Abstract
Background
Anemia at hemodialysis (HD) initiation is common. Correcting low hemoglobin (Hgb) before HD initiation may improve survival by avoiding potential harms of chronic anemia, high doses of erythropoiesis-stimulating agents (ESAs) and intravenous (IV) iron in the early HD period, and/or rapid Hgb rise.
Methods
We included 4604 incident HD patients from 21 countries in the Dialysis Outcomes and Practice Patterns Study Phases 4–5 (2009–15). Because low Hgb at HD start may reflect comorbidity or ESA hyporesponse, we restricted our analysis to the 80% of patients who achieved Hgb ≥10 g/dL 91–120 days after HD start (Month 4).
Results
About 53% of these patients had Hgb <10 g/dL in Month 1 (<30 days after HD start); they were younger with a similar comorbidity profile (versus Hgb ≥10 g/dL). Month 1 Hgb was associated with first-year HD mortality (adjusted hazard ratio for 1 g/dL higher Hgb was 0.89; 95% confidence interval: 0.81–0.97), despite minimal differences in Month 4 Hgb. Patients with lower Hgb in Month 1 received higher doses of ESA, but not IV iron, over the first 3 months of HD. Results were consistent when excluding catheter users or adjusting for IV iron and ESA dose over the first 3 months.
Conclusions
Even among patients with Hgb ≥10 g/dL 3 months later, anemia at HD initiation was common and associated with elevated mortality. A more proactive approach to anemia management in advanced chronic kidney disease (CKD) may thus improve survival on HD, though long-term prospective studies of non-dialysis CKD patients are needed.
Keywords: anemia, chronic kidney disease, hemodialysis, hemoglobin, mortality
INTRODUCTION
Most chronic kidney disease (CKD) patients suffer from anemia due to deficiencies in iron and erythropoietin, often resulting in fatigue, weakness and an increased risk of cardiovascular complications [1, 2]. A substantial proportion of CKD patients reach end-stage kidney disease (ESKD) with very low hemoglobin (Hgb) levels [3]. The most likely causes include: (i) uremic intoxication inhibiting erythropoiesis; (ii) lack of pre-ESKD nephrology care; (iii) lack of adequate anemia treatment, despite nephrologist care, including insufficient correction of iron deficiency, as illustrated by low levels of serum ferritin or transferrin saturation (TSAT); or (iv) lack of responsiveness to anemia treatment, often due to poor general health or acute illness. Regular treatments with intravenous (IV) iron and erythropoiesis-stimulating agents (ESAs) are standard for thrice-weekly incenter hemodialysis (HD) patients, but are more difficult to carry out for non-HD CKD patients who receive care intermittently.
ESA and IV iron therapies are effective in raising Hgb levels and avoiding blood transfusions [4–7]. However, concerns regarding ESA toxicity emerged following a number of randomized clinical trials (RCTs) [8–11]. Studies have consistently demonstrated harmful effects of administering large doses of ESA to reach and maintain higher Hgb levels [12], resulting in clinically acceptable Hgb targets that are now generally in the range of 10–12 g/dL [1, 13–16]. The practice of starting anemia therapy at 9.5–10.0 g/dL, based on results from the Time to Reconsider Evidence for Anemia Treatment (TREAT) trial (glomerular filtration rate 20–60 mL/min/1.73 m2) [11], may not be directly applicable to the dialysis transition period. Furthermore, two recent studies showed conflicting results regarding whether there may be a benefit [17] or no benefit [18] to starting dialysis therapy with a higher Hgb level. Controversy also remains in identifying optimal strategies for iron supplementation in non-dialysis CKD and ESKD [19–23]. In the non-dialysis CKD setting, the efficacy of IV iron to raise or sustain Hgb levels has been well-established, but most RCTs do not have sufficient follow-up to evaluate long-term safety [24]. In the HD setting, large cohort studies of IV iron and mortality have yielded mixed results [25–30]. While the recently published Proactive IV irOn Therapy in haemodiALysis patients (PIVOTAL) RCT [31] demonstrated non-inferiority of a proactive high IV iron dose strategy, results may not be generalizable to all Dialysis Outcomes and Practice Patterns Study (DOPPS) countries—especially not the USA, where average iron doses and ferritin levels are greater than even the high IV iron dose PIVOTAL arm [32].
ESA and IV iron doses among long-term HD patients have likely been titrated based on individual patient responsiveness to treatment, but doses in the early HD period may more likely be driven by nephrologist practice patterns and regional guidelines [1, 13–15]. Some nephrologists may choose to administer very high doses of ESA and/or large bolus doses of IV iron to quickly achieve Hgb increases and avoid the symptoms and potential consequences of severe anemia. However, ESA labels warn that rapid Hgb increases may increase risk of adverse cardiovascular events [33, 34]. It is also possible that patients who start HD with low Hgb experience worse outcomes on HD irrespective of HD treatment strategy.
We hypothesize that management of anemia before the start of dialysis improves survival after HD start by avoiding the potential harms of chronic anemia, high doses of ESA and IV iron in the early months of HD, and/or a rapid Hgb rise. To test this hypothesis, we will investigate: (i) the association between anemia at HD start and all-cause mortality through 1 year of HD and (ii) mortality rates for different doses of IV iron and ESA during the early dialysis period.
MATERIALS AND METHODS
Data source
The DOPPS is a large, international prospective cohort study of patients aged ≥18 years treated with incenter HD in 21 countries. Maintenance HD patients were randomly selected from national samples of dialysis facilities in each country; detailed information is included in prior publications [35, 36] and at http://www.dopps.org. Study approval and patient consent were obtained as required by national and local ethics committee regulations. This analysis included DOPPS Phase 4 (2009–11) and Phase 5 (2012–15) patients who enrolled in the DOPPS within 30 days after initiating HD.
Variables
Information on age, sex, post-dialysis weight, body mass index (BMI), comorbid conditions [diabetes, hypertension, congestive heart failure (CHF), peripheral vascular disease (PVD) and cancer] and catheter use was abstracted from medical records at DOPPS enrollment. Monthly data on medication prescriptions (ESA and IV iron) were also abstracted from medical records. To convert ESA doses to units of IV epoetin, we used conversion factors of 250:1 for darbepoetin [37] 208:1 (250/1.2) for pegylated epoetin-β [38] and 1.15:1 for subcutaneous injections [39]. Laboratory values (Hgb, TSAT, ferritin, albumin and phosphorus) are described as ‘Month 1’ when measured 0–30 days after starting HD (first measurement recorded in DOPPS) and ‘Month 4’ when measured 91–120 days after starting HD.
Study design
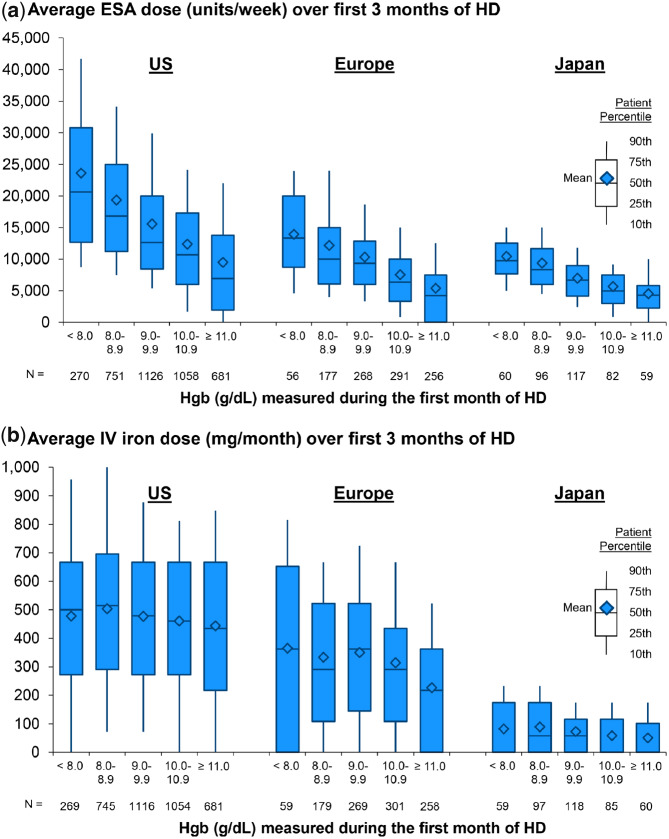
In our primary analysis, we estimated the effect of Month 1 Hgb on all-cause mortality from Months 4 to 12. We restricted our analysis to patients with Hgb ≥10.0 g/dL in Month 4 to facilitate a comparison of mortality rates between patients with similar Hgb levels 4 months after HD initiation (Figure 1). This allows us to investigate whether the hypothesized harm of starting HD with low (versus higher) Hgb is sustained even after a rapid Hgb increase into target range. While Hgb targets, specifically upper targets, are controversial [16] and have varied over time and by region [1, 13–15], we chose a restriction of ≥10 g/dL at Month 4. Patients with low Hgb at HD start due to severe comorbidity or a lack of responsiveness to pre-ESKD anemia therapy are less likely to experience an Hgb increase to ≥10 g/dL in the subsequent months; those with Hgb ≥10 g/dL after 4 months on HD would have theoretically been able to start HD with a higher Hgb, with active anemia management prior to starting HD. By excluding patients poorly responsive to anemia treatment after dialysis start, our study is thus designed to include an exposure variable that should mostly reflect differences in pre-ESKD anemia care. Among 5726 patients with Hgb measured in Month 4, we excluded 1122 (20%) with Hgb <10 g/dL. The remaining 4604 patients were eligible for the primary analysis (Figure 2); 447 from DOPPS Phase 4 and 4157 from DOPPS Phase 5, when more emphasis was placed on enrolling incident HD patients.
FIGURE 1.
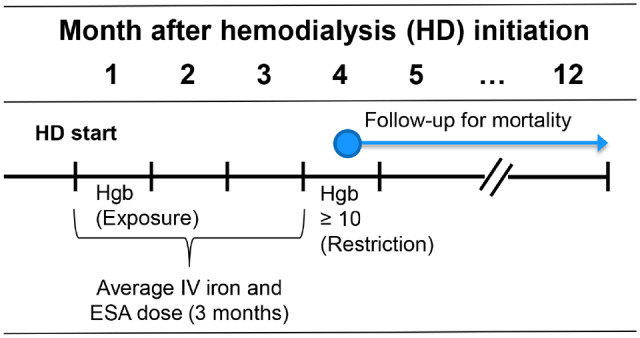
Illustration of study design and timing of data collection. Hgb values in Month 1 were measured 0–30 days after starting HD. Hgb values in Month 4 were measured 91–120 days after starting HD. The primary exposure is Month 1 Hgb. The primary analysis was restricted to 4604 patients with Hgb ≥10 g/dL in Month 4, with mortality follow-up beginning after Hgb measurement in Month 4, and ending after 12 months on HD. A secondary analysis of ESA and IV iron doses and mortality was restricted to 5959 patients with no Hgb restriction in Month 4.
FIGURE 2.
Flow diagram of patient selection with inclusion/exclusion criteria.
Statistical analysis
Cox regression was used to estimate the effect of Month 1 Hgb on all-cause mortality, restricted to patients with Month 4 Hgb ≥10.0 g/dL. Left-truncated Cox models were used, with time on dialysis as the time scale; time at risk started after the Hgb measurement in Month 4 and ended at the time of death, 7 days after leaving the facility due to transfer or change in kidney replacement therapy modality, loss to follow-up, end of study phase or 1 year after initiating HD (whichever event occurred first). Hgb was categorized to explore the functional form of association with mortality. Cox models were stratified by DOPPS phase and country, and by dialysis chain affiliation in the USA. Within-facility clustering was accounted for by using robust sandwich covariance estimators. Covariates selected for adjustment included age, sex, BMI, history of five comorbidities (diabetes, hypertension, CHF, PVD and cancer), catheter use at study enrollment, serum albumin and phosphorus in Months 1 and 4, and Hgb in Month 4.
We performed several sensitivity analyses to test the robustness of the findings. First, we additionally adjusted for ESA and IV iron doses over the first 3 months of HD to investigate the role of these potential mediators on causal pathways between low Hgb at HD initiation and mortality. Second, we used Hgb measured in the month prior to initiating HD as the exposure rather than Month 1 Hgb. Third, we excluded patients dialyzing with a catheter at study enrollment as a proxy for minimal pre-ESKD nephrology care. Fourth, we additionally adjusted for an indicator of >1 month of pre-ESKD nephrology care in a subset of 29% of patients for whom the data were available. Fifth, we varied the Month 4 Hgb restriction from ≥10.0 to ≥10.5 and ≥11.0 g/dL, because some ESA hyporesponsive patients could be treated to Hgb ≥10.0 g/dL, but not readily to higher Hgb levels. Sixth, we excluded patients not receiving any ESA therapy during the first 3 months of HD, an indicator of likely endogenous erythropoietin production in most patients.
In a secondary analysis not restricted to Month 4 Hgb ≥10 g/dL, we illustrated the distribution of ESA and IV iron doses over the first 3 months of HD therapy by Month 1 Hgb, TSAT and ferritin across three regions (Europe, Japan and USA). Countries outside of these three regions were included in the mortality models but were not shown in these descriptive analyses due to small sample sizes. In this population, we also estimated the effects of average ESA and IV iron doses administered over the first 3 months of HD on all-cause mortality. Cox regression models were implemented as in the primary analysis, with additional adjustment for Hgb, TSAT and ferritin in Month 1, but without adjustment for Month 4 Hgb, a potential mediator. We repeated this analysis in a subgroup of patients with Hgb <10.0 g/dL in Month 1 to better inform optimal treatment practices for patients who initiated HD with low Hgb.
We used multiple imputation, assuming data were missing at random, to impute missing covariate values using the Sequential Regression Multiple Imputation Method by IVEware [40]. Results from 20 such imputed data sets were combined for the final analysis using Rubin’s formula [41]. The proportion of missing data was <10% for all covariates, with the exception of Month 1 laboratory measures (ferritin 28%; TSAT 27%; albumin 16%; phosphorus 11%) and the five comorbidities (10–21%). All analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics
Our primary analysis included 4604 patients with Hgb ≥10.0 g/dL in Month 4 after starting HD. Among these patients, 53% had Hgb <10.0 g/dL in Month 1, including 6% with Hgb <8.0 g/dL. Also, 20% had Hgb ≥11.0 g/dL in Month 1, including 7% with Hgb ≥12.0 g/dL and 2% with Hgb ≥13.0 g/dL. The mean Hgb in Month 4 was between 11.3 and 11.6 g/dL across the five groups of Month 1 Hgb (from <8.0 to ≥11.0 g/dL). Patients with lower Hgb in Month 1 were younger, more likely to dialyze with a catheter and had higher ESA doses over the subsequent 3 months, but only marginally higher IV iron doses (Table 1). Patients with lower Hgb in Month 1 also had lower TSAT, higher serum ferritin and lower serum albumin in Month 1, but all of these differences were minimized by Month 4. The prevalence of comorbidities varied minimally by Month 1 Hgb.
Table 1.
Patient characteristics by Hgb in Month 1 after starting HD, restricted to patients with Hgb ≥10.0 g/dL in Month 4 after starting HD
| Patient characteristic | All | Hgb (g/dL) in Month 1 after HD start |
||||
|---|---|---|---|---|---|---|
| <8.0 | 8.0–8.9 | 9.0–9.9 | 10.0–10.9 | ≥11.0 | ||
| Patients, n (%) | 4604 | 283 (6) | 822 (18) | 1260 (28) | 1209 (27) | 897 (20) |
| Baseline characteristics and treatments | ||||||
| Age (years) | 64.1 ± 14.5 | 59.2 ± 16.1 | 62.7 ± 14.9 | 64.4 ± 14.7 | 65.1 ± 14.2 | 64.9 ± 13.7 |
| Sex (% male) | 60 | 56 | 60 | 57 | 60 | 66 |
| Post-dialysis weight (kg) | 80.4 ± 22.8 | 75.9 ± 22.9 | 78.3 ± 22.1 | 80.7 ± 23.3 | 80.6 ± 21.3 | 81.8 ± 23.5 |
| BMI (kg/m2) | 28.1 ± 6.9 | 26.8 ± 6.3 | 27.2 ± 6.5 | 28.4 ± 7.2 | 28.3 ± 6.6 | 28.4 ± 7.0 |
| Catheter use (%) | 59 | 65 | 63 | 60 | 59 | 51 |
| Hemodiafiltration (%) | 3 | 2 | 3 | 3 | 3 | 4 |
| Single pool Kt/V | 1.33 ± 0.35 | 1.28 ± 0.37 | 1.33 ± 0.37 | 1.35 ± 0.35 | 1.33 ± 0.35 | 1.33 ± 0.35 |
| Laboratory values <30 days after starting HD | ||||||
| Hgb (g/dL) | 9.9 ± 1.3 | 7.4 ± 0.5 | 8.5 ± 0.3 | 9.5 ± 0.3 | 10.4 ± 0.3 | 11.8 ± 0.8 |
| TSAT (%) | 20.5 ± 9.8 | 18.9 ± 9.3 | 19.4 ± 10.1 | 20.2 ± 9.5 | 20.4 ± 9.4 | 22.6 ± 10.2 |
| Ferritin (ng/mL) | 337 ± 334 | 402 ± 365 | 366 ± 368 | 336 ± 325 | 331 ± 333 | 305 ± 305 |
| Serum albumin (g/dL) | 3.4 ± 0.5 | 3.1 ± 0.5 | 3.3 ± 0.6 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 |
| Serum phosphorus (mg/dL) | 4.7 ± 1.5 | 5.0 ± 2.0 | 4.7 ± 1.5 | 4.6 ± 1.4 | 4.6 ± 1.4 | 4.8 ± 1.5 |
| Laboratory values 91–120 days after starting HD | ||||||
| Hgb (g/dL) | 11.5 ± 1.0 | 11.3 ± 1.0 | 11.5 ± 1.0 | 11.4 ± 1.0 | 11.4 ± 0.9 | 11.6 ± 1.1 |
| TSAT (%) | 26.6 ± 11.7 | 26.6 ± 12.5 | 25.7 ± 12.4 | 26.4 ± 11.4 | 26.7 ± 11.4 | 28.0 ± 11.9 |
| Ferritin (ng/mL) | 428 ± 377 | 388 ± 361 | 402 ± 352 | 439 ± 398 | 454 ± 372 | 424 ± 398 |
| Serum albumin (g/dL) | 3.7 ± 0.4 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.4 | 3.7 ± 0.4 |
| Serum phosphorus (mg/dL) | 5.2 ± 1.5 | 5.5 ± 1.6 | 5.4 ± 1.7 | 5.2 ± 1.5 | 5.2 ± 1.4 | 4.9 ± 1.3 |
| Anemia treatment during first 3 months of HD | ||||||
| ESA use (%, any during 3 months) | 92 | 98 | 98 | 98 | 91 | 77 |
| ESA dose (1000 U/week) | 12.7 ± 10.4 | 19.6 ± 13.6 | 16.7 ± 11.0 | 13.8 ± 10.1 | 10.8 ± 8.3 | 8.0 ± 8.9 |
| IV iron use (%, any during 3 months) | 86 | 86 | 87 | 87 | 86 | 82 |
| IV iron dose (mg/month) | 416 ± 292 | 445 ± 308 | 449 ± 301 | 428 ± 286 | 412 ± 281 | 364 ± 291 |
| Comorbid conditions (%) | ||||||
| Diabetes | 62 | 60 | 61 | 63 | 63 | 59 |
| Hypertension | 87 | 87 | 88 | 85 | 88 | 88 |
| CHF | 27 | 25 | 25 | 30 | 27 | 26 |
| PVD | 16 | 16 | 14 | 18 | 16 | 17 |
| Cancer (non-skin) | 11 | 9 | 12 | 12 | 12 | 9 |
Mean ± standard deviation or % shown; mean ESA and IV iron doses averaged >3 months and treat nonusers as 0 dose; note columns do not sum to the total due to 133 (3%) patients missing Month 1 Hgb data.
Hgb and mortality
Among the 4604 patients, we observed 277 deaths and a mortality rate of 0.105/year during follow-up. Patients with lower Month 1 Hgb—those who experienced a rapid Hgb increase to ≥10.0 g/dL during the subsequent 3 months—had higher mortality rates than patients who started HD with higher Hgb (Table 2). Compared with Hgb ≥11.0 g/dL, the adjusted hazard ratio (HR) [95% confidence interval (CI)] was 1.99 (1.18–3.38) for Hgb <8.0 g/dL and ranged from 1.18 to 1.35 for Hgb 8.0–10.9 g/dL (Table 2, Model 3). As a continuous variable, Month 1 Hgb was inversely associated with mortality (adjusted HR for 1 g/dL higher Hgb = 0.89; 95% CI: 0.81–0.97; P for trend = 0.01). In sensitivity analyses, the HR for 1 g/dL higher Hgb was generally consistent when: (i) adjusting for potential mediators, ESA and IV iron doses over the subsequent 3 months (HR = 0.89); (ii) using Hgb measured in the month prior to initiating HD as the exposure (HR = 0.90); (iii) excluding catheter users (HR = 0.84); (iv) adjusting for >1 month of pre-ESKD nephrology care (HR = 0.87); (v) varying the Month 4 Hgb restriction of ≥10.0 to ≥10.5 (HR = 0.87) and to ≥11.0 (HR = 0.89) g/dL; and (vi) excluding patients not treated with ESA during the first 3 months of HD (HR = 0.89).
Table 2.
HR of mortality for Hgb measured in Month 1 after starting HD, by level of covariate adjustment, among patients with Hgb ≥10.0 g/dL in Month 4 after starting HD
| Exposure | N (%) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Hgb (g/dL) in Month 1 after HD start, categories | ||||
| <8.0 | 283 (6) | 1.81 (1.07–3.05) | 2.10 (1.25–3.54) | 1.99 (1.18–3.38) |
| 8.0–8.9 | 822 (18) | 1.23 (0.81–1.86) | 1.30 (0.87–1.95) | 1.23 (0.83–1.84) |
| 9.0–9.9 | 1260 (28) | 1.52 (1.06–2.18) | 1.39 (0.96–2.01) | 1.35 (0.93–1.95) |
| 10.0–10.9 | 1209 (27) | 1.28 (0.89–1.85) | 1.26 (0.86–1.84) | 1.18 (0.81–1.73) |
| ≥11.0 | 897 (20) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Hgb (g/dL) in Month 1 after HD start, continuous | ||||
| Per 1 g/dL | – | 0.91 (0.83–0.99) | 0.88 (0.81–0.97) | 0.89 (0.81–0.97) |
HR (95% CI) of all-cause mortality in left-truncated Cox model (vintage time scale) from Month 4 to Month 12 of HD; all Cox models stratified by DOPPS phase, country and US dialysis chain affiliation; Model 1: unadjusted; Model 2: adjusted for age, sex, BMI, five comorbidities (diabetes, hypertension, CHF, PVD and cancer) and catheter use; Model 3: further adjusted for serum albumin and phosphorus in Months 1 and 4, and Hgb in Month 4.
Description of ESA and IV iron dosing
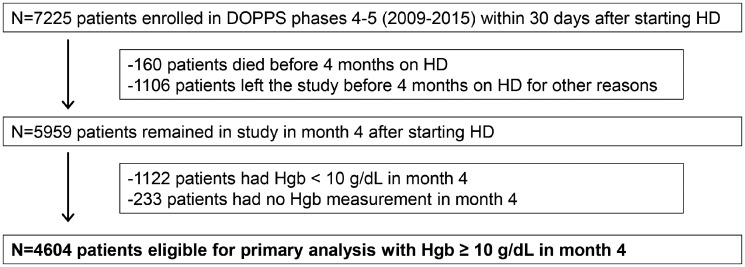
We also studied 5959 patients who survived to Month 4 (to measure ESA and IV iron doses administered over the first 3 months of HD), but not restricting to patients with Hgb ≥10.0 g/dL in Month 4, to illustrate the variation in dosing patterns. Patients with lower Month 1 Hgb had greater ESA doses over the subsequent 3 months, reflecting indication for the treatment and/or pre-ESKD ESA hyporesponsiveness (Figure 3a). Patients with lower Month 1 Hgb had greater IV iron doses over the subsequent 3 months in Europe, but not in the USA, where a median dose of 450–500 mg/month was observed regardless of Hgb level (Figure 3b). IV iron doses in the first 3 months of HD were higher among patients initiating HD with lower TSAT and lower ferritin in all regions (Supplementary data, Figure S1). However, relatively high IV iron doses were still observed among many patients with ferritin levels ≥800 ng/mL. Overall, ESA and IV iron doses in the first 3 months of HD were highest in the USA, followed by Europe, and lowest in Japan across all strata.
FIGURE 3.
Distribution of (a) ESA dose and (b) IV iron dose administered over the first 3 months of HD therapy, by region and Hgb measured during the first month of HD. In contrast to primary analysis, these data were not restricted to patients with Hgb ≥10 g/dL in Month 4 after starting HD. Europe: Belgium, France, Germany, Italy, Spain, Sweden and UK. Data from other regions (Canada, Australia, New Zealand, China, Russia, Turkey and Gulf Cooperation Council) were suppressed in this figure due to small sample sizes, but were included in all other analyses.
Anemia treatment and mortality
Among this population of 5959 patients who survived to Month 4, 92% were prescribed an ESA, and 83% were prescribed IV iron at some point during the first 3 months on HD. The associations of both ESA and IV iron doses over the first 3 months of HD on all-cause mortality from Month 4 to Month 12 are shown in Table 3. We observed elevated mortality at only very high ESA doses; the adjusted HR (95% CI) was 1.43 (1.02–2.01) for >25 000 (12% of patients) versus 5000–10 000 units/week. In a subgroup analysis of 3378 patients with Month 1 Hgb <10.0 g/dL, the pattern was similar. The adjusted association between IV iron dose and mortality was U-shaped, with the lowest mortality rate observed for patients receiving 200–399 mg/month in both the whole sample and subgroup with Month 1 Hgb <10.0 g/dL (Table 3).
Table 3.
HR of mortality for ESA and IV iron doses over the first 3 months of HD without restricting to patients with Hgb ≥10 g/dL in Month 4, overall and among a subset of patients with Hgb <10.0 g/dL in Month 1 of HD
| All HD patients |
||||
|---|---|---|---|---|
| Restricted to subset with Hgb <10.0 g/dL in first month of HD | ||||
| Exposure | N (%) | Adjusted HR | N (%) | Adjusted HR |
| (95% CI) | (95% CI) | |||
| Average ESA dose (units/week) over first 3 months on HD | ||||
| None | 479 (8) | 0.94 (0.59–1.51) | 103 (3) | 0.63 (0.22–1.82) |
| <5K | 711 (12) | 0.84 (0.54–1.29) | 253 (8) | 0.56 (0.25–1.27) |
| 5K–10K | 1543 (26) | 1 (Ref.) | 836 (25) | 1 (Ref.) |
| 10K–15K | 1203 (21) | 0.89 (0.64–1.24) | 773 (23) | 0.85 (0.56–1.27) |
| 15K–25K | 1223 (21) | 0.97 (0.71–1.33) | 818 (25) | 0.80 (0.53–1.20) |
| >25K | 699 (12) | 1.43 (1.02–2.01) | 537 (16) | 1.54 (1.01–2.36) |
| Average IV iron dose (mg/month) over first 3 months on HD | ||||
| None | 1008 (17) | 1.50 (1.05–2.15) | 549 (17) | 1.74 (1.08–2.79) |
| <200 | 741 (13) | 1.08 (0.72–1.62) | 407 (12) | 1.25 (0.74–2.10) |
| 200–399 | 1157 (20) | 1 (Ref.) | 605 (18) | 1 (Ref.) |
| 400–599 | 1359 (23) | 1.11 (0.78–1.57) | 783 (24) | 1.39 (0.88–2.18) |
| ≥600 | 1604 (27) | 1.24 (0.87–1.76) | 975 (29) | 1.29 (0.80–2.07) |
HR (95% CI) of all-cause mortality in left-truncated Cox model (vintage time scale) from Month 4 to Month 12 of HD; Cox models stratified by DOPPS phase, country and US dialysis chain affiliation. Adjustments: age, sex, BMI, five comorbidities (diabetes, hypertension, CHF, PVD and cancer), catheter use, serum albumin and phosphorus in Month 1 and Month 4, Hgb, TSAT and ferritin in Month 1. Note this analysis was not restricted to patients with Hgb ≥10 g/dL in Month 4.
DISCUSSION
Among patients with Hgb levels ≥10.0 g/dL in Month 4 of HD, 53% of patients had Hgb <10.0 g/dL in Month 1 after HD initiation, and lower Hgb in Month 1 of HD was associated with a higher mortality rate in Months 4–12. While we expected to observe a greater mortality rate for patients who initiated HD with low Hgb, this elevated rate was still observed among patients achieving Hgb ≥10.0 g/dL in the early HD period. In secondary analyses not restricted to Hgb ≥10.0 g/dL in Month 4 of HD, we found a U-shaped association between IV iron dose over the first 3 months of HD and mortality, and elevated mortality for patients receiving the largest doses of ESA (>25 000 U/week).
Our primary result (Table 2, Model 3) is consistent with our hypothesis that management of anemia before the start of dialysis improves survival after HD start, although there are many possible explanations for these findings. One potential explanation is that intense anemia treatment and/or a rapid Hgb rise in the early HD period may be responsible for the elevated mortality rate. Patients who started HD with the lowest Hgb levels received the largest ESA doses over the first 3 months of HD. However, additional adjustment for ESA and IV iron doses (potential mediators) had minimal impact on the association between Hgb at HD initiation and mortality, thus making this explanation unlikely, though we did not conduct a formal mediation analysis [42]. Another possibility is that anemia treatment prior to ESKD has long-term benefits compared with initiating anemia therapy after HD start. Among patients with Hgb ≥10.0 g/dL in Month 4 of HD, mean Hgb levels in Month 4 were similar for patients who initiated HD with Hgb ≥11.0 (11.6 g/dL) versus Hgb <8.0 (11.3 g/dL) g/dL. The subsequent mortality rate was twice as high for patients who started HD with Hgb <8.0 versus ≥11.0 g/dL, despite also having a similar comorbidity profile (Table 1). This striking difference in mortality may point to a lingering effect of untreated anemia in CKD. We also found an 18–35% greater mortality rate between Hgb 8.0–10.9 and ≥11.0 g/dL, though surprisingly minimal difference within the range of 8.0–10.9 g/dL. It is possible that a ‘step function’ exists, with low-, medium- and high-risk groups, though our study is not powered to detect the exact Hgb cutpoints. In addition, a noncausal explanation is that the association may be biased due to residual confounding, possibly because patients with higher Hgb at HD start adhered more to prescribed treatments and recommendations and/or received better overall quality of care before HD initiation. We adjusted for catheter use at study enrollment as a proxy for this latter potential confounder, and also excluded catheter users in a sensitivity analysis.
The relation between anemia management during the transition period to ESKD and post-dialysis outcomes is challenging to assess and often requires an innovative study design. Mc Causland et al. [18] performed a post hoc follow-up analysis of TREAT among the subset (only 15%) of randomized patients who reached ESKD [11]. Mean Hgb at dialysis start was higher in the darbepoetin intervention (11.3 g/dL) versus the control (9.5 g/dL) group, but no all-cause mortality benefit (HR = 1.16; 95% CI: 0.69–1.93) was observed from HD start (when anemia therapy switched from protocol-driven to physician-driven) through 6 months; differences with our study may be explained by the study design and/or selection criteria. An increased stroke rate was observed in the intervention group, although paradoxically, it is likely ESA doses were much higher (by indication) during the at-risk period in the control group. The authors concluded that there is no apparent benefit from starting dialysis therapy with a higher Hgb level, but acknowledged other factors (e.g. avoiding transfusions) should help inform whether to treat mild-to-moderate anemia with ESA in CKD patients preparing for dialysis. A recent study by Wetmore et al. [17], using United States Renal Data System (USRDS) data, in contrast to Mc Causland et al. [18], drew similar conclusions as our study when analyzing US Medicare patients >66 years old at dialysis initiation. The 1-year mortality rate was slightly lower (HR = 0.88) among patients treated with ESA prior to dialysis start and who maintained Hgb ≥9.0 g/dL during the 3 months before and after dialysis start versus patients with Hgb <9.0 g/dL before dialysis start who then received ESA after HD start and experienced an Hgb increase to ≥9.0 g/dL. It is encouraging that the current DOPPS study achieved similar findings to the USRDS study while using a different data source (international sample with no age restriction) and a different analytical approach to address the question of interest.
Results from large observational studies [43, 44] are consistent with our finding that patients receiving the highest ESA doses in the early HD period had worse survival. Following patients soon after HD initiation may better capture dosing patterns (practice preferences) before patients transition to a more individualized steady-state dosing protocol, at which point observed associations between ESA dose and mortality are more likely attributable to confounding factors that drive ESA dose requirements than a causal effect [45, 46].
While our main focus was on Hgb levels, we also investigated IV iron dosing. IV iron dose over the first 3 months of HD was generally greater in patients who initiated HD with low levels of ferritin and TSAT, as expected. Patients starting HD with lower (versus higher) Hgb received more IV iron in the next 3 months, but this pattern was not observed in the USA, where a high median dose of 450–500 mg/month was observed regardless of Hgb at HD start. This may reflect the practice of bolus IV iron dosing in many US facilities, where doses of at least 100 mg are administered in consecutive HD sessions [47, 48]. Michels et al. [49] found that a low-dose (versus bolus dose) IV iron strategy in the early HD period may be beneficial by reducing ESA doses and risk of mortality. While we did not assess specific dosing patterns, we similarly observed a greater mortality rate at high doses of IV iron that would generally characterize a bolus dosing strategy, with the lowest mortality rate observed at 200–399 mg/month. Our results were also consistent with Kuo et al. [50] who demonstrated that a low-dose IV iron strategy was optimal for incident HD patients. The recent PIVOTAL trial randomized patients to a proactive high IV iron dose (400 mg/month IV iron; discontinue if ferritin >700 ng/mL or TSAT >40%) or reactive low IV iron dose (100–400 mg/month IV iron; discontinue if ferritin >200 ng/mL and TSAT >20%) and demonstrated non-inferiority of the high IV iron dose strategy [31]; however, these ‘high’ doses were lower than the median dose of 450–500 mg/month we observed in the USA, and the upper ferritin threshold of 700 ng/mL in the ‘proactive’ arm was lower than the median serum ferritin levels observed in the USA [32]. Generalizability of these findings in the context of the high levels of serum ferritin and IV iron dosing observed in the USA remains an open question.
This analysis had some limitations. First, to address potential residual confounding due to better pre-ESKD care (unrelated to anemia), we adjusted for catheter use at study enrollment, a proxy for lack of pre-ESKD nephrology care. Regarding potential residual confounding due to health status, it is reassuring that the distribution of key risk factors (e.g. serum albumin at Month 4) and prevalence of comorbidities was similar by Hgb level at HD initiation (Table 1). Second, we were unable to determine history of anemia therapy—including usage or dosage—prior to HD start, which would have helped inform the cause(s) of initiating HD with low Hgb (e.g. lack or treatment or hyporesponsiveness); this limitation prompted us to restrict to patients with Hgb ≥10 g/dL in Month 4, excluding patients whose low Month 1 Hgb was likely due to poor general health or ESA hyporesponsiveness, thus using low Hgb at HD start as a proxy for lack of pre-ESKD anemia treatment. Third, data on potentially important variables were not available for a majority of patients, including transfusions, C-reactive protein (not measured in North America) and residual kidney function. Finally, our exposure variable was Hgb measured within 30 days after initiating HD (median = 13 days), but Hgb could have changed in the days immediately after starting HD. We thus performed a sensitivity analysis in a subset of patients for which data were available on Hgb levels immediately prior to starting HD.
This analysis also had some key strengths. We considered mortality as the primary outcome rather than a surrogate outcome, such as change in Hgb, which is often used in smaller RCTs with short follow-up to demonstrate effectiveness, but not safety, of pre-ESKD anemia therapies. Furthermore, the large international DOPPS sample reflected a wide range of anemia management practices in the early HD period. In particular, we observed that IV iron prescription patterns did not appear to be driven by Hgb levels in the USA, where doses were very high in the first 3 months of HD (median 450–500 mg/month).
In this study, we found that, even among patients who achieved Hgb ≥10.0 g/dL within 4 months of starting HD, low Hgb at HD initiation was common (53% had <10.0 g/dL) and was associated with elevated mortality. Compared with Hgb ≥11.0 g/dL at HD start, we observed a 2-fold greater mortality rate for Hgb <8.0 g/dL and an 18–35% greater mortality rate for Hgb 8.0–10.9 g/dL. A more proactive approach to anemia management in advanced CKD may thus improve first-year survival on HD, though long-term prospective studies examining anemia treatments and adverse events starting in the nondialysis CKD setting are needed.
Supplementary Material
ACKNOWLEDGEMENTS
Heather Van Doren, senior medical editor at Arbor Research Collaborative for Health, provided editorial assistance on this manuscript.
FUNDING
The Dialysis Outcomes and Practice Patterns Study (DOPPS) Program is supported by Amgen, Kyowa Hakko Kirin and Baxter Healthcare. Additional support for specific projects and countries is provided by AstraZeneca, the European Renal Association-European Dialysis and Transplant Association, Fresenius Medical Care Asia-Pacific Ltd, Fresenius Medical Care Canada Ltd, the German Society of Nephrology, Janssen, the Japanese Society for Peritoneal Dialysis, Keryx, Kidney Care UK, MEDICE Arzneimittel Pütter GmbH & Co KG, Proteon and Vifor Fresenius Medical Care Renal Pharma. Public funding and support is provided for specific DOPPS projects, ancillary studies, or affiliated research projects by: Australia: the National Health and Medical Research Council; Canada: Cancer Care Ontario (CCO) through the Ontario Renal Network (ORN); France: French National Institute of Health and Medical Research (INSERM); Thailand: Thailand Research Foundation (TRF), Chulalongkorn University Matching Fund, King Chulalongkorn Memorial Hospital Matching Fund and the National Research Council of Thailand (NRCT); the UK: National Institute for Health Research (NIHR) via the Comprehensive Clinical Research Network (CCRN); and the US: the National Institutes of Health and the Patient-Centered Outcomes Research Institute. All support is provided without restrictions on publications.
AUTHORS’ CONTRIBUTIONS
Each author contributed to conceiving and/or designing the work that led to the submission, acquiring data and/or playing an important role in interpreting the results, drafting/revising the manuscript and approving the final version. A.K., H.M., S.W., N.L.F., D.E.S., R.L.P. and B.M.R. contributed to the research idea and study design. S.W., R.L.P. and B.M.R. were responsible for data acquisition. A.K., H.M., S.W., N.L.F., R.V., S.H.J., M.M.S., D.E.S., M.I., R.L.P. and B.M.R. were responsible for data analysis/interpretation. A.K., H.M., N.L.F., D.E.S., R.L.P. and B.M.R. were responsible for statistical analysis. H.M., N.L.F., R.V., S.H.J., M.M.S., D.E.S., M.I., R.L.P. and B.M.R. were responsible for supervision or mentorship.
CONFLICT OF INTEREST STATEMENT
S.W. is an employee of Vifor Pharma Int. in Glattbrugg, Switzerland. The remaining authors declare they have no financial interests.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 2.National Institute of Diabetes and Digestive and Kidney Diseases. Anemia in Chronic Kidney Disease. 2014. https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd/anemia (3 March 2018, date last accessed)
- 3.United States Renal Data System. 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017 [Google Scholar]
- 4. Eschbach JW, Egrie JC, Downing MR. et al. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. N Engl J Med 1987; 316: 73–78 [DOI] [PubMed] [Google Scholar]
- 5. Cody JD, Hodson EM.. Recombinant human erythropoietin versus placebo or no treatment for the anaemia of chronic kidney disease in people not requiring dialysis. Cochrane Database Syst Rev 2016; 1: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besarab A, Amin N, Ahsan M. et al. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 2000; 11: 530–538 [DOI] [PubMed] [Google Scholar]
- 7. Coyne DW, Kapoian T, Suki W. et al. ; DRIVE Study Group. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 2007; 18: 975–984 [DOI] [PubMed] [Google Scholar]
- 8. Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 9. Drüeke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 10. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 11. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 12. Phrommintikul A, Haas SJ, Elsik M. et al. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet 2007; 369: 381–388 [DOI] [PubMed] [Google Scholar]
- 13. Kliger AS, Foley RN, Goldfarb DS. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 2013; 62: 849–859 [DOI] [PubMed] [Google Scholar]
- 14. Locatelli F, Bárány P, Covic A. et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement. Nephrol Dial Transplant 2013; 28: 1346–1359 [DOI] [PubMed] [Google Scholar]
- 15. Tsubakihara Y, Nishi S, Akiba T. et al. 2008 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 2010; 14: 240–275 [DOI] [PubMed] [Google Scholar]
- 16. Shah HH, Fishbane S.. Is there an established hemoglobin target range for patients undergoing chronic dialysis? Semin Dial 2018; 31: 415–419 [DOI] [PubMed] [Google Scholar]
- 17. Wetmore JB, Li S, Yan H. et al. Predialysis anemia management and outcomes following dialysis initiation: a retrospective cohort analysis. PLoS ONE 2018; 13: e0203767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mc Causland FR, Claggett B, Burdmann EA. et al. Treatment of anemia with darbepoetin prior to dialysis initiation and clinical outcomes: analyses from the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). Am J Kidney Dis 2018; 73: 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Del Vecchio L, Locatelli F.. Clinical practice guidelines on iron therapy: a critical evaluation. Hemodial Int 2017; 21: S125–S131 [DOI] [PubMed] [Google Scholar]
- 20. Collister D, Rigatto C, Tangri N.. Anemia management in chronic kidney disease and dialysis: a narrative review. Curr Opin Nephrol Hypertens 2017; 26: 214–218 [DOI] [PubMed] [Google Scholar]
- 21. Macdougall IC, Bircher AJ, Eckardt KU. et al. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2016; 89: 28–39 [DOI] [PubMed] [Google Scholar]
- 22. Charytan DM, Pai AB, Chan CT. et al. Considerations and challenges in defining optimal Iron utilization in hemodialysis. J Am Soc Nephrol 2015; 26: 1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdougall IC. Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin Kidney J 2017; 10 (Suppl 1): i16–i24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shepshelovich D, Rozen-Zvi B, Avni T. et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis 2016; 68: 677–690 [DOI] [PubMed] [Google Scholar]
- 25. Bailie GR, Larkina M, Goodkin DA. et al. Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int 2015; 87: 162–168 [DOI] [PubMed] [Google Scholar]
- 26. Kalantar-Zadeh K, Regidor DL, McAllister CJ. et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3070–3080 [DOI] [PubMed] [Google Scholar]
- 27. Miskulin DC, Tangri N, Bandeen-Roche K. et al. Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feldman HI, Joffe M, Robinson B. et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15: 1623–1632 [DOI] [PubMed] [Google Scholar]
- 29. Tangri N, Miskulin DC, Zhou J. et al. Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: a comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant 2015; 30: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hougen I, Collister D, Bourrier M. et al. Safety of intravenous iron in dialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2018; 13: 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macdougall IC, White C, Anker SD. et al. ; PIVOTAL Investigators and Committees. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019; 380: 447–458 [DOI] [PubMed] [Google Scholar]
- 32.The DOPPS Practice Monitor (DPM), April 2018. http://www.dopps.org/dpm (14 January 2019, date last accessed)
- 33. Unger EF, Thompson AM, Blank MB. et al. Erythropoiesis-stimulating agents - time for a reevaluation. N Engl J Med 2010; 362: 189–192 [DOI] [PubMed] [Google Scholar]
- 34. Singh AK. The FDA’s perspective on the risk for rapid rise in hemoglobin in treating CKD anemia: Quo Vadis. Clin J Am Soc Nephrol 2010; 5: 553–556 [DOI] [PubMed] [Google Scholar]
- 35. Young EW, Goodkin DA, Mapes DL. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 2000; 57 (Suppl 74): S-74–S-81 [Google Scholar]
- 36. Pisoni RL, Gillespie BW, Dickinson DM. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44 (Suppl 2): 7–15 [DOI] [PubMed] [Google Scholar]
- 37. Bock HA, Hirt-Minkowski P, Brünisholz M. et al. ; Swiss EFIXNES trial investigators. Darbepoetin alpha in lower-than-equimolar doses maintains haemoglobin levels in stable haemodialysis patients converting from epoetin alpha/beta. Nephrol Dial Transplant 2008; 23: 301–308 [DOI] [PubMed] [Google Scholar]
- 38. Choi P, Farouk M, Manamley N. et al. Dose conversion ratio in hemodialysis patients switched from darbepoetin alfa to PEG-epoetin beta: AFFIRM study. Adv Ther 2013; 30: 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McFarlane PA, Pisoni RL, Eichleay MA. et al. International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int 2010; 78: 215–223 [DOI] [PubMed] [Google Scholar]
- 40. Raghunathan TE, Solenberger PW, Van Hoewyk J.. IVEware: Imputation and Variance Estimation Software: User Guide. Ann Arbor, MI: Institute for Social Research, University of Michigan, 2002 [Google Scholar]
- 41. Little RJA, Rubin DB.. Statistical Analysis with Missing Data. New York, NY: Wiley, 1987 [Google Scholar]
- 42. VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press, 2015 [Google Scholar]
- 43. Zhang Y, Thamer M, Kaufman JS. et al. High doses of epoetin do not lower mortality and cardiovascular risk among elderly hemodialysis patients with diabetes. Kidney Int 2011; 80: 663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suttorp MM, Hoekstra T, Mittelman M. et al. Treatment with high dose of erythropoiesis-stimulating agents and mortality: analysis with a sequential Cox approach and a marginal structural model. Pharmacoepidemiol Drug Saf 2015; 24: 1068–1075 [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Thamer M, Stefanik K. et al. Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 2004; 44: 866–876 [PubMed] [Google Scholar]
- 46. Bradbury BD, Danese MD, Gleeson M. et al. Effect of epoetin alfa dose changes on hemoglobin and mortality in hemodialysis patients with hemoglobin levels persistently below 11 g/dL. Clin J Am Soc Nephrol 2009; 4: 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brookhart MA, Freburger JK, Ellis AR. et al. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 2013; 24: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kshirsagar AV, Freburger JK, Ellis AR. et al. Intravenous iron supplementation practices and short-term risk of cardiovascular events in hemodialysis patients. PLoS ONE 2013; 8: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Michels WM, Jaar BG, Ephraim PL. et al. Intravenous iron administration strategies and anemia management in hemodialysis patients. Nephrol Dial Transplant 2017; 32: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuo KL, Hung SC, Liu JS. et al. Iron supplementation associates with low mortality in pre-dialyzed advanced chronic kidney disease patients receiving erythropoiesis-stimulating agents: a nationwide database analysis. Nephrol Dial Transplant 2015; 30: 1518–1525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.