Abstract
Background
Although patient-reported outcome measures (PROMs) are gaining increased interest in developing health care quality and are a useful tool in person-centered care, their use in routine care is still limited. The aim of this study is to describe the implementation and initial results of PROMs via the Swedish Renal Registry (SRR) on a national level.
Methods
After testing and piloting the electronic PROM application, nationwide measures were carried out in 2017 for completing the RAND-36 questionnaire online or by paper in a wide range of chronic kidney disease (CKD) patients (Stages 3–5, dialysis and transplantation) through the SRR. Cross-sectional results during the first year were analyzed by descriptive statistics and stratified by treatment modality.
Results
A total of 1378 patients from 26 of 68 renal units (39%) completed the questionnaire. The response rate for all participating hemodialysis units was 38.9%. The CKD patients had an impaired health profile compared with a Swedish general population, especially regarding physical functions and assessed general health (GH). Transplanted patients had the highest scores, whereas patients on dialysis treatment had the lowest scores. The youngest age group assessed their physical function higher and experienced fewer physical limitations and less bodily pain than the other age groups but assessed their GH and vitality (VT) relatively low. The oldest age group demonstrated the lowest health profile but rated their mental health higher than the other age groups. The older the patient, the smaller the difference compared with persons of the same age in the general population.
Conclusions
Nationwide, routine collection of PROMs is feasible in Sweden. However, greater emphasis is needed on motivating clinical staff to embrace the tool and its possibilities in executing person-centered care. CKD patients demonstrate impaired health-related quality of life, especially regarding limitations related to physical problems, GH and VT/energy/fatigue.
Keywords: chronic kidney disease, epidemiology, health-related quality of life, patient-centered care, person-centered care
INTRODUCTION
When evaluating and improving health care in chronic diseases, symptoms, function in daily life and well-being are important patient outcomes [1]. Health-related quality of life (HRQoL) is a significant key indicator of how a condition affects the patient’s life and HRQoL assessments can therefore identify possible problem areas related to health experiences. Interventions aimed at giving feedback and discussing the HRQoL outcomes face-to-face with the individual patient have been studied within oncology and diabetes care [2, 3]. Person-centered care and patient partnership have been increasingly highlighted as an important factor in improving health care quality [4]. Patient-reported outcome measures (PROMs) have the potential to be a useful tool in achieving this.
PROMs embrace both symptoms and HRQoL, which are important measures in renal care. HRQoL refers to functioning and well-being in physical, mental and social dimensions of life as a subjective experience, which varies over time and illness trajectory [5]. It is affected not only by health status and treatment, but also by factors like age, psychosocial aspects, culture, self-care ability, illness perception [6] and care framing [7, 8].
Impaired HRQoL is well described among patients with chronic kidney disease (CKD) and found to correlate with factors such as symptom burden [9], hypertension, anemia, nutritional status, dialysis modality, depression, cognitive dysfunction and frailty [10–14], and also education, depression, exercise habits [15], proteinuria [16] and inflammatory status [17]. Low HRQoL scores in dialysis patients have proved to be strong and independent predictors of hospitalization and mortality [18–20]. Previous research has also shown that patients with a kidney transplant experience better HRQoL [21, 22], that deteriorating HRQoL is seen in early CKD stages [15, 23, 24] and that the transition to commencing dialysis treatment has been identified as a vulnerable period with impaired HRQoL scores [25, 26]. Moreover, patients with CKD often suffer from comorbidities and complications such as cardiovascular disease and diabetes, which can be assumed to influence HRQoL.
Although PROMs are increasingly used in research, their use is still limited in routine care [27]. Fundamental prerequisites for carrying out a systematic implementation of PROMs in clinical practice are appropriate measures; informed, trained and motivated staff; an implementation plan and a technical solution for electronic PROMs (ePROMs) online. In renal care, it is also of special interest to carry out assessments through the illness trajectory, that is, during the different CKD stages and renal replacement therapy (RRT) modalities.
This report describes the preparation, roll-out and initial results of PROM through the RAND 36-Item Health Survey (RAND-36), a PROM questionnaire assessing HRQoL, which was launched in 2017 by the Swedish Renal Registry (SRR) both online and as a conventional paper version for patients in renal care in Sweden.
MATERIALS AND METHODS
The SRR
The SRR, started in 1991, is a national registry for renal failure. Today the SRR captures epidemiological information regarding renal care and outcomes from the entire disease trajectory starting with renal biopsies, continuing with CKD Stages 3–5 and on to RRT. Currently the SRR contains 13 000 patients with CKD Stages 3–5, 3200 patients on hemodialysis (HD), 900 on peritoneal dialysis (PD) and 5600 with a kidney transplant [28]. Since the start >65 000 patients have been registered. In Sweden, there are 68 units and departments providing specialized nephrology care and all 68 deliver data to the SRR. Recently the SRR decided to incorporate the RAND-36 PROM questionnaire into the registry, offering an opportunity for data collection online.
The RAND-36 questionnaire
The RAND-36 questionnaire was developed by the RAND Corporation. It is a self-administered generic HRQoL questionnaire that is not specific to any disease or treatment group. It is equivalent to the 36-item Short Form Health Survey (SF-36) questionnaire and covered by a conceptual model of HRQoL, measuring function and well-being [29]. The questionnaire includes 36 items that yield an eight-dimensional profile measured on a 100-point scale; a higher score indicates a better perceived health state. The eight dimensions are physical functioning (PF), role limitations caused by physical problems role physical (RP), bodily pain (BP), general health (GH), vitality/energy/fatigue (VT), social functioning (SF), mental health/emotional well-being (MH) and role limitations caused by MH/emotional problems role emotional (RE). The items refer to perceived health status during the last 4 weeks. An additional item refers to the patient’s assessment of his/her health transition (HT) during the last 24 months.
Mean scores of the SF-36 in a general Swedish population (n = 8930) 15–93 years of age are PF 87.9, RP 83.2, BP 74.8, GH 75.8, VT 68.8, SF 88.6, RE 85.7 and MH 80.9 [30]. A validation of the Swedish version of the RAND-36, comprising dialysis patients, showed evidence supporting reliability as well as responsiveness [31]. The algorithms for BP and GH differ between the RAND-36 and SF-36. However, these differences are negligible at the group level, which allows reliable comparisons of results from both questionnaires.
Launching ePROM in the SRR
The process of preparation and roll-out of the ePROM is described in Table 1. This process included issues such as developing and testing the information technology (IT) solution, patient login for online PROM completion and an instant feedback graph (Supplementary data, Figure S1), pilot tests and interviews, drafting information packages to patients and medical staff and implementation activities. The SRR urged participating clinical units to ask all their patients to complete the questionnaire at least once a year.
Table 1.
Preparation and roll-out of ePROM in the SRR
| Choice of appropriate PROM for HRQoL in renal patients, given criteria like psychometric outcomes | Validation of the Swedish version of the RAND-36 in collaboration with the Swedish PROM center. Eighty-four dialysis patients from five clinics participated [31] |
| Development and pilot testing of the online version of the RAND-36 questionnaire | Technically and legally secure and safe system for login and response procedures. Approved and tested IT solution with patient login through the SRR website or the Swedish National Health Guide System (‘Vårdguiden 1177’). Pilot tests on 10 patients, including interviews, feedback on individual results, adjustments and retests |
| Drafting patient information | Information package for clinical use on the SRR web page describing the questionnaire, response instructions, objectives, clinical use and responsible receiver |
| Development and testing of feedback graph to patient and medical staff | The patient receives a feedback graph (Supplementary data, Figure S1) online instantly after completing the questionnaire. The graph describes the individual health profile together with explainatory text of the different health domains and index scores and can also be seen and used by medical staff |
| Developing a technical solution for group-level outcome data in the SRR database | Instant online results on request |
| Implementation in clinical practice, information and education of medical staff within renal care about the motives, use and ways to handle the RAND-36 questionnaire | Continuous activities from the lead board of the SRR: workshops, presentations, professional meetings and discussions, articles in national renal periodicals, information package on the SRR homepage including materials like user’s guide and guidelines for local implementation, checklists, information letter to managers, office support. Information to renal patients through the Swedish Kidney Patient Association. Collaboration with the Swedish Association of Renal Nurses |
Data collection
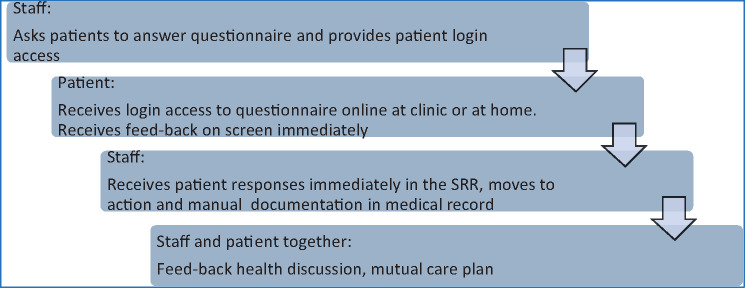
All renal clinics in Sweden (n = 68) were invited to implement and use the PROM via the SRR. Distribution of questionnaires, login information and collection of answers were organized by local nurses at each unit. The patients received a personal login that could be used on any computer, laptop, e-book reader or smartphone. They were asked to complete the questionnaire at the clinic or at home and they could opt between online or paper format, if that was considered more feasible. The flow chart of the ePROM roll-out is shown in Figure 1. PROM responses were extracted from the SRR database along with other relevant variables [age, sex, estimated glomerular filtration rate (eGFR), treatment modality at the time of completion of the questionnaire]. We selected the eGFR closest before the date of questionnaire completion.
FIGURE 1.
Flow chart for the PROM process.
Statistical analysis
Patient characteristics were described by means, medians and proportions according to their underlying distribution. The different domains of the RAND-36 were then assessed graphically and through descriptive statistics overall and stratified by age, sex and current treatment modality. Means and corresponding 95% confidence intervals were computed. Differences between groups were assessed by nonparametric statistics. All analyses were performed in Stata 15 (StataCorp, College Station, TX, USA).
Ethical considerations
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden. According to Swedish law, health care quality registries can be used for research. Patients have the right to opt out, but no additional individual consent is required for specific research projects.
RESULTS
PROM implementation in clinical practice
The pilot tests showed that the patients had no technical problems with the online version and that it took between 3 and 10 min to complete the questionnaire online [31]. The pilot interviews showed that the patients appreciated the feedback on their individual results online as well as by nursing staff. During the implementation phase, that is, the first year of use (2017), the RAND-36 questionnaire was completed by 1378 patients from 26/68 (39%) renal units. From HD units, 474/1220 (38.9%) patients completed the questionnaire; the proportion of patients from the 23 participating HD units varied from 1.8% to 97.6% (Supplementary data, Table S1). The response rate in the CKD, PD and transplant groups were not analyzed as the denominators for these groups were largely unknown.
PROM results
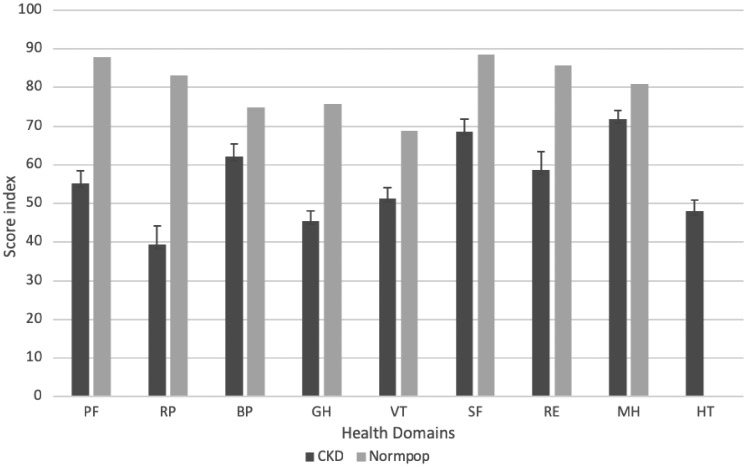
The questionnaire was answered by 604 patients in CKD Stages 3–5, 474 patients on HD treatment, 237 patients with a kidney transplant and 63 patients on PD treatment (Table 2 and Supplementary data, Table 2). About one-third (35.6%) of the participants were female, which is congruent with the overall proportion in the SRR. The mean age was 67.4 years; the transplant group was the youngest (58.3 years) and the CKD Stages 3–5 group was the oldest (70.8 years). Diabetes and cardiovascular disease were common among the participants. The mean eGFR in CKD patients not on dialysis (CKD-ND) was 23.3 mL/min/1.73 m2 and in the kidney transplant group was 44.1 mL/min/1.73 m2. Overall, the renal patient group showed an impaired health profile compared with that of the Swedish general population, especially regarding limitations related to physical problems, GH and VT (Figure 2).
Table 2.
Characteristics of participating patients
| Characteristics | Values |
|---|---|
| Sex | |
| Women, n (%) | 491 (35.6) |
| Age (years), mean (SD) | |
| All participants (n = 1378) | 67.4 (14.4) |
| CKD-ND (n = 604) | 70.8 (13.4) |
| TX (n = 237) | 58.3 (12.3) |
| HD (n = 474) | 67.6 (14.8) |
| PD (n = 63) | 68.1 (13.0) |
| Age group (years), n (%) | |
| 20–44 | 111 (8.1) |
| 45–64 | 383 (27.8) |
| 65–74 | 425 (30.8) |
| ≥75 | 459 (33.3) |
| Primary kidney disease, n (%) | |
| Hypertension | 307 (22.3) |
| Other | 258 (18.7) |
| Glomerulonephritis | 247 (17.9) |
| Diabetic nephropathy | 243 (17.6) |
| Uremia unspecified | 163 (11.8) |
| Polycystic kidney, adult type | 110 (8.0) |
| Pyelonephritis | 40 (2.9) |
| Renal vascular disease | 10 (0.7) |
| Comorbidity, n (%) | |
| Diabetes (n = 1349) | 402 (29.8) |
| Ischemic heart disease (n = 1350) | 229 (17.0) |
| Heart disease, other (n = 927) | 181 (19.5) |
| Malignancy, except skin cancer (n = 1349) | 145 (20.2) |
| Cerebrovascular disease (n = 1350) | 93 (6.9) |
| Peripheral vessel (n = 1347) | 87 (6.5) |
| eGFR (mL/min/1.73 m2), mean (SD) | |
| CKD-ND (n = 574) | 23.3 (10.9) |
| TX (n = 213) | 44.1 (18.0) |
CKD-ND: CKD not on RRT; TX: kidney transplant. eGFR, estimated glomerular filtration rate, ml/min/1.73 m2 by Modification of Diet in Renal Disease equation.
FIGURE 2.
Unadjusted health domains (mean and confidence interval) of the RAND-36 in CKD (n = 1378, ages 21–93 years) and the SF-36 in the Swedish general population (n = 8930, ages 15≥80 years). PF: physical functioning; RP: role physical; BP: bodily pain; GH: general health; VT: vitality; SF: social functioning; RE: role emotional; MH: mental health; HT: health transition.
Treatment groups
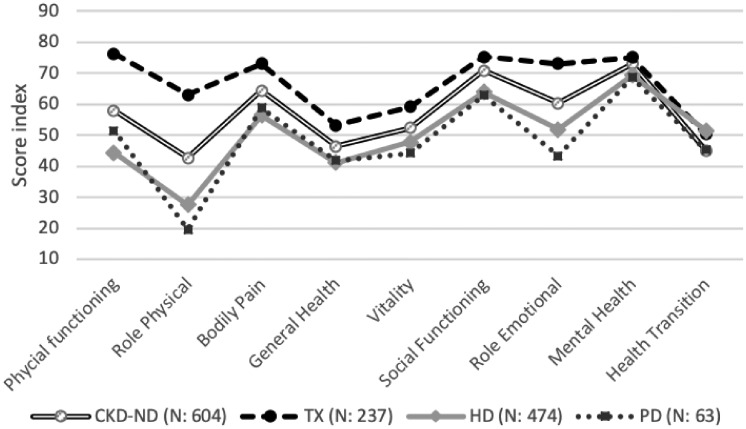
When comparing the different patient groups, their health profiles differed, mainly within the areas of physical and emotional limitations (Figure 3). Transplanted patients assessed their HRQoL higher, whereas patients on dialysis treatment had the most impaired scores. However, in the HD group, 34.2% of the patients assessed their health as being improved compared with 1 year earlier, as compared with all other groups where the assessed health improvement ranged from 14% to 20%.
FIGURE 3.
Undajusted mean values RAND-36 health domains in treatment groups. CKD-ND (not on RRT): CKD Stages 3–5; TX: kidney transplant.
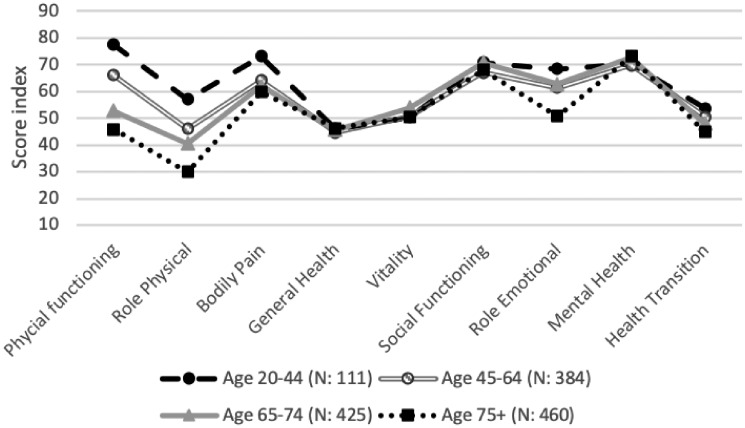
Age groups
When dividing the results in age groups, some differences emerged, especially regarding physical function and physical and emotional limitations (Figure 4). The youngest age group (ages 20–44 years) assessed their physical function higher and experienced fewer physical limitations and less BP than the other age groups. However, this age group also assessed their GH and their VT relatively low. In contrast, the oldest age group (≥75 years) demonstrated the lowest scores in five of the nine health areas (PF, RP, BP, RE, HT) but rated their MH higher than the other age groups. Comparisons between mean values of age groups in the SRR and the Swedish general population revealed that the older the patients, the smaller the difference compared with persons of the same age in the general population (Supplementary data, Figure S2)
FIGURE 4.
Mean values of the RAND-36 health domains in age groups.
DISCUSSION
This description of a nationwide invitation to collect PROMs depicts the potential to assess HRQoL through a quality registry. The SRR, with its complete follow-up of patients from CKD Stages 3–5 through RRT offers a unique opportunity to follow the illness trajectory over time. It may be a valuable complement to other medical outcome measures. It also depicts the challenges in accomplishing a nationwide implementation and reveals the difficulties in collecting PROMs in routine care. A sample of 1378 of 22 710 patients was indeed low coverage, and the patient selection may have been biased. However, the distribution of age, sex, primary kidney disease and comorbidity did not differ much between responding patients on HD and all patients (responders and nonresponders) at participating HD units (Supplementary data, Table S2). To implement a new routine and to reach continuous and systematic data collection at all renal units is a substantial challenge. Despite facilitating actions to inform and incentivize staff, more and repeated efforts for training the staff are required to enhance implementation. Handling PROMs is new to most clinical units. Previous research indicates that concerns regarding routine use among clinicians need to be addressed for successful implementation [32]. Factors contributing to engaging clinicians in the systematic use of PROMs have been identified as the use of a relevant, evidence-based and IT-supported questionnaire; a person-centered culture at the participating units; a systematic feedback approach to patients and active leadership and enthusiasm from respected clinicians [33]. For successful implementation, clinical staff needs to understand and feel ‘what’s in it for me?’ It is therefore important to underline motives and benefits like getting systematic knowledge about and follow-up of self-reported health and its relation to health care results and improvement; getting the opportunity to compare different patient and treatment groups; gaining a complementary perspective that contributes to a more holistic view and using a tool to highlight the patients’ perspective and provide a starting point for a health dialogue that could contribute to increased patient participation and person-centered care. Besides analyzing the results from the RAND-36 at an aggregate level, it creates the potential to enable individual follow-up, including feedback dialogues, with the health care staff. This ensures the patient’s perspective and participation and forms a basis for further care planning. In renal care—as in other chronic diseases—retaining or increasing HRQoL and well-being constitutes a care goal. Attention to the patients’ HRQoL could facilitate accomplishing individually tailored interventions to enhance or maintain physical fitness/function and psychosocial well-being, as well as support self-management. Systematic implementation of monitoring and discussing HRQoL would not only facilitate communication and improve understanding of the patients’ perspective in a more person-centered approach, but will also provide a tool to screen for and prioritize potential problems.
The online application has its pros and cons: the immediate feedback online was appreciated by the participating pilot patients. The online mode is time saving for the staff, as less time is required for manual registering tasks. On the other hand, not all patients have access to or feel comfortable with computers and the internet. Thus it is necessary to offer patients a paper questionnaire as well, if needed. However, as people are becoming more accustomed to utilizing electronic devices, we believe that the future of PROMs lies in the development of electronic medical records that will be automatically linked to ePROMs. This would enable caregivers, as well as patients, to look at the results at medical appointments and thus facilitate patient-centered care.
During this first year of implementation, 39% of all renal clinics participated. The response rate in the HD population was low, with a large variation between the participating units. A low response rate was also seen when implementing PROMs among HD patients within the SRR [34], where the response rate initially was 29%. Clearly this highlights the challenge and the efforts needed to succeed in implementing new routines and systematic and nationwide collection of PROMs.
The patients investigated in this report had considerably lower scores compared with the Swedish general population. These results are in line with previous findings, that is, CKD has a negative impact on HRQoL, especially in the physical domains [14, 23]. The most affected HRQoL dimensions—limitations related to physical problems, GH and VT—require recognition and management. The transplant group, however, demonstrated higher scores. This is not surprising, since transplant is considered a favorable treatment, with fewer disturbances of well-being and everyday life. Previous findings also show that patients with a kidney transplant experience higher HRQoL scores [21, 22]. Our findings could also be explained by the facts that this may constitute a selected and younger group.
The dialysis groups showed impaired scores, especially in the role limitation domains (RP, RE). Besides assuming an impact from the kidney disease and treatment itself, it may also be partly explained by the fact that the treatment is time consuming. An interesting finding was that in the HD group, about a third of the patients reported that their health was improved during the last year. This may reflect that being stabilized and better treated for uremia in HD can improve HRQOL. It was earlier demonstrated that patients initiating dialysis had lower HRQoL scores, highlighting that the transitional state just before dialysis is started may be an exceptionally vulnerable time [26]. However, it may also reflect that people experiencing better health are more prone to respond. In this report, the HD group seemed to be a bit better off than the PD group. This must be interpreted with caution, as the group sizes and age distribution differed and the PD group was rather small. Some studies indicate that PD treatment is related to better HRQoL [35, 36]. However, earlier reviews have found no difference between the two treatment groups [37, 38].
In our study, we also found some differences related to age. The youngest age group had higher scores on PF, physical limitations and pain, but had lower scores on GH and VT. The oldest age group had the lowest scores in several health domains but had higher scores than the other age groups in MH. These findings have been seen in earlier research [39] and may be related to divergent health expectations and the ability to accept and adapt to impaired health [40], where younger patients may have increased expectations of self-efficacy and a normal lifestyle. For the future and with more patient data, it would be interesting to analyze age differences within the treatment groups.
When interpreting any HRQoL instrument, one must consider its limitations. Ceiling and floor effects may skew the results. The individuals’ assessment of their health status is subjective and affected by surrounding factors like cultural aspects and environmental changes, which should be considered when interpreting and comparing results [41]. The group sizes of the patients versus the Swedish general population were disproportionate. Another considerable limitation was the low coverage. In this report, a nationwide, systematic procedure had not yet been reached. Besides implementation-related difficulties, nonresponse may have been due to frailty, cognitive function and language. At the time of this investigation, the SRR had access to the RAND-36 in only three languages, which also may have affected our results. Furthermore, another limitation is that data regarding the Swedish general population are somewhat out of date.
CONCLUSIONS
The recently implemented PROM application in the SRR allows nationwide collection of HRQoL data in CKD patients. However, greater emphasis is needed in motivating clinical staff to embrace the tool and its possibilities in executing person-centered care. CKD patients demonstrated impaired HRQoL, especially regarding limitations related to physical problems, GH and VT. Initial results indicate that HRQoL differs between treatment modalities and age groups. With the challenges in collecting data kept in mind, we are still hopeful for future possibilities regarding PROMs through national quality registries.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to the patients for their participation in completing the questionnaire and to the health care staffs at all Swedish renal care units for their efforts in implementing the RAND-36 into the clinical work.
FUNDING
This work was supported by a grant for strategic research from Karolinska University Hospital and Center for Innovative Medicine (CIMED) funding from the Stockholm City Council (to M.E.).
AUTHORS’ CONTRIBUTIONS
A.P. and M.E. contributed to the study concept and design and drafting the manuscript. A.P., M.E. and M.S. were responsible for data collection, statistical analysis and interpretation of results and critical revision of important intellectual content and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Sullivan M. The new subjective medicine: taking the patient's point of view on health care and health. Soc Sci Med 2003; 56: 1595–1604 [DOI] [PubMed] [Google Scholar]
- 2. de Wit M, Delemarre-van de Waal HA, Bokma JA. et al. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabetes Care 2008; 31: 1521–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luckett T, Butow PN, King MT.. Improving patient outcomes through the routine use of patient-reported data in cancer clinics: future directions. Psychooncology 2009; 18: 1129–1138 [DOI] [PubMed] [Google Scholar]
- 4. Eckardt KU, Bansal N, Coresh J. et al. Improving the prognosis of patients with severely decreased glomerular filtration rate (CKD G4+): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2018; 93: 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Revicki DA, Osoba D, Fairclough D, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res 2000; 9: 887–900 [DOI] [PubMed] [Google Scholar]
- 6. Broadbent E, Petrie KJ, Main J. et al. The brief illness perception questionnaire. J Psychosom Res 2006; 60: 631–637 [DOI] [PubMed] [Google Scholar]
- 7. Arvidsdotter T, Marklund B, Taft C. et al. Quality of life, sense of coherence and experiences with three different treatments in patients with psychological distress in primary care: a mixed-methods study. BMC Complement Altern Med 2015; 15: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brannstrom M, Boman K.. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail 2014; 16: 1142–1151 [DOI] [PubMed] [Google Scholar]
- 9. Voskamp PWM, van Diepen M, Evans M. et al. The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: effect and importance in the EQUAL study. Nephrol Dial Transplant 2018; 10.1093/ndt/gfy167 [DOI] [PubMed] [Google Scholar]
- 10. Campbell KL, Ash S, Bauer JD.. The impact of nutrition intervention on quality of life in pre-dialysis chronic kidney disease patients. Clin Nutr 2008; 27: 537–544 [DOI] [PubMed] [Google Scholar]
- 11. Finkelstein FO, Wuerth D, Finkelstein SH.. Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney Int 2009; 76: 946–952 [DOI] [PubMed] [Google Scholar]
- 12. Leaf DE, Goldfarb DS.. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int 2009; 75: 15–24 [DOI] [PubMed] [Google Scholar]
- 13. Soni RK, Weisbord SD, Unruh ML.. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spiegel BM, Melmed G, Robbins S. et al. Biomarkers and health-related quality of life in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol 2008; 3: 1759–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chin HJ, Song YR, Lee JJ. et al. Moderately decreased renal function negatively affects the health-related quality of life among the elderly Korean population: a population-based study. Nephrol Dial Transplant 2008; 23: 2810–2817 [DOI] [PubMed] [Google Scholar]
- 16. Kelley K, Aricak OT, Light RP. et al. Proteinuria is a determinant of quality of life in diabetic nephropathy: modeling lagged effects with path analysis. Am J Nephrol 2007; 27: 488–494 [DOI] [PubMed] [Google Scholar]
- 17. Farag YMK, Keithi-Reddy SR, Mittal BV. et al. Anemia, inflammation and health-related quality of life in chronic kidney disease patients. Clin Nephrol 2011; 75: 524–533 [DOI] [PubMed] [Google Scholar]
- 18. Mapes DL, Lopes AA, Satayathum S. et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2003; 64: 339–349 [DOI] [PubMed] [Google Scholar]
- 19. Lowrie EG, Curtin RB, LePain N. et al. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 2003; 41: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 20. Thong MSY, Kaptein AA, Benyamini Y. et al. Association between a self-rated health question and mortality in young and old dialysis patients: a cohort study. Am J Kidney Dis 2008; 52: 111–117 [DOI] [PubMed] [Google Scholar]
- 21. Butt Z, Yount SE, Caicedo JC. et al. Quality of life assessment in renal transplant: review and future directions. Clin Transplant 2008; 22: 292–303 [DOI] [PubMed] [Google Scholar]
- 22. Ogutmen B, Yildirim A, Sever MS. et al. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc 2006; 38: 419–421 [DOI] [PubMed] [Google Scholar]
- 23. Mujais SK, Story K, Brouillette J. et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol 2009; 4: 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perlman RL, Finkelstein FO, Liu L. et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis 2005; 45: 658–666 [DOI] [PubMed] [Google Scholar]
- 25. Korevaar JC, Jansen MA, Merkus MP. et al. Quality of life in predialysis end-stage renal disease patients at the initiation of dialysis therapy. Perit Dial Int 2000; 20: 69–75 [PubMed] [Google Scholar]
- 26. Pagels AA, Soderkvist BK, Medin C. et al. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 2012; 10: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aiyegbusi OL, Kyte D, Cockwell P. et al. A patient-centred approach to measuring quality in kidney care: patient-reported outcome measures and patient-reported experience measures. Curr Opin Nephrol Hypertens 2017; 26: 442–449 [DOI] [PubMed] [Google Scholar]
- 28.Svenskt Njurregister (Swedish Renal Registry). Svenskt Njurregister Årsrapport 2017. Jönköping: Svensk Njurmedicinsk Förening (SNF); 2017. https://www.medscinet.net/snr/rapporterdocs/Svenskt%20Njurregister%202017,%20rev%20171114.pdf (18 January 2019, date last accessed)
- 29. Ware JE. Conceptualizing disease impact and treatment outcomes. Cancer 1984; 53: 2316–2323 [DOI] [PubMed] [Google Scholar]
- 30. Sullivan M, Karlsson J, Taft C. et al. SF-36 hälsoenkät: svensk manual och tolkningsguide = (Swedish manual and interpretation guide). 2. uppl. ed. Göteborg: Sahlgrenska sjukhuset, Sektionen för vårdforskning; 2002. x, ca 200 s. med var. pag. p
- 31. Orwelius L, Nilsson M, Nilsson E. et al. The Swedish RAND-36 Health Survey - reliability and responsiveness assessed in patient populations using Svensson's method for paired ordinal data. J Patient Rep Outcomes 2017; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aiyegbusi OL, Kyte D, Cockwell P. et al. Patient and clinician perspectives on electronic patient-reported outcome measures in the management of advanced CKD: a qualitative study. Am J Kidney Dis 2019; 10.1053/j.ajkd.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 33. Ovretveit J, Zubkoff L, Nelson EC. et al. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care 2017; 29: 874–879 [DOI] [PubMed] [Google Scholar]
- 34. Nimmo A, Bell S, Brunton C. et al. Collection and determinants of patient reported outcome measures in haemodialysis patients in Scotland. QJM 2018; 111: 15–21 [DOI] [PubMed] [Google Scholar]
- 35. Brown EA, Johansson L, Farrington K. et al. Broadening options for long-term dialysis in the elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 2010; 25: 3755–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Makkar V, Kumar M, Mahajan R. et al. Comparison of outcomes and quality of life between hemodialysis and peritoneal dialysis patients in Indian ESRD population. J Clin Diagn Res 2015; 9: Oc28–Oc31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ho YF, Li IC.. The influence of different dialysis modalities on the quality of life of patients with end-stage renal disease: a systematic literature review. Psychol Health 2016; 31: 1435–1465 [DOI] [PubMed] [Google Scholar]
- 38. Zazzeroni L, Pasquinelli G, Nanni E. et al. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Pressure Res 2017; 42: 717–727 [DOI] [PubMed] [Google Scholar]
- 39. van Loon IN, Bots ML, Boereboom FTJ. et al. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol 2017; 18: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Griva K, Yu Z, Chan S. et al. Age is not a contraindication to home-based dialysis–quality-of-life outcomes favour older patients on peritoneal dialysis regimes relative to younger patients. J Adv Nurs 2014; 70: 1902–1914 [DOI] [PubMed] [Google Scholar]
- 41. Terada I, Hyde C.. The SF-36: an instrument for measuring quality of life in ESRD patients. EDTNA ERCA J 2002; 28: 73–76, 83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.