Abstract
Background
The COVID-19 outbreak in late December 2019 has quickly emerged into pandemic in 2020. We aimed to describe the epidemiology and clinical characteristics of hospitalized COVID-19 patients, and to investigate the potential risk factors for COVID-19 severity.
Method
1663 hospitalized patients with laboratory-confirmed diagnosed COVID-19 from Tongji Hospital between January 14, 2020, and February 28, 2020 were included in the present study. Demographic information, exposure history, medical history, comorbidities, signs and symptoms, chest computed tomography (CT) scanning, severity of COVID-19 and laboratory findings on admission were collected from electronic medical records. Multivariable logistic regression was used to explore the association between potential risk factors with COVID-19 severity.
Results
In the present study, the majority (79%) of 1663 COVID-19 patients were aged over 50 years old. A total of 2.8% were medical staff, and an exposure history of Huanan seafood market was document in 0.7%, and 7.4% were family infection. Fever (85.8%), cough (36.0%), fatigue (23.6%) and chest tightness (11.9%) were the most common symptoms in COVID-19 patients. As of February 28, 2020, of the 1663 patients included in this study, 26.0% were discharged, 10.2% were died, and 63.8% remained hospitalized. More than 1/3 of the patients had at least one comorbidity. Most (99.8%) patients had abnormal results Chest CT, and the most common manifestations of chest CT were local patchy shadowing (70.7%) and ground-glass opacity (44.8%). On admission, lymphocytopenia was present in 51.1% of the patients, mononucleosis in 26.6%, and erythrocytopenia in 61.3%. Most of the patients had increased levels of C-reactive protein (80.4%) and D-dimer (64.4%). Compared with non-severe patients, severe patients had more obvious abnormal laboratory results related to inflammation, coagulation disorders, liver and kidney damage (all P < 0.05). Older age (OR = 2.37, 95% CI: 1.47–3.83), leukocytosis (OR = 2.37, 95% CI: 1.47–3.83), and increased creatine kinase (OR = 2.37, 95% CI: 1.47–3.83) on admission were significantly associated with COVID-19 severity.
Conclusion
Timely medical treatment and clear diagnosis after the onset might be beneficial to control the condition of COVID-19. Severe patients were more likely to be to be elder, and tended to have higher proportion of comorbidities and more prominent laboratory abnormalities. Older age, leukocytosis, and increased creatine kinase might help clinicians to identify severe patients with COVID-19.
Keywords: Epidemiological, Clinical characteristics, Risk factors, COVID-19
Introduction
Since early December 2019, several pneumonia cases of unknown origin were identified in Wuhan, Hubei province, China [1,2]. The pathogen has been identified as a novel coronavirus and belongs to β-coronavirus genus that has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by International Committee on Taxonomy of Viruses [3]. Additionally, the pneumonia caused by SARS-CoV-2 has been named as coronavirus disease 2019 (COVID-19) by World Health Organization (WHO) [4]. COVID-19 has soon become a serious worldwide health problem and was declared as pandemic in March 2020 [[5], [6], [7], [8]]. By June 16, 2020, 7.94 million cases were confirmed globally, including 84.82 thousand cases in China and 434.80 thousand patients have died from this viral infection worldwide, 4.65 thousand in China [7]. This novel virus shared 79.5% sequence identify to severe acute respiratory syndrome coronavirus (SARS-CoV), and spread from person to person [[9], [10], [11]].
Many studies have reported the clinical characteristics of COVID-19 patients [1,5,[12], [13], [14]], but the outbreak is still progressing, an updated analysis of cases in a relative large sample might help identify the defining the epidemiology and clinical features of COVID-19.
In the present study, we aimed to describe the epidemiology and clinical characteristics of 1663 hospitalized COVID-19 patients at single-center hospital, and to explore the associations between potential risk factors with COVID-19 severity.
Materials and methods
Data collection
We obtained the medical records for 1663 hospitalized patients with laboratory-confirmed diagnosed COVID-19 from Tongji Hospital between January 14, 2020, and February 28, 2020. Admission criteria: the patient has clinical symptoms, a positive nucleic acid test, or CT suggests viral pneumonia. Demographic information, exposure history (Huanan seafood market exposure and family infection), medical history, comorbidities, signs and symptoms, chest computed tomography (CT) scanning, severity of COVID-19 and laboratory findings on admission were collected from electronic medical records. More detailed information on fundamentals and method has been previously reported elsewhere [15]. The study was approved by Tongji Hospital Ethics Committee.
Severity of COVID-19 was defined according to the diagnostic and treatment guideline (Version 5–6) published by the government [16,17]. Severe COVID-19 was defined if they meet one of following criteria: (1) Respiratory distress with respiratory frequency ≥30 breaths per min with shortness of breath or difficulty breathing; (2) Oxygen saturation ≤93% at rest; (3) Artery partial pressure of oxygen (PaO2)/inspired oxygen fraction (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa).
SARS-CoV-2 nucleic acid detection
Throat swab specimens were collected for the SARS-CoV-2 viral nucleic acid detection and stored in 5 mL virus preservation solution. Virus RNA was extracted with 24 h on Tianlong PANA9600 automatic nucleic acid extraction system (Tianlong, China). The open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) genes were simultaneously tested with RT-PCR. RT-PCR assay was performed on Tianlong Gentier 96E real-time PCR system with the following conditions: incubation at 50 °C for 15 min, pre-denaturation at 95 °C for 15 min, 45 cycles of denaturation at 94 °C for 15 s, and extension at 55 °C for 45 s (collecting fluorescence signal). A Ct-value less than 40 for both genes was defined as a positive test, and a Ct-value of 40 or more was defined as a negative test.
Statistics analysis
Shapiro-Wilk Test was used to test data normality. Two-tailed t-test was conducted to test difference in means between no-severe and severe groups, and Mann Whitney U test was to test difference in skewed parameters. Chi-square tests or Fisher's exact test, when appropriate, was used for categorical variables. Multivariate logistic regression model was conducted to explore the associations of potential risk factors with COVID-19 severity. SAS version 9.4 (SAS Institute, Cary, North Carolina, USA) was used to conduct all statistical analyses. Two-sided statistical tests considered to be significant at p values below 0.05.
Results
Epidemiological characteristics
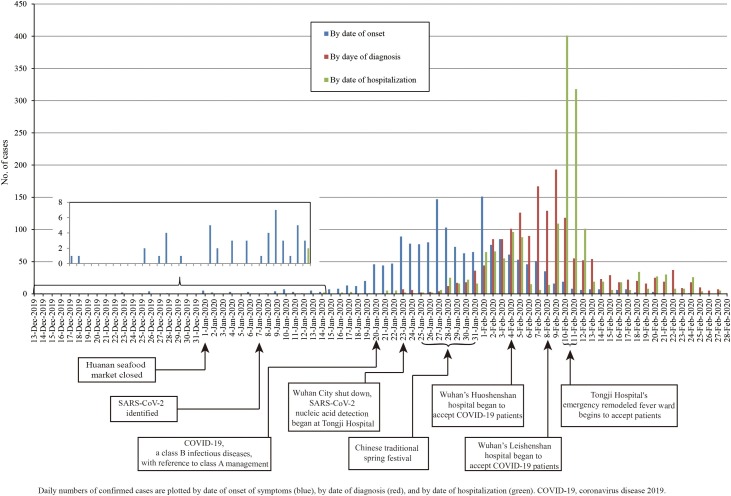
On January 20, COVID-19 was included in the legal category B infectious diseases in China, with reference to category A management. COVID-19 was included in the legal category B infectious diseases according to Law of the People's Republic of China on the Prevention and Treatment of Infectious Diseases. Category A management: patients and carriers of pathogens shall be treated in isolation, and the period of isolation shall be determined according to the results of medical examinations; for suspected patients, be isolated and treated in designated places before diagnosis; for patients in medical institutions, pathogen carriers, and close contacts of suspected patients, conduct medical observations and take other necessary preventive measures in designated places. During the traditional Chinese New Year, the number of onset cases was increasing, reaching the highest on February 1, and then gradually decreasing. SARS-CoV-2 confirmed positive patients gradually increased from January 23, reached the highest on February 9, and then gradually declined. The number of COVID-19 patients admitted in the hospital gradually increased from January 25 to February 4, and then decreased. The inpatients reached the peak on February 10–11 (Fig. 1 ).
Fig. 1.
Epidemic curve of the confirmed cases of hospitalized COVID-19 at Tongji Hospital.
Daily numbers of confirmed cases are plotted by date of onset of symptoms (blue), by date of diagnosis (red), and by date of hospitalization (green). COVID-19, coronavirus disease 2019.
Baseline characteristics of the non-severe (n = 799) and severe (n = 864) COVID-19 patients were presented in Table 1 . In total 1663 hospitalized patients infected with COVID-19 (825 females, 838 males) were included in the present study. The median age of the all patients was 64.0 years (IQR, 52.0−71.0), and 79% of them were over 50 years old. A total of 2.8% were medical staff, and an exposure history of Huanan seafood market was document in 0.7%, and 7.4% were family infection. The median interval from onset of symptoms to COVID-19 confirmation was 9.0 days (IQR, 5.0−14.0), the median interval from onset of symptoms to hospital admission was 10.0 days (IQR, 6.0−14.0), the median interval from onset of symptoms to death was 18.0 days [(IQR, 11.0–23.0), data not shown], and the length of hospital stay was 17.0 days (IQR, 13.0−19.0). Of 1663 patients, 433 (26.0%) people improved and discharged, 169 (10.2%) died, and 1061 (63.8%) were still in hospital by date of February 28, 2020. Among the whole patients, 39.0% had at least one comorbidity, and hypertension (20.9%), diabetes (14.7%), and coronary heart disease (7.9%) were the most common coexisting diseases. Compared with non-severe patients, the severe patients were more likely to be male, older and tended to have a longer time from onset of symptoms to hospital admission, from onset of symptoms to COVID-19 confirmation, and shorter time of hospital stay. In addition, severe patients had higher proportion of comorbidity such as hypertension, diabetes and coronary heart disease (all P < 0.01).
Table 1.
Baseline characteristics of patients infected with COVID-19.
| All patient | Disease Severity |
p value | ||
|---|---|---|---|---|
| Non-severe | Severe | |||
| N | 1663 | 799 | 864 | |
| Age | ||||
| Median (IQR) | 64.0(52.0−71.0) | 61.0(47.0−69.0) | 66.0(57.0−73.0) | <0.0001 |
| Age groups (years), n (%) | <0.0001 | |||
| <30 | 38(2.3) | 26(3.3) | 12(1.4) | |
| 30−49 | 311(18.7) | 202(25.3) | 109(12.6) | |
| 50−69 | 826(49.7) | 378(47.3) | 448(51.9) | |
| ≥70 | 488(29.3) | 193(24.2) | 295(34.1) | |
| Women, n (%) | 825(49.6) | 415(51.9) | 410(47.5) | 0.07 |
| Occupation, n (%) | <0.0001 | |||
| Others | 896(53.9) | 476(59.6) | 420(48.6) | |
| Medical staff | 46(2.8) | 33(4.1) | 13(1.5) | |
| Retirees | 568(34.2) | 216(27.0) | 352(40.7) | |
| Farmers / workers | 153(9.2) | 74(9.3) | 79(9.1) | |
| Exposure history, n (%) | ||||
| Huanan seafood market exposure | 11(0.7) | 6(0.8) | 5(0.6) | 0.67 |
| Family infection | 123(7.4) | 58(7.3) | 65(7.5) | 0.84 |
| Time from onset to diagnosis, Median (IQR), days | 9.0(5.0−14.0) | 9.0(4.0-14.0) | 10.0(5.0-15.0) | 0.007 |
| Time from onset to admission, Median (IQR), days | 10.0(6.0−14.0) | 9.0(6.0−14.0) | 10.0(7.0−15.0) | 0.01 |
| Length of hospital stay, Median (IQR), days | 17.0(13.0−19.0) | 18.0(15.0−20.0) | 17.0(11.0−18.0) | <0.0001 |
| Clinical outcomes at data cutoff, n (%) | <0.0001 | |||
| Hospitalization | 1061(63.8) | 519(65.0) | 542(62.7) | |
| Improvement and discharge | 433(26.0) | 280(35.0) | 153(17.7) | |
| Death | 169(10.2) | 0(0.0) | 169(19.6) | |
| Comorbidity, n (%) | ||||
| Hypertension | 347(20.9) | 142(17.8) | 205(23.7) | 0.003 |
| Diabetes | 245(14.7) | 85(10.6) | 160(18.5) | <0.0001 |
| Coronary heart disease | 131(7.9) | 46(5.8) | 85(9.8) | 0.002 |
| Chronic obstructive pulmonary disease | 62(3.7) | 25(3.1) | 37(4.3) | 0.21 |
| Cerebrovascular disease | 57(3.4) | 24(3.0) | 33(3.8) | 0.36 |
| Chronic liver disease | 38(2.3) | 16(2.0) | 22(2.6) | 0.46 |
| Chronic renal disease | 31(1.9) | 16(2.0) | 15(1.7) | 0.69 |
| Cancer | 19(1.1) | 6(0.8) | 13(1.5) | 0.15 |
Data are medians (IQR) or number (%). COVID-19, coronavirus disease 2019; IQR, inter-quartile ranges. The percentages were calculated column-wise.
Symptomatic and radiological characteristics
Of 1663 patients, the main clinical symptoms were fever (85.8%), cough (36.0%), fatigue (23.6%) and chest tightness (11.9%), followed by diarrhea (4.6%), dyspnea (4.4%), loss of appetite (4.2%), myalgia (3.4%), dizziness/headache (1.7%), pharyngalgia (1.4%), nausea (1.1%), vomiting (1.1%), runny nose (0.2%), and stuffy nose (0.1%). Compared with non-severe patients, the severe patients had a higher proportion of symptoms such as fever, cough, fatigue, chest tightness and loss of appetite (all P < 0.05). We observed similar results after further excluding the patients with underlying diseased. Among patients without fever, the main clinical symptoms were cough (57.2%), chest tightness (23.3%), and fatigue (17.8%) (Supplementary Table 2). Cluster analysis found that clinical symptoms could be divided into three categories. First category: fever; second category: cough; third category: fatigue, chest tightness, diarrhea, dyspnea, loss of appetite, myalgia, dizziness/headache, pharyngalgia, nausea, vomiting, runny nose, and stuffy nose (Supplementary Fig. 1).
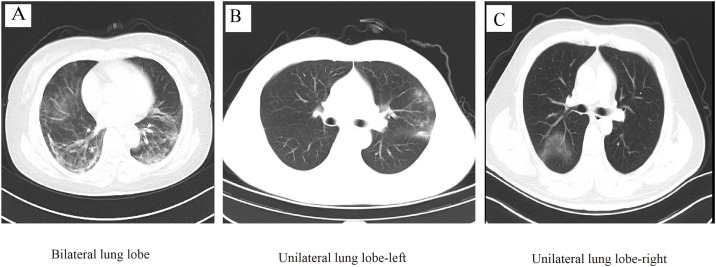
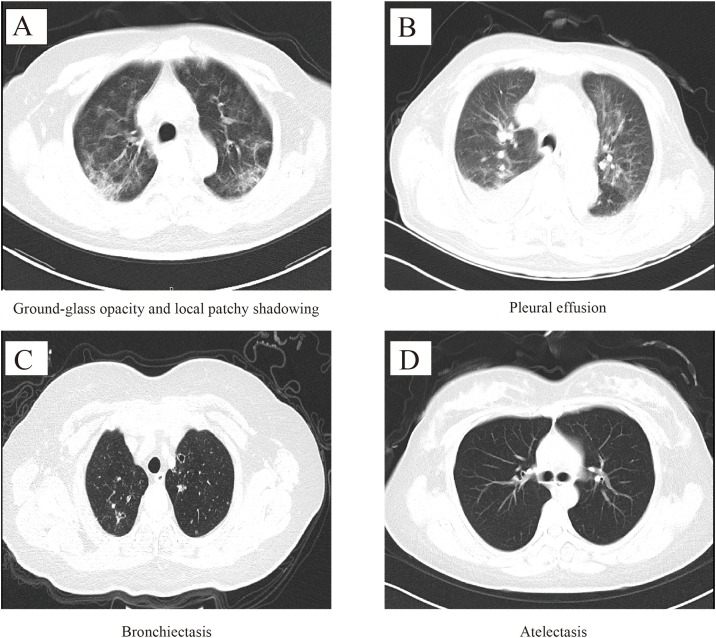
Due to 188 missing CT results on admission (CT done in other hospitals), 1475 were included in the radiological characteristics analysis. Among 1475 patients, 99.8% patients had abnormal results (91.4% in bilateral lung lobe, 5.3% in unilateral lung lobe-left, and 3.1% in unilateral lung lobe-right, Table 2 , Fig. 2 A–C). The most common manifestations of chest CT were local patchy shadowing (70.7%, Fig. 3 A) and ground-glass opacity (44.8%, Fig. 3A), followed by pleural effusion (14.6%, Fig. 3B), bronchiectasis (1.6%, Fig. 3C) and atelectasis (0.4%, Fig. 3D). Compared with non-severe patients, the severe patients had a higher proportion of symptoms such as pleural effusion and bronchiectasis (all P < 0.01).
Table 2.
Symptomatic and radiological characteristics of patients infected with COVID-19.
| All patient | Disease Severity |
p value | ||
|---|---|---|---|---|
| Non-severe | Severe | |||
| Signs and symptoms, n (%) | ||||
| N | 1663 | 799 | 864 | |
| Fever | 1427(85.8) | 671(84.0) | 756(87.5) | 0.04 |
| Cough | 598(36.0) | 252(31.5) | 346(40.1) | 0.0003 |
| Fatigue | 392(23.6) | 168(21.0) | 224(25.9) | 0.02 |
| Chest tightness | 198(11.9) | 71(8.9) | 127(14.7) | 0.0003 |
| Diarrhea | 76(4.6) | 33(4.1) | 43(5.0) | 0.41 |
| Dyspnea | 73(4.4) | 30(3.8) | 43(5.0) | 0.22 |
| Loss of appetite | 69(4.2) | 25(3.1) | 44(5.1) | 0.04 |
| Myalgia | 57(3.4) | 26(3.3) | 31(3.6) | 0.71 |
| Dizziness/headache | 29(1.7) | 12(1.5) | 17(2.0) | 0.47 |
| Pharyngalgia | 24(1.4) | 12(1.5) | 12(1.4) | 0.85 |
| Nausea | 18(1.1) | 11(1.4) | 7(0.8) | 0.26 |
| Vomiting | 18(1.1) | 11(1.4) | 7(0.8) | 0.26 |
| Runny nose | 4(0.2) | 1(0.1) | 3(0.4) | 0.36 |
| Stuffy nose | 1(0.1) | 0(0.0) | 1(0.1) | – |
| Chest CT images, n (%) | ||||
| N | 1475 | 763 | 712 | |
| Lesion distribution | ||||
| Bilateral lung lobe | 1348(91.4) | 691(90.6) | 657(92.3) | 0.24 |
| Unilateral lung lobe-left | 78(5.3) | 39(5.1) | 39(5.5) | 0.75 |
| Unilateral lung lobe-right | 46(3.1) | 30(3.9) | 16(2.3) | 0.06 |
| Upper lobe+Lower lobe | 919(62.3) | 478(62.6) | 441(61.9) | 0.70 |
| Upper lobe | 179(12.1) | 87(11.4) | 92(12.9) | 0.39 |
| Lower lobe | 374(25.4) | 195(25.6) | 179(25.1) | 0.82 |
| Normal lung | 3(0.2) | 3(0.4) | 0(0.0) | – |
| Image characteristics | ||||
| Ground-glass opacity | 661(44.8) | 334(43.8) | 327(45.9) | 0.41 |
| Local patchy shadowing | 1043(70.7) | 542(71.0) | 501(70.4) | 0.78 |
| Pleural effusion | 216(14.6) | 88(11.5) | 128(18.0) | 0.0005 |
| Bronchiectasis | 24(1.6) | 5(0.7) | 19(2.7) | 0.002 |
| Atelectasis | 6(0.4) | 3(0.4) | 3(0.4) | 1.00 |
COVID-19, coronavirus disease 2019. The percentages were calculated column-wise.
Fig. 2.
Lesion distribution of Chest CT images in COVID-19 patients.
Fig. 3.
Image characteristics of chest CT in COVID-19 patients.
Laboratory findings
As Table 3 shown, most of patients had normal white blood cell count (81.2%), with 9.2% increased and 9.6% decreased cases on admission. Decreased levels of lymphocyte count (51.1%), lymphocyte percentage (47.0%), albumin (50.1%), hemoglobin (59.3%) and increased levels of lactate dehydrogenase (76.9%), procalcitonin (51.0%), alanine aminotransferase (20.6%), andaspartate aminotransferase (21.0%), had been observed. C-reactive protein was increased in 90.4% of the patients, and 64.4% of the patients had increased D-dimer, and some patients had prolonged prothrombin time (22.0%), activated partial thromboplastin time (28.9%), and thrombin time (6.9%).
Table 3.
Laboratory findings of patients infected with COVID-19.
| All patient | Disease Severity |
Reference | p value | ||
|---|---|---|---|---|---|
| Non-severe | Severe | ||||
| White blood cell count, ×10^9/L | 5.5(4.4−7.2) | 5.3(4.2−6.7) | 5.8(4.6−7.6) | 3.5−9.5 | <0.0001 |
| Increased | 140/1528(9.2) | 42/727(5.8) | 98/801(12.2) | <0.0001 | |
| Decreased | 147/1528(9.6) | 73/727(10.0) | 74/801(9.2) | 0.60 | |
| Lymphocyte count, ×10^9/L | 1.1(0.8−1.4) | 1.1(0.8−1.5) | 1.0(0.7−1.4) | 1.1−3.2 | 0.004 |
| Increased | 6/1528(0.4) | 4/727(0.6) | 2/801(0.3) | 0.43 | |
| Decreased | 780/1528(51.1) | 346/727(47.6) | 434/801(54.2) | 0.01 | |
| Lymphocyte percentage (%) | 20.8(13.4−28.3) | 21.8(14.6−29.3) | 19.5(11.8−27.4) | 20−50 | <0.0001 |
| Increased | 13/1528(0.9) | 7/727(1.0) | 6/801(0.8) | 0.65 | |
| Decreased | 718/1528(47.0) | 307/727(42.2) | 411/801(51.3) | 0.0004 | |
| Monocyte count, ×10^9/L | 0.5(0.4−0.6) | 0.5(0.4−0.6) | 0.5(0.4−0.6) | 0.1−0.6 | 0.62 |
| Increased | 406/1528(26.6) | 179/727(24.6) | 227/801(28.3) | 0.10 | |
| Decreased | 12/1528(0.8) | 3/727(0.4) | 9/801(1.1) | 0.12 | |
| Monocyte percentage (%) | 8.4(6.6−10.8) | 8.7(6.9−10.9) | 8.2(6.3−10.6) | 3.0−10.0 | 0.002 |
| Increased | 486/1528(31.8) | 247/727(34.0) | 239/801(29.8) | 0.08 | |
| Decreased | 63/1528(4.1) | 18/727(2.5) | 45/801(5.6) | 0.002 | |
| Neutrophil count, ×10^9/L | 3.8(2.7−5.3) | 3.6(2.6−4.9) | 3.9(2.8−5.7) | 1.8−6.3 | <0.0001 |
| Increased | 242/1528(15.8) | 79/727(10.9) | 163/801(20.4) | <0.0001 | |
| Decreased | 95/1528(6.2) | 50/727(6.9) | 45/801(5.6) | 0.31 | |
| Neutrophil percentage (%) | 68.9(60.2−78.2) | 67.4(59.0−75.9) | 70.8(60.8−79.7) | 40.0−75.0 | <0.0001 |
| Increased | 502/1528(32.9) | 202/727(27.8) | 300/801(37.5) | <0.0001 | |
| Decreased | 18/1528(1.2) | 11/727(1.5) | 7/801(0.9) | 0.25 | |
| Platelet count, ×10^9/L | 225.0(167.0−303.0) | 213.0(165.0−294.0) | 237.0(172.0−312.0) | 125−350 | 0.006 |
| Increased | 224/1523(14.7) | 87/723(12.0) | 137/800(17.1) | 0.005 | |
| Decreased | 130/1523(8.5) | 56/723(7.8) | 74/800(9.3) | 0.29 | |
| Hemoglobin, g/L | 127.0(116.0−137.0) | 128.0(118.0−138.0) | 126.0(115.0−137.0) | 130−175 | 0.003 |
| Decreased | 906/1528(59.3) | 408/727(56.1) | 498/801(62.2) | 0.02 | |
| Alanine aminotransferase, U/L | 23.0(15.0−37.0) | 22.0(14.0−37.0) | 24.0(15.0−37.0) | 4−41 | 0.09 |
| Increased | 298/1445(20.6) | 139/672(20.7) | 159/773(20.6) | 0.96 | |
| Aspartate aminotransferase, U/L | 26.0(19.0−38.0) | 25.0(19.0−36.0) | 27.0(20.0−39.0) | 4−40 | 0.02 |
| Increased | 303/1445(21.0) | 128/672(19.1) | 175/773(22.6) | 0.09 | |
| Albumin, g/L | 34.9(31.8−38.3) | 35.5(32.6−38.9) | 34.5(31.0−37.9) | 35−52 | <0.0001 |
| Decreased | 723/1443(50.1) | 306/672(45.5) | 417/771(54.1) | 0.001 | |
| Creatinine, umol/L | 68.0(57.0−83.0) | 67.0(56.0−82.0) | 68.0(58.0−84.0) | 59−104 | 0.17 |
| Increased | 119/1438(8.3) | 44/667(6.6) | 75/771(9.7) | 0.03 | |
| Urea, mmol/L | 4.3(3.4−5.6) | 4.1(3.2−5.2) | 4.6(3.5−6.1) | 1.7−8.3 | <0.0001 |
| Increased | 113/1438(7.9) | 27/667(4.1) | 86/771(11.2) | <0.0001 | |
| Lactate dehydrogenase, U/L | 272.5(218.0-345.0) | 263.0(210.0-326.0) | 282.0(227.0-372.0) | 135−214 | <0.0001 |
| Increased | 1110/1444(76.9) | 495/673(73.6) | 615/771(79.8) | 0.005 | |
| Creatine kinase, U/L | 65.5(45.5−113.5) | 65.5(46.0−108.0) | 65.5(44.5−121.5) | 18−198 | 0.50 |
| Increased | 50/588(8.5) | 15/284(5.3) | 35/304(11.5) | 0.007 | |
| C-reactive protein, mg/L | 23.2(4.3−62.3) | 21.0(4.1−54.0) | 25.4(4.8−72.7) | 0.1−3.0 | 0.02 |
| Increased | 1173/1459(80.4) | 546/689(79.3) | 627/770(81.4) | 0.29 | |
| Prothrombin time, s | 13.8(13.2−14.4) | 13.7(13.2−14.3) | 13.9(13.3−14.6) | 11.5−14.5 | 0.0002 |
| Increased | 332/1511(22.0) | 126/711(17.7) | 206/800(25.8) | 0.0002 | |
| APTT, s | 38.8(35.6−42.7) | 38.9(35.8−42.8) | 38.7(35.6−42.5) | 29−42 | 0.49 |
| Increased | 399/1382(28.9) | 188/647(29.1) | 211/735(28.7) | 0.89 | |
| D-dimer, mg/L | 0.7(0.4−1.6) | 0.6(0.4−1.4) | 0.9(0.4−1.9) | 0−0.5 | 0.008 |
| Increased | 956/1484(64.4) | 411/698(58.9) | 545/786(69.3) | <0.0001 | |
| Thrombin time, s | 16.7(15.9−17.5) | 16.4(15.8−17.4) | 16.8(15.9−17.8) | 14−19 | 0.0002 |
| Increased | 95/1382(6.9) | 43/647(6.7) | 52/735(7.1) | 0.75 | |
| Procalcitonin, ng/mL | 0.06(0.03−0.09) | 0.05(0.03−0.08) | 0.06(0.04−0.1) | 0.02−0.05 | 0.003 |
| Increased | 655/1285(51.0) | 279/618(45.2) | 376/667(56.4) | <0.0001 | |
Data are medians (IQR) or No./total No. (%).COVID-19, coronavirus disease 2019, APTT, activated partial thromboplastin time; IQR, inter-quartile ranges.
Compared to non-severe patients, severe individuals tended to have higher levels of white blood cell count, neutrophil count/ percentage, platelet count, aspartate aminotransferase, urea, lactate dehydrogenase, and procalcitonin (all P < 0.05, Table 3). In addition, severe patients had lower levels of lymphocyte count/percentage, monocyte percentage, hemoglobin, and albulmin (all P < 0.05, Table 3). Prothrombin time was longer in severe patients than in non-severe patients. In the present study, we observed that non-severe and severe patients had higher levels of D-dimer. Compared to non-severe patients, severe individuals had higher levels of D-dimer, and the median (IQR) of D-dimer was 0.6 (0.4–1.4) mg/L in non-severe population, and 0.9 (0.4–1.9) mg/L in severe participants.
Logistic regression for COVID-19 severity with its potential risk factors
Age (<65, ≥65, years), gender (female, male), time from onset to admission history of hypertension (yes/no) and diabetes (yes/no), lymphopenia (<1.1, ≥1.1, ×10^9/L), leukocytosis (<9.5, ≥9.5, ×10^9/L), increased alanine aminotransferase (<40, ≥41, U/L), increased lactate dehydrogenase (<214, ≥214, U/L), increased creatine kinase, and increased D-dimer (<0.5, ≥0.5, mg/L) were included in the logistic regression for COVID-19 severity. As Table 4 presented, compared with the patients aged <65, patients aged ≥65 had higher odds of COVID-19 severity risk [OR = 1.73, 95% CI: (1.18−2.53)]. Leukocytosis [OR = 2.30, 95% CI: (1.25−4.25)] and increased creatine kinase [OR = 2.09, 95% CI: (1.06−4.09)] tended to have higher odds of COVID-19 severity risk.
Table 4.
Logistic regression model for COVID-19 severity with its potential risk factors, odds ratios (95% confidence intervals).
| OR | 95%CI | p value | |
|---|---|---|---|
| Age (years) | |||
| <65 | reference | – | – |
| ≥65 | 1.73 | (1.18−2.53) | 0.005 |
| Gender (male) | 0.91 | (0.64−1.30) | 0.60 |
| Time from onset to admission, days | |||
| <10 | reference | – | – |
| ≥10 | 0.94 | (0.66−1.35) | 0.74 |
| History of hypertension | 1.26 | (0.70−2.27) | 0.45 |
| History of diabetes | 1.16 | (0.72−1.89) | 0.54 |
| Lymphopenia | 1.03 | (0.66−1.61) | 0.89 |
| Leukocytosis | 2.30 | (1.25−4.25) | 0.008 |
| Increased alanine aminotransferase | 0.84 | (0.54−1.31) | 0.44 |
| Increased lactate dehydrogenase | 1.44 | (0.93−2.22) | 0.10 |
| Increased creatine kinase | 2.09 | (1.06−4.09) | 0.03 |
| Increased D-dimer | 1.17 | (0.78−1.76) | 0.45 |
COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval. Lymphopenia (<1.1, ≥1.1, ×10^9/L), leukocytosis (<9.5, ≥9.5, ×10^9/L), increased alanine aminotransferase (<40, ≥41, U/L), increased lactate dehydrogenase (<214, ≥214, U/L), increased creatine kinase, and increased D-dimer (<0.5, ≥0.5, mg/L).
Discussion
In the present study, the majority (79%) of 1663 COVID-19 patients were aged over 50 years old. A total of 2.8% were medical staff, and an exposure history of Huanan seafood market was document in 0.7%, and 7.4% were family infection. Fever (85.8%), cough (36.0%), fatigue (23.6%) and chest tightness (11.9%) were the most common symptoms in COVID-19 patients. A few people had symptoms such as diarrhea, dyspnea, loss of appetite, myalgia, dizziness/headache, pharyngalgia, nausea, vomiting, runny nose, and stuffy nose. Cluster analysis found that clinical symptoms could be divided into three categories: fever, cough and others (fatigue, chest tightness, diarrhea, dyspnea, loss of appetite, myalgia, dizziness/headache, pharyngalgia, nausea, vomiting, runny nose, and stuffy nose).
As of February 28, 2020, of the 1663 patients included in this study, 26.0% were discharged, 169 died (10.2%), and 63.8% remain hospitalized. More than 1/3 of the patients had at least one comorbidity. Most (99.8%) patients had abnormal results Chest CT, and the most common manifestations of chest CT were local patchy shadowing (70.7%) and ground-glass opacity (44.8%). On admission, lymphocytopenia was present in 51.1% of the patients, mononucleosis in 26.6%, and erythrocytopenia in 61.3%. Most of the patients had increased levels of C-reactive protein.
Daily numbers of COVID-19 confirmed cases by date of onset and by date of diagnosis were similar to previous studies [5]. During the traditional Chinese New Year (January 25, 2020–January 31, 2020), the number of onset cases was increasing, reaching the highest on February 1, which might be related to the large people movement during the Spring Festival. On the ninth day after the Spring Festival, SARS-CoV-2 confirmed positive patients reached the highest on February 9, which was consistent with previous findings that median incubation period of COVID-19 was 4 days (IQR, 2–7). Wuhan City shut down was conducive to COVID-19 prevention and control. The establishment of Wuhan’s Huoshenshan hospital, Wuhan’s Leishenshan hospital, and Wuhan Fangcang Hospital were of great significance to the prevention and control of epidemics. To prevent COVID-19, we should not go to densely populated areas.
The median age (64.0 y, IQR, 52.0−71.0) of all patients in the present study were older than other studies, which might be associated with the fact that more serious patients were admitted to Tongji hospital. Moreover, severe patients tended to be elder, when compared to non-severe patients, which was consistent with the previous findings [12]. In the present study, 2.8% of the patients were medical staff, and the percentage is lower than that reported by Wang et al., which might due to the size of the sample and policy of the hospital [14]. In the present study, we found that severe and non-severe patients had a significant difference regarding the median days from symptom onset to hospital admission and from onset of symptoms to COVID-19 confirmation, which might indicate that timely medical treatment after the onset might be beneficial to control the condition.
Hypertension, diabetes mellitus and cardiovascular diseases were the most common underlying diseases, consistent with other previous studies [[12], [13], [14]]. In addition, we found severe patients had higher proportion of comorbidity such as hypertension, diabetes and coronary heart disease, which was consistent with Guan’s report based on 1099 patients from 552 hospitals. However, Zhang et al. did not observed the similar findings, which might be associated with study sample size, different age and gender of the study subjects.
In line with previous study [[12], [13], [14],18], of 1663 patients in the present study, the main clinical symptoms were fever, cough, fatigue and chest tightness. We found serious patients had a higher proportion of symptoms such as fever, cough, fatigue, chest tightness and loss of appetite, which was consistent with the study conducted by Guan et al. based on 1099 patients. We still observed that serious patients had a higher proportion of symptoms such as fever, cough, and chest tightness after further excluding the patients with underlying diseased. However, several previous researches [[12], [13], [14]] did not found the significant difference. The most common manifestations of chest CT were local patchy shadowing and ground-glass opacity, which were consistent with previous studies [12,19]. Additionally, we observed that the severe patients had a higher proportion of symptoms such as pleural effusion and bronchiectasis, when compared with non-severe patients, which had not been reported yet.
Consistent with previous studies [[12], [13], [14],18], most of patients had normal white blood cell count, decreased levels of lymphocyte count/percentage, hemoglobin, and albulmin, increased levels of lactate dehydrogenase, C-reactive protein, increased D-dimer, procalcitonin, alanine aminotransferase, and aspartate aminotransferase. These abnormal laboratory findings showed sustained inflammatory response, disturbed coagulation mechanism, liver and kidney damage after patients infected [12]. Compared to non-severe patients, severe individuals tended to have higher levels of white blood cell count, neutrophil count/ percentage, platelet count, aspartate aminotransferase, urea, lactate dehydrogenase, D-dimer, and procalcitonin, which indicated severe patients might have more obvious inflammation, disturbed coagulation mechanism, liver and kidney damage. Previous study found that five COVID-19 patients younger than 50 years had a large-vessel stroke [20], which indicated that COVID-19 might increase the risk of cardiovascular disease. In the present study, we also found that non-severe and severe patients had higher levels of D-dimer. Additionally, we observed that leukocytosis and increased creatine kinase were positively associated with COVID-19 severity.
The strengths of present study included the relatively large sample size, and the ability to provide more information of epidemiological and clinical characteristics of patients infected with COVID-19. It would enable us to investigate the associations between potential risk factors and COVID-19 severity with moderate statistical power. Different from previous studies [15,21,22], the present study was the first comprehensive analysis of 1663 hospitalized patients with laboratory-confirmed diagnosis of COVID-19 from January 14, 2020 to February 28, 2020 in Tongji Hospital. Nonetheless, some limitations should be taken into consideration. Firstly, the present study was performed in single-center thus the findings may not be representative of general population. Secondly, we have not yet collected information on treatments in the present study.
Conclusions
In the present study, the majority of all COVID-19 patients were aged over 50 years old. Fever, cough, fatigue and chest tightness were the most common symptoms in COVID-19 patients. As of February 28, 2020, of the 1663 patients included in this study, 26.0% were discharged, 169 died (10.2%). Most patients had abnormal results Chest CT, and the most common manifestations of chest CT were local patchy shadowing and ground-glass opacity. Most patients with COVID-19 had higher levels of D-dimer, which might increase the risk of cardiovascular disease. Severe patients were more likely to be to be elder, and tended to have higher proportion of comorbidities and more prominent laboratory abnormalities. Older age, leukocytosis, and increased creatine kinase might help clinicians to identify severe patients with COVID-19.
Authors’ contributions
Caizheng Yu, Qing Lei, Wei Liu, and Wengang Li designed the study, interpreted data, and wrote the first draft of the paper; Caizheng Yu, Qing Lei, Wenkai Li and Xiong Wang took responsibility for the accuracy of the data analysis; Caizheng Yu, Qing Lei, Wei Liu, and Wengang Li performed data collection and designed the study’s analytic strategy. All authors have read and approved the final manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation (grant NSFC-51807078).
Conflict of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank all study subjects for participating in the present study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2020.07.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
Cluster diagram of clinical symptoms of patients with COVID-19.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus disease (COVID-19) outbreak https://www.who.int.
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020:2648. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Wong J.E.L., Leo Y.S., Tan C.C. COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 2020;323:1243–1244. doi: 10.1001/jama.2020.2467. [DOI] [PubMed] [Google Scholar]
- 7.Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 8.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C., Lei Q., Li W., Wang X., Liu W., Fan X. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. 2020;59:168–175. doi: 10.1016/j.amepre.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.New coronavirus pneumonia diagnosis and treatment plan (Fifth Edition) http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml.
- 17.New coronavirus pneumonia diagnosis and treatment plan (Sixth Edition) http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml.
- 18.Weijie Guan, Zhengyi Ni, Yu Hu, Wen-hua Liang, Chunquan Ou. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxley T.J., Mocco J., Majidi S., Kellner C.P. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cluster diagram of clinical symptoms of patients with COVID-19.