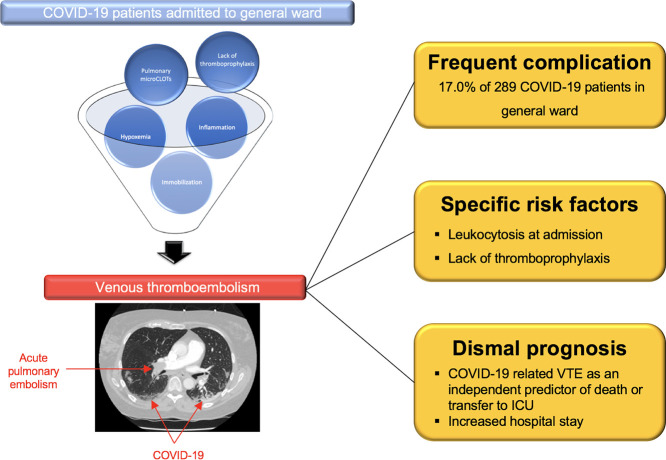
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Venous thromboembolism, Acute pulmonary embolism, Deep vein thrombosis
Highlights
-
•
Venous thromboembolism (VTE) is a frequent complication in COVID-19 patients.
-
•
Single-center study of COVID-19 patients admitted to general ward.
-
•
17.0% of patients with VTE
-
•
Lack of thromboprophylaxis and leukocytosis were independent risk factors of VTE.
-
•
VTE is independently associated with worse in-hospital outcomes.
While acute respiratory infections are known to increase thrombosis risk [1], the incidence of venous thromboembolic events (VTE) in patients with coronavirus disease 2019 (COVID-19) is higher than with other pathogens [2,3]. A variety of potential risk factors for thrombosis particularly present in critically-ill patients have been proposed including severe hypoxemia, thrombo-inflammation, endothelial dysfunction, pulmonary micro-CLOTs on top of recognized features such as immobilization, respiratory failure, mechanical ventilation and central venous catheter use [4]. Conversely, few studies have reported the incidence of VTE in patients hospitalized in regular hospital wards. We sought to describe the rate, predictive factors and prognosis of VTE in consecutive COVID-19 patients who have been admitted to conventional medical departments in Strasbourg University Hospital, the tertiary care center for the first large cluster of COVID-19 cases in France.
In this retrospective cohort study, we included data from all consecutive patients with confirmed COVID-19 infection who were admitted to one of the nine conventional medical departments in Strasbourg University Hospital (two centers: Nouvel Hôpital Civil and CHU Hautepierre) between February 25 and April 19, 2020. A confirmed case of COVID-19 was defined by a positive result of a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab and/or by typical findings of COVID-19 at chest computed tomography (CT) (bilateral and peripheral ground glass opacities and/or alveolar consolidations). Medical management including thromboprophylaxis was left at the discretion of the treating physician. When decided, thromboprophylaxis was achieved with standard preventive treatment (Enoxaparin at 0.4 mL per day, Fondaparinux at 2.5 mg per day or unfractionated heparin at 200 IU per hour) or reinforced preventive treatment (Enoxaparin at 0.4 mL twice per day). Venous thromboembolism risk was evaluated on admission to hospital via the Padua Prediction Score and the Improve score. When suspected, VTE were diagnosed using appropriate imaging tests.
The primary outcome was a confirmed diagnosis of any VTE including acute pulmonary embolism (APE), deep vein thrombosis (DVT) or cerebral venous thrombosis (CVT). The secondary endpoints included COVID-19 related in-hospital death, transfer to ICU, the composite of in-hospital death or transfer to ICU, hospital length of stay and maximal oxygen flow rate during hospitalization. Univariable and multivariable logistic regression was used to identify risk factors of COVID-19 associated VTE. The impact of VTE was assessed using both univariable and multivariable logistic regression model. Variables with a univariable p < 0.05 were considered for subsequent multivariable models. A two-tailed p value < 0.05 was considered significant. Statistical analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The present study was approved by the research ethics committee of Strasbourg Hospital (authorization CE-2020-57) and waived the need of informed consent.
Among 289 consecutive patients (mean age 62.2 ± 17.0 years, 171 (59.2%) male) admitted to general wards with confirmed COVID-19 between February 25 and April 19, 2020, 273 (94.8%) patients had RT-PCR confirmed COVID-19 infection and 251 (94.7%) presented CT features of COVID-19 pneumonia (Supplementary Fig. 1). VTE imaging tests were performed in 100 patients (34.6%) and VTE were evidenced in 49 patients (17.0%). APE was diagnosed in 42 patients (14.5%), CVT in 3 patients (1.0%) and DVT in 12 patients (4.2%) (Table 1 ).
Table 1.
Outcomes of the study population stratified by concomitant in-hospital venous thromboembolic events.
| Outcomes | Global Cohort (n = 289) |
VTE (n = 49) |
No VTE (n = 240) |
p value |
|---|---|---|---|---|
| Venous thromboembolic event | 49 (17.0) | 49 (100.0) | – | – |
| Acute pulmonary embolism | 42 (14.5) | 42 (85.7) | – | – |
| Cerebral venous thrombosis | 3 (1.0) | 3 (6.1) | – | – |
| Deep vein thrombosis | 12 (4.2) | 12 (24.5) | – | – |
| Transfer to ICU or in-hospital death – no. (%) | 90 (31.0) | 23 (47.9) | 67 (27.9) | 0.010 |
| In-hospital death – no. (%) | 24 (8.3) | 6 (12.2) | 18 (7.5) | 0.265 |
| Time from admission to death – days | 9.1 ± 5.8 | 13.3 ± 6.0 | 7.7 ± 5.2 | 0.038 |
| Transfer to ICU – no. (%) | 72 (25.0) | 21 (43.8) | 51 (21.3) | 0.002 |
| Time from admission to ICU transfer – days | 1.9 ± 1.9 | 2.2 ± 1.9 | 1.7 ± 1.9 | 0.337 |
| Discharged alive – no. (%) | 236 (88.7) | 35 (83.3) | 201 (89.7) | 0.284 |
| Maximal oxygen flow rate – L/min | 5.6 ± 4.9 | 6.6 ± 5.0 | 5.4 ± 4.9 | 0.126 |
| Length of stay – days | 12.1 ± 7.5 | 16.5 ± 8.7 | 11.4 ± 7.1 | <0.001 |
Data are presented as mean ± standard deviation in case of any other indication.
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit.
While a high risk of VTE on the basis of a Padua score of 4 or more was equally prevalent in VTE and no-VTE patients, Improve score was higher in VTE patients (1.55 ± 1.08 vs 1.10 ± 1.13; p = 0.013). Long-term anticoagulant therapy prior to hospitalization was less common in VTE patients than in patients without VTE (2.0% vs 12.9%, p = 0.024). From symptoms onset, time to hospital admission was longer in VTE patients in comparison with no-VTE patients (8.6 ± 6.4 vs 6.9 ± 4.4, p = 0.023) (Supplementary Table A1).
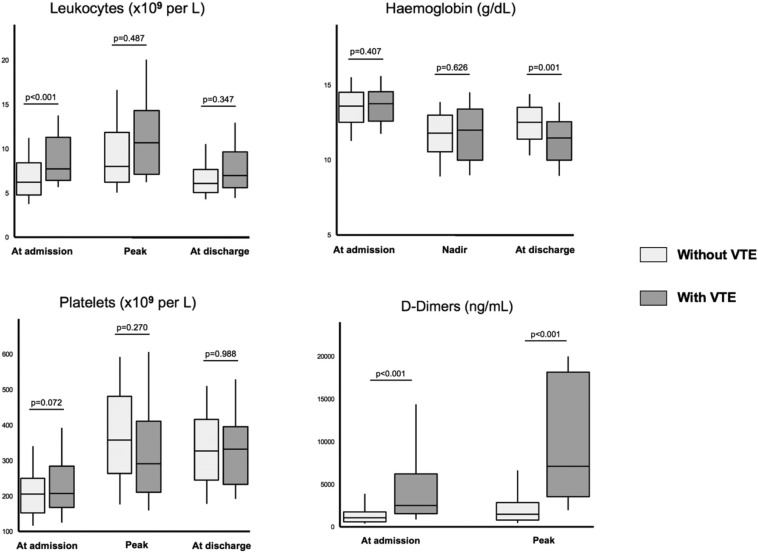
VTE patients demonstrated increased leukocyte count at the time of hospital admission (Fig. 1 ). Baseline D-Dimers levels were increased in VTE patients (5200 ± 5527 vs 1841 ± 2473, p < 0.001). A higher D-Dimer peak during hospital stay was evidenced in VTE patients (8821 ± 6851 vs 2701 ± 3463, p < 0.001). At discharge, VTE patients had a lower hemoglobin level (11.3 ± 2.1 vs 12.4 ± 1.7, p = 0.001). CRP values were similar between the two groups at any time between admission and discharge (Supplementary Table A2).
Fig. 1.
Leukocytes, hemoglobin, platelets and D-Dimers in COVID-19 patients during hospitalization.
Biological parameters at admission, Nadir/peak values and discharge in COVID-19 patients. Higher leukocyte count and D-Dimer levels were evidence at baseline in VTE patients as compared to non VTE patients. Similarly, higher D-Dimer peak and lower hemoglobin level at discharge were observed in VTE patients.
Abbreviations: COVID-19, coronavirus disease 2019; VTE, venous thromboembolism.
After multivariable analysis, time from symptom onset to admission (odds ratio (OR) 1.07, confidence interval (CI) 95% [1.00–1.16], p = 0.045), Improve score (OR 1.37, CI95% [1.02–1.83], p = 0.032), leukocyte count at admission (OR 1.16, CI95% [1.06–1.27], p = 0.001) and lack of thromboprophylaxis (OR 27.85, CI95% [9.35–82.95], p < 0.001) were associated with VTE occurrence (Supplementary Table A3). The composite of death or transfer to ICU occurred in 90 patients (31.0%) and was almost two-fold higher in VTE patients (47.9% vs 27.9%, p = 0.01). VTE patients experienced increased length of stay but in-hospital death did not reach statistical significance between the two subsets of patients (Table 1). After multivariable analysis, VTE (OR 3.24, CI95% [1.52–6.92], p = 0.002), fever at admission (OR 5.57, CI95% [1.47–21.01], p = 0.011), lymphopenia (OR 0.34, CI95% [0.15–0.75], p = 0.007) and the extent of COVID-19 evaluated by chest CT (OR 1.50, CI95% [1.04–2.15], p = 0.026) were evidenced as independent risk factors of death or transfer to ICU (Supplementary Table A4).
Several reports have stressed the high prevalence and negative impact of VTE in patients with COVID-19 admitted to the ICU [2,3]. While the mechanisms involved in thrombosis among ICU patients remain unclear, coagulation disorders, endothelial dysfunction, hypoxemia, pulmonary intravascular coagulation and thrombo-inflammation have been frequently proposed as the main underlying features of VTE on top of common risks factors [4]. In our study, we reported a high prevalence of VTE among non-ICU patients (17.0%), consistent with incidences reported in the literature [4]. Among them, 48.8% were considered at high risk of VTE on the basis of a Padua score ≥4. Interestingly, a Padua score ≥4 was not associated with an increased risk of VTE. As compared to the average annual incidence in general population (117 VTE per 100,000 person-years) [5] and in-hospital (960.5 VTE per 10,000 person-years) [6], our study patients experienced an estimated rate of 1695 per 10,000 population during a 2-month study period.
The absence of association observed between general risk factors for VTE and adverse thrombotic events in our cohort was in line with previous studies [2,4]. Preliminary reports suggested that hemostatic abnormalities including D-Dimers and mild thrombocytopenia were associated with a higher risk of VTE in critically ills [4]. Except for D-Dimer, VTE and non-VTE ward patients showed similar baseline haemostasis parameters and this may be explained in part by their intrinsic lower severity at the time of admission. Likewise, no impact of lupus anticoagulant on thrombotic events could be established.
The independent relationship observed in our study between leukocytosis and VTE strengthened the role of leukocytes in clot formation. Attenuation of thrombus formation by neutrophil depletion as well as high neutrophil deoxyribonucleic acid (DNA) content in venous thrombi in mice provided the initial clues that neutrophils act as a major determinant in thrombus growth [7]. Extended works using intravital microscopy to monitor clot formation have nicely demonstrated that the swift recruitment of tissue factor harbored by leukocytes derived microparticles is mandatory to thrombus growth [8]. Other experimental models have demonstrated that a cross talk between platelet monocytes and neutrophils was responsible for the initiation and amplification of vein thrombosis [7]. Evidences were provided that thrombus-resident neutrophils were indispensable for subsequent deep venous thrombosis propagation by binding factor XII and by supporting its activation through the release of neutrophil extracellular traps (NETs) [7]. In COVID-19 infection, it is likely that drastic activation of endothelial cells by hypoxia and cytokine storm would lead to surface expression of adhesion receptors facilitating the binding of circulating leukocytes and microparticles. Subsequent activation of the leukocytes induces expression of tissue factor and NETs that trigger thrombosis [7].
Our study also stressed the view that the lack of thromboprophylaxis was a major determinant of VTE in non-ICU COVID-19 patients. In our cohort, 89.3% of COVID-19 patients were treated with pharmacologic thromboprophylaxis during hospital stay. In line with similar reports [4,9], a sizeable proportion of thrombotic complications occurred despite thromboprophylaxis and clearly underlined the need of an optimized anti-thrombotic strategy. Appropriate anticoagulation during hospital stay seems to be associated with improved survival [10]. Several RCTs are already underway to determine the optimal anticoagulation regimen in both ICU and non-ICU patients with COVID-19 (NCT04344756, NCT04345848).
Our study has some limitations. First, the retrospective nature of the data collection should be acknowledged. Second, this analysis was conducted in a large university hospital located in Strasbourg cluster with a sustained disease transmission in the region and potentially increased COVID-19 severity. Third, as we did not have a systematic standardized assessment of thromboembolic events, this may underestimate the prevalence and risks of VTE. Finally, we cannot exclude the possibility of confounding due to different therapeutic doses of pharmacological prophylaxis.
Our study confirms previous observations suggesting that VTE are frequent and associated with a dismal prognosis among patients with COVID-19 hospitalized in non-ICU units. The dramatic impact of lack of thromboprophylaxis on VTE occurrence reinforces the need to investigate the optimal anticoagulation regimen in these patients.
Funding sources
This work was supported by GERCA (Groupe pour l'Enseignement, la prévention et la Recherche Cardiologique en Alsace).
Declaration of competing interest
None.
Acknowledgements
The authors would like to formally acknowledge the commitment of all front-line healthcare workers during the COVID-19 crisis in the University Hospital of Strasbourg as they commit themselves to difficult work and also put themselves at risk of infection. Strasbourg's University Hospital is proud of the commitment of all nurses, including newly graduated nurses, medical students, health support staff and volunteers.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2020.07.033.
Appendix A. Supplementary data
Supplementary material
References
- 1.Santos-Gallego C.G., Badimon J.J. The sum of two evils: pneumonia and myocardial infarction: is platelet activation the missing link? J. Am. Coll. Cardiol. 2014;64:1926–1928. doi: 10.1016/j.jacc.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchandot B., Sattler L., Jesel L. COVID-19 related coagulopathy: a distinct entity? J. Clin. Med. 2020;9:1651. doi: 10.3390/jcm9061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heit J.A. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J. Thromb. Thrombolysis. 2006;21:23–29. doi: 10.1007/s11239-006-5572-y. [DOI] [PubMed] [Google Scholar]
- 6.Heit J.A., Melton L.J., Lohse C.M. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin. Proc. 2001;76:1102–1110. doi: 10.4065/76.11.1102. [DOI] [PubMed] [Google Scholar]
- 7.von Brühl M.-L., Stark K., Steinhart A. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross P.L., Furie B.C., Merrill-Skoloff G., Chou J., Furie B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J. Leukoc. Biol. 2005;78:1318–1326. doi: 10.1189/jlb.0405193. [DOI] [PubMed] [Google Scholar]
- 9.Llitjos J.-F., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material