Abstract
This study evaluated the topical delivery of nordihydroguaretic acid (NDGA), a molecule that can potentially alleviate cutaneous damage caused by exposure to arsenic warfare chemicals. N-acetylcysteine (NAC 0.2% w/v) was added as an antioxidant, preventing the oxidation of NDGA to toxic quinones. A 24 h study was performed to arrive at a minimum concentration of NDGA needed to deliver maximum drug. A solution of 3% w/v delivered the maximum amount of drug at the end of 24 h (37.45 ± 4.32 μg). Short duration studies were carried out to determine the time needed to saturate skin with NDGA. There was no significant difference in the skin concentrations for 24 h and 8 h (14.89 ± 2.36 μg), due to skin saturation. However, there was significant difference in the amount of drug delivered to the epidermis (12.29 ± 1.87 μg) and dermis (2.54 ± 0.56 μg) at the end of 8 h. Solution of NDGA was applied on UV treated skin to assess changes in drug delivery. In vivo studies revealed that 3% NDGA was non-toxic for topical administration.
Keywords: Nordihydroguaretic acid, N-acetylcysteine, Skin saturation, Short duration study, UV treated Skin
1. Introduction
Arsenic, a member of the Group V of the periodic table is known for being a highly toxic element [1]. Historically, its compounds have been used for a variety of purposes like pesticides, medicines, doping agent in semiconductors and as an additive to glass and wool. However, lewisite, the lethal organoarsenic compound was specially synthesized for its use in World War I/II [2]. These toxic organo-arsenicals are commonly known as vesicants. They are rapidly absorbed through skin and mucous membrane causing painful inflammation including edema, erythema and blisters. Signs of burning and erythema occur in a few minutes of exposure to these lewisites and large, fluid filled sacs become visible on skin within 24 h after exposure [3]. The levels of reactive oxygen species (ROS) is also observed to increase rapidly followed by an inflammatory response [4]. There are reports of arsenic accumulating predominantly in the epidermis and causing extensive necrosis to the epidermal layers [5].
However, arsenic exposure is not just limited to the catastrophic warfare chemicals. Arsenicals and their derivatives contaminate the water table and soil, eventually accumulating in the food chain. Soil contamination paves way for entry of inorganic arsenic in the ground water that eventually leads to the human population consuming arsenic unknowingly [6]. In several countries, arsenic contaminated ground water is a major concern [7]. Skin being the primary organ where arsenic toxicity is often manifested first, there is a need to alleviate the cutaneous effects exerted by arsenicals.
Nordihydroguaretic acid (NDGA), is polyphenol with a o-dihydroxy structure. This catechol obtained from the creosote bush, Larrea tri-dentate, has a wide range of biological applications [8]. NDGA, commonly known as Masoprocol, is a known inhibitor of arachidonic acid 5-lipoxygenase activity and ROS [9]. These properties of NDGA might be the reason for a marked reduction in inflammation. This phyto-chemical being a potent ROS inhibitor in addition to its free radical scavenging activity has proven to inhibit proliferation of skin carcinogenesis by interfering with benzyol peroxide-meditated promotion of 7,12-dimethylbenz(a)-Anthracene, a powerful carcinogen. It is also a potent anti-tumor protector in other organs too [10].
This polyphenolic compound, having a Xlog P of 4.3 and an experimental log P of 5.8 [11,12], has also proven to be effective in treating actinic keratosis. It is a condition where the skin is photodamaged by ultraviolet radiation (UV). Another study showed that treatment with NDGA was found to be as effective as 5-flurouracil for treating actinic keratosis [13].
Considering the biological activity of NDGA on skin, we aimed to deliver this drug topically to potentially mitigate the cutaneous toxicity of arsenicals. The aim of this study was to achieve topical delivery of NDGA in an appropriate vehicle that would maximize delivery with minimum dose. Hence, various concentrations were tested to determine the minimum dose that exhibits maximum passive delivery into skin. Shorter delivery time is ideal for treating arsenic toxicity. Hence, short duration studies were performed to analyze the delivery of NDGA in the epidermis and dermis. We also investigated the changes in the pattern of drug delivery on multiple applications at various time-points. Since NDGA has been previously used for treating solar keratoses [13], it was interesting to demonstrate if there are changes in the cutaneous delivery of NDGA in UV-damaged skin. In vivo studies in SKH-1 hairless mice were carried out to assess the safety for topical NDGA formulation.
NDGA being a polyphenol is prone to oxidation, the products of oxidation being toxic quinones. This oxidation can inhibit its therapeutic effects. Thus N-acetyl cysteine (NAC) was used as an antioxidant to protect NDGA from oxidation. NAC has been also shown to alleviate arsenic toxicity by inhibiting the extracellular reactive oxygen species [14]. Studies have shown that NAC restores the skin barrier properties and plays an active role in reducing erythema [15]. Thus, the combined treatment is likely to be beneficial in mitigating the cutaneous injury caused by arsenicals.
The aim of the study was to achieve passive delivery of NDGA using NAC as a functional excipient. Effects of UV damage on skin were characterized by measuring the Transepidermal water loss (TEWL) and skin resistance values.
2. Materials and methods
2.1. Materials
NDGA was obtained from Tokyo Chemical Industries (TCI America). Methanol was procured from Pharmaco-aaper (Brookfield, CT, USA), propylene glycol (PG) and ethanol (Et) from EKI Chemicals (Joilet, IL, USA) while, dimethyl sulfoxide (DMSO) from Gaylord Chemical Company L.L.C (AL, USA). Dermatomed human cadaver skin (thickness ~750 μm) was purchased from Science Care (Pheonix, AZ, USA). Hairless mice were procured from Charles River Laboratories. Phosphate Buffered Saline (10X PBS) was obtained from Fisher Scientific (Fairlawn, NJ, USA). Syringe filters, (0.22 μm) were bought from Cell treat Scientific Products (Shirley, MA, USA).
2.2. Methods
2.2.1. Solubility studies
Solubility of NDGA in deionized water, 10 mM phosphate buffered saline (1XPBS), Ethanol, 1XPBS: PEG 400, isopropyl alcohol (IPA) and DMSO was investigated. Excess drug was added to each of the solvent compositions to ensure saturation. These mixtures were left at room temperature on a shaker for 24 h, at a speed of 150 rpm. After 24 h, the solutions were subjected to centrifugation at 13,400 rpm for 15 min. The supernatant was diluted as necessary with methanol and filtered using 0.22 μm syringe filters, to remove any particulate contamination and subsequently analyzed using the High Performance Liquid Chromatography (HPLC) method described in section 2.2.10.
2.2.2. Stability studies
To prevent oxidative degradation of NDGA, NAC was added as an antioxidant. For evaluating the efficacy of NAC, concentrations of 0.1%, 0.2%, 0.3% and 0.4%w/v were added to a solution of 5% w/v NDGA in methanol: water (80:20). NAC being highly hydrophilic, was added to the water phase and then this solution was added to methanol containing NDGA. The samples were left for a stability study for three days at 25 °C (covered) after which they were analyzed for stability on the HPLC.
2.2.3. Skin preparation
Dermatomed human skin was stored at −80 °C in a deep freezer. This skin was thawed using 1X PBS (pH 7.4) at 37 °C and cut in pieces of appropriate sizes to be mounted on Franz diffusion cells.
2.2.4. Skin integrity assessment
Skin integrity was ensured by measurement of electrical resistance. Any potential damage to skin that may have been caused during the surgical removal, storage and/or preparation would in turn affect permeation of the drug. The physical integrity of the stratum corneum is reflected by the electrical properties of the skin. Therefore, electrical resistance of the skin was measured [16]. The procedure for measuring skin resistance was carried out using silver/silver chloride (Ag/AgCl) electrodes, 34410A digital multimeter (Agilent Technologies, CA, USA) and an arbitrary waveform generator (20 MHz Function, Agilent 33220A). Receptor contained 5 mL whereas, the donor contained 300 μL of 1X PBS respectively. Vertical Franz diffusion cells were used for this study. Skin pieces mounted on Franz cells were left to equilibrate for 30 min after which Ag wire was placed in the donor and the AgCl electrode was placed in the receptor through the sampling arm. The load resistor (RL) was connected in series with the skin and the voltage drop (VS) across the skin and circuit (VO) was shown on the multimeter. Electrical resistance of the skin was calculated using the formula:
Here, RL and VO were 100 kΩ and 100 mV respectively.
Skin samples having resistance higher than 10 kΩ were selected for the permeation studies.
2.2.5. In-vitro permeation study setup
Vertical Franz diffusion cells with a receptor capacity of 5 mL were used for performing in vitro permeation studies to deliver NDGA. The effective area of diffusion was 0.64 cm2. The temperature in the receptor compartment was maintained at 37 °C with the help of a re-circulating water bath. The skin temperature was thus maintained at 32 °C. Franz cells were cleaned with an optimized protocol after which 5 mL receptor solution containing 1XPBS: PEG 400 (70:30) was added to maintain sink conditions. Skin pieces having adequate resistance were selected and mounted on the Franz cells with the side having the stratum corneum facing upwards. Solution containing NDGA was added to the donor chamber and the samples (300 μL) were withdrawn from the sampling arm of the receptor at pre-determined points. The receptor was replaced with equal volume of fresh buffered receptor solution after sampling. Samples were analyzed using HPLC where, the results were reported as an average ± SE (n = 4) for all test groups. This protocol was followed for all the studies to investigate the topical delivery of NDGA.
2.2.5.1. Defining an optimum concentration for NDGA.
For determining the minimum concentration necessary to achieve the maximum delivery of NDGA in skin, a series of concentrations were studied investigated. Solutions of 1%, 2%, 3%, 4% and 5%w/v concentrations were made by dissolving adequate amount of NDGA in a solvent composition of DMSO, isopropyl alcohol, Propylene glycol and water in the ratio 20: 15: 10: 55. A volume of 12.5 μL was added to the donor chamber. This was followed by sampling from the receptor at 0, 1, 2, 4, 8, 22 and 24 h. The samples were then analyzed using the HPLC.
2.2.5.2. Effect of short duration studies on delivery of NDGA.
Results from section 3.3.1 suggested that 3% w/v solution is optimum for delivering NDGA in skin. For determining the time during which adequate amounts of NDGA would be delivered, several short duration studies were performed. A 3% w/v solution of NDGA was used for this study. The first study was carried out for 2 h, with sampling points at 0 h, 30 min, 1 h and 2 h. The second study was carried out for 4 h where the samples were withdrawn at 0 h, 30 min, 1 h, 2 h and 4 h. The third study was performed for 8 h with sampling points at 0 h, 30 min, 1 h, 2 h, 4 h and 8 h. These short duration studies were performed for assessing the quick delivery of NDGA in skin.
2.2.5.3. Effects of multiple dosing on delivery of NDGA.
For replicating a real-life situation where there are chances of formulation getting rubbed off and there is a need to reapply the formulation, a multiple dose short duration study was performed. Results from section 3.3.2 suggested that the skin saturates after 8 h. Thus, multidose study was carried out for 8 h. A 3% w/v solution of NDGA was used for this study. The first study was performed with dosing at 0 h and 2 h with sampling points at 0 h, 30 min, 1, 2, 4 and 8 h. For the second study, dosing was carried out for 0 h and 4 h with sampling intervals as the previous study. Before applying the second dose, the residual formulation from the previous dose was gently wiped with two cotton buds.
2.2.5.4. Delivery of NDGA on UV damaged skin.
A solar simulator (Newport® Oriel instruments) was used to treat skin with UV. Skin groups (n = 4) were treated with UV for 10 min, 20 min and 30 min each with the lamp setting at 40 W. The skin was then mounted onto the Franz diffusion cells and left to equilibrate for 10 min after which 3% NDGA solution was added to the donor. NDGA was dissolved in the vehicle mentioned in section 2.2.5.1.
2.2.6. Effect of UV on barrier properties of skin
2.2.6.1. Transepidermal water loss (TEWL).
VapoMeter™ by Delfin technologies was used to calculate the TEWL. TEWL values were calculated prior to treating skin with UV and after the treatment was complete. Skin was thawed and left for equilibration on the Franz cell for 10min. The skin pieces were then removed from the Franz cell and gently wiped with Kimwipes™. VapoMeter™ was then placed on the skin, ensuring all the permeation area was covered with no contact with surrounding air. Skin was then treated with UV light and TEWL after treatment was measured with the procedure mentioned above.
2.2.6.2. Skin resistance changes.
For evaluating the effect of UV treatment on skin resistance, skin resistance was measured prior to and after treating skin with UV. Skin was thawed and left for equilibration on the Franz cell for 10 min. Initial skin resistance was measured after which the skin was dabbed with Kimwipes™. The skin pieces were then subjected to a UV treatment. On completion of the treatment, skin was placed on the Franz diffusion cells and left to equilibrate for 10 min and skin resistance was measured using the setup mentioned in 2.2.4.
2.2.6.3. Scanning electron microscopy.
The Phenom™ field emission SEM system (Nanoscience Instruments Inc., Pheonix, USA) was used to study the changes in skin surface after before and after treating skin with UV radiation.
2.2.7. ATR-FTIR studies for compatibility of NAC and NDGA
Compatibility of NAC and NDGA was tested using ATR-FTIR, IRAffinity-1S model (Shimadzu Scientific Instruments, Columbia, MD, USA). Samples were prepared by dissolving NAC, NDGA and mixture of NAC and NDGA in deionized water. The samples were placed on the diamond crystal and ATR-FTIR spectra was scanned and recorded from 4000 to 800 cm−1 at room temperature.
2.2.8. In vivo assessment for topical safety of NDGA
Female SKH-1 hairless mice of age 8–10 week were used. Before cutaneous application of NDGA, mice were anesthetized with ketamine and xylazine (100 mg/kg ketamine and 5 mg/kg xylazine) by in-traperitoneal injection. The anesthetized animals were randomly divided into two groups; group-I (n = 4) received vehicle, while group-II (n = 5) received NDGA (100μl applied on the dorsal side skin area of 8 cm2 at the dose of 6mg/mouse). NDGA was prepared using DMSO: isopropyl alcohol: PG: water (ratio 20:15:10:55). All mice were euthanized at 24 h and skins were harvested for histochemical analysis. Just before euthanizing, 0.5 ml blood was also withdrawn from heart puncture and collected in serum separator tubes. Serum was isolated by centrifugation at the speed of 7500 rpm for 15 min. Serum chemistry was done using the VetScan Comprehensive Diagnostic Profile reagent rotor with the VetScan Chemistry Analyzers (Abaxis, Union City, CA). All animal studies were done as per the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All animal protocols were approved by the Institutional Animal Care and Use Committee (APN −20130) at the University of Alabama at Birmingham.
2.2.9. Skin extraction protocol
The skin was subjected to an optimized wash protocol that would ensure removal of unabsorbed drug from the skin. After the wash protocol was complete, epidermis and dermis were separated with the help of forceps and scissors. The separated dermis and epidermis were subjected to extraction using methanol: water (80:20) after shaking on horizontal shaker for 4 h. NAC was added to the extraction solvent to prevent NDGA from degrading while the extraction was being carried out.
2.2.10. Quantitative analysis
Quantitative estimation of NDGA was carried out using a reverse phase high performance liquid chromatography system (RP-HPLC). A 2996 photodiode array detector was employed with a Waters Alliance 2695 separations module (Milford, MA, USA). An Eclipse Plus 5μ C18 100A, 150*4.6 mm column (Agilent, USA) was used for an isocratic elution at a flow rate set at 1 mL/min. The mobile phase used was methanol: buffer (65:30) with the buffer having a pH of 6. Run time for each sample was 10 min with the retention time for NDGA at 6 min. Standards for receptor samples were prepared in 1XPBS: PEG 400 (70:30) with 0.2% NAC as an antioxidant to prevent drug degradation. Standards for skin extractions were prepared in methanol: water (80:20) with 0.2%NAC added as an antioxidant. The wavelength used for detecting NDGA was 271 nm. The limit of detection was 0.025 μg while, the limit of quantification was 0.1 μg. Concentrations in the range of 0.025–100 μg/mL depicted linearity with an R2 of 0.9999. NAC did not interfere with the analysis of NDGA and there was no interference observed from the skin components that may have leached in the skin and receptor samples while analyzing the same.
2.3. Data analysis
Data analysis was performed using GraphPad Prism 8. Non-parametric tests were used for the statistical analysis and p < 0.05 considered for establishing a significant difference between test groups.
3. Results
3.1. Solubility studies
Solubility of NDGA in water was found to be 0.08 mg/mL, whereas in 1XPBS it was 0.05 mg/mL. Solubility in 1X PBS was not enough for maintaining sink conditions in the receptor. Hence, solubility in various ratios of 1XPBS: PEG 400 was determined. 1X PBS: PEG 400 in the ratio of 70: 30 had a solubility of 0.5 mg/mL. This solubility was appropriate for maintaining the sink conditions and was hence chosen as the receptor media. Solubility of NDGA was found to be 15 mg/mL in propylene glycol while in DMSO and ethanol, the observed solubility was more than 100 mg/mL.
3.2. Stability studies
NDGA was found to degrade over time on exposure to light and air. A concentration of 0.2% w/v was found to be effective in protecting NDGA from degrading. Addition of NAC did not interfere with the NDGA peak (Fig. 1). The oxidation of NDGA was thus prevented by addition of NAC.
Fig. 1.

Chromatogram for a three-day stability study for NDGA. (a) NDGA solution without antioxidant NAC. (b) NDGA solution with 0.2% w/v NAC.
3.3. In vitro permeation of NDGA
3.3.1. Optimum concentration of NDGA
There was significant difference in the epidermal and dermal delivery of NDGA for all concentrations 5%, 4% and 3%, the results of which have been shown in Fig. 2. However, there was no significant difference in the total skin concentrations for 5% w/v (31.33 ± 2.47 μg), 4% w/v (30.53 ± 3.17 μg) and 3% w/v (37.45 ± 4.32 μg). The lowest effective concentration was found to be 3% w/v. Concentrations 2% and 1% delivered significantly lower amount of drug (5.06 ± 0.71 μg and 6.65 ± 0.49 μg respectively). Hence, a concentration of 3% w/v was chosen for all other studies. No significant difference was observed in the amount of NDGA in epidermis and dermis for 2% w/v and 1% w/v (Fig. 2). Thus, a concentration of 2% and 1% w/v of NDGA did not saturate the skin, and allowed diffusion of drug from epidermis to dermis resulting in similar amount of drug delivered in both layers of skin. Analysis of receptor samples showed, no drug was delivered to the receptor compartment in this study.
Fig. 2.

Delivery of NDGA in 24 h, from an exposure area of 0.64 sq. cm.; data expressed as mean ± SE (n = 4)
*indicates significant difference (p < 0.05); unpaired student’s t-test.
3.3.2. Effect of duration of study on permeation of NDGA
For determining the time needed for NDGA to saturate skin, we carried out several time dependent studies. A 3% w/v solution was used for this study. There was significant difference in the total amount of drug delivered at the end of 2 h and 24 h (shown in Fig. 3). However, there was no significant difference in the total drug delivered at the end of 4 h (13.05 ± 0.34 μg), 8 h (14.83 ± 2.36 μg) and 24 h (37.45 ± 4.32 μg). This signifies that the skin is saturating at early time points. Significant difference in the epidermal and dermal concentrations were found only at the end of 8 h and 24 h (Fig. 4). Analysis of the receptor samples showed that the drug was not delivered to the receptor.
Fig. 3.

Total amount of NDGA delivered at varying study durations (exposure area 0.64 sq.cm); data expressed as mean ± SE (n = 4)
*indicates significant difference (p < 0.05); Kruskal Wallis.
Fig. 4.

Epidermal and dermal amounts of NDGA at varying study durations with an exposure area of 0.64 sq.cm; data expressed as mean ± SE (n = 4)
*indicates significant difference (p < 0.05); unpaired student’s t-test.
3.3.3. Effects of multiple dosing on delivery of NDGA
There was no significant difference in the epidermal concentrations of NDGA when dosing was carried out at 0 h, 2 h and 0 h, 4 h. Similarly, there was no significant difference in the dermal concentrations for dosing at 0 h, 2 h and 0 h, 4 h. However, there was significant difference in the concentrations of NDGA in the epidermis and dermis for both dosing intervals (Fig. 5). The data is shown in the graph below (Fig. 5). Thus, it can be inferred that the skin is saturated by a single application. Analysis of the receptor samples showed that the drug was not delivered to the receptor.
Fig. 5.

Amount of NDGA delivered in the epidermis and dermis by different dosage intervals (area of exposure 0.64 sq.cm); data expressed as mean ± SE (n = 4)
*indicates significant difference (p < 0.05); unpaired student’s t-test.
3.3.4. Delivery of NDGA through UV damaged skin
UV radiation did not seem to have any effect on the permeation characteristics of dermatomed human skin. There was no significant difference in the total amount of NDGA delivered treating skin with UV light for 0 min, i.e no exposure (14.83 ± 2.36 μg), 10 min (30.42 ± 2.47 μg), 20 min (28.19 ± 3.46 μg) and 30 min (20.37 ± 1.84 μg) each. There was no significant difference found in the epidermal concentrations of NDGA at the end of 8 h for 0 min (12.29 ± 1.87), 10 min (19.32 ± 2.18 μg), 20 min (19.33 ± 2.86 μg) and 30 min (12.69 ± 0.88 μg) respectively. There was no significant difference in the dermal delivery for 0 min (5.118 ± 1.77 μg), 10 min (11.1 ± 1.94), 20 min (8.87 ± 1.21 μg) min and 30 min (7.67 ± 1.28 μg) of NDGA at the end of 8 h for all the treatment durations. Analysis of the receptor samples showed there was no receptor delivery for NDGA.
3.3.5. Effects of UV treatment on skin barrier properties
3.3.5.1. Transepidermal water loss.
TEWL is the amount of water lost passively through the skin layers to the environment due to a vapor pressure gradient. The amount of TEWL increased after increasing the time of skin exposure to UV. Significant difference was observed in the TEWL values for treatment durations of 30 min and all other treatment groups (Fig. 6).
Fig. 6.
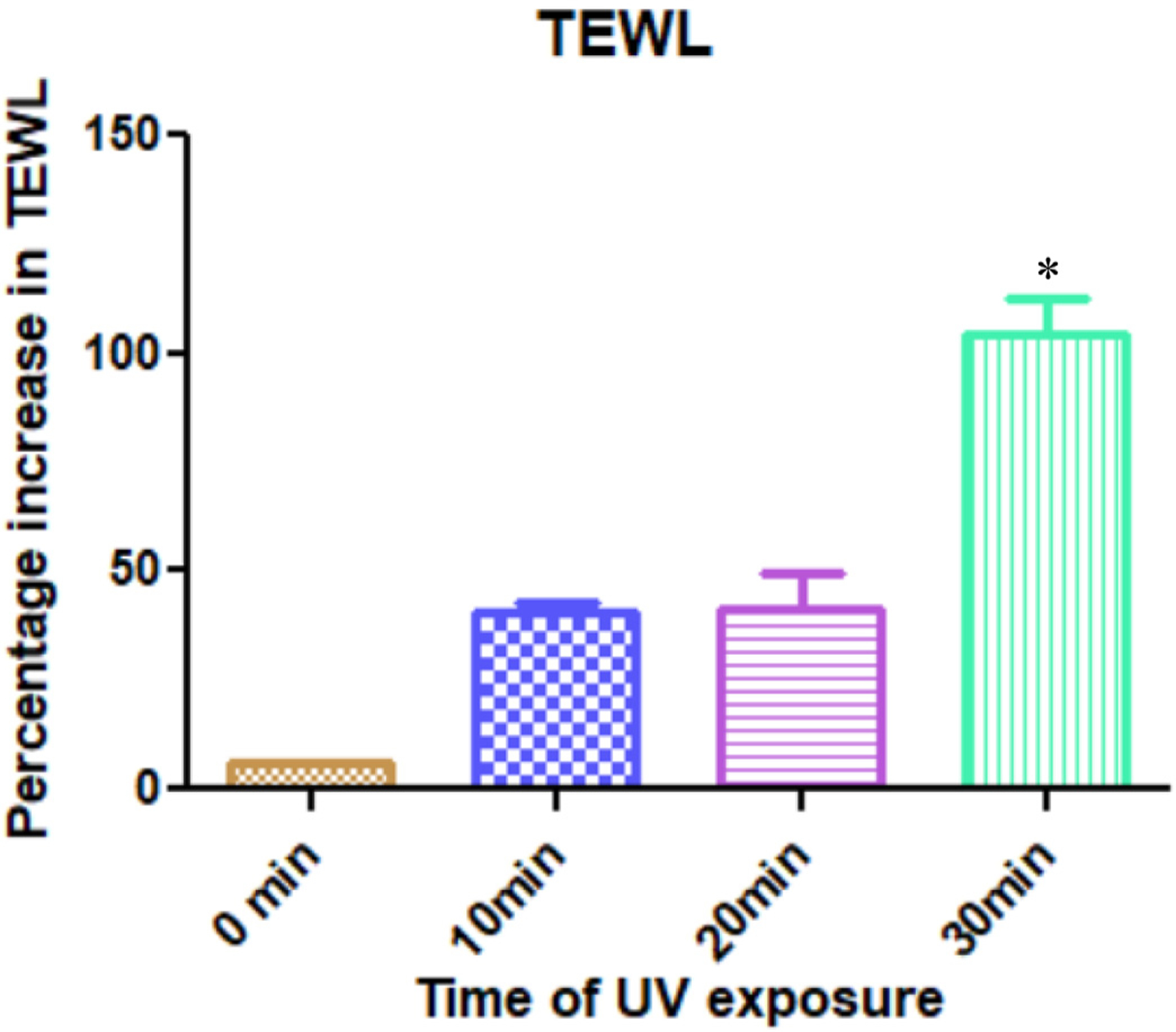
UV treatment on skin led to an increase in the TEWL of skin. The percentage increase in TEWL is depicted in this figure; data expressed as mean ± SE (n = 4)
* indicates significant difference as compared to the other test groups (p < 0.05); Kruskal Wallis.
3.3.5.2. Skin resistance.
Electrical resistance of the skin is generally attributed to the intactness of the stratum corneum. Thus, changes in the skin electrical resistance values can give an overall understanding of the structural integrity of the stratum corneum. There was no significant difference in the resistance of skin before and after treatment with UV radiation (Fig. 7).
Fig. 7.

Percentage drop in resistance on treating skin with UV; data expressed as mean ± SE (n = 4).
Scanning electron microscopy
3.4. ATR-FTIR studies for compatibility of NAC and NDGA
The ATR-FTIR studies showed that NAC and NDGA were compatible. NDGA shows a characteristic aromatic C–H bend between 1700 cm−1 to 1650 cm−1 [17]. This is visible in pure NDGA spectra and the spectra of mixture of NDGA and NAC. NDGA is a polyphenolic compound. Hence, there is a typical C–O stretch visible between 1110 cm−1 to 1000 cm−1 [17] for NDGA alone and mixture of NDGA and NAC. Thus, presence of NAC did not affect the properties of NDGA (see Fig. 9).
Fig. 9.

ATR- FTIR study for compatibility of NAC and NDGA.
3.5. In vivo assessment for topical safety of NDGA
The topical application of NDGA did not induce cutaneous erythema, edema or irritation in these animals (Fig. 10A). We observed these effects up to 24 h following NDGA treatment. The skin biopsies at this time were taken to assess the inflammatory as well as toxic responses from other tissues. The histology of control and NDGA treated skin sections did not differ significantly. Particularly, we did not observe hyperplasia and infiltration of lymphocytes or mast cell de-granulation in skin histology as compared to respective controls. As shown in Fig. 10B, we did not observe any significant change between vehicle-treated control and NDGA treatment groups.
Fig. 10.

Topical administration of NDGA for assessing cutaneous and systemic toxicity.
(A) Hematoxylin and eosin (H & E) staining of mouse skin sections at 24 h following topical application of vehicle or NDGA (6mg/mouse at the area of 8 cm2) application. Mice photographs representing gross skin changes. Inset representing magnification of 4X images. (B) Quantitative determination of serum chemistry parameters in vehicle-treated controls vs NDGA-treated animals.
4. Discussion
4.1. Topical delivery of NDGA
Topical delivery of NDGA was investigated for assessing its use for treating arsenic exposure induced inflammation and the subsequent keratoses that may occur. This route can help in a faster delivery in skin on cutaneous exposure to arsenicals. The rationale behind this study was to deliver NDGA into skin using a suitable vehicle, that can potentially be useful in a chemical warfare-like situation. It has been reported by Flora et al. that exposure to toxic arsenicals like lewisites can lead to a variety of skin diseases, many of them heading towards carcinogenicity [1]. Spontaneous skin reaction on exposure to the vesicants is edema and erythema within minutes while, blistering begins in about 6–8 h. The dermal epidermal separation begins alongside the blistering [18]. However, due to the tendency of arsenicals accumulating in the epidermis and causing extensive damage in the epidermal layers, the target site for NDGA delivery is the epidermis [1]. Thus, immediate delivery of NDGA can potentially relieve the arsenic exposed victims from cutaneous harm. Application of NDGA within minutes of exposure to the toxic arsenic gases may prevent progression of possible skin cancers arising due to arsenic exposures. It has been reported in literature that NDGA is effective in treating pre-cancerous lesions [19]. Thus, NDGA may prevent the formation and/or progression of lesions.
4.2. Choice of solvents for a suitable vehicle
We carried out solubility studies to incorporate a suitable solvent for dissolving NDGA. Solubility for NDGA was low in water (section 3.1) and thus various solvents and their combinations were tested. NDGA showed highest solubility in DMSO (section 3.1). FDA approves the use of DMSO as a solvent in pharmaceutical formulations. DMSO, the organosulphur compound has been used as an excipient in Pennsaid® and Pennsaid 2%. Thus, DMSO was chosen as a primary solvent to dissolve NDGA (20% v/v). However, addition of water to the DMSO-NDGA solution led to precipitation of NDGA. Thus, various concentrations of isopropyl alcohol were tried for effectively solubilizing NDGA along with DMSO. There are several formulations in the market using isopropyl alcohol as an excipient [20]. Inactive Ingredient (IIG) database from the FDA approves the use of isopropyl alcohol up to 78.34% w/v in a topical formulation. A concentration of 15% v/v was incorporated in the vehicle. Propylene glycol was added as a co-solvent as well as a surfactant that would prevent precipitation of NDGA upon addition of water. Propylene glycol is a common pharmaceutical cosolvent and is safe for topical use [21]. It has a lipophilic carbon chain and hydrophilic –OH moieties that impart surfactant-like properties, thus improving drug solubility. Propylene glycol was thus used (10% v/v) in the vehicle. Water (55% v/v) was added to make the desired volume. Thus, a homogenous mixture of DMSO, isopropyl alcohol, propylene glycol and water was used as a vehicle for topical delivery of NDGA.
4.3. Compatibility of NDGA and NAC
NAC was used as an antioxidant to prevent NDGA from degradation. However, to check the compatibility of NDGA with NAC we carried out ATR-FTIR studies. Results from section 3.4 showed that the NDGA peak did not shift after incorporation of NAC in the solution. This shows that, there were no changes to the structure of NDGA, and NAC can be incorporated in the formulation along with NDGA. Addition of NAC to the formulation would also reduce the toxic effects of the oxidation products of NDGA [22].
4.4. NDGA delivery in skin
Concentration study was carried out to determine the difference in NDGA delivery at several concentrations. NDGA at concentrations 10% or higher can be irritant to the skin [23]. Hence, concentrations of 5% and lower were used for delivery of NDGA in skin. Concentrations lower than 5% were effective in delivering drug in skin with no significant difference between 3%, 4% or 5% NDGA. However, the drug delivered by 1% or 2% formulations was significantly lower than the 3%–5% w/v solutions. Thus, the lowest concentration needed to deliver adequate amount NDGA in skin was 3% w/v. This concentration was selected for subsequent investigations.
For determining the amount of time needed for adequate delivery of NDGA delivery in skin, we performed several short duration studies. There was no significant difference in the delivery of NDGA in skin at the end of 4 h, 8 h and 24 h. It can thus be inferred that the skin saturated at the early time points. There was a significant difference in the epidermal and dermal delivery only at 8 h and 24 h respectively. This was interesting because there no significant difference in the NDGA amounts delivered in epidermis and dermis at 2 h and 4 h. We can infer from this finding that the dermis saturated earlier than the epidermis, and this may be the reason why no significant difference was found in the dermal concentrations at the end of 2 h, 4 h, 8 h and 24 h.
The study duration was reduced from 24 h to 8 h, as no significant difference in skin delivery was observed for both test groups. Also, blistering begins in about 6–8 h after arsenic exposure. Thus, assessing NDGA delivery in skin before the onset of blistering was essential. Faster delivery in skin is desired for a symptomatic relief from arsenic exposure. This suggests that on application of NDGA immediately after exposure to arsenicals, skin delivery would be ensured within a span of 8 h before the lewisite blistering worsens. The results of this experiment were thus promising.
However, about 80% of the drug was retained in the epidermis. Higher affinity of NDGA for the epidermis may be because the lipo-philicity of NDGA. Section 3.1 shows that NDGA has very low aqueous solubility. Epidermis is more lipophilic than the dermis, which makes it a good environment for the hydrophobic NDGA to be retained. Dermis being a relatively hydrophilic layer of the skin would not have allowed diffusion of the hydrophobic drug from the epidermis. This would be the reason why NDGA was largely retained in the epidermis. This, however, is in favor of combating arsenic toxicity. Arsenic exposure leads to accumulation of arsenic in the epidermis causing extensive necrosis of the epidermal layers [24]. Hence, there is a need for NDGA to be delivered to the epidermis.
Topical dosage forms applied on skin tend to be rubbed off on clothes after an extended application. This problem can be addressed by reapplying the formulation on the necessary area. Hence, we carried out multi-dose studies that can determine changes that may occur in the delivery of NDGA in such conditions. The results obtained were interesting. There was no significant difference in the delivery of NDGA after multiple dosing at various time points. This can be because the epidermis was saturated with NDGA and thus did not allow diffusion of drug from the formulation into skin after reapplication. Thus, multiple dosing did not significantly increase the amount of NDGA delivered in skin.
Initial symptoms of arsenic exposure resemble that of a sunburn [1]. For mimicking skin damaged by arsenic exposure, skin was treated with UV light for determining any changes in the skin resulting in subsequent changes in the skin delivery of NDGA. There was no significant difference in the delivery of NDGA in UV damaged and normal untreated skin. SEM images too, did not show any morphological changes on the skin surface after UV treatment (Fig. 8). Electrical resistance of the skin is one the important parameters that determines the drug delivery into and across skin. The reason for no difference in delivery would be related to the fact that there was no significant drop in skin resistance after UV treatment. Stratum corneum imparts resistance to skin because of its lower water content. UV treatment dehydrated the skin (seen in the increase of TEWL values section 3.3.5.1) but did not depict any resistance change as, the structural integrity of the stratum corneum was not disturbed. Physical damage to skin causes disruption of the stratum corneum which decreases the resistance as lower layers of skin are exposed. But UV treatment only dehydrated the skin without physically damaging the stratum corneum. Thus, there was no resistance drop observed. There have been reports of UV exposure increasing the TEWL of skin but not significantly increasing the delivery across skin [25]. Also, there have been reports where skin treated with UV did not have a significant impact on the physical intactness of the stratum corneum [26]. However, UV treatment caused significant increase in the TEWL of skin. Our results were thus in agreement to the studies reported. Interestingly, TEWL has also been indicated as a good measure of functioning of cutaneous barrier on exposure to arsenicals. Arsenic exposure to skin causes a significant immediate rise in the TEWL values. It has been reported in literature that arsenic exposed skin had greater TEWL than normal skin [27]. Thus, UV-treated skin was predicted to be a good model for evaluating permeation of NDGA after an immediate exposure to arsenicals. The UV study shows that application of NDGA immediately after exposure to arsenicals can have a similar in vitro delivery to normal, uncompromised skin.
Fig. 8.

SEM images of skin (a) Without UV treatment; (b) 10 min UV treatment; (c) 20 min UV treatment; (d) 30 min UV treatment. Scale bar = 200 μm.
4.5. In vivo studies
For demonstrating the safety of topical application of NDGA, we assessed its impact on parameters depicting cutaneous and systemic organ toxicities in SKH-1 hairless murine model. This is a susceptible animal strain, frequently used for investigating cutaneous toxicity of chemicals and radiation. We have previously used this model in our laboratory for investigating the effect of UVB on the skin as well as for demonstrating the impact of various novel drugs to block the toxicity of UVB [28]. The data thus obtained, suggested that topically administered NDGA does not manifest any significant cutaneous toxicity (Fig. 10A). Assessment of systemic toxicity was carried out using a panel of parameters by conducting serum chemistry for the serum samples. These predictive biomarkers are routinely used to depict the toxicity of any chemical or any other agent on liver, kidney and other systemic organs function. As there was no significant difference in the control and NDGA treated groups, we can infer that 3% w/v NDGA may be non-toxic for topical use.
5. Conclusions
The overall results obtained in this study are leading in the direction which aims to reduce the symptoms and possible skin carcinogenic effects of arsenic exposed individuals. Intact dermatomed human skin provided a good model for delivering NDGA after immediate exposure to arsenicals. The in vitro permeation data of NDGA shows that NDGA is delivered to the epidermal layers. Short duration studies demonstrate that NDGA delivery is feasible before blistering spreads. Both single dosing and multiple dosing were effective for skin delivery. UV exposure to skin did not significantly impact the delivery of NDGA into skin.
Acknowledgements
This project was partly funded by NIH/NINDS U01 NS095678.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.: Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jddst.2020.101773.
References
- [1].Flora SJS, Arsenicals: toxicity, their use as chemical warfare agents, and possible remedial measures, Handb. Toxicol. Chem. Warf. Agents (2015) 171–191, 10.1016/B978-0-12-800159-2.00015-4 second ed.. [DOI] [Google Scholar]
- [2].Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, Athar M, Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype, Sci. Rep 6 (2016) 34865, 10.1038/srep34865(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Srivastava RK, Traylor AM, Li C, Feng W, Guo L, Antony VB, Schoeb TR, Agarwal A, Athar M, Cutaneous exposure to lewisite causes acute kidney injury by invoking DNA damage and autophagic response, Am. J. Physiol. Physiol 314 (2018) F1166–F1176, 10.1152/ajprenal.00277.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rao CV, Pal S, Mohammed A, Farooqui M, Doescher MP, Asch AS, Yamada HY, Biological effects and epidemiological consequences of arsenic exposure, and reagents that can ameliorate arsenic damage in vivo, Oncotarget 8 (2017) 57605–57621, 10.18632/oncotarget.17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li C, Srivastava RK, Athar M, Biological and environmental hazards associated with exposure to chemical warfare agents: arsenicals, Ann. N. Y. Acad. Sci 1378 (2016) 143, 10.1111/nyas.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huq SMI, Joardar JC, Parvin S, Correll R, Naidu R, Arsenic contamination in food-chain : transfer of arsenic into, Food Mater. Groundwater Irrigat. 24 (2017) 305–316. [PMC free article] [PubMed] [Google Scholar]
- [7].Rahman M, Vahter M, Wahed MA, Sohel N, Yunus M, Streatfield PK, El Arifeen S, Bhuiya A, Zaman K, Chowdhury AMR, Ekström EC, Persson LÅ, Prevalence of arsenic exposure and skin lesions. A population based survey in Matlab, Bangladesh, J. Epidemiol. Community Health 60 (2006) 242–248, 10.1136/jech.2005.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lü J-M, Nurko J, Weakley SM, Jiang J, Kougias P, Lin PH, Yao Q, Chen C, Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update, Med. Sci. Monit. Int. Med. J. Exp. Clin. Res 16 (2010) RA93. [PMC free article] [PubMed] [Google Scholar]
- [9].Gao P, Zhai F, Guanand L, Zheng J, Nordihydroguaiaretic acid inhibits growth of cervical cancer SiHa cells by up-regulating p21, Oncol. Lett 2 (2011) 123–128, 10.3892/ol.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang L-CS, Chuang H, Kapoor M, Hsieh C-Y, Chou S-C, Lin H-H, Chen Y-W, Chang C-C, Hwu J-R, Liang Y-C, others, Development of nordihydroguaiaretic acid derivatives as potential multidrug-resistant selective agents for cancer treatment, RSC Adv. 5 (2015) 107833–107838, 10.1039/C5RA18827J. [DOI] [Google Scholar]
- [11].Nordihydroguaiaretic-acid, chemical and physical properties, https://pubchem.ncbi.nlm.nih.gov/compound/Nordihydroguaiaretic-acid#section=Chemical-and-Physical-Properties, Accessed date: 18 April 2020.
- [12].Mascoprocol, Experimental properties, https://www.drugbank.ca/drugs/DB00179, Accessed date: 18 April 2020.
- [13].Gonzales M, Bowden GT, Nordihydroguaiaretic acid-mediated inhibition of ultraviolet B-induced activator protein-1 activation in human keratinocytes, Mol. Carcinog 34 (2002) 102–111, 10.1002/mc.10052. [DOI] [PubMed] [Google Scholar]
- [14].Ghani S, Khan N, Koriyama C, Akiba S, Yamamoto M, N-acetyl-L-cysteine reduces arsenite-induced cytotoxicity through chelation in U937 monocytes and macrophages, Mol. Med. Rep 10 (2014) 2961–2966, 10.3892/mmr.2014.2612. [DOI] [PubMed] [Google Scholar]
- [15].Redondo P, Bauzá A, Topical N-acetylcysteine for lamellar ichthyosis, Lancet 354 (1999) 1880, 10.1016/S0140-6736(99)04245-2. [DOI] [PubMed] [Google Scholar]
- [16].Puri A, Murnane KS, Blough BE, Banga AK, Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydro-chloride, Int. J. Pharm 528 (2017) 452–462, 10.1016/j.ijpharm.2017.06.041. [DOI] [PubMed] [Google Scholar]
- [17].Sigma-Aldrich, Ir-Spectrum-Table, https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html, Accessed date: 18 April 2020.
- [18].Li C, Srivastava RK, Weng Z, Croutch CR, Agarwal A, Elmets CA, Afaq F, Athar M, Molecular mechanism underlying pathogenesis of lewisite-induced cutaneous blistering and inflammation: chemical chaperones as potential novel antidotes, Am. J. Pathol 186 (2016) 2637–2649, 10.1016/j.ajpath.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jian-Ming L, Jacobo N, Sarah M W, Jun J, Panagiotis K, Peter H L, Qizhi Y, Changyi C, Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update, Med. Sci. Mon. Int. Med. J. Exp. Clin. Res 16 (2010) 93–100, 10.1111/j.1600-6143.2008.02497.x.Plasma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Isopropyl alcohol, https://www.ewg.org/skindeep/ingredients/703198-isopropyl_alcohol/, Accessed date: 18 April 2020.
- [21].Bendas B, Schmalfuf3 U, Neubert R, International journal of pharmaceutics Influence of propylene glycol as cosolvent on mechanisms of drug transport from hydrogels, Int. J. Pharm 116 (1995) 19–30, 10.1016/0378-5173(94)00267-9. [DOI] [Google Scholar]
- [22].Billinsky JL, Krol ES, Nordihydroguaiaretic acid autoxidation produces a schi-sandrin-like dibenzocyclooctadiene lignan, J. Nat. Prod 71 (2008) 1612–1615, 10.1021/np8001354. [DOI] [PubMed] [Google Scholar]
- [23].Lambert J, Dorr R, Timmermann B, Nordihydroguaiaretic acid: a review of its numerous and varied biological activities, Pharm. Biol 42 (2004) 149–158, 10.1080/13880200490512016. [DOI] [Google Scholar]
- [24].Li C, Srivastava RK, Weng Z, Croutch CR, Agarwal A, Elmets CA, Afaq F, Athar M, Molecular mechanism underlying pathogenesis of lewisite-induced cutaneous blistering and inflammation: chemical chaperones as potential novel antidotes, Am. J. Pathol 186 (2016) 2637–2649, 10.1016/j.ajpath.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hung CF, Chen WY, Hsu CY, Aljuffali IA, Shih HC, Fang JY, Cutaneous penetration of soft nanoparticles via photodamaged skin: lipid-based and polymer-based nanocarriers for drug delivery, Eur. J. Pharm. Biopharm 94 (2015) 94–105, 10.1016/j.ejpb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- [26].Biniek K, Levi K, Dauskardt RH, Solar UV radiation reduces the barrier function of human skin, Proc. Natl. Acad. Sci. Unit. States Am 109 (2012) 17111–17116, 10.1073/pnas.1206851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nguon N, Cléry-Barraud C, Vallet V, Elbakdouri N, Wartelle J, Mouret S, Bertoni M, Dorandeu F, Boudry I, Time course of lewisite-induced skin lesions and inflammatory response in the SKH-1 hairless mouse model, Wound Repair Regen. 22 (2014) 272–280, 10.1111/wrr.12147. [DOI] [PubMed] [Google Scholar]
- [28].Chaudhary SC, Singh T, Talwelkar SS, Srivastava RK, Arumugam A, Weng Z, Elmets CA, Afaq F, Kopelovich L, Athar M, Erb-041, an estrogen receptor-$β$ agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by down-regulating the WNT signaling pathway, Canc. Prev. Res 7 (2014) 186–198, 10.1158/1940-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]


