cytokine production and inhibit cytokine actions No antiviral effect is known |
French trial as an adjunct to HCQ MERS-CoV: large retrospective analysis – no advantage |
| Convalescent plasma | Plasma from recovered COVI-19 patients | Convalescent COVID-19 patients may have high titre antibodies (titre > 1:320). | Trials to treat severe/life-threatening disease (not allowed for prevention) |
3. The Hype
Of all the potential therapies against SARS-CoV-2 infection, antimalarials – namely chloroquine (CQ) and hydroxychloroquine (HCQ) – have been the focus of tremendous public attention [14], [15], [16], [17], [18], [19]. There have been sharp differences of opinion as to the role of these two drugs in the prevention and treatment of SARS-CoV-2 infection. On one extreme, these drugs have been touted as the ‘biggest game-changers in the history of medicine’ [17], [20], while at the other end these drugs have been trolled as ‘useless and dangerous’ [18], [21], [22].
There have been interesting developments related to the role of CQ and HCQ for COVID-19 in the United States. Taking leads from the French trial [23], President Donald Trump has given several press briefings, including Tweets, in support of the drug. These have not been corroborated by his experts and the Food and Drug Administration (FDA). Despite this, on 29 March 2020, the FDA issued emergency authorization for the use of HCQ for hospitalized teen and adult patients with COVID-19 [24]. This move was supported by the White House, despite scant evidence. The US Government made huge quantities of HCQ available, procured from pharma companies and other countries, including India [24], [25]. Of late, the FDA has revoked the emergency use authorization for CQ/HCQ outside the clinical trials. India has also taken an interesting position on the role of CQ/HCQ for COVID-19. The National Task Force for COVID – constituted by the Indian Council for Medical Research on 22 March 2020 – recommended the use of HCQ for prophylaxis of SARS-CoV-2 infection for healthcare workers and household contacts of COVID-19 patients [15]. The decision was based on available preclinical and clinical data and anecdotal reports [15], [26]. Several events have surfaced amongst all of this controversy, mainly based on the hope that HCQ may be effective against the pandemic that is ravaging the world. Hoarding of these drugs has left a drug shortage in the market [27]. Patients with systemic lupus erythematosus and other autoimmune disorders who are on lifelong HCQ therapy find it difficult to get their daily supplies [28]. Several deaths have been reported due to the self-use of HCQ to prevent coronavirus infection and consequent drug-related cardiotoxicity [29], [30]. Pharmaceuticals have received bulk orders, and large quantities of the drug have been supplied to many countries, including the USA [31], [32].
4. Pharmacology of 4-aminoquinolines
Chloroquine was discovered in 1934 as an anti-malarial drug and is on the WHO Model List of Essential Medicines 2019 [33]. Hydroxyquinoline was developed during the Second World War and is more potent than CQ with less severe side effects [34]. Both CQ (C18H26ClN3; molecular mass 320 g/mol) and HCQ (C18H26ClN3O; molecular mass 336 g/mol) are 4-aminoquinolines (4AQs) [35]. HCQ differs from CQ by one hydroxy group (OH). They resemble each other in their pharmacokinetics, mode of action, indications, and type of drug toxicity. CQ is administered as phosphate, whereas HCQ is administered as sulfate. Given differing molecular weights of CQ and HCQ (320 versus 336, respectively) equivalent doses of the two drugs are different. 4AQs retinopathy occurs more often with CQ than HCQ [36]. HCQ is the only currently available molecule in the US market. However, CQ is available and continues to be used in most other countries. Both drugs are cheap, easily available and easy to administer [33]. Both CQ and HCQ are weak bases and occur as enantiomers (R and S isomers). After oral intake, the drugs are quickly absorbed in the upper intestinal tract, with high bioavailability (0.7‐0.8) and a large volume of distribution in the blood. The drug half-life is comparatively long (40–60 days). Both CQ and HCQ are lysosomotropic and get deposited in acidic vesicles – namely lysosomes and endosomes – and bind to melanin (skin and eyes). CQ and HCQ are 60% bound to plasma proteins and following administration are dealkylated in the liver via cytochrome p450 (CYP) into active metabolites. Both the parent drug and the active metabolites are excreted by the kidneys and faeces [35], [37] (Table 2 ).
Table 2.
Pharmacology of chloroquine and hydroxychloroquine.
| Parameter | Chloroquine | Hydroxychloroquine |
|---|---|---|
| Discovery year | 1934 | 1946 |
| Basic compound | 4-aminoquinoline | 4-aminoquinoline |
| Drug class | Anti-malarial | Anti-malarial |
| Drug formula | C18H26ClNO3 | C18H26ClNO3O |
| Molecular weight | 320 g/mol | 336 g/mol |
| Chemical nature | Weak base | Weak base |
| Salt for therapeutics | Phosphate | Sulfate |
| Availability | 250 mg (150 mg base); 500 mg (300 mg base) |
200 mg (155 mg base) |
| Brand name | Aralen (US) | Plaquenil (US) |
| Absorption | Upper intestinal tract; 2–4 hr; 89%; not affected by food |
Upper intestinal tract; 2–4 hr; 74%; not affected by food |
| Bioavailability | 0.7–0.8 | 0.7–0.8 |
| Distribution | Large: 60 000 L | Large: 47 257 L |
| Terminal half-life | 45 ± 15 d | 41 ± 11 d |
| Residence time | ≈ 900 h | ≈ 1300 h |
| Metabolism | Unmetabolized 62%; rest is dealkylated in liver; enzyme cytochrome 450; active metabolite desethylchloroquine 39% |
Unmetabolized 58%; rest is dealkylated in liver; enzyme cytochrome 450; active metabolites desethylchloroquine (18%) and desethylhydroxychloroquine (16%) |
| Clearance | Kidney (51%) and liver | Kidney (21%) and liver |
| Toxicity | ||
| Animal | 2–3 times more toxic than chloroquine (albino rats) | Safer |
| Cardiac | Same | Same |
| Ophthalmic | More (≈ 20% in 5–7 years) | Less (≈ 1% in 5–7 years) |
| Drug-drug interaction | Same | Same |
| Pregnancy and lactation | Safe | Safe |
| Indication | ||
| Malaria treatment | Yes | Yes |
| Malaria prophylaxis | Yes | Yes |
| Rheumatology | Not recommended | Drug of choice |
| Status for COVID-19 | ||
| In vitro antiviral activity | Less potent in vitro | Hydroxychloroquine is more potent in vitro than chloroquine |
| Treatment | Used in studies | Used in studies |
| Prophylaxis | - | Yes (ICMR) |
4.1. Indications of 4AQs
Both CQ and HCQ have been used for a long time in the treatment of malaria and are now being extensively used in rheumatic diseases. Despite the lack of evidence, hydroxychloroquine is being used as a desperate attempt for prophylaxis and treatment of COVD-19.
4.1.1. Malaria
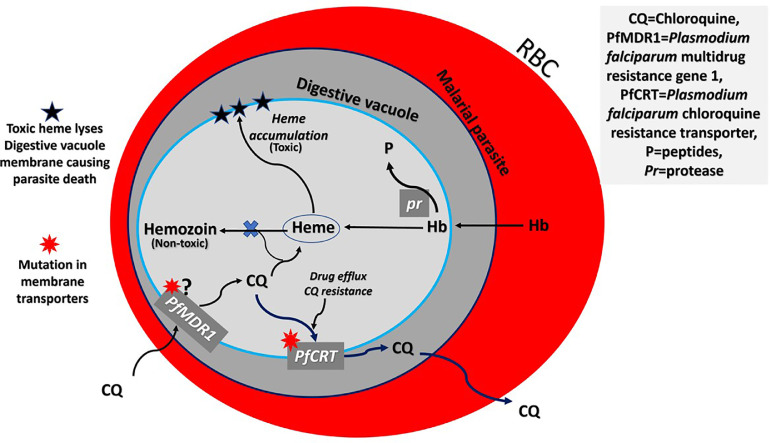
Both CQ and HCQ have been used in the treatment and prophylaxis of malaria [35], [38]. As antimalarials, they have marked schizonticidal and gametocidal activity and work against an asexual form of the malarial parasite in the stage of its life cycle within the red blood cells. The drugs do not act against the intrahepatic forms of the parasite. The mechanism of action of CQ and HCQ is related to their lysosomotropic property. The drugs accumulate within the food vacuole (lysosome-like organelle) of the parasite and prevent the conversion of toxic heme (released from digestion of hemoglobin by parasite proteases) into non-toxic hemozoin (the malarial pigment) (Figure 1 ). The parasite detoxifies heme in the food vacuole via a biocrystallization process in which heme is sequestered into large insoluble crystals, namely hemozoin. The drugs bind heme and prevent it from being incorporated into crystals. The accumulated free heme lyses membranes and leads to parasite death [39]. Of late, as a result of extensive mass use of these drugs, there has been an emergence and spread of resistance and its use has become limited to regions with no known resistance [40]. The chloroquine resistance is due to decreased accumulation of chloroquine in the food vacuole. Drug resistance is primarily mediated by mutant forms of the chloroquine resistance transporter (PfCRT), which cause efflux of chloroquine from the digestive vacuole [41], and possibly the multidrug resistance 1 (PfMDR1) gene [42]. These drugs are not recommended for the treatment of Plasmodium falciparum due to widespread resistance to it.
Figure 1.
Mode of action of chloroquine in malaria and the mechanism of chloroquine drug resistance. Chloroquine (CQ) accumulates in the food vacuole of the parasite. The drug inhibits the formation of hemozoin (non-toxic) from the heme (toxic) released by the digestion of hemoglobin (Hb). The accumulated heme lyses membranes and leads to parasite death. Chloroquine résistance is due to a decreased accumulation of chloroquine in the food vacuole. The drug resistance is primarily mediated by mutant forms of the chloroquine resistance transporter (PfCRT) that causes efflux of chloroquine from the digestive vacuole.
4.1.2. Rheumatic diseases
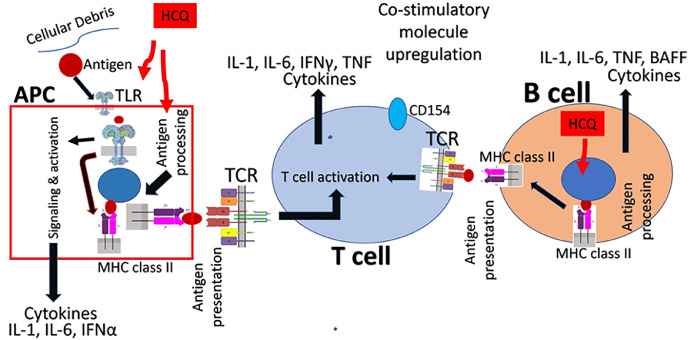
Antimalarial drugs currently have a major therapeutic role in Rheumatology. HCQ is preferred to CQ, as such patients need long-term therapy and HCQ has a lower incidence of retinopathy when compared with CQ [43]. HCQ is used in active rheumatoid arthritis (early mild disease or adjuvant therapy to other disease-modifying anti-rheumatic drugs – the DMARDs), systemic and discoid lupus erythematosus, Sjogren's syndrome, sarcoidosis, antiphospholipid syndrome, and photosensitive dermatosis [44], [45], [46], [47], [48], [49]. The drug has become a cornerstone in managing patients with systemic lupus erythematosus [50]. The therapeutic effect of HCQ in rheumatic disorders is related to inhibition of various processes in innate and adaptive immunity (Figure 2 ). The drug has an immunoregulatory effect and downregulates pro-inflammatory cytokines, namely: interleukin 1 (IL-1), interleukin-6 (IL-6), interferons (IFNα and IFNγ), tumor necrosis factor (TNF), and B-cell activating factor (BAFF). The drug is lysosomotropic and accumulates within lysosomes and endosomes and raises their pH. The drug inhibits lysosomal enzymes and inhibits autophagy pathway and endocytosis. This, in turn, downregulates autoantigen presentation (major histocompatibility complex (MHC) class II-mediated), T-cell activation, differentiation, and expression of co-stimulatory molecules (such as CD154) and release of cytokines. In endosomes, the drug prevents toll-like receptor (TLR) signaling and cGAS-STING signaling, and downregulates the production of proinflammatory cytokines [37], [51], [52].
Figure 2.
Basis of hydroxychloroquine (HCQ) use in rheumatic diseases. The drug in antigen processing cells (APC) – namely plasmacytoid dendritic cells, monocytes, macrophages, and B cells – interferes with toll-like receptor (TLR)-mediated activation, signaling and cytokine production. In APC such as plasmacytoid dendritic cells and B cells, the drug inhibits antigen processing and subsequent major histocompatibility complex (MHC) class II-mediated antigen presentation to T cells. This prevents T cell activation, production of proinflammatory molecules and reduces the production of cytokines. Abbreviations: IL-1, interleukin 1; IL-6, interleukin-6; IFNγ, interferons; TNF, tumor necrosis factor; BAFF, B-cell activating factor.
4.1.3. COVID-19
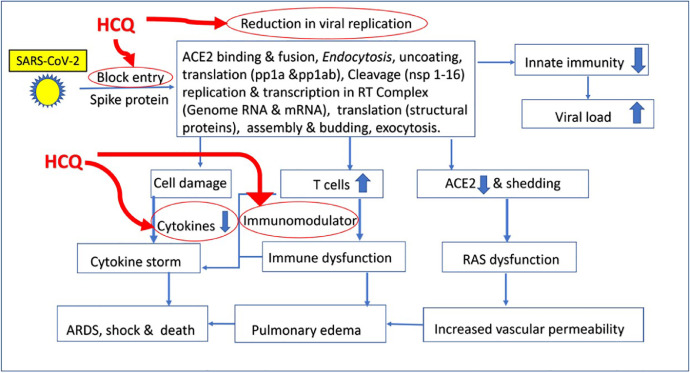
Both CQ and HCQ have several effects that can potentially prevent SARS-CoV-2 infection and also reduce its progression (Figure 3 ). The drugs may interfere with the entry of the virus into cells. Coronaviruses highjack the ACE2 receptors for its entry into the cell [53]. The SARS-CoV-2 receptor binding domain (RBD) has much more affinity (15-fold) to bind ACE2 compared with SARS-CoV RBD, resulting in much higher infectivity. Both drugs are known to interfere in the glycosylation of ACE2 [54]. This can make spike protein-ACE2 binding less efficient and impede the entry of the virus into the cells. The drugs are lysosomotropic, are weak bases, enter the cell organelle – namely acidic endosomes and lysosomes – and increase their pH [55]. This can interfere with viral activity in many ways. The virus fusion process within the host cell and replication can be prevented. Within antigen processing cells, drugs can interfere with antigen processing and MHC class II-mediated antigen presentation. This, in turn, can interfere with T-cell activation, expression of CD154, and downregulate cytokine production. Both drugs disrupt TLR-nucleic acid sensor cGAS and downregulate pro-inflammatory genes [37].
Figure 3.
Proposed sites of action of hydroxychloroquine in SARS-CoV-2 infection. The flow diagram shows stages of SARS-CoV-2 infection in the human host and subsequent mechanism of effects leading to target organ damage. The possible sites where HCQ may act are shown by the red arrows. Abbreviations: HCQ, hydroxychloroquine; ARDS, acute respiratory distress syndrome; ACE2 Angiotensin-converting enzyme 2; pp1a & pp1ab polyprotein 1a & polyprotein 1ab; nsp non-structural protein; RAS Renin-angiotensin system.
5. Systematic review
The use of CQ and HCQ in COVID-19 has been a focus of tremendous public attention. To scrutinize this, a systematic review was performed to identify studies where CQ and HCQ had been used to treat COVID-19. A PRISMA checklist was used to conduct a systematic review [56]. MEDLINE (National Library of Medicine, Bethesda, MD, USA) and EMBASE (Elsevier, New York, NY, USA) were searched from inception to 18 April 2020, using keywords chloroquine AND COVID-19, and hydroxychloroquine AND COVID-19, to find articles providing information on the efficacy and safety of these formulations in patients with SARS-CoV-2 infection. The search also included articles related to in vitro studies. There was no language barrier employed and searches were expanded using a snowballing method to retrieve relevant papers. Several clinical trial registries were also searched to identify published results from ongoing trials. Articles available in press and media were scrutinized to search the published source articles. Articles were also searched in preprint repositories, namely medRxiv (Cold Spring Harbor Laboratory), ResearchGate (Berlin, Germany), figshare (UK), etc. The systematic review protocol was not registered due to the urgency of the matter. Two contributors mentioned in the acknowledgements (AS, MK) independently screened the databases and the trial registries, and extracted relevant information. Discrepancies and doubts about the relevance of the sources were solved by consensus with inputs from all the three of us (AS, MK, MSK).
The initial search identified 293 articles (188 from PubMed, 80 EMBASE and 25 from other sources). Several trials are being undertaken, which include amongst others: a) WHO mega trial, b) Columbia University trial, c) University of Minnesota, New York, trial, and d) 23 registered ongoing trials in China. However, none of these trials’ results are currently available. Following the screening of titles and abstracts and removing duplicates, 17 articles about HQ and/or HCQ in coronavirus infection were evaluated (Table 3 ). This included four in vitro studies and 13 clinical trials. Two of these trials evaluated the prophylactic use of HCQ in COVID-19 [57], [58].
Table 3.
In vitro studies and clinical trials of chloroquine and hydroxychloroquine in SARS-CoV-2 infection.
| Author [reference] | Drug | Study group | Design/experiments | Outcome |
|---|---|---|---|---|
| In vitro studies | ||||
| Vincet [59] | CQ | Vero E6 cell model | SARS-CoV | Viral inhibition |
| Wang [60] | CQ | Vero E6 cell model | SARS-CoV-2 | Viral inhibition at entry and post-entry infection |
| Liu [61] | CQ, HCQ | Vero E6 cell model | SARS-CoV-2 | Viral inhibition, CQ more potent than HCQ |
| Yao [62] | CQ, HCQ | Vero E6 cell model | SARS-CoV-2 | Viral inhibition, HCQ more potent than CQ, HCQ dose estimation done |
| Therapeutic clinical trials | ||||
| Gao [63] | CQ, HCQ | 100 patients | Observational with historical controls | Inhibits pneumonia progression, improves lung function, shortens disease course |
| Gautret [23] | HCQ ± AZT | 42 patients | Observational with historical controls | Hastens viral clearance at day 6 (70% vs 12.5%), AZT enhances viral clearance |
| Gautret [65] | HCQ ± AZT | 80 patients | Observational | Viral clearance at day 7- 83%, hospital stay- 4.6 days |
| CHEN [66] | HCQ | 30 patients | Small randomized study | No effect on viral clearance at day 7 (86.7% vs 93.3%) |
| Chen [67] | HCQ | 62 patients | Randomized | Significant effect on time to clinical recovery, body temperature recovery time, and the cough remission time |
| Magagnoli [68] | HCQ, HCQ + AZT | 368 patients | Retrospective | Mortality HCQ 27%, HCQ + AZT 22.1%, controls 11.4%. Need for ventilation: no difference in three groups |
| Borba [69] | HCQ | 400 patients (interim analysis 80 patients) | Parallel double blind with two dosage regimens planned and terminated after interim analysis | Mortality higher (17%) with higher HCQ dosage regimen |
| Recovery trial [72] | HCQ | 4674 patients (interim analysis) | Large randomized controlled | 28 mortality 25.7% vs 23.5%, no effect on hospital stay |
| Tang [72] | HCQ | 150 patients | Randomized | Viral clearance day 28 (85.4% vs 81.3%) |
| Molina [70] | HCQ + AZT | 11 patients | Observational | Viral clearance at day 6: 20% |
| Mahevas [71] | HCQ | 181 patients | Observational with historical controls | Transfer to ICU within 7 days: 20.2% vs 22.1%, death 2.8% vs 4.8% |
| Prophylactic clinical trials | ||||
| Boulware [58] | HCQ | 821 asymptomatic with high-risk exposure | Large randomized double-blind study | Post-exposure incidence 11.8% vs 14.3% |
| Chatterjee [57] | HCQ (four doses) | Healthcare workers | Case control study | Significant decline in chances of getting infected (AOR 0.44; 95% CI 0.22–0.88) |
| CQ, chloroquine; HCQ, hydroxychloroquine; AZT, azithromycin | ||||
5.1. In vitro studies
There are several in vitro studies performed on Vero E6 cells that have evaluated the antiviral efficacy of CQ and HCQ against coronaviruses. All of these studies have shown strong antiviral activity of CQ and HCQ against coronaviruses. The authors believe that antiviral activity was due to the well-known lysosomotropic property of the drug, causing high endosomal pH and interfering with virus cell fusion. Besides, (CQ/HCQ) the drug was found to interfere with virus entry due to interference with the terminal glycosylation of ACE2. Vincent et al. [59] studied in vitro antiviral properties of CQ on SARS-coronavirus, namely SARS-CoV on a primitive Vero E6 model: CQ showed a strong inhibitory effect on the virus. Recently, Wang et al [60] showed low micromolecular concentrations of CQ to be highly effective with high selectivity index in blocking SARS-CoV-2 infection in vitro, both at entry and post-entry stages of infection. Seven drugs were tested in the experiments, which included ribavirin, penciclovir, favipiravir, nafamostat, nitazoxanide, remdesivir, and CLQ. Two of the seven drugs – namely remdesivir and CQ – potently blocked virus infection at low molecular concentration and showed a high selectivity index. The fact that the drugs demonstrated anti-viral effects at low concentrations could show a favorable clinical response in human infection. Anti-viral activity of CQ at entry and post-entry of infection suggests that drugs can be used both for prophylaxis as well as curative reasons. Liu et al. [61] compared the antiviral potency in vitro of CQ and HCQ against SARS-CoV-2, the causative agent of COVID-19. The results showed that HCQ was less potent than CQ in its anti-SARS-CoV-2 activity. However, Yao et al. [62] reported on the anti-SARS-CoV-2 activity of HCQ and CQ and found that HCQ was more potent than CQ in its antiviral activity. Based on a physiologically-based pharmacokinetic model, they calculated the therapeutic dose of HCQ as 400 mg given twice on the first day followed by 200 mg twice daily for 4 days for SARS-CoV-2 infection. A recent study has shown that CQ does not block SARS-CoV-2 infection of the TMPRSS2-positive lung cell line Calu-3 [100]. It is known that SARS-CoV-2 spike protein attaches to the ACE2 receptors and inserts its genetic material in to the cell. Next, the virus is absorbed in to endosomes. The green monkey kidney cells need cathepsin L for the virus to successfully infect them. In lung cells, however, an enzyme called TMPRSS2 (on the cell surface) is necessary. Cathepsin L requires an acidic environment to function and allow the virus to infect the cell, while TMPRSS2 does not. In the green monkey kidney cells, CQ/HCQ decrease the acidity, which then disables the cathepsin L enzyme, blocking the virus from infecting the monkey cells. In human lung cells, which have very low levels of cathepsin L enzyme, the virus uses the enzyme TMPRSS2 to enter the cell. TMPRSSR2 is not controlled by acidity, CQ/HCQ cannot block the SARS-CoV-2 from infecting the lungs or stop the virus from replicating. This is a possible explanation of lack of efficacy of CQ/HCQ in COVID-19 related lung disease in clinical trials.
5.3. Clinical studies
The above-mentioned in vitro studies have convincely shown that CQ and HCQ have strong antiviral activity against coronavirus. However, the same is not true for clinical trials. There is currently no published convincing randomized controlled trial to show that CQ and/or HCQ is beneficial in prophylaxis and/or treatment of SARS-CoV-2 infection. However, the results of six trials and anecdotal reports were evaluated.
Gao et al. [63] reported on the efficacy and safety of CQ or HCQ for the treatment of COVID-19-associated pneumonia in 100 patients enrolled from 10 hospitals. CQ/HCQ was superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus-negative conversion, and shortening the disease course. Severe adverse reactions to CQ/HCQ were not noted in the aforementioned patients. The authors supported their conclusions on an audio transcript of the news (in Chinese) briefing held by the State Council of China on 17 February 2020. Reference was also made of the Chinese Clinical Trial Registry, which enrolled these patients and which the authors had accessed on 18 February 2020. The evidence of such data in the trial registries was reviewed and none was available or found. Therefore, it is impossible to comment on or confirm the validity of the above observations, conclusions and recommendations.
Gautret et al. [23] recruited 42 patients with COVID-19 to evaluate the role of HCQ. Six of the treated patients were excluded (one death, three ICU admissions, one adverse drug reaction, and one refusal to therapy). Thirty-six patients were therefore included in the final analysis. This included 20 patients who received HCQ 200 mg TID PO. Six of the 20 patients in the HCQ group also received azithromycin to treat infections and supplement the antiviral activity of HCQ. Sixteen patients in the control group were recruited from other hospitals or those who refused drug treatment. Of the 36 patients who were evaluated, six had no symptoms, 22 complained of throat symptoms and eight had a pneumonic disease. The primary endpoint of the study was viral clearance (throat swab negative by RT-PCR) at the sixth day after inclusion. The secondary endpoint included serial viral load, clinical follow-up and adverse drug reactions. Viral clearance at day 6 (primary outcome) occurred in 14 (70%) patients in the HCQ-treated group compared with two (12.5%) in the control group (P < 0.001). HCQ-treated patients had faster viral clearance than the control group. All six patients for whom azithromycin was added to HCQ cleared the virus on day 6. This trial was fraught with major issues. Enrolment of the patients was not randomized, which is essential for making dependable comparisons. Six of the treated patients who had poor outcomes were excluded from the analysis, thus skewing the outcome. The protocol for virus testing was faulty, as viral clearance was declared only at 6 days and not beyond. Six patients received HCQ with azithromycin. Both drugs can cause prolonged QTc intervals and enhance the chances of deaths related to Torsade de Pointes (TdP) [64]. The paper did not undergo peer review and was published within 24 hours of submission.
Recently, Gautret et al. [65] reported on an observational study of 80 patients with relatively mildly infected patients with SARS-CoV-2 who received HCQ 200 mg TDS PO x 10 days plus azithromycin 500 mg QD PO x 1 day, followed by 250 mg QD PO x 4 days. One patient died and the second patient was admitted to ICU. All other patients had rapid clinical improvement, with a rapid fall in nasopharyngeal viral load (negative at day 7 in 83% and day 8 in 93%). Viral cultures from respiratory samples became negative in 97.5% by day 5. The mean hospital stay was 5 days. This study had a major limitation as it was an observational single-arm study, and without a comparative group who did not receive the drug, it is not possible to comment on the beneficial effects of the drug, especially in a cohort of patients with mild clinical disease.
Chen et al. [66] reported a pilot study on the role of HCQ in COVID-19 patients. The study was published in the Chinese journal J. Zhejiang Univ. (Med Sci) and only the abstract was available in English. The authors recruited 30 patients with COVID-19. Patients were randomized into two groups: 15 received 400 mg HCQ per day for 5 days along with supportive treatment, while another 15 patients received supportive treatment alone. The primary endpoint was negative throat swabs for SARS-CoV-2 by RT-PCR at day 7. One patient having HCQ treatment developed severe disease. There was no significant difference in the two groups in the percentage of viral clearance (86.7% vs. 93.3%; P > 0.05) or median duration of viral clearance (4 days vs. 2 days; P > 0.05). Both groups performed equally when assessed by clinical and radiologic parameters. The authors concluded that the prognosis of COVID-19 patients enrolled in this study was good and further studies need to be undertaken with a larger sample size to evaluate the role of HCQ in SARS-CoV-2 infection. The trial was published in Chinese and reproduced in English. The sample size was so small that the power of the study was very low. It is unknown whether the published data were peer-reviewed for ethical, scientific and statistical errors, etc.
Chen et al. [67] posted results of a randomized trial as a preprint in medRxiv. Sixty-two patients with COVID-19 were randomized into two groups: 31 patients received HCQ 400 mg QD x 5 days in addition to supportive treatment, while another 31 patients received supportive treatment alone. Patients in the HCQ group performed significantly better in time to clinical recovery, body temperature recovery time and cough remission time. Radiological improvement in pneumonia occurred in 25/31 (80.6%) in the HCQ group and 17/31 (54.8%) in the control group. Four patients progressed to severe illness and all belonged to the control group. It was concluded that HCQ treatment in COVID-19 shortened clinical recovery and promoted the absorption of pneumonia. It is difficult to evaluate the results of this trial as it was an open-labeled trial amenable to bias and had been published as a preprint and not gone through a review process.
Magagnoli et al. [68] performed a retrospective analysis of data from patients hospitalized with COVID-19 and posted the results as a preprint in medRxiv. A total of 368 patients were evaluated, who fell into three groups: HCQ alone (n = 97), HCQ plus azithromycin (n = 113), and no HCQ (n = 158). Two primary outcomes were evaluated: death and the need for mechanical ventilation. The death rates in HCQ alone, HCQ plus azithromycin and no HCQ were 27.8%, 22.1% and 11.4%, respectively. The need for mechanical ventilation occurred in 13.3%, 6.9% and 14.1% in the three groups, respectively. The death rates in the HCQ group were higher than the no HCQ group (P < 0.030), while there was no difference in the need for ventilation in three groups. In this study, the authors found no evidence that the use of HCQ, either with or without azithromycin, reduced the risk of mechanical ventilation in patients hospitalized with Covid-19. An association of increased overall mortality was identified in patients treated with HCQ alone. This large retrospective study in a large cohort of patients cast doubts on the efficacy of HCQ in COVID-19. The results of randomized controlled trials are highly anticipated to clarify this controversy.
The CloroCovid-19 study was initiated in Manaus, Amazonas, Brazil as a parallel, double-blind, randomized, phase IIb trial with two doses of CQ: high dose (600 mg CQ BID PO x 10 days or total dose 12 g) vs. low dose (450 mg BID PO x 1 day, followed by 450 mg QD PO for 4 days or total dose 2.7 g) [69]. All patients received ceftriaxone and azithromycin. Of a pre-defined 440 patients sample size, 81 patients were enrolled and the study had to be terminated. High-dose CQ was associated with a high occurrence of long QTc (25%) and higher mortality (17%) than the low dose. The fatality rate in patients on CQ was similar to those of historical controls not using CQ. One of the 14 patients who were tested showed negative RT-PCR for SARS-CoV-2 in respiratory samples on day 5.
The results from several therapeutic trials have recently been reported in the interim period [69], [70], [71], [72]. However, none of these have shown conclusive evidence for or against the use of HCQ in COVID-19. The two trials on the prophylactic role of HCQ to protect healthcare workers [57] or individuals with high-risk exposure [58] have shown contradictory results. A randomized, double-blind, placebo-controlled trial testing HCQ as postexposure prophylaxis was conducted [58]. The incidence of new illness compatible with Covid-19 did not differ significantly between participants receiving HCQ (49 of 414 [11.8%]) and those receiving placebo (58 of 407 [14.3%]). Side effects were more common with HCQ than with placebo (40.1% vs. 16.8%), but no serious adverse reactions were reported. The authors concluded that after high-risk or moderate-risk exposure to Covid-19, HCQ did not prevent occurrence of Covid-19. The trial had major limitation as the vast majority of the participants, including health care workers, were unable to access testing and diagnosis of COVID-19 was based on symptoms compatible with COVID-19. In contrast, ICMR conducted a case-control study to evaluate pre-prophylaxis role of HCQ in health-care workers [57]. It was concluded that consumption of four or more maintenance doses of HCQ was associated with a significant decline in the odds of getting infected (AOR: 0.44; 95% CI: 0.22-0.88); a dose-response relationship existed between frequency of exposure to HCQ and such reductions.
There have been several anecdotal reports about the efficacy of HCQ in COVID-19 available online and secondary to media reports. Reports from Jaipur, India, were of an Italian couple and two doctors with severe COVID-19 who received a combination of drugs – including lopinavir/ritonavir, oseltamivir and CQ – and made an eventual recovery [73], [74]. Medanta Hospital, New Delhi, reported on 14 Italian tourists with COVID-19 who were treated with lopinavir, azithromycin and CQ, and made an eventual recovery [75]. These and other anecdotal reports on the use of CQ are non-contributory. The drug was used in combination with antiviral drugs and there was no evidence that CQ was effective for SARS-CoV-2 infection in these patients.
It is believed that multiple well-designed and conducted randomized clinical trials are needed to evaluate the role of CQ/HCQ in SARS-CoV-2 infection. The studies should include patients with a broad range of clinical presentations, including asymptomatic carriers, mildly symptomatic, severe disease, and those with cytokine storm syndrome with acute respiratory distress syndrome (ARDS) and on ventilator support. Patients with cardiac and hepatic disease should be included in trials. The trials should have varying drug dosages. Trials should be randomized, blinded with placebo in the control arm, and should have an adequate sample size. The trials should be registered and under constant review for exceptional results for early termination. Also, trials need to be performed to evaluate the prophylactic role of chloroquine; they also need to be randomized controlled studies with adequate sample size. Once these trials are available, only then can the role of CQ and HCQ in SARS-CoV-2 infection be defined. At present there is so much speculation and it is impossible to make guesses as to the role of these drugs in COVID-19. As of June 1, 2020, there are a remarkable 203 COVID-19 registered trials with HCQ, 60 of which are focussed on prophylaxis. The Healthcare Worker Exposure Response and Out- comes of Hydroxychloroquine [HERO-HCQ] pre-exposure trial alone involves 15,000 health care workers [103]. The preliminary results of coveted RECOVERY Trial have been recently published as a preprint [101]. A total of 1542 patients hospitalised with COVID-19 were randomised to HCQ and compared with 3132 patients randomised to usual care alone. There was no significant difference in the primary endpoint of 28-day mortality (HCQ 25.7% vs. usual care 23.5%; hazard ratio 1.11 [95% confidence interval 0.98-1.26]; p = 0.10). There was also no evidence of beneficial effects on hospital stay duration or other outcomes. These data convincingly rule out any meaningful mortality benefit of HCQ in patients hospitalised with COVID-19. Recently WHO, based on solidarity trial interim results, has discontinued the trial’s HCQ arm [102]. The interim trial results showed that HCQ produce little or no reduction in the mortality of hospitalized COVID-19 patients when compared to standard of care.
5.4. Drug toxicity
Both CQ and HCQ have been widely used and had a reasonable margin of safety. The drugs function as immunomodulators and have no immunosuppressant activity [76]. Thus, the use of these drugs is not associated with a higher risk of infections and or cancers [37], [77]. Common adverse reactions include nausea, vomiting, diarrhea, and abdominal discomfort [78]. However, these drugs can cause cardiotoxicity, myopathy and retinopathy [79], [80], [81], [82] (Table 4 ).
Table 4.
Adverse drug reactions of hydroxychloroquine therapy.
| Common |
| Abdominal pain, anorexia, nausea, vomiting, diarrhea, headaches, emotional lability, skin reactions, tinnitus, dizziness, vertigo, alopecia, hair color changes |
| Uncommon |
| Hypoglycemia, bone marrow disorders, acute hepatic failure, angioedema, photosensitivity reaction, severe cutaneous adverse reactions (scars) |
| Caution |
| G6PD deficiency (hemolysis), moderate-to-severe hepatic impairment and renal impairment (monitor blood levels), alcoholism, psoriasis (severe flare-up of psoriasis), pregnancy and lactation (crosses placenta and secreted in milk; however, regarded as generally safe to use) |
| Major toxicity |
| Acute use: cardiotoxicity (long QT syndrome, Torsade de Pointes, sudden cardiac deaths) Chronic use: retinopathy, myopathy and neuropathy |
Of all the adverse drug reactions, cardiac toxicity has recently gained paramount importance. These drugs are known to prolong the QTc interval [79], [80], [83]. This is due to the drug-related block of the inward rectifier potassium ion channel (Kir2.1) [84]. This impairs ventricular repolarization, broadens cardiac action potential, and hence the long QT interval. Drug-induced QT/QTc per se is asymptomatic; however, it can lead to Torsade de Pointes (TdP), which is a potentially lethal polymorphic ventricular tachycardia. Patients with TdP present with episodes of near syncope, syncope with or without convulsions, and sudden cardiac death. Ventricular tachycardia in TdP has a characteristic initiation sequence. It starts with early afterdepolarization, which fires a premature ventricular beat followed by a long pause. Next, a sinus beat with a longer QT interval occurs. The T wave of the sinus beat is interrupted by a ventricular premature beat that is the first beat of the polymorphic ventricular tachycardia.
Myopathy and, to a lesser extent, neuropathy are well-documented complications of therapy with CQ/HCQ and other antimalarial agents [81]. CQ typically produces a vacuolar myopathy characterized by a progressive proximal weakness that rapidly resolves with discontinuation of the drug. Anti-malarial myopathy is an poorly recognized entity and should be suspected if patients on CQ/HCQ show elevated muscle enzymes. Muscle biopsy for histological examination and ultrastructural studies confirms the diagnosis [85]
Retinopathy is the most dreaded complication of CQ/HCQ [82]. Retinopathy is more commonly associated with CQ than HCQ. The drugs bind to the retinal pigment melanin and cause toxic retinal damage by disrupting lysosomal degradation in the retinal pigment epithelium. The disease causes bilateral paracentral visual field defects and depigmentation of the paracentral retinal pigment epithelium. Later there is progressive development of bull's eye maculopathy and paracentral scotoma, which may progress to severe visual loss. Factors that predispose to retinopathy include: a) prolonged therapy (> 5 years); b) a dose of > 5 mg/kg actual body weight per day; c) a high cumulative dose (> 600–1000 g); d) stage 3–5 chronic kidney disease; and e) co-medication with tamoxifen. Patients on long-term CQ and HCQ therapy need regular screening, as per protocol, to detect retinal toxicity at an early stage. The drug should be stopped at the first sign of toxicity.
5.5. Drug interactions
Both CQ and HCQ have major drug interactions that are clinically important. These are broadly classified into the following groups: a) drugs that cause QT prolongation and potentiate cardiotoxicity of CQ and HCQ; b) drugs that inhibit cytochrome 450 enzymes and increase drug levels and toxicity of CQ and HCQ; c) CQ and HCQ inhibit P-glycoprotein (P-gp), an energy dependent effluent transporter, and increase levels of drugs eliminated through P-gp; d) CQ and HCQ compete with the metabolism of other drugs and increase their bioavailability; e) CQ and HCQ absorption may be affected by drugs that bind it in the gut or alter stomach pH (Table 5 ). The CQ/HCQ drug interactions include several commonly prescribed drugs, and clinicians need to be aware of this. CQ and HCQ are generally safe in pregnancy. The drug crosses the placenta; however, there have been no adverse effects on the foetus. The drug is secreted in milk but causes no adverse effects on neonates. Overall, these drugs are considered safe to use during pregnancy and breastfeeding [86]. Both CQ and HCQ can cause hemolysis in patients with G6PD deficiency, and such patients on drugs to need close monitoring.
Table 5.
Chloroquine and hydroxychloroquine drug interactions.
| Drugs that cause QT interval prolongation | ||
| Macrolides (erythromycin, clarithromycin and azithromycin) | Have additive/synergistic effects on QT interval prolongation, increase chances of toxic arrhythmias, polymorphic ventricular fibrillation and death | |
| Quinolones (ciprofloxacin and levofloxacin) | ||
| Anti-arrhythmic (amiodarone and sotalol) | ||
| Antifungal (ketoconazole and fluconazole) | ||
| Antidepressants (amitriptyline and dothiepin) | ||
| Anti-emetics (ondansetron, granisetron and dolasetron) | ||
| Drugs that inhibit cytochrome 450 enzyme | ||
| Cimetidine | Increases CQ and HCQ levels and possible toxicity | |
| Diltiazem and verapamil | ||
| Fluoxetine (Prozac), paroxetine (Paxil) | ||
| Metronidazole (Flagyl) | ||
| Drugs that are eliminated through the P-glycoprotein (P-gp) eliminator pathway (CQ and HCQ Inhibit of P-gp) | ||
| Digoxin | Increases serum levels of digoxin and ciclosporin and needs close monitoring | |
| Ciclosporin | ||
| Drugs that compete with the metabolism of CQ and HCQ | ||
| Metoprolol | Increases bioavailability of metoprolol | |
| Tamoxifen | Increases chances of retinopathy | |
| Methotrexate | Reduces absorption of methotrexate and reduces methotrexate hepatotoxicity | |
| Drugs which reduce the absorption of CQ and HCQ either by binding or altering gastric pH | ||
| Antacids, kaolin and proton pump inhibitors | Reduce absorption of CQ and HCQ; maintain a 4-hour period between intake of two classes of drugs | |
Azithromycin is a macrolide that has been known to cause prolongation of the QT/QTc interval and a higher risk of cardiac death [87], [88]. The FDA has issued a warning that azithromycin can lead to potentially fatal arrhythmias and advised to discourage the use of this antibiotic among patients with underlying heart disease and/or those with known electrolyte imbalance [89]. Therefore, the concomitant use of CQ or HCQ and azithromycin, as has been used in a French trial, puts patients at a higher risk of cardiotoxicity and should be avoided [23], [59], [90]. It is believed that if antibiotic/antibiotics are indicated in patients on CQ and/or HCQ, an alternative antibiotic with no cardiac effects and drug interaction should be chosen.
Other drugs used in managing COVID-19 patients may show drug interaction with CQ/HCQ [91]. Lopinavir/ritonavir strongly inhibits CYP3A4 and has a large number of significant drug interaction concerns. Remdesivir in vitro studies appear to be a substrate for the drug-metabolizing enzymes CYP2C8, CYP2D6 and CYP3A4, as well as a substrate for organic anion transporting polypeptides 1B1 (OATP1B1) and P-glycoprotein (P-gp) transporters. The clinical relevance of these in vitro assessments has not yet been established, and whether remdesivir would be a clinically relevant victim or perpetrator of drug interactions is still unknown. Interleukin-6 pathway inhibitors such as tocilizumab, sarilumab and siltuximab are being studied for their potentially beneficial ability to limit the cytokine response that may be seen in some patients with COVID-19. One unique drug interaction consideration with these drugs is their effect on drug metabolism.
6. Clinical guidelines for the safe use of HCQ in COVID-19
Although HCQ has had a substantial margin of safety [83], [92], its use in critically sick patients like those with COVID-19 has caused several instances of serious cardiac events and deaths [21]. These events are more likely to occur under three circumstances: a) with higher doses of the drug [69]; b) with concomitant use of azithromycin, which potentiates the effect on QT interval [64], [87]; and c) in patients who have underlying co-morbid conditions, which can predispose such patients to long QT intervals and TdP [80], [83]. A 21-point risk score based on 10 parameters to predict QT interval prolongation has been developed and validated [93]: patients with low risk (≤ 6) have a 15% chance of long QT interval, which increases to 37% with a moderate-risk (7–10) score and 73% in those with a high-risk score (≥ 11).
It has been proposed that a stepwise action plan be followed while HCQ is used in patients with COVID-19 (Table 6 ) [93], [94], [95]. This takes into account drug allergy and the possibility of underlying congenital long QT syndrome; both of which are an absolute contraindication to HCQ therapy. As HCQ has drug interactions with many commonly used drugs, these need to be identified and the non-essential drug should be stopped during HCQ therapy. Here, concomitant use of azithromycin is of great significance and if the two drugs are used together, patients need to be followed intensively for long QT syndrome. A patient's risk score for QTc prolongation should be calculated and if the score is ≥ 11, the chances of long QTc are substantial and the drug is contraindicated. It is recommended that all COVID-19 patients who are potential candidates for HCQ therapy should have their QTc interval determined. A QTc interval of ≥ 500 ms is a contraindication for HCQ therapy. While the patient is on the drug, determination of QTc by telemetry or interval ECG should be performed and drug dosage altered or stopped as per protocol. Patients should have a correction of electrolyte imbalance and the use of loop diuretics should be monitored to reduce the chances of cardiotoxicity. In the case of TdP with stable tachycardia developing following HCQ, magnesium sulfate 1–2 g IV over 15 minutes is indicated. If patients do not respond, isoproterenol 2–10 mcg/minute infusion or pacing to a rate of 100–120 depolarizations/minute, as required to suppress PVC, usually terminates TdP [93].
Table 6.
Step-wise actions to be followed for starting patients on chloroquine or hydroxychloroquine.
| Purpose: To prevent long QTc interval, Torsade de Pointes causing polymorphic ventricular tachycardia and sudden cardiac deaths. | ||
|---|---|---|
| Step | Particulars | Action |
| 1 | Check: known drug allergy congenital long QT syndrome (occurs in 1:7000) |
Drug contraindicated |
| 2 | Check: drug interaction refer to Table 4, Table 5 for a list of drugs that have a drug interaction with CQ or HCQ |
Stop non-essential drugs that have a drug interaction |
| 3 | Check: the risk score for QTc prolongation total score: 21. Score ≤ 6 (risk low, chance 15%); 7–10 (risk moderate, chance 37%); ≥ 11 (risk high, chance 73%) Risks include: age ≥ 68 years - 1, female gender - 1, concomitant loop diuretic - 1, serum K+ ≤ 3.5 mEq/L - 2, admission QTc ≥ 450 ms - 2, acute MI - 2, sepsis - 3, heart failure - 3, one QTc-prolonging drug - 3, ≥ 2 QTc-prolonging drugs – 3, additional points i.e. 6 |
Drug contraindicated when risk is high (score ≥ 11) |
| 4 | Calculate baseline QTc: {QT interval - start of the Q wave to the end of the T wave; QTc = QT / √ RR, use an app to calculate QTc. Normal ≤ 430 ms (male)/≤ 450 ms (female)}(75) |
Drug contraindicated if baseline QTc ≥ 500 ms |
| 5 | Monitor: serum K+ and Mg+ use of loop diuretics |
Correct electrolyte imbalance |
| 6 | Plan: interval ECG (12 hourly) or cardiac telemetry in sick patients interval ECG in ambulatory subjects |
Reduce dose if QTc prolongs, stop the drug if QTc ≥ 500 ms |
| In case of Torsade de Pointes: stable tachycardia - give magnesium sulfate 1–2 g IV x 15 min not responsive - give isoproterenol 2–10 mcg/min infusion or pacing to a rate of 100–120 depolarizations/minute as required to suppress PVC, usually terminates Torsade de Pointes |
- | |
Tisdale JE, et al. Circ Cardiovasc Qual Outcomes 2013;6:479–87.
6.1. The scenario as of today
The role of antimalarial drugs – namely CQ and HCQ – in the management of COVID-19 patients is a dynamic phenomenon and will rapidly change as soon as the results of randomized drug trials are available. Few facts are currently clear. Both CQ and HCQ have the potential to have antiviral properties based on the site of action. These drugs are immunomodulators and downregulate cytokine production. Both these actions can mitigate the effects of SARS-CoV-2 virus in target organs, namely the lungs, heart, liver, and gut. There is convincing evidence in vitro that both CQ and HCQ have strong antiviral properties. The million-dollar question is whether these in vitro studies will transform into a clinical response [96], [97]. The results of preliminary large-scale randomized controlled trials have failed to show any survival benefit of such drug therapy in COVID-19 [101].[102]. Based on these results, as of today, most experts are against the use of HCQ in treatment and post-exposure prophylaxis of COVID-19. Inspite of this, the advocacy and widespread use of HCQ has been based on fear of SARS-CoV-2 infection and media and social forces rather than medical evidence and/or the global COVID-19 research agenda [103]. The second question which needs an answer is how safe are these drugs in a setting of COVID-19? Both CQ and HCQ have a narrow margin of safety. Thus, indiscriminate unsupervised use of these drugs as a prophylactic or therapeutic weapon can cause serious side effects, mostly related to cardiotoxicity [98]. It is believed that supervised use of CQ as a prophylactic agent in high-risk populations advocated by ICMR is a safe and appropriate step [57], [58]. If a drug shows good results, it will be a huge weapon with which to block the transmission of the virus. If the results are otherwise, the results could lead the direction on the use of antimalarials in COVID-19.
Funding: Professor MS Khuroo received grants from Dr. Khuroo Medical Trust (Registration number 273 CD 314), sponsored by Dr. Khuroo's family meant to help the needy, promote medical education and support cutting-edge scientific research.
Acknowledgements
The author is grateful to Ahmad A. Sofi (Burn Hall School, Srinagar) and Mohammad Khuroo (Indian School Al-Ghubrah, Muscat, Oman) for significant contributions in the preparation of this review. They were involved in software, resources, formal analysis, data curation, and conducted systematic review of articles about HCQ in COVID-19. They drew the sketches which formed basis of figures of the article.
References
- 1.Gates B. Responding to Covid-19 - A Once-in-a-Century Pandemic. New Engl J Med. 2020 doi: 10.1056/NEJMp2003762. https://www.ncbi.nlm.nih.gov/pubmed/32109012 [updated Feb 28]. Available from: [DOI] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; Geneva, Switzerland: 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(COVID-2019)-and-the-virus-that-causes-it [updated March 11Available from: [Google Scholar]
- 3.WHO . World Health Organization; Geneva, Switzerland: 2020. Coronavirus disease (COVID-19) Pandemic: World Health Organization.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [updated March 11]. Available from: [Google Scholar]
- 4.Legido-Quigley H, Asgari N, Teo YY, Leung GM, Oshitani H, Fukuda K, et al. Are high-performing health systems resilient against the COVID-19 epidemic. Lancet. 2020;395(10227):848–850. doi: 10.1016/S0140-6736(20)30551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebrahim SH, Ahmed QA, Gozzer E, Schlagenhauf P, Memish ZA. Covid-19 and community mitigation strategies in a pandemic. BMJ. 2020;368:m1066. doi: 10.1136/bmj.m1066. [DOI] [PubMed] [Google Scholar]
- 6.Smith T, Bushek J, Prosser T. Elsevier; Amsterdam, The Netherlands: 2020. COVID-19 Drug Therapy – Potential Options. Clinical Drug Information | Clinical Solutions.https://www.elsevier.com/__data/assets/pdf_file/0007/988648/COVID-19-Drug-Therapy_Mar-2020.pdf [updated March 26, 2020. Available from: [Google Scholar]
- 7.Anonymous. Life Sciences; New York, USA: 2020. Treatments for COVID-19: Drugs being tested against the coronavirus.https://www.livescience.com/coronavirus-covid-19-treatments.html [updated March 23]. Available from: [Google Scholar]
- 8.CDC . Centers for Disease Control and Prevention; Atlanta, Georgia, USA: 2020. Information for Clinicians on Therapeutic Options for COVID-19 Patients.https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html [updated March 21Available from: [Google Scholar]
- 9.Diederich S. EurekAlert, AAAS; Washington, DC, USA: March 5, 2020. Preventing spread of SARS coronavirus-2 in humans: Göttingen infection researchers identify potential drug.https://www.eurekalert.org/pub_releases/2020-03/dpp-pso030520.php Available from: [Google Scholar]
- 10.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . World Health Organization; Geneva, Switzerland: 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [updated March 13 Available from: [Google Scholar]
- 12.CDC . Centers for Disease Control and Prevention; Atlanta, Georgia, USA: 2020. Interim Clinical Guidance for Management of Patients with Coronavirus infection (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [updated March 7Available from: [Google Scholar]
- 13.FDA . Food & Drug Administration; MD, USA: 2020. Coronavirus Disease 2019 (COVID-19)https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/coronavirus-disease-2019-covid-19 [updated March 28Available from: [PubMed] [Google Scholar]
- 14.Cortez MF, Che C. Bloomberg; London, UK: 2020. Malaria Drug Chloroquine No Better Than Regular Coronavirus Care, Study Finds.https://www.bloomberg.com/news/articles/2020-03-25/hydroxychloroquine-no-better-than-regular-covid-19-care-in-study [updated March 25Available from: [Google Scholar]
- 15.Kumar C. Business Today; Lower Parel, Mumbai: 2020. Coronavirus outbreak: ICMR recommends the use of hydroxy-chloroquine for critical COVID-19 cases.https://www.businesstoday.in/current/economy-politics/coronavirus-outbreak-icmr-recommends-use-hydroxy-chloroquine-critical-covid-19-cases/story/399005.html [updated March 23 Available from: [Google Scholar]
- 16.Goodman J. BBC; London, UK: 2020. Coronavirus and chloroquine: Has it been approved in US.https://www.bbc.com/news/51980731 Reality Check [updated March 21]. Available from: [Google Scholar]
- 17.Schneider L. For Better Science; Germany: March 26, 2020. Chloroquine genius Didier Raoult to save the world from COVID -19.https://forbetterscience.com/2020/03/26/chloroquine-genius-didier-raoult-to-save-the-world-from-covid-19/ Available from: [Google Scholar]
- 18.Haseltine WA. Forbes; New Jersey, USA: 2020. Hydroxychloroquine Is Ineffective In Treatment Of Patients Hospitalized With Covid-19, According To Small Controlled Trial From Shanghai.https://www.forbes.com/sites/williamhaseltine/2020/03/25/hydroxychloroquine-is-ineffective-in-treatment-of-patients-hospitalized-with-covid-19-according-to-small-controlled-trial-from-shanghai/#5f03cad96092 [updated March 25Available from: [Google Scholar]
- 19.Acharjee S. India Today Insight. India Today; Noida, Uttar Pradesh, India: March 24, 2020. Covid-19: The bitter truth about using hydroxychloroquine as a preventive drug.https://www.indiatoday.in/india-today-insight/story/covid-19-the-bitter-truth-about-using-hydroxychloroquine-as-a-preventive-drug-1659116-2020-03-24 Available from: [Google Scholar]
- 20.McLaughlin EC. CNN Health; New York, USA: 2020. Chloroquine and hydroxychloroquine: what to know about the potential coronavirus drugs.https://edition.cnn.com/2020/03/23/health/chloroquine-hydroxycholoroquine-drugs-explained/index.html updated March 24Available from: [Google Scholar]
- 21.Guastalegname M, Vallone A. Could chloroquine /hydroxychloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanath A. 2020. Coronavirus crisis: A doctor explains why malaria drugs can’ protect you from Covid-19: Scroll in [March 23.https://scroll.in/pulse/957018/explainer-malaria-drugs-cant-protect-you-from-covid-19-there-is-no-scientific-evidence-ye Available from: [Google Scholar]
- 23.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Diamond D. March 29, 2020. Coronavirus: FDA issues emergency authorization of anti-malaria drug for coronavirus care: Politico, Arlington County, Virginia, USA.https://www.politico.com/news/2020/03/29/fda-emergency-authorization-anti-malaria-drug-155095 Available from: [Google Scholar]
- 25.Richardson V. The Washington Times; Washington, DC, USA: March 30, 2020. FDA gives emergency approval for antimalarial drugs in treating coronavirus patients.https://www.washingtontimes.com/news/2020/mar/30/fda-gives-emergency-approval-antimalarial-drugs-tr/ Available from: [Google Scholar]
- 26.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natarajan D. 24 March 2020. Indians Are Buying Chloroquine to Fight the Coronavirus. They Should Not Be.https://science.thewire.in/health/coronavirus-covid-19-chloroquine/ Available from: [accessed. [Google Scholar]
- 28.Owens B. Excitement around hydroxychloroquine for treating COVID-19 causes challenges for rheumatology. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman B. 20 March 2020. Man dies after self-medicating with chloroquine phosphate.https://www.axios.com/chloroquine-coronavirus-death-09c91a91-4fe7-472c-9de9-79b890aa8fff.html Available from: [accessed. [Google Scholar]
- 30.Anonymous. Assam doctor dies after taking an anti-malaria drug to prevent coronavirus. https://www.sentinelassam.com/guwahati-city/assam-doctor-dies-after-taking-anti-malaria-drug-to-prevent-coronavirus/.
- 31.Chandna H. 22 March 2020. Indian firms Ipca, Zydus Cadila get big US orders for chloroquine to fight COVID-19.https://theprint.in/world/indian-firms-ipca-zydus-cadila-get-big-us-orders-for-chloroquine-to-fight-covid-19/385859/ Available from: [accessed 22 March 2020] [Google Scholar]
- 32.Mahase E. Covid-19: six million doses of hydroxychloroquine donated to the US despite lack of evidence. BMJ. 2020;368:m1166. doi: 10.1136/bmj.m1166. [DOI] [PubMed] [Google Scholar]
- 33.WHO . World Health Organization; Geneva: 2019. Model List of Essential Medicines, 21st List, 2019.https://apps.who.int/iris/handle/10665/325771?show=full License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 34.McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(1A):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 35.Tanenbaum L, Tuffanelli DL. Antimalarial agents. Chloroquine, hydroxychloroquine, and quinacrine. Arch Dermatol. 1980;116(5):587–591. doi: 10.1001/archderm.116.5.587. [DOI] [PubMed] [Google Scholar]
- 36.Kishimoto M, Deshpande GA, Yokogawa N, Jbuyon JP, Okada M. Use of Hydroxychloroquine in Japan. J Rheumatol. 2012;39(6):1296. doi: 10.3899/jrheum.111569. [DOI] [PubMed] [Google Scholar]
- 37.Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 38.Moore T. In: Harrison's Principles of Internal Medicine Volume 1. 20th ed. Jameson JLF, Kasper AL, Hauser D, Longo SL, Loscalzo DN, editors. MacGraw Hill; New York, USA: 2020. Agents Used to Treat Parasitic Diseases; pp. 1556–1568. [Google Scholar]
- 39.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79(1):55–87. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 40.Antony HA, Parija SC. Antimalarial drug resistance: An overview. Trop Parasitol. 2016;6(1):30–41. doi: 10.4103/2229-5070.175081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ecker A, Lehane AM, Clain J, Fidock DA. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012;28(11):504–514. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, White NJ, et al. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother. 1999;43(12):2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finbloom DS, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985;12(4):692–694. [PubMed] [Google Scholar]
- 44.Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon C, Amissah-Arthur MB, Gayed M, Brown S, Bruce IN, D'Cruz D, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford) 2018;57(1):e1–e45. doi: 10.1093/rheumatology/kex286. [DOI] [PubMed] [Google Scholar]
- 46.Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vivino FB, Carsons SE, Foulks G, Daniels TE, Parke A, Brennan MT, et al. New Treatment Guidelines for Sjogren's Disease. Rheum Dis Clin North Am. 2016;42(3):531–551. doi: 10.1016/j.rdc.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett ML. Antimalarials in dermatology. Curr Probl Dermatol. 2000;12:257–259. [Google Scholar]
- 49.Cherney K, Carter A. Rheumatoid Arthritis Medication List. Healthline. 2016 July 8. [Google Scholar]
- 50.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 51.Minie AJM, Sasaki T, Woodward JJ, Elkon KB. Antimalarial Drugs as Immune Modulators: New Mechanisms for Old Drugs. Annu Rev Med. 2017;68:317–330. doi: 10.1146/annurev-med-043015-123453. [DOI] [PubMed] [Google Scholar]
- 52.Wallace DJ, Linker-Israeli M, Hyun S, Klinenberg JR, Stecher V. The effect of hydroxychloroquine therapy on serum levels of immunoregulatory molecules in patients with systemic lupus erythematosus. J Rheumatol. 1994;21(2):375–376. [PubMed] [Google Scholar]
- 53.Kannan S, Shaik Syed Ali P, Sheeza A, Hemalatha K. COVID-19 (Novel Coronavirus 2019) - recent trends. Eur Rev Med Pharmacol Sci. 2020;24(4):2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 54.Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet. 1996;31(4):257–274. doi: 10.2165/00003088-199631040-00003. [DOI] [PubMed] [Google Scholar]
- 56.Khuroo MS, Khuroo NS, Khuroo MS. Diagnostic accuracy of point-of-care tests for hepatitis C virus infection: a systematic review and meta-analysis. PloS one. 2015;10(3) doi: 10.1371/journal.pone.0121450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee P, Anand T, Singh KJ, Rasaily R, Singh R, Das S, et al. 2020 June 13. Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19 2020 [updated Cited.http://www.ijmr.org.in/preprintarticle.asp?id=285520 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in the treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 64.Simpson TF, Kovacs RJ, Stecker EC. 2020. Ventricular Arrhythmia Risk Due to Hydroxychloroquine-Azithromycin Treatment For COVID-19: Am Coll Cardiol.https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19?fbclid=IwAR1Him9RU1NWw2hGeYKAVt9ojULup1wlq-KPAni3U39UXq68eTtlsQGPwIA Available from: [accessed 29 March 2020] [Google Scholar]
- 65.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Jun LD, Liu L, Liu P, Xu Q, Xia L, Ling Y, et al. A pilot study of hydroxychloroquine in the treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49(1) doi: 10.3785/j.issn.1008-9292.2020.03.03. 0- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Z, Hu P, Zhang Z, Jiang S, Han S, Yan D, et al. 2020. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial.https://doiorg/101101/2020032220040758 [Google Scholar]
- 68.Magagnoli J, Narendran S, Pereira F, Cummings MJ, Hardin JW, Sutton S, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv preprint. 2010 doi: 10.1016/j.medj.2020.06.001. https://doiorg/101101/2020041620065920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borba MG, Sobo C-T. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with the severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study) medRxiv preprint. 2020 https://doiorg/101101/2020040720056424 [Google Scholar]
- 70.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahevas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020 2020.04.10.20060699. [Google Scholar]
- 72.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open-label, randomized controlled trial. BMJ. 2020 doi: 10.1136/bmj.m1849. 369:m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anonymous. Rajasthan turns into a learning ground for the treatment of corona. Times of India. 2020 21 March. [Google Scholar]
- 74.Anonymous. Rajasthan: Two Covid-19 patients from Bhilwara critical. Jaipur News Times of India. 2020 22 March. [Google Scholar]
- 75.Chandra H. Paracetamol, chloroquine & Google translator: How Medanta treated Italians with coronavirus. The Print. 2020 25 March. [Google Scholar]
- 76.WHO . World Health Organization Malaria Policy Advisory Committee Meeting; Geneva: March 22-24, 2017. The cardiotoxicity of antimalarials.https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-se Available from: [Google Scholar]
- 77.Lazarus MN, Robinson D, Mak V, Moller H, Isenberg DA. Incidence of cancer in a cohort of patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2006;45(8):1012–1015. doi: 10.1093/rheumatology/kei281. [DOI] [PubMed] [Google Scholar]
- 78.Srinivasa A, Tosounidou S, Gordon C. Increased Incidence of Gastrointestinal Side Effects in Patients Taking Hydroxychloroquine: A Brand-related Issue. J Rheumatol. 2017;44(3):398. doi: 10.3899/jrheum.161063. [DOI] [PubMed] [Google Scholar]
- 79.Costedoat-Chalumeau N, Hulot JS, Amoura Z, Leroux G, Lechat P, Funck-Brentano C, et al. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford) 2007;46(5):808–810. doi: 10.1093/rheumatology/kel402. [DOI] [PubMed] [Google Scholar]
- 80.Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf. 2018;41(10):919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 81.Abdel-Hamid H, Oddis CV, Lacomis D. Severe hydroxychloroquine myopathy. Muscle Nerve. 2008;38(3):1206–1210. doi: 10.1002/mus.21091. [DOI] [PubMed] [Google Scholar]
- 82.Jorge A, Ung C, Young LH, Melles RB, Choi HK. Hydroxychloroquine retinopathy - implications of research advances for rheumatology care. Nat Rev Rheumatol. 2018;14(12):693–703. doi: 10.1038/s41584-018-0111-8. [DOI] [PubMed] [Google Scholar]
- 83.WHO. The cardiotoxicity of antimalarials. WHO Evidence Review Group Meeting, 13–14 October 2016, Varembé Conference Centre, Geneva, Switzerland [March 22-24, 2017]. Geneva: World Health Oranization. Available from: WHO/HTM/GMP/MPAC/2017.2.
- 84.Rodriguez-Menchaca AA, Navarro-Polanco RA, Ferrer-Villada T, Rupp J, Sachse FB, Tristani-Firouzi M, et al. The molecular basis of chloroquine block of the inward rectifier Kir2.1 channel. Proc Natl Acad Sci U S A. 2008;105(4):1364–1368. doi: 10.1073/pnas.0708153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casado E, Gratacos J, Tolosa C, Martinez JM, Ojanguren I, Ariza A, et al. Antimalarial myopathy: an underdiagnosed complication? A prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65(3):385–390. doi: 10.1136/ard.2004.023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arguin PM, Mali S. Centers for Disease Control and Prevention; 2011. Infectious diseases related to travel.http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/malaria.htm Malaria. Atlanta, Georgia, USANov 08. Available from: [Google Scholar]
- 87.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu ZK, Yuan J, Li M, Sutton SS, Rao GA, Jacob S, et al. Cardiac risks associated with antibiotics: azithromycin and levofloxacin. Expert Opin Drug Saf. 2015;14(2):295–303. doi: 10.1517/14740338.2015.989210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nordqvist J. Medical News Today; Brighton, East Sussex, UK: 2013. FDA Warns Antibiotic Zithromax Can Cause Irregular Heart Activity.https://www.medicalnewstoday.com/articles/257624#1 13 March 13. Available from: [Google Scholar]
- 90.Fossa AA, Wisialowski T, Duncan JN, Deng S, Dunne M. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg. 2007;77(5):929–938. [PubMed] [Google Scholar]
- 91.Streetman DS, Nemerovski CW. 2020. Drug Interaction Concerns for Potential COVID-19 Treatments.https://www.drugtopics.com/clinical-news/drug-interaction-concerns-potential-covid-19-treatments [updated May 22, 2020]. Available from: [Google Scholar]
- 92.Ursing J, Rombo L, Eksborg S, Larson L, Bruvoll A, Tarning J, et al. High-Dose Chloroquine for Uncomplicated Plasmodium falciparum Malaria Is Well Tolerated and Causes Similar QT Interval Prolongation as Standard-Dose Chloroquine in Children. Antimicrob Agents Chemother. 2020;64(3) doi: 10.1128/AAC.01846-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woosley RL. Cardiac Complications of Chloroquine-Based Therapy. Expert Opinion / Commentary. 2020 https://www.practiceupdate.com/content/cardiac-complications-of-chloroquine-based-therapy/98054 Elsevier[updated 20 March 2020]. Available from: [Google Scholar]
- 95.Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3(5):241–253. doi: 10.1177/2042098612454283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rathi S, Ish P, Kalantri A, Kalantri S. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taccone FS, Gorham J, Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, Barbhaiya C, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nature Med. 2020 doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 99.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 Jul 17. doi: 10.1056/NEJMoa2021436. Online ahead of print. PMID: 32678530. [DOI] [PMC free article] [PubMed]
- 100.Hoffmann M., Mösbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber HH, Krüger N, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 101.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 20151852. [Google Scholar]
- 102.WHO. “Solidarity” clinical trial for COVID-19 Treatments. July 20, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [accessed 11 August 2020].
- 103.Cohen M.S. Hydroxychloroquine for the Prevention of Covid-19 — Searching for Evidence. New Engl J Med. 2020;383(6):585–586. doi: 10.1056/NEJMe2020388. [DOI] [PMC free article] [PubMed] [Google Scholar]