Abstract
Anti-low-density lipoprotein receptor-related lipoprotein 2 (LRP2) nephropathy/anti-brush border antibody disease is rare and characterized by tubular basement membrane, Bowman’s capsule and glomerular subepithelial immune deposits on kidney biopsy. No reported cases have occurred in patients with lymphoproliferative disorders or monoclonal gammopathies. We present two cases of anti-LRP2 nephropathy that occurred in patients with progressive low-grade B-cell lymphoma and had concurrent kidney infiltration by lymphoma on biopsy. We speculate that underlying immune dysregulation related to lymphoma may contribute to the development of this rare autoimmune kidney disease in some patients.
Keywords: anti-brush border antibody, anti-LRP2 nephropathy, chronic lymphocytic leukemia, kidney biopsy, LDL receptor-related protein 2, lymphoplasmacytic lymphoma, megalin, renal pathology, Waldenstrom macroglobulinemia
INTRODUCTION
Anti-low-density lipoprotein receptor-related lipoprotein 2 (LRP2 also known as megalin) nephropathy/anti-brush border antibody (ABBA) disease was recently characterized and appears rare, with <20 cases reported in the literature [1–5]. Patients are ≥65 years of age, have acute kidney injury and subnephrotic range proteinuria and progress to end-stage renal disease. Kidney biopsies show prominent tubular basement membrane (TBM) immune deposits composed of polytypic immunoglobulin G (IgG) and complement 3 (C3), acute tubular injury, interstitial inflammation and tubulitis, glomerular immune deposits with a segmental membranous pattern, reactivity of immune deposits with LRP2 and circulating antibodies against LRP2 [1–5].
CASE REPORTS
Case 1
The patient is a 74-year-old man with 5-year history of Waldenstrom’s macroglobulinemia/lymphoplasmacytic lymphoma (LPL) with a baseline creatinine of 1.28 mg/dL that increased to 3.83 mg/dL over 7 months. He had a new subnephrotic range proteinuria (2 g/g urine protein:creatinine ratio) and anemia with hemoglobin of 10.3 g/dL. Serum protein electrophoresis (SPEP) demonstrated IgM monoclonal protein (1.42 g/dL) and elevated kappa free light chain (7.56 mg/dL, κ:λ ratio of 3.62). C3 levels were low. A computed tomography scan showed no lymphadenopathy or splenomegaly. Bone marrow biopsy showed hypercellular marrow with 60–70% involvement by kappa-restricted LPL with MYD88 L265P mutation.
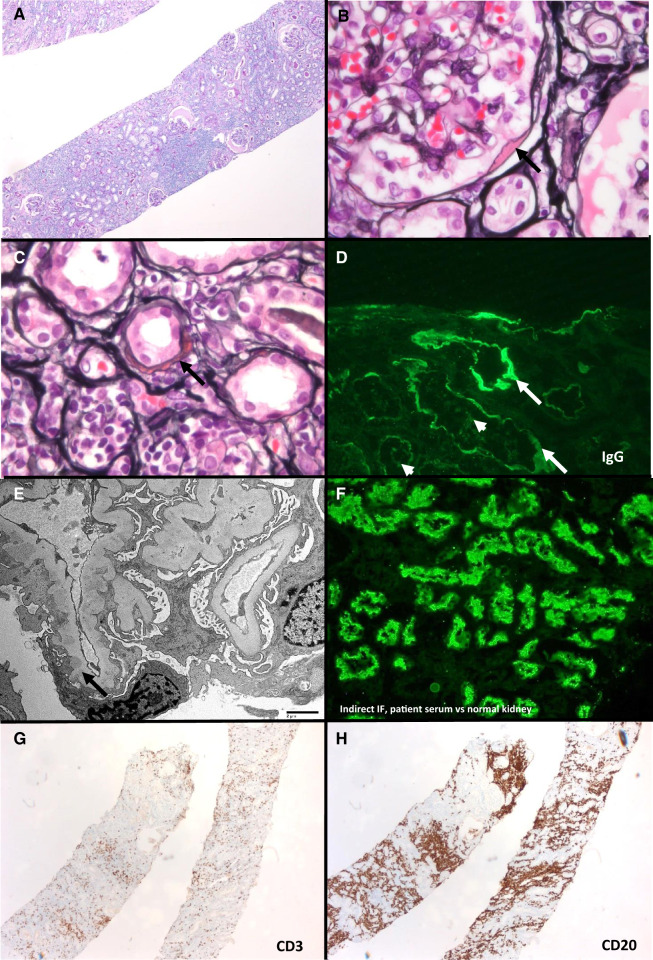
Kidney biopsy (Figure 1, Table 1) demonstrated 32 glomeruli that were patent and had segmental membranous features. The tubulointerstitium had a diffuse inflammatory infiltrate, acute tubular injury, foci of mild tubulitis and occasional large TBM deposits. There was moderate tubular atrophy and interstitial fibrosis. The infiltrate was primarily of small CD20-positive B cells with aggregates of kappa-restricted CD138-positive plasma cells. B-cell receptor gene rearrangement studies were monoclonal for IgH and Igκ. Immunofluorescence revealed segmental granular peripheral capillary wall and Bowman’s capsule staining for polytypic IgG and C3. There was widespread granular to chunky, near-circumferential staining of TBMs for polytypic IgG and C3. Tubular brush borders had focal reactivity with polytypic IgG. IgG subclasses showed IgG4-dominant staining followed by IgG1; IgG2 and IgG3 were negative. Ultrastructural studies demonstrated TBM and subepithelial immune deposits, with associated podocyte foot process effacement. No mesangial or subendothelial immune deposits were present. Anti-LRP2 was positive in TBM deposits and Bowman’s capsule and indirect immunofluorescence of the patient’s serum demonstrated reactivity with normal kidney tubular brush borders (ABBAs) at a titer of 1:1000.
FIGURE 1.
Anti-LRP2 nephropathy with concurrent kidney infiltration by LPL (Case 1). (A) Kidney biopsy with diffuse lymphoplasmacytic inflammatory infiltrate (periodic acid–Schiff, ×50). (B) Immune deposit along Bowman’s capsule (Jones, ×200). (C) Partially circumferential TBM immune deposit; tubules have acute injurious changes with attenuation of their cytoplasm and surrounding lymphoplasmacytic inflammation (Jones, ×400). (D) Granular TBM (arrows) and focal tubular brush border (arrowheads) staining for IgG. (E) Segmentally distributed subepithelial immune deposits (transmission electron microscopy, direct magnification ×1900). (F) Indirect immunofluorescence of patient’s serum reacting with normal kidney tubular brush borders (image courtesy of Dr Chris Larsen, Arkana Labs). (G) Scattered CD3-positive T-cell infiltrate (×50). (H) CD20-positive B cells, compatible with involvement by LPL (50×).
Table 1.
Summary of kidney biopsy findings in anti-LRP2 nephropathy and renal lymphom
| Light microscopy | ||||
|---|---|---|---|---|
| Glomeruli | Tubules | Interstitium | ||
| Case 1 |
|
|
|
|
| Case 2 | Segmental membranous features |
|
|
|
|
| ||||
| Immunofluorescence microscopy | ||||
|
| ||||
| Glomeruli | Tubules | IgG subclass staining | LRP2 | |
|
| ||||
| Case 1 | Segmental granular peripheral capillary wall and Bowman’s capsule staining for IgG, kappa and lambda light chains and C3 |
|
IgG4 > IgG1; IgG2 and IgG3 negative | Anti-LRP2 positive in TBM and Bowman’s capsule deposits but not glomerular tuft |
| Case 2 | Segmental granular peripheral capillary wall and Bowman’s capsule staining for IgG, kappa and lambda light chains and C3 |
|
IgG1 > IgG2 = IgG4; IgG3 negative | Anti-LRP2 positive in TBM and Bowman’s capsule deposits but not glomerular tuft |
|
| ||||
| Electron microscopy | ||||
| Glomeruli | Tubules | |||
|
| ||||
| Case 1 | Segmental subepithelial immune deposits | TBM immune deposits | ||
| Case 2 | Segmental subepithelial immune deposits | TBM immune deposits | ||
LPL, lymphoplasmacytic lymphoma.
He was diagnosed with anti-LRP2 nephropathy and renal parenchymal infiltration by LPL and was treated with bortezomib, dexamethasone and rituximab. He had hematologic response with improvement of peripheral blood counts but progressed to dialysis in <1 month.
Case 2
The patient is a 70-year-old man with a history of hypertension, hypothyroidism, diabetes and 2 years of chronic lymphocytic leukemia (CLL). Creatinine progressed from a baseline of 1.4 to 2.11 mg/dL over 15 months and continued to increase to 2.4 mg/dL. He had hematuria and subnephrotic range proteinuria (0.47 g/g urine protein:creatinine ratio). Peripheral white blood count was 39000/mm3, progressively rising from 19000/mm3 10 months prior. He had elevated kappa free light chain and an elevated κ:λ ratio. SPEP was negative and urine protein electrophoresisurine protein electrophoresis demonstrated monoclonal kappa light chain.
Kidney biopsy (Figure 2, Table 1) revealed four glomeruli that were patent and had segmental membranous features. There was acute tubular injury; mild tubulointerstitial inflammation; large, wedge-shaped TBM immune deposits and a background of moderate tubular atrophy and interstitial fibrosis. Kappa-restricted CD20-positive B cells had aberrant coexpression of CD5 and were clustered along the capsule and in interstitial aggregates. Tissue for immunofluorescence contained corticomedullary junction without glomeruli. There was bright, granular to chunky TBM staining for polytypic IgG and C3, with tubular brush border staining for IgG. IgG subclasses revealed IgG1 dominance and lesser staining for IgG2 and IgG4; IgG3 was negative. Electron microscopy revealed TBMs and segmental subepithelial deposits without mesangial or subendothelial deposits. There was granular LRP2 staining in TBM and Bowman’s capsule deposits, and indirect immunofluorescence of the patient’s serum demonstrated reactivity with normal kidney tubular brush borders at a titer of 1:100.
FIGURE 2.
Anti-LRP2 nephropathy with concurrent kidney infiltration by CLL/small lymphocytic lymphoma (SLL) (Case 2). (A) Wedge-shaped TBM immune deposit (Jones, ×400). (B) Widespread TBM. (C) Focal brush border staining for IgG. (D) Immune deposits embedded within TBM and bulging toward the luminal aspect (transmission electron microscopy, direct magnification ×590). (E) TBM immune deposits stained with LRP2 (image courtesy of Dr Nidia Messias, Arkana Labs). (F) Capsular and patchy interstitial infiltrate (periodic acid–Schiff, ×100) is composed of (G) CD20-positive B cells (×200) with (H) a kappa light chain restriction by in situ hybridization studies (×200); lambda light chain in situ hybridization is negative (inset, ×200), consistent with a monoclonal B-cell population.
The patient was diagnosed with anti-LRP2 nephropathy and kidney infiltration by CLL. Given the evidence of progressive CLL, he was treated with rituximab. Two months after initiating treatment his creatinine had declined to 2.2 mg/dL and the ABBA titer dropped to 1:10.
DISCUSSION
The novel finding in this report is the coexistence of anti-LRP2 nephropathy—a rare and recently characterized entity—with renal infiltration by low-grade B-cell lymphoma. We document a temporal association between the progression of low-grade B-cell lymphoma and the development of a polyclonal IgG-mediated autoimmune disease targeting LRP2/megalin, a transmembrane endocytic glycoprotein in the proximal tubule responsible for reuptake of filtered proteins [6].
Anti-LRP2 nephropathy has not been reported in patients with lymphoproliferative disorders, and the relationship between the processes is unclear. Anti-LRP2 immune deposits were polyclonal, reacting with multiple IgG heavy chains and kappa and lambda light chains. Polyclonal tubular brush border staining was present—representing a reaction with the pathogenic antibody—providing additional evidence against the possibility of a monoclonal anti-LRP2 antibody directly produced by B-cell lymphoma. Despite polyclonality, a potential connection between anti-LRP2 nephropathy and lymphoid neoplasia warrants future consideration, given its relative rareness and concurrent renal infiltration by B-cell lymphoma in 2 of 15 reported cases (including this report). Underlying immune dysregulation related to lymphoma may contribute to the development of autoimmune disease, as described for immune-mediated cytopenias, neurologic disorders, systemic vasculitis, inflammatory arthritis and numerous other paraneoplastic entities [7].
Like other published cases of anti-LRP2 nephropathy, TBM and Bowman’s capsule immune deposits reacted with anti-LRP2 antibodies, but glomerular basement membrane deposits did not. This corresponds with the known expression of LRP2 in tubular and parietal epithelial cells also recently demonstrated by single-cell RNA sequencing studies. In our interpretation of this publically available online dataset, there is no definitive expression of LRP2 in podocytes. However, irregular, low-level potential expression of LRP2 in some cells that cluster as podocytes is seen, and we cannot entirely exclude the possibility of LRP2 expression in some podocytes [8, 9]. Thus, lack of detection of LRP2 in glomerular immune deposits could be related to a low level of antigen present and/or the focal and segmental nature of glomerular immune complex deposition in anti-LRP2 nephropathy. Alternately, a mechanism of retrograde exosomal flow from proximal tubules or adjacent parietal epithelial cells may account for the commonly observed subepithelial immune deposits.
Finally, we have observed anti-LRP2 nephropathy in 3 of 224 (1.3%) native kidney biopsies performed in patients ≥65 years of age at our institution in 1 year [1]. We postulate that this disease may be more common than previously recognized and/or has an emerging immune trigger. Prior to recognition of the target antigen, LRP2/megalin, some cases were likely incompletely classified, potentially as unusual variants of membranous nephropathy with TBM and Bowman’s capsular deposits [10] and/or IgG4 tubulointerstitial nephritis [1].
In summary, we present the first two cases of anti-LRP2 nephropathy diagnosed in association with a clinically progressing low-grade B-cell lymphoma that had concurrent lymphomatous kidney infiltration on biopsy. We highlight an important diagnostic pitfall and raise the possibility of lymphoma-related immune dysregulation as a contributor to the development of this rare autoimmune kidney disease in some patients.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Arkana Laboratories for performing the anti-LRP2 staining and indirect immunofluorescence studies and Drs Nidia Messias and Chris Larsen in particular for contributing images.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
REFERENCES
- 1. Dinesh KP, Raniele D, Michels K. et al. Anti-LRP2 nephropathy with abundant IgG4-positive plasma cells: a case report. Am J Kidney Dis 2019; 74: 132–137 [DOI] [PubMed] [Google Scholar]
- 2. Larsen CP, Trivin-Avillach C, Coles P. et al. LDL receptor-related protein 2 (megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol 2018; 29: 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrison EB, Kozlowski EJ, McPhaul JJ Jr.. Primary tubulointerstitial nephritis caused by antibodies to proximal tubular antigens. Am J Clin Pathol 1981; 75: 602–609 [DOI] [PubMed] [Google Scholar]
- 4. Rosales IA, Collins AB, do Carmo PA. et al. Immune complex tubulointerstitial nephritis due to autoantibodies to the proximal tubule brush border. J Am Soc Nephrol 2016; 27: 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas MF, Rabideau DP, Schwartz MM. et al. Evidence of autologous immune-complex nephritis. N Engl J Med 1981; 305: 1326–1329 [DOI] [PubMed] [Google Scholar]
- 6. Nielsen R, Christensen EI, Birn H.. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 2016; 89: 58–67 [DOI] [PubMed] [Google Scholar]
- 7. Jachiet V, Mekinian A, Carrat F. et al. Autoimmune manifestations associated with lymphoma: characteristics and outcome in a multicenter retrospective cohort study. Leuk Lymphoma 2018; 59: 1399–1405 [DOI] [PubMed] [Google Scholar]
- 8. Wilson PC, Wu H, Kirita Y. et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci USA 2019; 116: 19619–19625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu H, Uchimura K, Donnelly EL. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 2018; 23: 869–881.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Markowitz GS, Kambham N, Maruyama S. et al. Membranous glomerulopathy with Bowman’s capsular and tubular basement membrane deposits. Clin Nephrol 2000; 54: 478–486 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.