Abstract
Background and Purpose:
We aimed to compare functional and procedural outcomes of acute ischemic stroke patients with none-to-minimal (modified Rankin score, mRS, 0–1) and moderate (mRS 2–3) pre-stroke disability treated with mechanical thrombectomy (MT).
Methods:
Consecutive adult patients undergoing MT for an anterior circulation stroke were prospectively identified at two comprehensive stroke centers from 2012–2018. Procedural and 90-day functional outcomes were compared among patients with pre-stroke mRS 0–1 and 2–3 using Chi-squared, logistic, and linear regression tests. Primary outcome and significant differences in secondary outcomes were adjusted for pre-specified covariates.
Results:
Of 919 patients treated with MT, 761 were included and 259 (34%) patients had moderate pre-stroke disability. 90-day mRS 0–1 or no worsening of pre-stroke mRS was observed in 36.7% and 26.7% of patients with no-to-minimal and moderate pre-stroke disability, respectively (OR 0.63 [0.45–0.88], p=0.008; adjusted OR 0.90 [0.60–1.35], p=0.6). No increase in the disability at 90 days 22.4% and 26.7%. Rate of symptomatic intracerebral hemorrhage (7.3% vs 6.2%, p=0.65), successful recanalization (86.7% vs 83.8%, p=0.33), and median length of hospital stay (5 vs 5 days, p=0.06) were not significantly different. Death by 90-days was higher in patients with moderate pre-stroke disability (14.3% vs 40.3%, OR 4.06[2.82–5.86], p<0.001; adjusted OR 2.83[1.84, 4.37], p<0.001).
Conclusions:
One-third of patients undergoing MT had a moderate pre-stroke disability. The odds of maintaining pre-stroke functional status at 90-days and procedural success rates were not different between patients with no-to-minimal and moderate pre-stroke disability. However, patients with pre-stroke disability were more likely to die by 90 days.
Search Terms: All Cerebrovascular disease/Stroke, Infarction, Acute Ischemic Stroke, Mechanical Thrombectomy, Disability
BACKGROUND
Currently, the American Heart Association / American Stroke Association guidelines recommend offering a mechanical thrombectomy (MT) to patients with an acute ischemic stroke (AIS) involving a large anterior cerebral vessel occlusion but without a pre-stroke disability, defined as pre-stroke modified Rankin Score (mRS) of 0 or 1.1 This guideline reflects the patient selection criteria used in the initial MT efficacy clinical trials, which excluded patients with a pre-stroke mRS ≥2, except the DEFUSE 3 trial that allowed for inclusion of patients with pre-stroke mRS 2.2–6 Thus, there is a paucity of data for the utility of MT in AIS patients with pre-stroke disability.7 Overall, any worsening in the functionality of pre-stroke disabled patients incurs higher rates of institutionalization, mortality, and care costs.8 Exclusion of disabled patients from this highly-effective treatments may be counterproductive. However, before MT can be universally offered to disabled patients, a quantitative understanding of the efficacy and safety of MT in the pre-stroke disabled population is necessary. Here, we analyzed patients consecutively-treated with an MT at two US comprehensive stroke centers to understand 1) the composition of patients with no-to-minimal and moderate pre-stroke disability treated with an MT in practice and 2) the differences in functional and procedural outcomes between these two groups. Based on existing data comparing outcomes of intravenous thrombolysis in these two patient populations,9–12 we hypothesized that the proportion of patients who suffer a functional decline after an MT in these two groups of patients will be comparable to each other.
METHODS
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
We conducted an observational study of prospectively-identified, consecutive, acute ischemic stroke patients treated with an MT at two comprehensive stroke centers in the United States from 2012 to 2018 at one and from 2015 to 2018 at the second institution. The study was approved by each institution’s Review Board and formal patient consent was waived. All consecutive adult (≥18 years) patients treated with an MT for an anterior circulation large vessel occlusion stroke with a pre-stroke mRS ≤3 were included. Patients without a pre-stroke functional status assessment, those with a severe pre-stroke disability (mRS ≥4), and those with ≥2 points improvement in mRS from the pre-stroke baseline to 90 days were excluded. The latter criterion was motivated by concerns that such a drastic improvement in pre-stroke disability could represent either a transient pre-stroke disability or an interrater discrepancy in mRS scoring.
Study Variables
The primary study variable was pre-stroke disability, divided into two groups based on mRS: no-to-minimal (mRS 0–1) and moderate (mRS 2–3). Pre-stroke mRS score was ascertained primarily from the formal assessment of the pre-stroke functional status by an occupational or physical therapist, and when necessary, in conjunction with notes of other clinicians. The pre-stroke mRS scores were defined as: 0=no symptoms, 1=minimal but functionally independent and able to carry out all activities, 2=functionally independent but unable to carry out all activities, 3=functionally dependent but able to walk without assistance from another individual, 4= functionally dependent and requires significant assistance to ambulate, and 5=bedridden. Patients’ baseline characteristics and medical history, imaging variables, and MT procedural metrices were obtained from prospectively collected institutional databases.
Outcome Measures
The primary outcome measure was an excellent functional outcome, defined as 90-day mRS of 0 or 1 or no accumulation of disability. Secondary outcomes were no accumulation of disability at 90 days (defined as no increase in the pre-stroke mRS score at 90 days), patient-centered change in the functional baseline (defined as the mean change in the utility-weighted mRS), using the standard utility weights applied to pre- and post-stroke mRS,13 successful recanalization (defined by a modified thrombolysis in cerebral ischemia score, mTICI, ≥2b), symptomatic intracranial hemorrhage, length of hospital stay, and death at 90 days (mRS 6). Symptomatic hemorrhage was defined as hemorrhage associated with a 4-point increase in the baseline NIHSS score.14
Statistical Analysis
The baseline characteristics between the group of patients with no-to-minimal (mRS 0–1) and moderate (mRS 2–3) pre-stroke disability were compared using a Chi-squared test, t-test, or Mann-Whitney-U test, as appropriate. Chi-square and logistic regression were used to determine associations between pre-stroke disability with categorical outcomes and linear regression was used for continuous outcomes. The primary outcome and any statistically significant differences in the secondary outcomes were adjusted for the following co-variables using a logistic regression model: age, sex, initial glucose, NIHSS on presentation, time to recanalization, intravenous thrombolysis, Alberta Stroke Program Early CT (ASPECT) score, and successful recanalization. To explore for heterogeneity in the primary outcome, an institution-dependent interaction analysis was performed. A sensitivity analysis was undertaken to only include patients without any improvement in their 90-day mRS from baseline and those >80 years in age. In an exploratory analysis compared the outcomes of patients with pre-stroke mRS 0–2 vs 3. For any outcome that significantly correlated with pre-stroke disability on both univariate and multivariate analyses, propensity score matching (using nearest neighbor propensity score matching) was undertaken to balance the differences in covariables between the two groups of patients. All analyses and plots were generated using R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance α was set at < 0.05 for all statistical analyses. All p-values are 2‐sided, unless otherwise stated. All effect sizes are reported with 95% confidence intervals in addition to p-values.
RESULTS
Patient Characteristics
The workflow outlining patient selection for analysis is depicted in Figure 1. Briefly, a total of 919 patients were consecutively treated with an MT at two institutions, of which 761 met our inclusion and exclusion criteria for analysis. 90-day follow-up was available for 469/502 (93.4%) of patients with no-to-minimal (mRS 0–1) and 243/259 (94%) of those with moderate (mRS 2–3) pre-stroke disability (n=107, 41%,with pre-stroke mRS 2; n=152, 59%, with pre-stroke mRS 3). Patients with moderate pre-stroke disability had a higher median age and a lower proportion of them were males (Table 1). Hypertension, atrial fibrillation, anticoagulant, and antiplatelet use was more frequent in this group. Patients with moderate disability were less frequently treated with intravenous thrombolytics and had a higher baseline NIHSS and initial blood glucose. There were no significant differences in ASPECTS score, location of the large vessel occlusion, or time from stroke onset to recanalization. Yearly rate of MT and proportion of pre-stroke disabled patients among those are outlined in the Supplemental Figure I.
Figure 1. Workflow for patient selection.
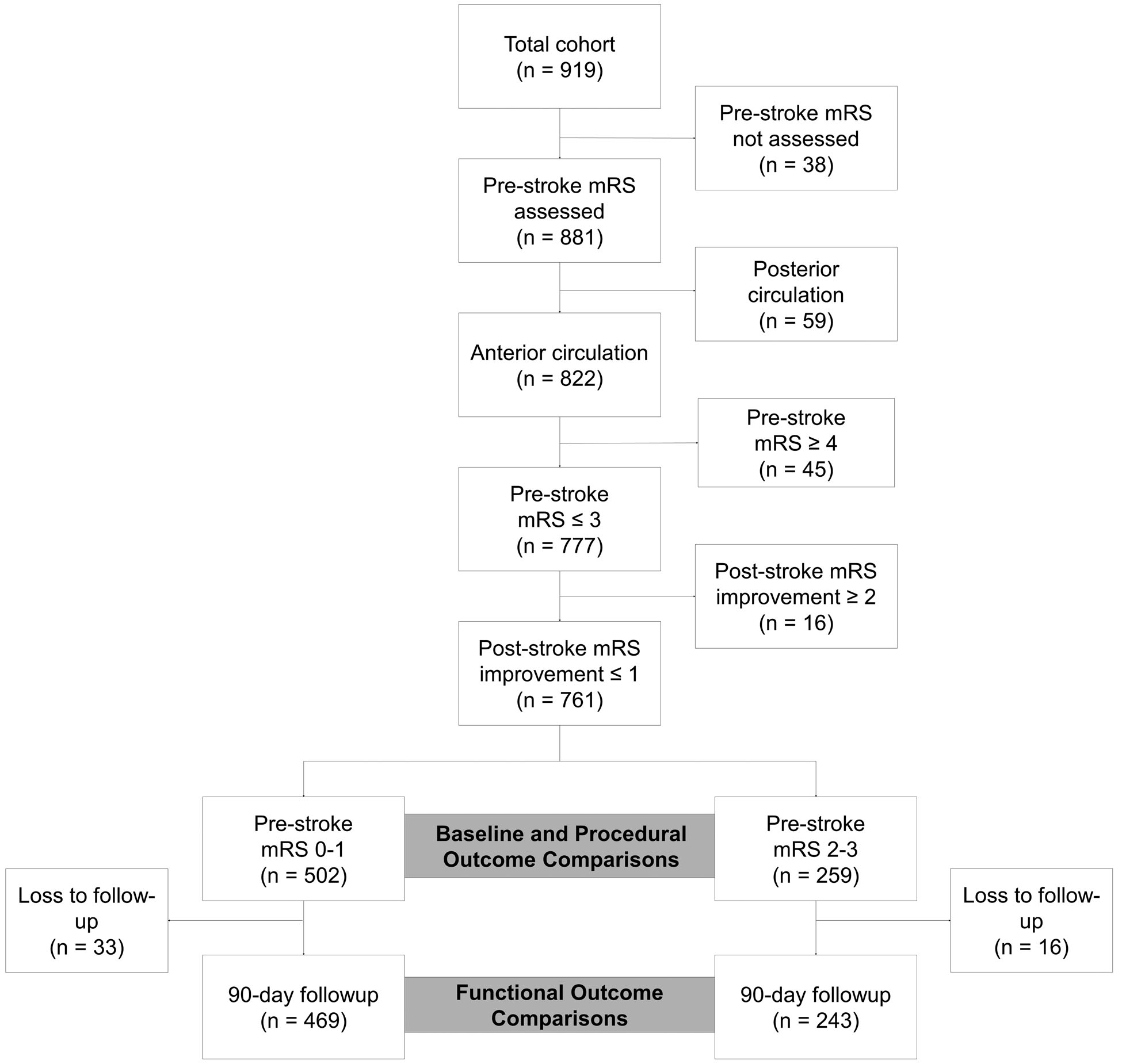
Table 1.
Comparisons of baseline demographic and stroke presentation characteristics between patients with and without moderate pre-stroke disability.
| mRS 0–1 (n=502) | mRS 2–3 (n=259) | P-value* | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age, year, median [IQR] | 67 [57–77] | 80 [67–88] | <0.001 |
| Males, n (%) | 268 (53.4) | 96 (37.1) | <0.001 |
| Hypertension, n (%) | 348 (69.3) | 221 (85.3) | <0.001 |
| Diabetes, n (%) | 112 (22.3) | 72 (27.8) | 0.11 |
| Atrial Fibrillation, n (%) | 170 (33.9) | 147 (56.8) | <0.001 |
| Antiplatelet use, n (%) | 187 (37.3) | 123 (47.5) | 0.004 |
| Anticoagulant use, n (%) | 63 (12.5) | 49 (18.9) | 0.001 |
| Stroke Presentation | |||
| Initial blood glucose, mg/dL, median [IQR] | 119 [103–145] | 127 [104–164] | 0.008 |
| NIHSS, median [IQR] | 15 [10–20] | 17 [12–22] | 0.001 |
| ASPECTS, median [IQR] | 9 [8–10] | 9 [8–10] | 0.66 |
| Proximal vessel occlusion, n (%) | 0.57 | ||
| A1/A2 | 1 (0.2) | 2 (0.8) | |
| ICA | 106 (21.1) | 48 (18.5) | |
| M1 | 310 (61.8) | 161 (62.2) | |
| M2 | 84 (16.7) | 47 (18.1) | |
| M3 | 1 (0.2) | 1 (0.4) | |
| Time from symptom onset to recanalization, minutes, median [IQR] | 289.5 [198.75–458.5] | 273.5 [190.0–431.5] | 0.35 |
| Intravenous thrombolysis, n (%) | 263 (52.4) | 113 (43.6) | 0.03 |
Non-parametric or Chi-square test
Primary Outcome: Excellent Functional Outcome
A greater proportion of patients with no-to-minimal pre-stroke disability achieved an excellent functional outcome (defined as 90-day mRS of 0–1, or no worsening of pre-stroke mRS) than those with moderate pre-stroke disability (172/469, 36.7%, vs. 65/243, 26.7%, respectively; odds ratio, OR, with 95% confidence interval: 0.63 [0.45–0.88], p=0.008; Table 2). This difference was not significant in a logistic regression model adjusted for covariables including age, sex, initial glucose, NIHSS on presentation, time to recanalization, intravenous thrombolysis, ASPECT score, and successful recanalization, yielding an adjusted OR of 0.90 [0.60–1.35], p=0.6. Given the imbalances in baseline demographics and stroke characteristics between no-to-minimal and moderate disability groups, a propensity score matched analysis was further pursued. Propensity matching resulted in 226 patients per group, where sex, hypertension, diabetes, atrial fibrillation, antiplatelet use, anticoagulant use, glucose, NIH stroke scale, ASPECT score, time to recanalization, stroke laterality, and intravenous thrombolytic administrated were well balanced. The only variable with an imbalance was age, which remained higher in the moderate disability group (mean 76.6 ± SD 14.3 vs. 74.2±11.3, p=0.04). Patients with moderate disability continued to have a similar odds of having an excellent functional outcome at 90 days in the propensity matched analysis with an OR 1.16 [0.75– 1.8], p= 0.49. Interestingly, among pre-stroke disabled patients, 6/41 (15%) with unsuccessful recanalization achieved an excellent functional outcome compared to 59/217 (29%) of those with successful recanalization (p=0.08; OR 2.32 [0.99–6.40], p=0.073; aOR 2.87 [1.02–10.36], p=0.07)
Table 2.
Unadjusted comparisons of outcomes between patients with and without moderate pre-stroke disability.
| mRS 0–1 | mRS 2–3 | Odds ratio* [95% CI] | P-value | Adjusted Odds Ratio| | [95%CI] | |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| mRS 0–1 or No disability accumulated, n/total (%) | 172/469 (36.7) | 65/243 (26.7) | 0.63 [0.45–0.88] | 0.008 | 0.90 [0.60–1.35] |
| Secondary Outcomes | |||||
| No disability accumulated, n/total (%) | 105/469 (22.4) | 65/243 (26.7) | 1.27 [0.88–1.81] | 0.20 | 1.90 [1.24–2.94] |
| Change in UW-mRS, mean±SD / total | −0.35±0.35 / 469 | −0.38±0.32 / 243 | −0.04 [−0.09–0.02]† | 0.17‡ | |
| Death, n/total (%) | 67/469 (14.3) | 98/243 (40.3) | 4.06 [2.82–5.86] | <0.001 | 2.95 [1.84–4.78] |
| Length of hospital stay, days, median [IQR] / total | 5 [3–8] / 502 | 5 [3–9] / 259 | 0.90 [−0.07–1.86]† | 0.06§ | |
| Procedural Outcomes | |||||
| Symptomatic ICH, n/total (%) | 36/496 (7.3) | 16/259 (6.2) | 0.84 [0.45–1.52] | 0.56 | |
| mTICI 2b-3, n/total (%) | 435/502 (86.7) | 217/259 (83.8) | 0.80 [0.53–1.22] | 0.28 |
Ratio of odds in mRS 2–3 to odds in mRS 0–1 group with corresponding p-value of a Fisher’s exact test are provided.
Numerical change in the outcome from mRS 0–1 to mRS 2–3 modeled by unadjusted linear regression.
T-test and
Mann-Whitney U test.
Adjusted for age, sex, initial glucose, NIHSS on presentation, time to recanalization, intravenous thrombolysis, ASPECT score, and successful recanalization
Secondary Outcomes
A total of 105/469 (22.4%) patients with no-to-minimal pre-stroke disability and 65/243 (26.7%) patients with moderate pre-stroke disability had no increase in their pre-stroke disability at 90 days. These proportions were not significantly different (p=0.2). Compared to patients with no-to-minimal pre-stroke disability, the odds of accumulating additional disability for patients with moderate pre-stroke disability did not significantly differ (OR 1.27 [0.88–1.81], p=0.2; Table 2; Figure 2). When this association was adjusted for the pre-specified covariables, an aOR of 1.90 [1.24–2.94], p=0.004, was obtained (Supplemental Table I). This significant result was very likely deemed to be a statistical manifestation of the suppressive effect of age and baseline NIHSS on the association of pre-stroke disability with the primary outcome. Patient with no-to-minimal pre-stroke disability experienced a similar mean change in utility weighted mRS at 90 days (−0.35±SD 0.35) as patients with moderate pre-stroke disability (−0.38 ±0.32). Numerical change in utility weights modeled by a linear regression revealed a minimal change (−0.04 [−0.09–0.02], p=0.17, Table 2). However, mortality at 90 days was significantly higher in patients with moderate pre-stroke disability compared to those with no-to-minimal pre-stroke disability (98/243, 40.3%, vs 67/469, 14.3%; OR 4.06 [2.82–5.86], p<0.001; Figure 2; Table 2). After adjusting with the pre-specified co-variables, this result remained unchanged (adjusted OR 2.83 [1.84–4.37], p<0.001; Supplemental Table II). Mortality at 90 days remained significantly higher in patients with moderate pre-stroke disability (OR 3.17 [2.07–4.95], p<0.001; adjusted OR 2.95 [1.84–4.78], p<0.001) in the propensity matched analysis.
Figure 2. Distribution of 90-day modified Rankin Score according to patients’ pre-stroke disability.
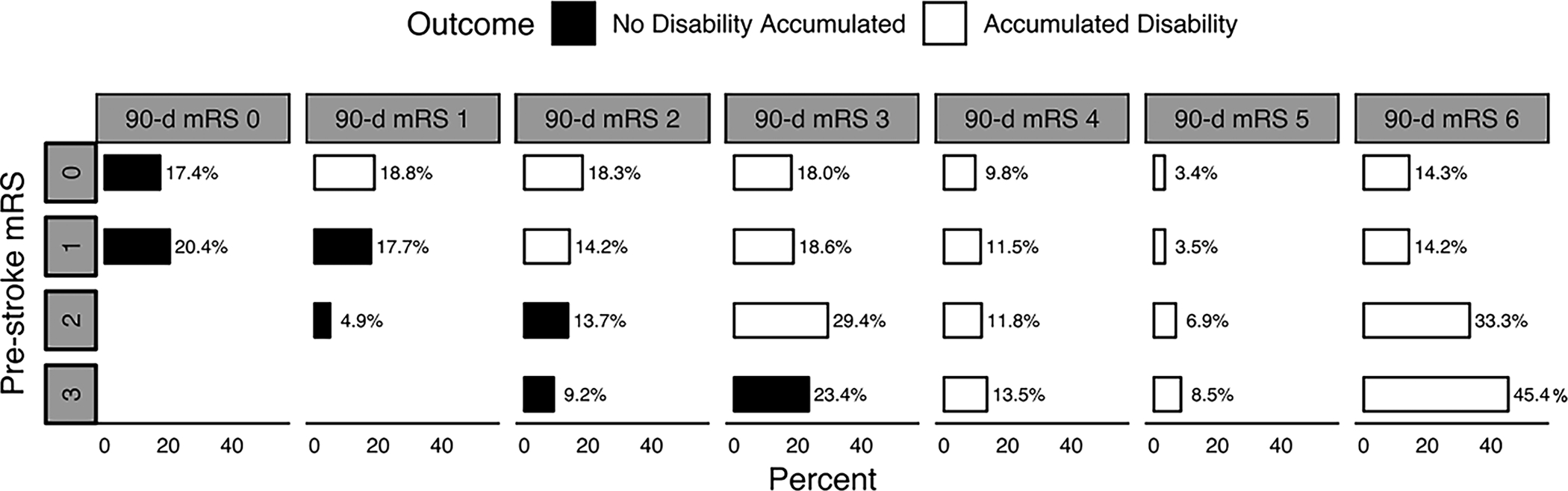
Solid blocks denote no accumulation of disability and open blocks denote presence of additional disability or death.
Patients with no-to-minimal disability experienced similar rates of symptomatic intracranial hemorrhage (36/496, 7.3%) compared to patients with moderate pre-stroke disability (16/259, 6.2%; OR 0.84 [0.45–1.52], p=0.65). Success rates of recanalization were also similar (435/502, 86.7%, vs. 217/259, 83.8%, respectively, OR 0.80 [0.53–1.22], p=0.28). Length of hospital stay was not significantly different between patient groups (5 [IQR: 3–8] vs. 5 [3–9] days, respectively, p=0.06). In a subanalysis excluding patients who died, which leads to a lower length of stay, the length of stay was significantly different although the distribution characteristics were similar (5 [3–8] vs. 5 [3–9] days, p=0.03).
Sensitivity analysis revealed a significant interaction according to the institution. The unadjusted ORs for the primary outcome (no increase in disability) in two subsets based on the institution were 1.6 [0.94–2.71], p=0.08, and 0.81 [0.47–1.37], p=0.05. A test for interaction yielded a p-value of 0.05 (Supplemental Figure II). In the sensitivity analysis including only those patients without any improvement in their 90-day mRS from baseline (n=720), no increase in pre-stroke disability at 90 days was observed in 82/479 (17%) patients with no-to-minimal pre-stroke disability and 47/241 (20%) patients with moderate pre-stroke disability (p=0.45). Overall, the OR of having no increase in the pre-stroke disability at 90 days remained in this population was similar to the overall population (unadjusted OR 1.17 [0.78–1.74], p=0.44; adjusted OR 1.97 [1.22–3.2]; p=0.006). Among patients >80 years in age (n=225), no increase in pre-stroke disability at 90 days was observed in 13/97 (15%) patients with no-to-minimal pre-stroke disability and 29/128 (24%) patients with moderate pre-stroke disability (p=0.12), resulting in an unadjusted OR of 1.8 [0.89–3.81], p=0.11 and adjusted OR of 2.33 [0.99, 5.73], p=0.45. Among patients with pre-stroke mRS 3, excellent functional outcome was observed in 46/152 (32.6%) of the patients compared to 191/609 (33.5%) of those with pre-stroke mRS 0–2 (unadjusted OR 0.96 [0.65–1.42], p=0.852. No increase in disability was observed in 46/152 (32.6%) of those with pre-stroke mRS 3 compared to 191/609 (33.5%) patients with pre-stroke mRS 0–2 (unadjusted OR 1.75 [1.16–2.63], p=0.007; adjusted OR 3.12 [1.89–5.26], p<0.001; Supplemental Table-III). Other outcomes among pre-stroke mRS 3 group were not significantly different.
DISCUSSION
In this observational study of consecutive acute ischemic stroke patients treated with an MT at two large comprehensive stroke centers, almost 1 in 3 patients had an existing pre-stroke disability and a distinct baseline characteristic profile. These practical data provided us the opportunity to study the effect of MT in patients with moderate pre-stroke disability. Despite their different baseline characteristics compared to patients with no-to-minimal pre-stroke disability, patients with moderate pre-stroke disability had similar odds of accumulating additional disability after an MT as measured on the mRS scale. In other words, the likelihood of retaining their functional baseline was similar to patients with no-to-minimal pre-stroke disability. However, patients with moderate pre-stroke disability experienced a significantly greater mortality at 90 days. Other common outcomes such as procedural success, length of hospital stay, and complications between these two groups were not significantly different. A trend towards better outcome was observed with successful recanalization among patients with a pre-stroke disability. Additionally, there was insufficient evidence that outcomes of patients with a pre-stroke mRS 3 were worse than those with a pre-stroke mRS 0–2.
Several characteristics of patients with moderate pre-stroke disability were different. Collectively, they were older with a higher proportion of females, had a greater number of comorbidities, and generally presented with higher NIHSS. This profile is consistent with prior population-based studies of patients with pre-stroke disability, although not treated with an MT.15, 16 Recent studies have shown that MT is highly effective in improving outcomes of older patients.17, 18 While pre-stroke disability, just like older age and female sex, is a risk factor for worse outcomes after stroke, our data strongly suggests that MT may remain effective in this group19, 20.
Patients with pre-stroke disability represent a significant proportion of the acute ischemic stroke population, ranging from 10–30% at the initial presentation.8, 11 Previous studies have shown that recanalization therapy with intravenous thrombolysis is efficacious in these patients.9–12 The current acute stroke guidelines recommend considering intravenous thrombolytic therapy for patients with pre-existing disability after considering the patient’s quality of life, goals of care, and social factors.1 A recent multi-society consensus statement recommended expanding patients eligible for mechanical thrombectomy on an institutional basis based on the finding that a significant proportion of patients treated with mechanical thrombectomy beyond guideline eligibility criteria have comparable outcomes to those that specifically meet guideline criteria.21, 22 These studies may provide a precedent for treatment of acute ischemic stroke patients with pre-stroke disability and a framework for the shared-decision making process for treatment considerations.
The currently available data evaluating outcomes of pre-stroke disabled, MT-treated patients are sparse and conflicted. Our results agree with one prior study which concluded that a substantial portion of patients with pre-existing disability have a good functional outcome at 90 days and that their functional outcomes were directly correlated with reperfusion status, suggesting these patients can benefit from MT.7 This study reports practices in Europe with different patient populations, cost per procedure, and stroke system dynamics than the United States.23–26
Here, we introduce a novel approach to defining a good clinical outcome after MT that uniquely considers patients’ pre-stroke disability. The conventional dichotomous definition of a good functional outcome (mRS 0–2) is disadvantageous to patients with a pre-stroke disability as it would require, for instance in patients with a pre-stroke mRS of 3, an improvement beyond their baseline functional status. Therefore, we posit that a good outcome in these patients must account for retention of the functional baseline, or no increase in disability, as defined in this study. Defined as such, any increase in mRS at 90-day mRS from the pre-stroke baseline is considered a bad outcome. This definition, though, unconventionally assigns a bad outcome to patients with a pre-stroke mRS 0 who are mRS 1 at 90 days post-MT. Therefore, we secondarily defined excellent functional outcome as 90-day mRS 0–1 or no increase in disability. Although this outcome occurred less frequently in patients with pre-stroke disability, an adjusted analysis revealed similar odds of having an excellent functional outcome between patients with and without pre-stroke disability. Lastly, utility-weighted mRS may also serve as an appropriate means to compare outcomes because it is a more patient-centered, value-based approach. Our data showed no differences in the change of utility-weighted mRS between patients with and without pre-stroke disability.
These results have implications for both research and clinical practice. Patients with pre-stroke disability are most often excluded from research studies and clinical trials. This not only limits research enrollment, but also perpetuates the vacuum of research on disabled patients. Although recent trials are increasingly including patients with a pre-stroke mRS 2, those with mRS 3 are still widely excluded from clinical research.27, 28 In practice, physicians are often faced with the dilemma of making MT-related treatment decisions for patients with pre-stroke disability. Plausible reasons for this dilemma include the current explicit guideline recommendations and the lack of data on the success and complications of MT in these patients, raising complexity surrounding the informed consent process. Together with prior European studies, our data may provide guidance, especially to the U.S. practitioners, in making MT-related decisions and having informed discussions about outcomes and procedural complications in patients with pre-stroke disability. Importantly, we hope these collective data serve to prompt a randomized trial to formally the effectiveness of MT in patients with pre-stroke disability.
Even if MT is offered, however, the cost effectiveness is unknown in patients with prior disability. The median length of incident hospitalization was similar between MT-treated patients with and without existing disability in our study. Inferentially, MT may be cost-effective in patients with pre-stroke disability, at least immediately.29 Further dedicated analyses with decision tree modelling to determine the cost-effectiveness of MT in this population is necessary and should account for indirect costs (e.g. caregiver costs). These analyses should account if the goals of care differences among these groups of patients explain the higher mortality in pre-stroke disabled patients despite no significant differences in symptomatic hemorrhage and recanalization rates.
While our study is powered by a large dataset, representing practices of two geographically-different, high-volume centers in the United States, some limitations deserve mention. Importantly, data herein should be interpreted with limitations inherent to the retrospective observational design of this study. Specifically, there is a risk of bias in our data for favoring selection of patients with pre-stroke disability perceived by the treating physician to most likely benefit from an MT. For instance, patients could be selected based on a perceived transient disability as opposed to a permanent disability, which could result in an improvement of pre-stroke disability by 90 days post-MT. To guard against this, we specifically excluded patients with an improvement of >1 point on the mRS score. An improvement of 1 point is well within the inter-rater reliability of the mRS scale.30 Additionally, our study may be underpowered to make concrete non-inferiority conclusions. Inter-institutional differences in our primary outcome, as highlighted by the interaction analysis, may slightly limit the generalizability of our results. Notably, we found a near-significant inter-institutional heterogeneity in the primary outcome with opposite directions of EVT treatment effect, underpinning the importance of differences in institution-level factors (such as operator expertise, patient population, post-EVT care etc.) in outcomes of disabled patients. Lastly, true treatment effect of MT in pre-stroke disabled patients can only be revealed by a direct comparison of treated vs untreated. Large, prospective, multicenter, randomized studies are necessary to address these limitations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Rut Thakkar for assistance in data collection.
SOURCES OF FUNDING
Dr. E. Mistry reports funding from National Institute of Health/ National Institute of Neurological Disorders and Stroke (K23NS113858).
DISCLOSURES
Dr. Froehler serves as a consultant for Medtronic, Stryker, Microvention, Cerenovus, Corindus, Genentech, Balt, and Viz.ai; and receives grant funding from Stryker, Medtronic, Microvention, Penumbra, Genentech, and EndoPhys. Dr. Chitale receives modest research funding from Cerenovus and Medtronic. Dr. Schrag reports grants from National Institutes of Health (NIH) 1K76AG060001, 1R03NS111486, and 1R21NS106510 outside the submitted work. Dr. Jasne reports other from medtronic outside the submitted work. Dr. Madsen has modest funding from NHLBI. Dr. Yaghi reports other from Medtronic outside the submitted work. Dr Jayaraman reports personal fees from Medtronic outside the submitted work. Dr. Khatri reports grants from Cerenovus, consulting fees from Lumosa, and grants from Nervive outside the submitted work. Dr. Mistry reports grants from NIH/National Institute of Neurological Disorders and Stroke (K23 113858) during the conduct of the study. Ms. Salwi and Espaillat have no disclosures. Drs. Cutting, Salgado, Fusco, Kirshner, Schrag, Jasne, Burton, MacGrory, Saad, Jayaraman, Madsen, Dakay, McTaggart, Yaghi, and A. Mistry have no disclosures.
REFERENCES
- 1.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:46–110 [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. New England Journal of Medicine. 2018;378:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. The New England Journal of Medicine. 2015;372:2296–2306 [DOI] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. New England Journal of Medicine. 2017 [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-Retriever Thrombectomy after Intravenous T-Pa Vs. T-Pa Alone in Stroke. New England Journal of Medicine. 2015;372:2285–2295 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. The New England Journal of Medicine. 2015;372:11–20 [DOI] [PubMed] [Google Scholar]
- 7.Goldhoorn R-JB, Verhagen M, Dippel DWJ, van der Lugt A, Lingsma HF, Roos YBWEM, et al. Safety and Outcome of Endovascular Treatment in Prestroke-Dependent Patients. Stroke. 2018;49:2406–2414 [DOI] [PubMed] [Google Scholar]
- 8.Ganesh A, Luengo-Fernandez R, Pendlebury ST, Rothwell PM. Long-Term Consequences of Worsened Poststroke Status in Patients with Premorbid Disability. Stroke. 2018;49:2430–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foell RBT, Silver B, Merino JG, Wong EH, Demaerschalk BM, Poncha F, et al. Effects of Thrombolysis for Acute Stroke in Patients with Pre-Existing Disability. Canadian Medical Association Journal. 2003;169. [PMC free article] [PubMed] [Google Scholar]
- 10.Gensicke H, Strbian D, Zinkstok SM, Scheitz JF, Bill O, Hametner C, et al. Intravenous Thrombolysis in Patients Dependent on the Daily Help of Others before Stroke. Stroke. 2016:450–456 [DOI] [PubMed] [Google Scholar]
- 11.Karlinski M, Kobayashi A, Czlonkowska A, Mikulik R, Vaclavik D, Brozman M, et al. Role of Pre-Existing Disability in Patients Treated with Intravenous Thrombolysis for Ischemic Stroke. Stroke. 2014;45:770–775 [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Coote S, Frost T, Dewey HM, Choi PMC. Acute Stroke Patients with Mild-to-Moderate Pre-Existing Disability Should Be Considered for Thrombolysis Treatment. Journal of Stroke and Cerebrovascular Diseases. 2018;27:2707–2711 [DOI] [PubMed] [Google Scholar]
- 13.Chaisinanunkul N, Adeoye O, Lewis RJ, Grotta JC, Broderick J, Jovin TG, et al. Adopting a Patient-Centered Approach to Primary Outcome Analysis of Acute Stroke Trials Using a Utility-Weighted Modified Rankin Scale. Stroke. 2015:2238–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rüdiger Vk, BJ P, CB CV, Andrew D, Mayank G, HM D, et al. The Heidelberg Bleeding Classification. Stroke. 2015;46:2981–2986 [DOI] [PubMed] [Google Scholar]
- 15.Gill Thomas M. MD, Gahbauer Evelyne A. MD, MPH, Lin Haiqun MD, Ph.D., Han Ling MD, Ph.D., Allore Heather G. PD. Comparisons between Older Men and Women in the Trajectory and Burden of Disability over the Course of Nearly 14 Years. Journal of the American Medical Directors Association. 2014;14:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallas MI, Rone-adams S, Echternach JL, Brass LM, Bravata DM. Dependence in Prestroke Mobility Predicts Adverse Outcomes among Patients with Acute Ischemic Stroke. Stroke. 2008:2298–2303 [DOI] [PubMed] [Google Scholar]
- 17.Mohlenburch M, Pfaff J, Schonenberger S, Nagel S, Bosel J, Herweh C, et al. Endovascular Stroke Treatment of Nonagenarians. American Journal of Neuroradiology. 2017;38:299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal M, Menon BK, Zwam WHV, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular Thrombectomy after Large-Vessel Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Five Randomised Trials. Lancet. 2016:1723–1731 [DOI] [PubMed] [Google Scholar]
- 19.de Ridder IR, Fransen PSS, Beumer D, Berkhemer OA, van den Berg LA, Wermer MJ, et al. Is Intra-Arterial Treatment for Acute Ischemic Stroke Less Effective in Women Than in Men. Interventional Neurology. 2016;5:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sennfält S, Norrving B, Petersson J, Ullberg T. Long-Term Survival and Function after Stroke. Stroke. 2019;50:53–61 [DOI] [PubMed] [Google Scholar]
- 21.Bhole R, Goyal N, Nearing K, Belayev A, Doss VT, Elijovich L, et al. Implications of Limiting Mechanical Thrombectomy to Patients with Emergent Large Vessel Occlusion Meeting Top Tier Evidence Criteria. Journal of Neurointerventional Surgery. 2017:225–228 [DOI] [PubMed] [Google Scholar]
- 22.Siegler JE, Messé SR, Sucharew H, Mehta T, Arora N, Starosciak AK, et al. Thrombectomy Is Safe for Dawn- and Defuse-3-Ineligible Patients Who Present within the Extended Window: A Subgroup Analysis from the Best Prospective Cohort Study. Stroke. 2019;50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevick LK, Ghali S, Hill MD, Danthurebandara V, Lorenzetti DL, Noseworthy T, et al. Systematic Review of the Cost and Cost-Effectiveness of Rapid Endovascular Therapy for Acute Ischemic Stroke. Stroke. 2017;48:2519–2526 [DOI] [PubMed] [Google Scholar]
- 24.Kim AS, Johnston SC. Temporal and Geographic Trends in the Global Stroke Epidemic. Stroke. 2013;44:123–125 [DOI] [PubMed] [Google Scholar]
- 25.Ovbiagele B, Nguyen-Huynh MN. Stroke Epidemiology: Advancing Our Understanding of Disease Mechanism and Therapy. Neurotherapeutics. 2011;8:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim AS, Nguyen-Huynh M, Johnston SC. A Cost-Utility Analysis of Mechanical Thrombectomy as an Adjunct to Intravenous Tissue-Type Plasminogen Activator for Acute Large-Vessel Ischemic Stroke. Stroke. 2011;42:2013–2018 [DOI] [PubMed] [Google Scholar]
- 27.Direct Transfer to an Endovascular Center Compared to Transfer to the Closest Stroke Center in Acute Stroke Patients with Suspected Large Vessel Occlusion (Racecat). Clinicaltrials.gov Identifier: NCT02795962.
- 28.Efficacy and Safety of Thrombectomy in Stroke with Extended Lesion and Extended Time Window (Tension). ClinicalTrials.gov Identifier: NCT03094715. [DOI] [PMC free article] [PubMed]
- 29.Zhai S, Gardiner F, Neeman T, Jones B, Gawarikar Y. The Cost-Effectiveness of a Stroke Unit in Providing Enhanced Patient Outcomes in an Australian Teaching Hospital. Journal of Stroke and Cerebrovascular Diseases. 2017;26:2362–2368 [DOI] [PubMed] [Google Scholar]
- 30.Wilson JTL, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the Modified Rankin Scale across Multiple Raters. Stroke. 2005;36:777–781 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


