Abstract
Background
Diabetic patients with kidney disease have a high prevalence of non-diabetic renal disease (NDRD). Renal and patient survival regarding the diagnosis of diabetic nephropathy (DN) or NDRD have not been widely studied. The aim of our study is to evaluate the prevalence of NDRD in patients with diabetes and to determine the capacity of clinical and analytical data in the prediction of NDRD. In addition, we will study renal and patient prognosis according to the renal biopsy findings in patients with diabetes.
Methods
Retrospective multicentre observational study of renal biopsies performed in patients with diabetes from 2002 to 2014.
Results
In total, 832 patients were included: 621 men (74.6%), mean age of 61.7 ± 12.8 years, creatinine was 2.8 ± 2.2 mg/dL and proteinuria 2.7 (interquartile range: 1.2–5.4) g/24 h. About 39.5% (n = 329) of patients had DN, 49.6% (n = 413) NDRD and 10.8% (n = 90) mixed forms. The most frequent NDRD was nephroangiosclerosis (NAS) (n = 87, 9.3%). In the multivariate logistic regression analysis, older age [odds ratio (OR) = 1.03, 95% CI: 1.02–1.05, P < 0.001], microhaematuria (OR = 1.51, 95% CI: 1.03–2.21, P = 0.033) and absence of diabetic retinopathy (DR) (OR = 0.28, 95% CI: 0.19–0.42, P < 0.001) were independently associated with NDRD. Kaplan–Meier analysis showed that patients with DN or mixed forms presented worse renal prognosis than NDRD (P < 0.001) and higher mortality (P = 0.029). In multivariate Cox analyses, older age (P < 0.001), higher serum creatinine (P < 0.001), higher proteinuria (P < 0.001), DR (P = 0.007) and DN (P < 0.001) were independent risk factors for renal replacement therapy. In addition, older age (P < 0.001), peripheral vascular disease (P = 0.002), higher creatinine (P = 0.01) and DN (P = 0.015) were independent risk factors for mortality.
Conclusions
The most frequent cause of NDRD is NAS. Elderly patients with microhaematuria and the absence of DR are the ones at risk for NDRD. Patients with DN presented worse renal prognosis and higher mortality than those with NDRD. These results suggest that in some patients with diabetes, kidney biopsy may be useful for an accurate renal diagnosis and subsequently treatment and prognosis.
Keywords: chronic kidney disease, diabetes mellitus, diabetic nephropathy, non-diabetic renal disease, renal biopsy
INTRODUCTION
Diabetes mellitus (DM) is one of the most important health problems in the world and it is dramatically increasing in frequency [1]. One of the reasons is the progressive ageing of the population, along with the increase in overweight, obesity, unhealthy diets and physical inactivity, factors that increase the prevalence of this type of chronic disease. Currently, there are about 500 million people affected by DM worldwide. It is expected that by the year 2045, this number will have increased to about 693 million [2]. This increase will be more relevant in underdeveloped countries, such as Southeast Asia, South and Central America or Africa, where it is expected to increase by up to 150% [2]. Furthermore, DM is an important cause of chronic disease. In fact, in the last two decades, DM proportion has escalated up to 79% as a cause of years lived with disability (YLD). In men, it is the second cause of YLD and in women, the third, surpassed by chronic lumbar pain in both sexes and headaches in the case of women [3].
Chronic kidney disease will develop in between 30% and 40% of patients with Type 1 or Type 2 diabetes [4, 5]. DM is now the first cause of progressive renal disease that leads to end-stage renal disease (ESRD) and subsequently the need of renal replacement therapy (RRT) [6–8]. In diabetic patients, the presence of albuminuria is an independent risk factor for mortality. This mortality increases exponentially when albuminuria and a decrease in glomerular filtration rate (GFR) are associated [9]. The coexistence of DM and other cardiovascular risk factors such as smoking, hypertension (HTA) and dyslipidaemia exponentially raises the cardiovascular mortality rate of these patients [7].
The renal involvement of patients with diabetes is very heterogeneous, and when biopsied almost two-thirds of them are diagnosed with non-diabetic renal disease (NDRD) [10–12]. This high prevalence of NDRD among biopsied patients with diabetes may be related to the fact that the renal biopsy in diabetic patients is usually performed to rule out other causes of renal disease [13, 14]. The role of renal biopsy in patients with diabetes has been under discussion for years [15]. In this context, cohort studies have been carried out with biopsied diabetic patients. Most studies are retrospective and unicentric with a variable cohort size [16, 17]. In these populations, the prevalence and risk factors of NDRD versus diabetic nephropathy (DN) have been studied [11, 12, 18–25]. As the renal prognosis is worse in DN than in NDRD patients, the diagnosis of renal injuries may be helpful to stratify our diabetic patients [19, 25–27]. However, until now, no differences have been found in survival between DN and NDRD patients [12].
The aim of our study is to analyse, with a multicentric study, the prevalence of NDRD in a cohort of biopsied patients with diabetes. In addition, we also plan to study whether clinical and analytical data may be useful to predict NDRD in patients with diabetes and renal involvement. Finally, we plan to find differences in renal and patient prognosis according to the diagnosis in the renal biopsy (DN versus NDRD).
MATERIALS AND METHODS
Patients
This retrospective cohort study was performed in 18 nephrology departments from the Spanish Group for the Study of Glomerular diseases (GLOSEN), the Catalonian Group for the Study of Glomerular diseases (GLOMCAT) and the Spanish Group of Diabetic Nephropathy (GEENDIAB). Data from renal biopsies performed in patients with diabetes from 2002 to 2014 were collected. Patient identification was performed by reviewing histopathological charts and clinical histories. The Medical Ethics Committee of Parc de Salut Mar, Barcelona, Spain approved the study protocol; the approval number is CEIC2013/5468/I.
Variables studied
A total of 112 variables were studied: 58 (51.8%) were clinical and 54 (48.2%) were laboratory data. Patient demographic characteristics were recorded (age, gender and race), along with history of HTA, dyslipidaemia, duration of DM, presence or absence of diabetic retinopathy (DR), diabetic neuropathy, ischaemic heart disease, previous stroke, peripheral vascular disease, malignancy, systemic disease, and treatment with renin–angiotensin–aldosterone system blockers (RAASB), oral antidiabetics, insulin, statin and aldosterone antagonists.
At the time of renal biopsy, weight, height, systolic blood pressure (BP) and diastolic BP were recorded. In terms of laboratory data, renal function [serum creatinine in milligram per deciliter and estimated glomerular filtration rate (eGFR) according to Modification of Diet in Renal Disease-4 in mL/min/1.73 m2], urea in milligram per decilitre, fasting blood glucose levels in milligram per deciliter, proteinuria (g/24 h), microhaematuria, autoimmune markers [antinuclear antibodies (ANAs), Anti-double stranded DNA (Anti-DsDNA), Anti-neutrophil cytoplasmic antibodies (ANCAs), Anti-Glomerular Basement Membrane (anti-GBM) and cryoglobulins] and viral serology (anti-Hepatitis C Virus (HCV), surface antigen of the hepatitis B virus (HBsAg) and anti-Human Immunodeficency Virus (anti-HIV)) were all examined.
The indications of renal biopsy such as nephrotic syndrome, acute kidney injury (AKI), nephrotic proteinuria in patients with diabetes and <5 years of evolution, nephrotic proteinuria without DR, abrupt decrease in eGFR, micro/macrohaematuria, signs or symptoms of systemic disease and proteinuria >1 g (excluded nephrotic) in patients with diabetes and <5 years of evolution were also recorded.
Renal biopsies were reviewed for this study at every participating centre. The morphological characteristics found in the biopsy (number of glomeruli, diffuse or nodular mesangial expansion, global or segmental sclerosis, percentage of glomerulosclerosis and increase of basement glomerular membrane) and the final diagnoses were collected. Based on the diagnoses, the renal biopsies were classified into three categories: isolated DN, NDRD or DN-superimposed NDRD (DN plus NDRD) [11].
Finally, the follow-up was assessed at 1, 3, 5 and 10 years post-renal biopsy. The variables evaluated were renal function (creatinine level and GFR), urea concentration, fasting blood glucose, 24-h proteinuria, microalbuminuria, urine protein/creatinine ratio, need for RRT and death.
Statistical analysis
Statistical analysis was performed using IBM’s SPSS Statistics version 20.0. The variables are expressed in mean and standard deviation and the qualitative variables in percentages. The distribution of variables was assessed using the Kolmogorov–Smirnov test. Univariate comparisons between groups were performed using a Chi-squared test for categorical variables and one-way analysis of variance test for comparing means. A multivariate analysis of variables considered as potential predictors of DN versus NDRD (dependent variable) was performed using binary logistic regression. Regarding the study of survival and the need for RRT, Kaplan–Meier curves and log-rank tests were performed. For the multivariate analysis, the Cox regression was performed to find the risk factors for mortality and for the start of RRT. A P < 0.05 was considered a statistically significant difference.
RESULTS
Baseline characteristics of the population
A total of 832 patients with diabetes and kidney biopsy were included in the study. The most relevant clinical and analytical data at the time of renal biopsy are summarized in Table 1. Patients were classified according to the pathological diagnosis: DN, NDRD or DN plus NDRD (mixed forms). The average age of the population was 61.7 ± 12.8 years old; however, the patients with NDRD were older (P < 0.05). About 74.6% (n = 621) of the patients were men. Some 7.1% of the total patients (n = 59) had Type 1 DM, and a significantly greater proportion was observed in the isolated DN group. In total, 722 patients (87%) had HTA. The predominant race was Caucasian (n = 646, 77.6%) in all groups. The average duration of DM was 10.8 ± 8.6 years, and patients with NDRD had a significantly shorter time of evolution of DM. About 26.6% of patients (n = 221) had DR, with a higher proportion in cases with DN (P < 0.05). The mean creatinine level was 2.8 ± 2.2 mg/dL and patients with NDRD had a significantly worse renal function (P < 0.05). The median (interquartile range 25–75) proteinuria was 2.7 (1.2–5.4) g/24 h and there were no differences between the three groups. Microhaematuria was observed in 34.6% (n = 288) of the cases, most frequently in the NDRD group. It is relevant to highlight that one-third of patients with isolated DN have microhaematuria.
Table 1.
Baseline characteristics of the population
| Characteristics | All patients | DN | NDRD plus DN | NDRD | P-value |
|---|---|---|---|---|---|
| Patients, n (%) | 832 (100) | 329 (39.5) | 90 (10.8) | 413 (49.6) | – |
| Age, years | 61.7 ± 12.8 | 59 ± 12.8 | 62.3 ± 12.1 | 63.7 ± 12.6 | <0.001 |
| Male sex, n (%) | 621 (74.6) | 245 (74.5) | 72 (80) | 304 (73.6) | 0.449 |
| Type 1 DM, n (%) | 59 (7.1) | 37 (11.2) | 8 (8.9) | 14 (3.4) | <0.001 |
| HTA, n (%) | 722 (87) | 293 (89.6) | 77 (85.6) | 352 (86) | 0.275 |
| Systolic BP, mmHg | 144 ± 25 | 150 ± 26 | 150 ± 25 | 138 ± 23 | <0.001 |
| Diastolic BP, mmHg | 77 ± 12 | 79 ± 13 | 77 ± 12 | 75 ± 12 | 0.015 |
| Race, n (%) | |||||
| Caucasian | 646 (77.6) | 234 (94) | 71 (94.7) | 341 (94.2) | |
| Black | 4 (0.5) | 2 (0.8) | 0 | 2 (2.7) | 0.069 |
| Asiatic | 7 (0.8) | 1 (0.4) | 0 | 6 (1.7) | |
| Unknown | 29 (3.5) | 12 (4.8) | 2 (2.7) | 15 (4.1) | |
| Duration of DM, years | 10.8 ± 8.6 | 12.2 ± 8.4 | 12.1 ± 9.3 | 9.4 ± 8.5 | <0.001 |
| DR, n (%) | 221 (26.6) | 145 (44.1) | 30 (33.3) | 46 (11.1) | <0.001 |
| Creatinine, mg/dL | 2.8 ± 2.2 | 2.6 ± 1.7 | 3.5 ± 3.1 | 2.9 ± 2.3 | 0.003 |
| eGFR (MDRD-4) | 38.2 ± 27.5 | 40.2 ± 26 | 32.7 ± 27.7 | 37.9 ± 28.3 | 0.133 |
| Proteinuria, g/24 h | 2.7 (1.2–5.4) | 3.2 (3.9–4.9) | 2.5 (2.8–4.7) | 2.4 (3.4–4.3) | 0.254 |
| Microhaematuria, n (%) | 288 (34.6) | 93 (33.7) | 38 (45.2) | 157 (47.9) | 0.001 |
| Fasting plasma glucose, mg/dL | 138.5 ± 64 | 143.2 ± 66.8 | 140.4 ± 65.3 | 134.3 ± 60.5 | 0.179 |
Statistical analysis: analysis of variance. MDRD, Modification of Diet in Renal Disease. Data are presented as mean ± SD or median (interquartile range, 25–75) unless otherwise indicated. Bold values: P <0.05. DM, diabetes mellitus; HTA, hypertension; BP, blood pressure; DR, diabetic retinopathy, eGFR, estimated glomerular filtration rate.
Note: Bold values are with statistical significance.
The markers of systemic disease are summarized in the Supplementary data, Table S1. Serologic testing positive for ANA and ANCA was found more commonly in patients with diabetes and NDRD at kidney biopsy (P < 0.05).
Indications for renal biopsy
The indications for renal biopsy are summarized in Table 2. The most frequent indication for renal biopsy in all studied patients was nephrotic syndrome (n = 261, 31.4%), followed by abrupt reduction of eGFR in patient with previous stable renal function (n = 173, 20.8%), AKI (n = 118, 14.2%), nephrotic proteinuria without DR (n = 89, 10.7%), signs or symptoms of systemic disease (n = 53, 6.4%), proteinuria >1 g with DM <5 years of evolution (n = 46, 5.5%), micro/macrohaematuria (n = 42, 5%) and nephrotic proteinuria with DM <5 years of evolution (n = 18, 2.2%). Interestingly in NDRD patients, haematuria, AKI and the suspicion of systemic disease were more frequent as a reason for renal biopsy (P < 0.001). Among the patients with haematuria, 69.1% were diagnosed with NDRD. AKI was both the indication for renal biopsy in 66.1% of the cases and the suspicion of systemic disease in 67.9% of NDRD as compared with DN patients (Table 2).
Table 2.
Indications of renal biopsy
| Indications | DN | NDRD plus DN | NDRD |
|---|---|---|---|
| Patients, n (%) | 329 (39.5) | 90 (10.8) | 413 (49.6) |
| Nephrotic syndrome or fast increase of proteinuria, n (%) | 138 (52.9) | 28 (10.7) | 95 (36.4) |
| Abrupt decrease in eGFR in patient with stable renal function, n (%) | 76 (43.9) | 21 (12.1) | 76 (43.9) |
| AKI, n (%) | 23 (19.5) | 17 (14.4) | 78 (66.1) |
| Nephrotic proteinuria without DR, n (%) | 36 (40.4) | 7 (7.0) | 46 (51.7) |
| Signs of symptoms of systemic disease, n (%) | 13 (24.5) | 4 (7.5) | 36 (67.9) |
| Proteinuria >1 g in DM with <5 years of evolution, n (%) | 16 (34.8) | 5 (10.9) | 25 (54.3) |
| Micro/macrohaematuria, n (%) | 11 (26.2) | 5 (11.9) | 26 (61.9) |
| Nephrotic proteinuria with DM <5 years of evolution, n (%) | 6 (33.3) | 1 (5.6) | 11 (61.1) |
| Others, n (%) | 10 (31.3) | 2 (6.3) | 20 (62.5) |
AKI, acute kidney injury.
Results of renal biopsy
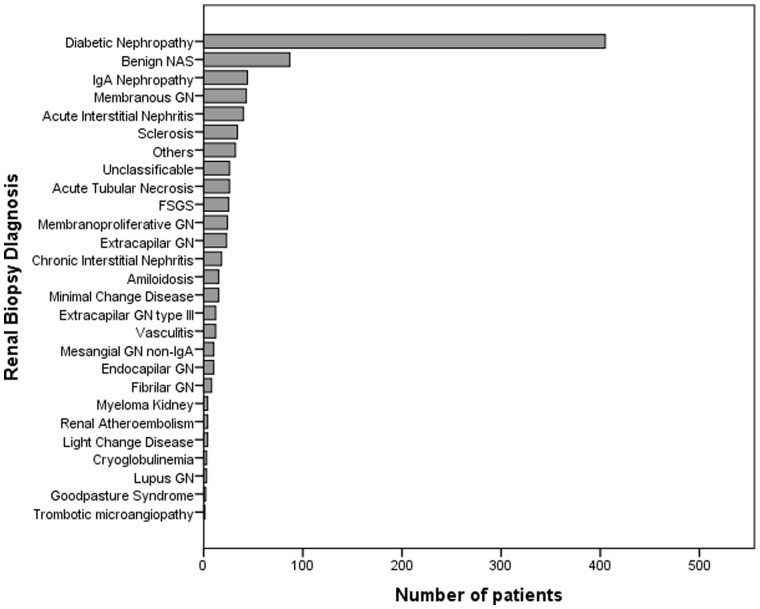
DN was diagnosed in 39.5% (n = 329) of patients, NDRD in 49.6% (n = 413) and DN-superimposed NDRD in 10.8% (n = 90). The most frequent NDRD was benign nephroangiosclerosis (NAS) (n = 87, 9.3%), followed by immunoglobulin A (IgA) nephropathy (IgAN) (n = 44, 4.7%), membranous nephropathy (MN) (n = 43, 4.6%), acute interstitial nephritis (AIN) (n = 40, 4.3%), acute tubular necrosis (n = 26, 2.8%), focal segmental glomerulosclerosis (n = 25, 2.7%) and extracapillary glomerulonephritis (n = 20, 2.1%). The other NDRD diagnoses were less represented in the cohort (Figure 1). About 3.7% (n = 34) of patients did not have a diagnosis because the glomeruli were sclerotic, and 2.8% (n = 26) were unclassifiable because they did not have enough material.
FIGURE 1.
Diagnosis from renal biopsy: distribution of the number of patients according to their diagnosis in renal biopsy. GN, glomerulonephritis; FSGS, focal segmental glomerulosclerosis.
Predictive factors for non-diabetic nephropathy
To identify the predictors of NDRD, a multivariate binary logistic regression analysis was performed including the variables with statistical significance in the bivaritate analyses: age, creatinine, the presence of microhaematuria, time duration of DM and the presence of DR (Table 3). The independent risk factors for NDRD were the presence of microhaematuria, older age and the absence of DR. The model’s discriminatory capacity obtained a receiver operating characteristic curve with an area under the curve of 0.721 (95% confidence interval 0.677–0.765) (Supplementary data, Figure S2).
Table 3.
Predictive factors for NDRD in diabetic patients
| Parameter | OR (95% CI) | P-value |
|---|---|---|
| Microhaematuria (yes/no) | 1.51 (1.03–2.21) | 0.033 |
| Age, years | 1.03 (1.02–1.05) | <0.001 |
| DR (yes/no) | 0.28 (0.19–0.42) | <0.001 |
| Time of duration of DM, years | 0.98 (0.96–1) | 0.086 |
Multivariate binary logistic regression analysis. Dependent variable: NDRD. OR: odds ratio; 95% CI: confidence interval of 95%.
Note: Bold values are with statistical significance.
Renal prognosis and survival
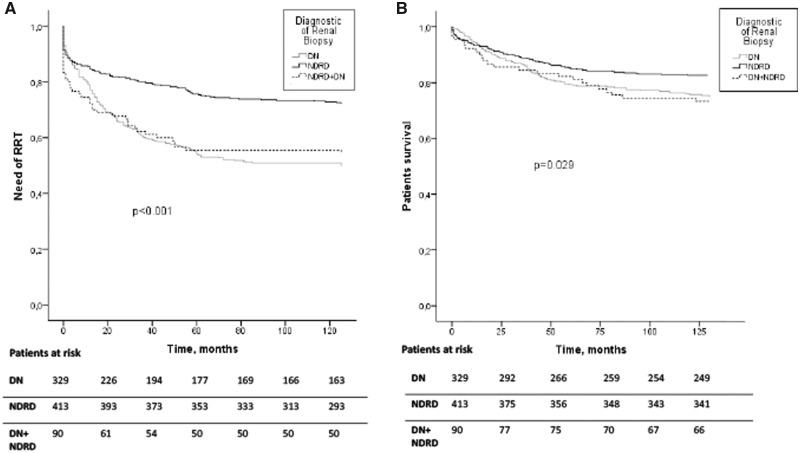
Actuarial survival analyses of the study cohort and renal prognosis (need for RRT) were performed. About 38.6% (n = 321) of the patients needed RRT during the follow-up period. Of these patients, 50.2% were affected by DN (n = 165), 27.8% (n = 115) of patients with NDRD and 45.6% (n = 41) with mixed forms. The overall mortality of the patients at 10-year post-renal biopsy was 21.6% (n = 180), of which 46.1% (n = 83) were diagnosed with DN, 40.6% (n = 73) with NDRD and 13.3% (n = 24) with mixed forms. Survival analysis using Kaplan–Meier curves showed that patients with DN or NDRD plus DN presented worse renal prognosis than NDRD (log-rank test P < 0.001) and higher mortality (log-rank test, P = 0.029; see Figure 2). It is important to mention that differences in renal prognosis are evident very early after renal biopsy. In the multivariate Cox analysis adjusted by sex, age, creatinine, proteinuria, treatment with RAASB, ischaemic heart disease, peripheral vascular disease, DR and diagnosis in renal biopsy of DN, older patients (P < 0.001), higher creatinine level (P < 0.001), higher proteinuria (P < 0.001), the presence of DR (P = 0.007) and the diagnostic of DN (P < 0.001) were identified as risk factors for RRT. In addition, older patients (P < 0.001), peripheral vascular disease (P = 0.002), higher creatinine level (P = 0.01) and the diagnostic of DN in renal biopsy (P = 0.015) were identified as independent risk factors for mortality (Table 4).
FIGURE 2.
Renal and patient survival curves in all groups studied. Analysis using Kaplan–Meier of the different groups divided according to renal diagnoses: NDRD, DN and mixed forms (DN plus NDRD). (A) Renal replacement therapy (RRT). (B) Mortality. The need of RRT (log-rank test P < 0.001) and mortality (log-rank test P = 0.029) were significantly increased in biopsy-proven DN patients.
Table 4.
Multivariate Cox regression analysis for need of RRT and mortality
| Variables | HR (95% CI) | P-value |
|---|---|---|
| Need of renal replacement therapy | ||
|
| ||
| Model 1: Age, sex, ischaemic heart disease, peripheral vascular disease, DR, creatinine, proteinuria, treatment with RAASB and DN | ||
| Age, years | 1.017 (1.006–1.028) | <0.001 |
| Sex (men versus women) | 1.251 (0.918–1.706) | 0.155 |
| Ischaemic heart disease (yes versus no) | 1.270 (0.907–1.777) | 0.164 |
| Peripheral vascular disease (yes versus no) | 1.209 (0.873–1.673) | 0.253 |
| DR (yes versus no) | 1.506 (1.121–2.024) | 0.007 |
| Creatinine, mg/dL | 1.276 (1.224–1.330) | <0.001 |
| Proteinuria, g/24 h | 1.060 (1.032–1.088) | <0.001 |
| Treatment with RAASB (yes versus no) | 1.076 (0.791–1.466) | 0.641 |
| DN (DN versus NDRD/NDRD plus DN) | 1.900 (1.425–2.533) | <0.001 |
|
| ||
| Mortality | ||
|
| ||
| Model 2: Age, sex, ischaemic heart disease, peripheral vascular disease, DR, creatinine, proteinuria, treatment with RAASB and DN | ||
| Age, years | 1.039 (1.022–1.057) | <0.001 |
| Sex (men versus women) | 1.305 (0.870–1.957) | 0.198 |
| Ischaemic heart disease (yes versus no) | 1.003 (0.643–1.563) | 0.99 |
| Peripheral vascular disease (yes versus no) | 1.878 (1.252–2.818) | 0.002 |
| DR (yes versus no) | 1.191 (0.787–1.802) | 0.409 |
| Creatinine, mg/dL | 1.100 (1.023–1.182) | 0.01 |
| Proteinuria (g/24 h) | 1.019 (0.981–1.059) | 0.322 |
| Treatment with RAASB (yes versus no) | 1.165 (0.773–1.755) | 0.466 |
| DN (DN versus NDRD/NDRD plus DN) | 1.591 (1.096–2.308) | 0.015 |
HR, hazard ratio; CI, confidence interval. DR, diabetic retinopathy; RAASB, renin angiotensin system blockade; DN, diabetic nephropathy. Bold values: P<0.05.
Note: Bold values are with statistical significance.
DISCUSSION
In our studied cohort of 832 patients with DM, the largest European study to date of renal biopsy findings in patients with diabetes, approximately two-thirds of the patients had NDRD as a unique or contributing cause of renal disease. The most frequent indication of renal biopsy was nephrotic syndrome. In our study, the first cause of NDRD was benign NAS. We found that microhaematuria, older age and absence of DR were independent predictors of NDRD in renal biopsy in diabetic patients. Our results reinforce the clinical belief that diabetic patients with microhaematuria, older age and without DR are more prone to develop NDRD. Regarding the renal prognosis, we found that patients with DN or DN plus NDRD presented a higher risk of needing RRT than patients with NDRD. In addition, older age, higher creatinine level, higher proteinuria, DR and DN involvement were identified as independent risk factors for the need of RRT. Patients with biopsy-proven DN or mixed forms also had a higher mortality rate when compared with those with NDRD. In addition, older age, higher creatinine level, the presence of peripheral vascular disease and the diagnostic of DN in renal biopsy were identified as risk factors for mortality.
Previous studies focused on renal biopsies of diabetic patients with >100 patients are summarized in Table 5 [11, 18, 19, 22, 26, 28–40]. We must take into consideration that most of these studies are retrospective and include <250 patients [12, 18, 19, 22–24, 26, 29, 32, 35, 40–43]. In our study, we strived to collect data of 832 kidney biopsies performed in 18 Spanish centres. For this purpose, three working groups participated in this study: GLOSEN, GLOMCAT and GEENDIAB. To our knowledge, this is the largest study performed in Europe.
Table 5.
Main characteristics, histological findings and predictive factors for NDRD in biopsied diabetic patients (with n >100 patients)
| Study (year) | Patients (n) | DN, n (%) | NDRD, n (%) | Mixed forms, n (%) | Most common NDRD, n (%) | Risk factors for NDRD |
|---|---|---|---|---|---|---|
| Current study | 832 | 329 (39.5) | 413 (49.6) | 90 (10.8) | Hypertensive nephrosclerosis (87, 9.7%), IgAN (44, 4.7%), MN (43, 4.6%) | Older age, microhaematuria, absence of DR |
| Liu et al. [28] | 1604 | 717 (44.7) | 787 (49.1) | 100 (6.2) | MN (630, 39.3%), IgAN (287, 17.9%), MCD (215, 13.4%) | – |
| Imtiaz et al. [22] | 206 | 74 (35.9) | 87 (42.2) | 45 (21.8) | – | Shorter duration of DM |
| Liu et al. [29] | 200 | 93 (46.5) | 107 (53.5) | – | IgAN (65, 32.7%), MN (37, 18.7%), FSGS (11, 5.6%) | Higher level of Hb, microhaematuria, shorter duration DM, lower BP, lower level of HbA1c |
| Zhuo et al. [40] | 210 | 14 (6.5) | 174 (82.9) | 22 (10.7) | IgAN (62, 28.7%), MN (35, 16.2%), FSGS (6, 2.8%) | – |
| Sharma et al. [11] | 620 | 227 (37) | 220 (36) | 164 (27) | ATN (109, 17.8), FSGS (69, 11.3%), hypertensive neprhosclerosis (70, 11.5%), IgAN (35, 5, 7%) | Shorter duration of DM |
| Byun et al. [26] | 110 | 41 (37.3) | 59 (53.6) | 10 (9.1) | IgAN (48, 43.5%), MN (16, 14.5%), crescentic GN (8, 7.2%) | Absence of DR, shorter duration of DM, lower level of proteinuria, lower level of creatinine |
| Oh et al. [30] | 126 | 50 (39.7) | 65 (51.6) | 11 (8.7) | IgAN (20, 16%), MN (15, 11.9%), FSGS (10, 7.6%), MPGN (6, 4.7%) | – |
| Chong et al. [31] | 110 | 69 (62.7) | 20 (18.2) | 21 (19.1) | AIN (54, 48.8%), hypertensive nephrosclerosis (27, 24.4%), MCD (8, 7.3%) | AKI, absence of DR |
| Haider et al. [32] | 567 | 207 (38.1) | 174 (32) | 162 (29.8) | FSGS (92, 17%), AIN (71, 13%), IgAN (49, 9%), MN (16, 3%) | – |
| Chang et al. [19] | 119 | 43 (36.2) | 64 (53.8) | 12 (10) | MN (39, 32.9%), MCD (19, 15.8%), FSGS (14, 11.8%), IgAN (14, 11.8%) | Higher level of Hb, absence of DR, shorter duration of DM |
| Bi et al. [33] | 220 | 120 (54.5) | – | 100 (45.5) | IgAN (75, 34%), MN (48, 22%), mesangial-proliferative GN (31, 14%) | Microhaematuria, higher level of proteinuria, absence of DR |
| Zhang et al. [34] | 130 | 96 (73.9) | 34 (26.1) | – | IgAN (22, 16.9%), MN (8, 6.15%) | – |
| Zhou et al. [35] | 110 | 60 (54.5) | 50 (45.5) | – | IgAN (37, 34%), MN (24, 22%), MPGN (15, 14%) | – |
| Pham et al. [18] | 232 | 64 (27.5) | 123 (53.2) | 45 (19.3) | FSGS (49, 21%), MCD (35, 15.3%), IgAN (35, 15.3%), MN (31, 13.3%) | – |
| Soni et al. [36] | 160 | 44 (27.5) | 68 (42.5) | 48 (30) | AIN (29, 18.1%), post-infectious GN (28, 17.2%), MN (18, 11.2%), FSGS (12, 7.7%) | – |
| Rychlík et al. [37] | 163 | 69 (42.4) | 77 (47.5) | 17 (10.1) | IgAN (25, 15%), MN (20, 12%), PICGN (19, 11.5%) | – |
| Mazzucco et al. [38] | 393 | 156 (39.7) | 169 (43) | 68 (17.3) | MN (91, 23.1%), IgAN (80, 20.3%), post-infectious GN (82, 20.9%), MCD (49, 12.4%) | – |
| Suzuki et al. [39] | 109 | 80 (73.3) | – | 29 (26.7) | IgAN (49, 44.8%), proliferative GN (41, 37.9%), MN (8, 6.9%), AIN (8, 6.9%), FSGS (4, 3.4%) | – |
FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; ATN, acute tubular necrosis; GN, glomerulonephritis; PICGN, pauci-inmune crescentic glomerulonephritis; MPGN, membrano-proliferative glomerulonephritis; Hb, haemoglobin. Note: Bold values are with statistical significance.
The indication of renal biopsy in general clinical practice in patients with diabetes is usually prompted by the clinical suspicion of NDRD [6, 10, 13, 14]. Thus, the indications for renal biopsy in our study correspond to those described in the literature: nephrotic syndrome, abrupt reduction of eGFR in a patient with previous stable renal function, renal failure, nephrotic proteinuria without DR, signs or symptoms of systemic disease, proteinuria >1 g with DM <5 years of evolution, micro/macrohaematuria and nephrotic proteinuria with DM <5 years of evolution. In concordance with our study, Liu et al. [28], in a Chinese cohort of biopsied diabetic patients, showed that the most frequent indication for kidney biopsy in patients with diabetes was nephrotic syndrome. In our study, we observed that nephrotic syndrome was more frequent in patients with isolated DN [12]. As expected, it is worthy of mention that haematuria, AKI and suspicion of systemic disease were mainly observed in NDRD patients. As in our study, according to Liu et al. [28], AKI and microhaematuria as indications of renal biopsy are associated with NDRD in diabetic patients.
The results of the renal biopsy diagnoses, namely DN, NDRD or mixed forms, in diabetic patients are variable: biopsy-proven DN is diagnosed in from 6.5% to 73.9%, NDRD from 18.2% to 82.9% and mixed forms from 4% to 45.5% [11, 18–20, 22, 26, 28–39]. In our study, the percentage of DN was 39.5%, NDRD 49.6% and mixed forms 10.8%. In concordance, Sharma et al. [11] and Liu et al. [28] found 44.7% and 37% of biopsy-proven DN, respectively. The most frequent NDRD in the previously published studies was IgAN [12, 21, 27, 33, 39, 40, 43, 44], followed by focal segmental glomerulosclerosis [18, 23, 32, 45], MN [19, 28, 38] and AIN [36, 41, 42]. Surprisingly, in our Spanish cohort, the most frequent cause of NDRD was benign NAS. Whether this difference may be related to a higher prevalence of HTA in our cohort of biopsied diabetic patients (87%) as compared with the other cohorts is unknown. As compared with our cohort, the percentage of HTA in the Chinese study was 50.8% [28]; however, in the rest of the studies, the prevalence of HTA was similar to our population [21, 22, 26–29, 33, 40, 41, 46].
In the present study, older age, presence of microhaematuria and absence of DR were identified as independent risk factors for NDRD. Previous studies also identified older age [12], presence of microhaematuria [21, 26, 29, 33, 46] and absence of DR [12, 19, 25, 26, 29, 33, 42] as risk factors for NDRD. The fact that microhaematuria and DR were associated with NDRD was expected; however, it is interesting to note that our study confirms that older age in patients with diabetes is a risk factor for NDRD. These results suggest that in older patients with diabetes, renal biopsy should be indicated under the minimal suspicion of non-diabetic kidney disease. In addition, some studies identified lower BP [46], elevated haemoglobin [19, 29], lower glycosylated haemoglobin (HbA1c) [29], elevated proteinuria [33] and decreased creatinine level [26] as risk factors for NDRD. In other studies, increased creatinine level [12, 42] and lower proteinuria [12, 21, 26] were also identified as independent risk factors for NDRD. Even though a shorter duration of DM has been identified as an independent risk factor for NDRD [11, 12, 19, 22, 24, 26, 27, 29, 43, 46], our study was not able to confirm it in the multivariate analysis. The discordance of these results regarding the DM duration may be in part related to the unreliability of this data, as patients frequently do not know when Type 2 diabetes started and their diagnosis may have been delayed. Fiorentino et al. [16] concluded, in a recent meta-analysis with a total of 48 studies (n = 4876) of biopsies performed in patients with diabetes, that a lower systolic BP, lower level of HbA1c, shorter duration of DM and absence of DR were predictors of NDRD. Higher creatinine level was a predictor of DN, and the higher level of creatinine and lower GFR were an indicator for the superimposed DN plus NDRD.
When we analysed the renal prognosis, we confirmed that biopsy-proven DN is a risk factor for ESRD. Previous studies had shown that patients with DN had worse renal prognosis [12, 19, 25–27]. Age, DR, serum creatinine, proteinuria and DN were identified as risk factors for ESRD. Wong et al. also identified a higher level of serum creatinine and proteinuria as risk factors for renal disease progression [27]. Proteinuria is a classic risk factor for ESRD in diabetic patients [1, 12]. Some studies with lower numbers of patients also found the presence of DR as an independent risk factor for ESRD [19]. Chang et al. performed two Cox regression models to identify the independent risk factors for ESRD. In the first model, higher creatinine level, higher systolic BP, longer duration of DM and the presence of DN in the renal biopsy were the identified risk factors. Surprisingly, in the second model, when the presence of DR was added, the duration of DM and the presence of DN were no longer considered risk factors for ESRD [19]. In our study, which included DR in the analysis, the presence of DN in the renal biopsy persists as an independent risk factor for ESRD. These results reinforce the knowledge that the diagnosis of NDRD in diabetic patients should be performed, proving that it is crucial, because these patients may benefit from specific treatments and thus achieve a better renal prognosis than patients with diabetes and biopsy-proven DN. As shown in our previous study, treatment with RAASB did not modify the renal prognosis in biopsied diabetic patients [27]. Probably, the RAASB confers a better renal protection in early stages of DM. The mean duration of DM in this cohort is ∼11 years, so the renal histological changes might be too advanced. Another reason for this may be possibly explained by a bias in patient selection, as renal biopsy in diabetic patients is currently performed under the suspicion of another NDRD, AKI or heavy proteinuria, among others.
Regarding patient survival, we found that biopsy-proven DN was a risk factor for mortality. There is only one study with diabetic patients in which patient survival was compared between DN and NDRD, and no differences were found between the two groups [12]. To our knowledge, our study is the first that identified older age, peripheral vascular disease, increased creatinine level and DN as risk factors for mortality in a biopsied cohort of patients diagnosed with diabetes. Older age, peripheral vascular disease and increased serum creatinine level are risk factors clearly related to the DM complications previously described for mortality in diabetic patients, indicating that these risk factors are maintained when kidney biopsy was performed [9]. More interestingly and newly found, we have now identified biopsy-proven DN as an independent risk factor for mortality. Our results regarding the increase in mortality in DN patients may be ascribed to two possible causes: (i) the known increased risk of mortality in diabetic kidney disease patients and (ii) NDRD can benefit from specific treatments that may be able to modify renal and patient prognosis.
Our study has several limitations. Overall, ∼78% of patients in this cohort are Caucasian; therefore, the renal histology and renal prognosis may be different from other populations. A high percentage of kidney biopsies were reported as unclassifiable, limiting the analyses. Furthermore, the glycated haemoglobin data, microalbuminuria and urine protein/creatinine ratio were eliminated from the analysis due to the high percentage of missed values.
In conclusion, the number of renal biopsies performed in patients with diabetes has been increasing in recent times. In our Spanish cohort, ∼60% of biopsies in patients with diabetes yielded a NDRD with or without DN. The identification of risk factors for NDRD of DM patients may help us identify patients at risk for NDRD and subsequently indicate the specific treatment for improving renal and patient prognosis. To our knowledge, this study is the first to demonstrate that diabetic patients with biopsy-proven DN have worse renal and survival prognoses. Further studies are necessary to determine the importance of renal biopsy when it comes to treatment and renal prognosis in daily clinical practice with diabetic patients.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the members of GLOSEN, GEENDIB and GLOMCAT for their participation with helping this study become a reality. S.B. performed this work within the basis of her thesis at the Department de Medicina of Universitat Autònoma de Barcelona.
FUNDING
The authors are current recipients of research grants from the FONDO DE INVESTIGACIÓN SANITARIA-FEDER, ISCIII, PI17/00257 and REDINREN, RD16/0009.
AUTHORS’ CONTRIBUTIONS
M.J.S. and X.F. collaborated on the original idea and study design. S.B. carried out the study as part of her doctoral thesis, with the supervision of M.J.S. and J.P. All authors contributed to the inclusion of patients in the cohort. S.B. and M.J.S. collaborated on the statistical analysis and wrote the article. All authors approved the final version of the submitted manuscript.
CONFLICT OF INTEREST STATEMENT
M.J.S. reports conflicts of interest with NovoNordisk, Janssen, Boehringer, Eli Lilly, AstraZeneca and Esteve.
REFERENCES
- 1. Espinel E, Fort J, Agraz I. et al. Renal biopsy in type 2 diabetic patients. J Clin Med 2015; 4: 998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation (IDF). Eighth Edition 2017. IDF Diabetes Atlas, 8th edition.2017, 1–150
- 3. James SL, Abate D, Abate KH. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guideline Development GroupBilo H, Coentrao L, Couchoud C. et al. Clinical practice guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant 2015; 30: ii1–ii142 [DOI] [PubMed] [Google Scholar]
- 5. Rossing K, Christensen PK, Hovind P. et al. Progression of nephropathy in type 2 diabetic patients. Kidney Int 2004; 66: 1596–1605 [DOI] [PubMed] [Google Scholar]
- 6. Suarez MLG, Thomas DB, Barisoni L. et al. Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes 2013; 4: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang S, Zhang XG, Cai GY. et al. Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: a meta-analysis. PLoS One 2013; 8: e64184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalunya GD. Registre de malalts renals de catalunya. Informe Estadístic 2016 [Google Scholar]
- 9. Afkarian M, Sachs MC, Kestenbaum B. et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bermejo S, Pascual J, Soler MJ.. The current role of renal biopsy in diabetic patients. Minerva Med 2018; 109: 116–125 [DOI] [PubMed] [Google Scholar]
- 11. Sharma SG, Bomback AS, Radhakrishnan J. et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 2013; 8: 1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bermejo S, Soler MJ, Gimeno J. et al. Factores predictivos de nefropatía no diabética en pacientes diabéticos. Utilidad de la biopsia renal. Nefrología 2016; 36: 535–544 [DOI] [PubMed] [Google Scholar]
- 13. Rocco MV, Berns JS.. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60: 850–886 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Microvascular complications and foot care: standards of medical care in Diabetes 2018. Diabetes Care 2018; 41: S105–S118 [DOI] [PubMed] [Google Scholar]
- 15. Bermejo S, Pascual J, Soler MJ.. The large spectrum of renal disease in diabetic patients. Clin Kidney J 2017; 10: 255–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiorentino M, Bolignano D, Tesar V. et al. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol Dial Transplant 2017; 32: 97–110 [DOI] [PubMed] [Google Scholar]
- 17. Anders HJ, Huber TB, Isermann B. et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018; 14: 361–377 [DOI] [PubMed] [Google Scholar]
- 18. Pham TT, Sim JJ, Kujubu DA. et al. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 2007; 27: 322–328 [DOI] [PubMed] [Google Scholar]
- 19. Chang TI, Park JT, Kyung KJ. et al. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract 2011; 92: 198–204 [DOI] [PubMed] [Google Scholar]
- 20. Zhuo L, Zou G, Li W. et al. Prevalence of diabetic nephropathy complicating non-diabetic renal disease among Chinese patients with type 2 diabetes mellitus. Eur J Med Res 2013; 18: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mak SK, Gwi E, Chan KW. et al. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant 1997; 12: 2588–2591 [DOI] [PubMed] [Google Scholar]
- 22. Imtiaz S, Drohlia M, Nasir K. et al. Analysis of renal diseases detected in renal biopsies of adult patients: a single-center experience. Saudi J Kidney Dis Transpl 2017; 28: 368. [DOI] [PubMed] [Google Scholar]
- 23. Mou S, Wang Q, Liu J. et al. Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract 2010; 87: 354–359 [DOI] [PubMed] [Google Scholar]
- 24. Horvatic I, Tisljar M, Kacinari P. et al. Non-diabetic renal disease in Croatian patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2014; 104: 443–450 [DOI] [PubMed] [Google Scholar]
- 25. Soleymanian T, Hamid G, Arefi M. et al. Non-diabetic renal disease with or without diabetic nephropathy in type 2 diabetes: clinical predictors and outcome. Ren Fail 2015; 37: 572–575 [DOI] [PubMed] [Google Scholar]
- 26. Byun JM, Lee CH, Lee SR. et al. Renal outcomes and clinical course of nondiabetic renal diseases in patients with type 2 diabetes. Korean J Intern Med 2013; 28: 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong TYH, Choi PCL, Szeto CC. et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care 2002; 25: 900–905 [DOI] [PubMed] [Google Scholar]
- 28. Liu D, Huang T, Chen N. et al. The modern spectrum of biopsy-proven renal disease in Chinese diabetic patients—a retrospective descriptive study. Peer J 2018; 6: e4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu MY, Chen XM, Sun XF. et al. Validation of a differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases and the establishment of a new diagnostic model. J Diabetes 2014; 6: 519–526 [DOI] [PubMed] [Google Scholar]
- 30. Oh SW, Kim S, Na KY. et al. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract 2012; 97: 418–424 [DOI] [PubMed] [Google Scholar]
- 31. Chong YB, Keng TC, Tan LP. et al. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail 2012; 34: 323–328 [DOI] [PubMed] [Google Scholar]
- 32. Haider DG, Peric S, Friedl A. et al. Kidney biopsy in patients with diabetes mellitus. Clin Nephrol 2011; 76: 180–185 [DOI] [PubMed] [Google Scholar]
- 33. Bi H, Chen N, Ling G. et al. Nondiabetic renal disease in type 2 diabetic patients: a review of our experience in 220 cases. Ren Fail 2011; 33: 26–30 [DOI] [PubMed] [Google Scholar]
- 34. Zhang PP, Ge YC, Li SJ. et al. Renal biopsy in type 2 diabetes: timing of complications and evaluating of safety in Chinese patients. Nephrology 2011; 16: 100–105 [DOI] [PubMed] [Google Scholar]
- 35. Zhou J, Chen X, Xie Y. et al. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant 2008; 23: 1940–1945 [DOI] [PubMed] [Google Scholar]
- 36. Soni SS, Gowrishankar S, Kishan AG. et al. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology 2006; 11: 533–537 [DOI] [PubMed] [Google Scholar]
- 37. Rychlík I, Jančová E, Tesař V. et al. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant 2004; 19: 3040–3049 [DOI] [PubMed] [Google Scholar]
- 38. Mazzucco G, Bertani T, Fortunato M. et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis 2002; 39: 713–720 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki D, Takano H, Toyoda M. et al. Evaluation of renal biopsy samples of patients with diabetic nephropathy. Intern Med 2001; 40: 1077–1084 [DOI] [PubMed] [Google Scholar]
- 40. Zhuo L, Ren W, Li W. et al. Evaluation of renal biopsies in type 2 diabetic patients with kidney disease: a clinicopathological study of 216 cases. Int Urol Nephrol 2013; 45: 173–179 [DOI] [PubMed] [Google Scholar]
- 41. Yaqub S, Kashif W, Hussain SA.. Non-diabetic renal disease in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl 2012; 23: 1000–1007 [DOI] [PubMed] [Google Scholar]
- 42. Kong W-Y, Cheah P-L, Tan S-Y. et al. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail 2012; 34: 323–328 [DOI] [PubMed] [Google Scholar]
- 43. Tone A, Shikata K, Matsuda M. et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract 2005; 69: 237–242 [DOI] [PubMed] [Google Scholar]
- 44. Schwartz MM, Lewis EJ, Leonard-Martin T. et al. ; The Collaborative Study Group. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. Nephrol Dial Transplant 1998; 13: 2547–2552 [DOI] [PubMed] [Google Scholar]
- 45. Tan J, Zwi LJ, Collins JF. et al. Presentation, pathology and prognosis of renal disease in type 2 diabetes. BMJ Open Diabetes Res Care 2017; 5: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilfred DC, Mysorekar VV, Venkataramana RS. et al. Nondiabetic renal disease in type 2 diabetes mellitus patients: a clinicopathological study. J Lab Physicians 2013; 5: 94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.