Abstract
Background
Experimental studies have shown fibroblast growth factor 23 (FGF23)-mediated upregulation of the distal tubule sodium/chloride (Na+Cl−) co-transporter leading to increased Na reabsorption, volume expansion and hypertension. However, data on the associations of FGF23 with renal Na regulation and blood pressure (BP) are lacking in young CKD patients.
Methods
FGF23 and other determinants of mineral metabolism, plasma renin activity (PRA), fractional excretion of Na (FENa) and BP, were analyzed at a single center in 60 patients aged 5–22 years with CKD Stages 1 (n = 33) and Stages 2–3 (n = 27) defined by cystatin C- and creatinine-based estimating equations (estimated glomerular filtration rate, eGFR). Associations between FGF23 and renal Na handling were explored by regression analysis.
Results
Median FGF23 levels were higher in CKD Stages 2–3 versus CKD 1 (119 versus 79 RU/mL; P < 0.05), with hyperparathyroidism [parathyroid hormone (PTH) >69 pg/mL] in only few subjects with CKD Stages 2–3. Median FENa was comparable in both subgroups, but with proportionally more values above the reference mean (0.55%) in CKD Stages 2–3 and 3-fold higher (1.6%) in CKD Stage 3. PRA was higher in CKD Stages 2–3 (P < 0.05). Meanwhile in CKD Stage 1, FGF23 did not associate with FENa, and in CKD Stages 2–3 FGF23 associated positively with FENa (r = 0.4; P < 0.05) and PTH (r = 0.45; P < 0.05), and FENa associated with FE of phosphate (r = 0.6; P < 0.005). Neither FGF23 nor FENa was associated with systolic or diastolic BP in either subgroup. The negative association of eGFR by cystatin with FENa remained the strongest predictor of FENa by multivariable linear regression in CKD Stages 2–3.
Conclusions
The elevated FGF23, FENa and PRA and the positive association of FGF23 with FENa do not suggest FGF23-mediated increased tubular Na reabsorption and volume expansion as causing hypertension in young patients with incipient CKD.
Keywords: blood pressure, CKD, FGF23, fractional sodium excretion
INTRODUCTION
Patients with chronic kidney disease (CKD) have a high cardiovascular mortality [1, 2]. Hypertension is a frequent comorbidity in both adults and children with CKD, contributing to CKD progression [3, 4] and cardiac-related morbidity and mortality [2]. Fibroblast growth factor 23 (FGF23), a phosphaturic hormone that suppresses 1,25(OH)2 vitamin D [1,25(OH)2D, calcitriol] synthesis, is a powerful predictor of adverse outcomes in patients with CKD [5]. FGF23 concentrations reach levels several hundred times the normal range in advanced CKD [6–8] and can exert direct stimulatory effects on the heart promoting left ventricular hypertrophy and fibrosis [9]. Whether these cardiac effects of FGF23 are the result of direct actions targeting the myocardial cells, or indirectly by stimulation of traditional cardiovascular risk factors like hypertension or activation of the renin–angiotensin–aldosterone system (RAAS) remains ill-defined and the subject of ongoing research [10]. Clinically, the association of hypertension with elevated levels of FGF23 has not been consistent, showing mostly a rather weak association in the absence of overt renal dysfunction [11], and hypertension may be absent in experimental models where FGF23 levels are elevated [12]. Of particular interest are the recent experimental data suggesting direct effects of FGF23 on the Na+Cl− co-transporter (NCC) of the renal distal tubular epithelium resulting in Na retention, blood volume expansion, hypertension and cardiac hypertrophy, advancing a novel explanation for the association between higher FGF23 and cardiovascular morbidity and mortality [13]. However, this formulation is based on observations in nonuremic models, and would imply a decrease in fractional excretion of Na (FENa) as CKD progresses, a pathophysiological sequence contradicting seminal studies clearly demonstrating an increase in FENa proportional to the decline in GFR with little, if any, increase in extracellular fluid or plasma volume in patients with CKD [14, 15], and increased FENa following high Na loads confirming that the changes in Na excretion are mostly the result of adjustments in the tubular Na reabsorption [16]. Furthermore, the use of thiazide diuretics that inhibit the NCC for management of hypertension and volume overload in CKD remains controversial [17]. However, more recent studies have shown antihypertensive effects of chlorthalidone in adults and children with CKD and resistant hypertension [18, 19]. Assessment of FENa, in a more pristine state prior to any diuretic usage, could further assist clinicians on the preferable type of diuretic use in these patients. Although FGF23 was unknown at the time the original studies were performed [14], they would presuppose increased FGF23 concentrations as FENa rose in the patients with progressive CKD. In one recent study of patients with moderate CKD, FGF23 levels were indeed associated positively with FENa, thus challenging the notion that FGF23 causes clinically significant Na retention [20]. However, the patients were older, mostly with chronic glomerulopathies known to be associated with proteinuria, the study included patients with diabetes mellitus and ischemic heart disease, and nearly one-half were receiving diuretics. These characteristics, particularly the use of diuretics, may confound the relationships between FGF23, renal tubular handling of Na, hypertension and blood volume. The discrepancies between the aforementioned experimental [13] and the clinical observations [14, 15] led us to hypothesize that in younger patients with moderate CKD, where chronic diuretic administration and other potential confounding cardiovascular risk factors are usually absent, FENa would not be reduced despite higher circulating FGF23 levels. Thus, we investigated the potential associations of circulating FGF23 and plasma renin activity (PRA) levels with tubular handling of Na and blood pressure (BP) measurements in a sample of young patients with mild to moderate incipient CKD.
MATERIALS AND METHODS
Routine clinical and biochemical data were analyzed in 60 children, adolescents and young adults with predialysis CKD at a single center of the University of Miami in Miami, Florida, USA. Eligible criteria for inclusion were CKD Stages 1–3 defined with estimated glomerular filtration rate (eGFR) determined by creatinine (cr) and cystatin C (cys) equations. Exclusion criteria were recipients of solid organ transplants, active nephrotic proteinuria with edema, acute kidney injury in the preceding 3 months, chronic tubulointerstitial nephritis and treatment with VDR activators, inorganic phosphate (Pi)-chelating agents or diuretics. To avoid potential confounding effects of other cardiovascular risk factors frequently prevalent in older patients, we excluded patients with diabetes, history of smoking, evidence of peripheral vascular disease, clinical or echocardiographic evidence of coronary heart disease and sustained poorly controlled hypertension. Additional exclusion criteria were congenital cardiomyopathy, primary myocardial disease or historic heart transplantation.
In addition to routine anthropometric parameters, systolic and diastolic BP (SBP and DBP) measurements were obtained with an automated oscillometric device (Dynamap; GE Healthcare, Waukesha, WI, USA) in the sitting position after 5 min of resting, and the average of three sequential measurements was recorded. These readings were indexed to age, gender and height percentile to define hypertension (>95th percentile) [21]. In patients >18 years, BPs >130/80 were recorded as >95th percentile. Plasma FGF23 was measured by a second-generation C-terminal enzyme-linked immunosorbent assay that recognizes both the intact FGF23 and its C-terminal fragments (Immutopics). Values in 33 local children aged 5–21 years with normal GFR were: median 97 RU/mL, range 55–220 RU/mL, lower quartile 70 RU/mL [22]. Circulating immunoreactive parathyroid hormone (iPTH) (intact assay), 25(OH) D (DiaSorin assay) and 1,25(OH)2D were measured as previously described [17, 22], and PRA was measured by liquid chromatography/tandem mass spectrometry (normal range 0.25–5.82 ng/mL/h). Fractional excretion of the analytes Na and Pi were calculated as (U analyte × P cr)/(P analyte × U cr) × 100 (where U = urinary; P = plasma). Nonfasting tubular reabsorption of phosphate (TP) indexed to GFR (TP/GFR), a reliable and reproducible indicator of the tubular threshold of phosphate in young patients, was calculated as (SPi – UPi × Scr/Ucr) (where S = serum; normal value 4.01 ± 0.53 mg/dL in ages >1 year) [23].
Statistical analyses
Data are expressed as mean ± SEM or SD, median (interquartile range) and log-transformed values for non-normally distributed measurements. Two-tailed Student’s t-test or Mann–Whitney (when not normally distributed) compared data between CKD groups. Correlations were assessed by Spearman or Pearson tests as appropriate, and multivariate linear regression analysis for correlates between FENa and the variables of interest. Excel (Microsoft Corp.) and Graph Pad Prism version 5.00 for Windows (Graph Pad Software, San Diego, CA, USA) were used, and P < 0.05 were considered significant.
RESULTS
Cohort characteristics by CKD stage
The clinical and biochemical characteristics of all 60 patients are displayed in Table 1. The patients were divided into two groups: those with normal eGFR >90 mL/min/1.73 m2 (equivalent to CKD Stage 1, n = 33), and those with eGFR >30 to <90 mL/min/1.73 m2 (equivalent to CKD Stages 2 and 3, n = 27). The median ages were similar, the majority were male, white (Hispanic and non-Hispanic) and most had non-glomerular forms of CKD. Obesity [body mass index (BMI) >95th percentile] and short stature (height <10th percentile) were more prevalent in patients with more advanced CKD stages. Systolic hypertension (values >95th percentile) was similarly observed in 33% of CKD Stage 1 and in 28% of CKD Stages 2 and 3 groups, but diastolic hypertension was less prevalent. FGF23 concentrations in CKD Stage 1 (79 RU/mL) were similar to those in normal healthy controls (97 RU/mL), while FGF23 levels in CKD Stages 2–3 were nearly 50% higher and significantly above those in CKD Stage 1 (P < 0.05). Although median PTH values were higher in CKD Stages 2 and 3 versus CKD Stage 1 (P < 0.05), hyperparathyroidism (levels >65 pg/mL) was detected in only four patients with CKD Stages 2 and 3 and in none with CKD Stage 1. The 1,25(OH)2 D concentrations were not significantly lower in CKD Stages 2 and 3, with levels <50 pg/mL (the lowest quartile in the reference patients with normal GFR) in nearly one-half of patients with CKD Stages 2 and 3 (43%) but only in 23% of those with CKD Stage 1. The 25(OHD) levels were similarly reduced (<30 ng/mL) in both groups. Serum Na, Ca, age-adjusted Pi and albumin were normal, and none of the patients displayed hyponatremia (Na <135 mEq/L) or hypoalbuminemia (albumin <3.5 g/dL). Values of TP/GFR and fractional urinary excretion of phosphate (FEPi) were similar in both subgroups (Table 1). However, FEPi >11.8%, the 75th percentile in the reference group, was documented nearly twice as much in CKD Stages 2 and 3 (33%) as in CKD Stage 1 (18%), with the highest median values (25%, range 14–70%) in CKD Stage 3. Similarly, FENa median values (Figure 1A) were comparable in both subgroups (0.54% versus 0.52% in CKD Stage 1 versus CKD Stages 2–3, respectively), but with proportionally more values greater than the reference mean (0.55%) and greater than the 75th percentile (0.8%) in CKD Stages 2 and 3 patients, with values exceeding the 97th percentile only in patients with GFR <60 mL/min/1.73 m2, and were 3-fold higher (1.64%) than those with CKD Stage 1 (Figure 1A). FENa values, potentially modifiable by dietary Na intake, were comparable in patients of White and non-White ethnicity (0.55 ± 0.42 and 0.62 ± 0.45%, respectively). PRA, an indicator of plasma volume status (Figure 1B), was significantly higher in the patients with reduced GFR compared with the values in those with normal GFR (3.0 versus 2.0 ng/mL/h, respectively; P < 0.05) and without any value <1.1 ng/mL/h, the 25th percentile of the reference group.
Table 1.
Characteristics of the patients by CKD stage
| Characteristic | CKD Stage 1 (n = 33) | CKD Stages 2 and 3 (n = 27) |
|---|---|---|
| (eGFR >90 mL/min/1.73 m2) | (eGFR >30–<90 mL/min/1.73 m2) | |
| Age, years | 15 (10–17) | 16 (13–17) |
| Gender, % male/female | 61/39 | 89/11 |
| Ethnicity, % white/AA-other | 52/48 | 41/59 |
| BMI, kg/m2 (% obesity) | 23±5.4 (3) | 24±6.7 (33) |
| Systolic BP percentile (% above 95th percentile) | 72.5±28 (33) | 61±38 (28) |
| Diastolic BP percentile (% above 95th percentile) | 68±23 (10) | 65±26 (11) |
| eGFRcr, mL/min/1.73 m2 | 109 (81–131) | 76 (65–102)* |
| eGFRcys, mL/min/1.73 m2 | 110 (99–118) | 76 (60–83)** |
| Plasma FGF23, RU/mL | 79 (66–125) | 119 (90–164)*** |
| Serum PTH, pg/mL | 26 (19–35) (n = 30) | 39 (23–58) (n = 23)*** |
| Serum 1,25(OH)2D, pg/mL | 61±22 (n = 31) | 53±18 (n = 21) |
| Serum 25(OHD), ng/mL | 27±6.0 | 29±9.0 |
| Serum sodium, mEq/L | 139±0.4 | 139±0.3 |
| Serum calcium, mg/dL | 9.7±0.4 | 9.7±0.5 |
| Serum phosphorus, mg/dL | 4.38±0.7 | 4.48±0.7 |
| Serum albumin, g/dL | 4.6±0.06 | 4.5±0.08 |
| TP/GFR, mg/dL | 3.84 (3.3–4.6) | 3.71 (3.4–4.40) |
| Urine FEPi, % | 9.2 (5.7–11.8) | 9.6 (6.6–14.2) |
Data are means±SD or medians (25–75th percentiles). AA-other, includes African-American and other non-White ethnicities; FEPi calculated as: (urine Pi × serum cr/urine cr × serum Pi) × 100; TP/GFR, tubular reabsorption of phosphate indexed to GFR; 25(OH)2D, 1,25-dihydroxyvitamin D; 25(OHD), 25-hydroxyvitamin D.
P < 0.005 versus CKD Stage 1.
P < 0.0001 versus CKD Stage 1.
P < 0.05 versus CKD Stage 1.
FIGURE 1.
Urinary FENa and PRA levels in different CKD stages. FENa overall values (A) were comparable in both subgroups, but with proportionally more values greater than the median (0.54%) and greater than 75th percentile (0.80%) in CKD Stages 2 and 3 patients, with values exceeding the 97th percentile (1.3%) only in patients with reduced GFR. Solid lines represent median with interquartile ranges, dashed line represents the reference 97th percentile. PRA measurements (B) were significantly higher in the patients with CKD Stages 2 and 3, and without any value <1.1 ng/mL/h, the 25th percentile of the reference group. Solid lines represent median with interquartile ranges, dashed line represents the 25th percentile of the reference group. *P < 0.05 compared with CKD Stage 1.
Associations between renal Na handling, renal function, Na regulating factors and BP
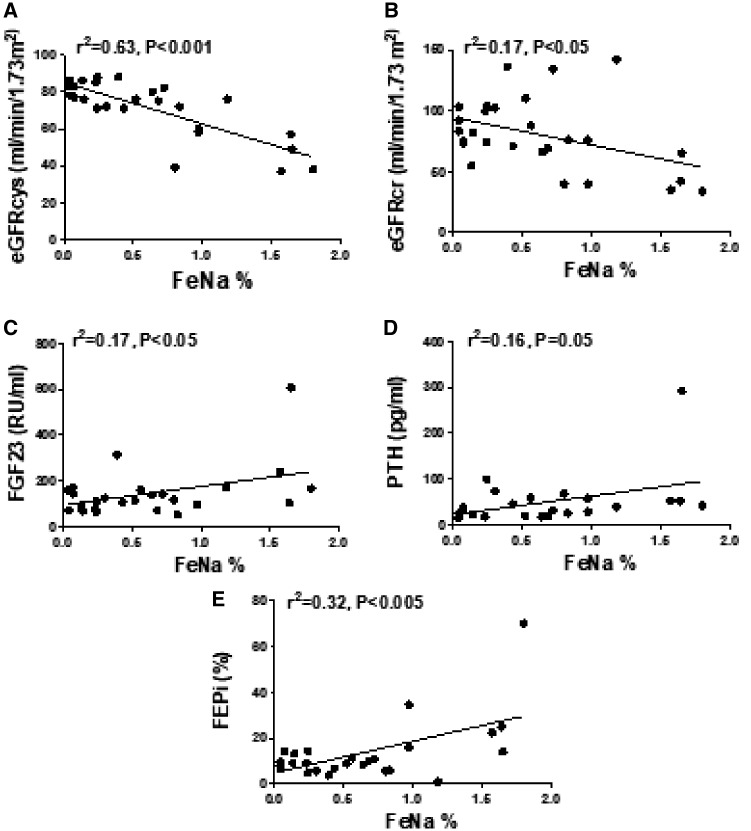
By initial analysis, in the patients with CKD Stages 2 and 3, FENa displayed the strongest negative association with estimated glomerular filtration by cystatin (eGFRcys) (P < 0.001) (Figure 2A). Lower eGFRcr also showed an inverse relationship with FENa (P < 0.05) (Figure 2B). Higher FENa displayed positive associations with higher FGF23 levels (P < 0.05) (Figure 2C), serum PTH (P = 0.05) (Figure 2D) and FEPi (P < 0.005) (Figure 2E). FENa also correlated positively with logFGF23 (data not shown) but did not correlate with serum Pi and Na, SBP and DBP percentiles, BMI percentiles and PRA. Plasma FGF23 and log10FGF23 both correlated positively with PTH (both r = 0.45, both P < 0.05), but they did not correlate with serum Ca, Pi, Na, eGFRs, FEPi, TP/GFR, 1,25(OH)2D or PRA, and had no apparent effect on BP measurements. FEPi was strongly and inversely associated with lower eGFRcys (r = −0.6; P < 0.005), eGFRcr (r = −0.5; P < 0.005), TP/GFR (r = −0.6; P < 0.005) and 1,25(OH)2D levels (r = −0.7; P < 0.05), and as stated above and in Figure 2, FEPi correlated positively with FENa. The serum Pi correlated positively with TP/GFR (r = 0.8; P < 0.005). The SBP percentile did not correlate with BMI, eGFRs, blood concentrations of Na, Pi, 1,25(OH)2D, PTH, FGF23 or PRA, or with urine FEPi, TP/GFR and FENa in any of the two CKD subgroups. By multivariate linear regression analysis with FENa as the dependent variable and factors capable of affecting FENa as independent variables, all significant associations on the bivariate analysis were markedly attenuated, whereas eGFRcys remained as the strongest predictor of FENa in patients with CKD Stages 2 and 3 (Table 2). In patients with CKD Stage 1, the only variable significantly associating with FENa was the eGFRcr (r = −0.36; P < 0.05).
FIGURE 2.
Associations between FENa and markers of renal function and mineral homeostasis in patients with incipient CKD. FENa correlated inversely with eGFRcys (A) and eGFRcr (B), and positively with FGF23 (C), circulating PTH (D) and FEPi (E).
Table 2.
Multivariate regression analysis of determinants of FENa in patients with CKD Stages 2 and 3
| Coefficients | Standard error | t-statistic | P-value | Lower 95% | Upper 95% | |
|---|---|---|---|---|---|---|
| Intercept | 1.86 | 0.499 | 3.726 | 0.001 | 0.822 | 2.899 |
| eGFRcys | −0.02 | 0.006 | −3.056 | 0.005 | −0.033 | −0.006 |
| eGFRcr | −0.001 | 0.002 | −0.433 | 0.668 | −0.006 | 0.004 |
| FGF23 | 0.001 | 0 | 1.691 | 0.105 | 0 | 0.002 |
| FEPi | 0.009 | 0.006 | 1.478 | 0.154 | −0.003 | 0.022 |
In the above model including potential modulators of FENa, eGFRcys remained as the strongest factor associated with FENa in patients with moderately reduced GFR.
DISCUSSION
In this study, urine FENa associated strongly and positively with FGF23 concentrations and negatively with a lower eGFR. In addition, neither FGF23 nor FENa was associated with BP or with PRA. These findings do not support renal Na-conserving properties mediated by FGF23, and do not provide indirect evidence of blood volume expansion, at least not in young patients with early stages of CKD.
FGF23, a bone-derived potent phosphaturic hormone that regulates Pi metabolism and 1,25(OH)2D metabolism, suppresses Pi resorption in proximal tubules by downregulating Na-dependent phosphate co-transporter type II a/c (Npt2a/c) [24]. The highest FEPi in our patients with higher FGF23 levels and lower eGFR are in agreement with the rise of FGF23 levels as the earliest change of mineral homeostasis in incipient CKD [25] and with the known phosphaturic properties of FGF23 exhibited prior to the development of overt hyperparathyroidism [26]. Similarly, the tendency toward lower levels of 1,25(OH)2D in patients with reduced eGFR and higher FGF23 levels are in line with the suppressive effects of FGF23 on renal 1,25(OH)2D synthesis [24] and with the expected findings in incipient CKD [26].
FGF23 may function not only as a phosphaturic hormone, but, according to the aforementioned experimental studies, also as an Na-conserving hormone [13]. The physiological significance of the fact that FGF23 is bestowed with both phosphate-wasting and Na-conserving activity simultaneously has not been studied thoroughly in patients with CKD. In the present study, FGF23 levels associated positively with FENa and negatively with eGFR. These relationships, coupled with the strong inverse relationships of both FENa and FEPi with the eGFR, the positive relationship of FENa with FEPi and the highest FENa values in patients with reduced GFR, suggest that both urinary Na and Pi fractional excretion tend to increase as renal function decreases without necessarily large fluctuations in Na and Pi intake [14, 27]. Although we did not quantify dietary phosphorus and Na intake, urinary FENa values were comparable in patients of different ethnicities with likely diverse dietary habitual Na and Pi intakes, thus it is improbable that the patients with the lower GFR, as a group, were selectively consuming larger amounts of these ions to account for the higher fractional excretion values. Dietary Pi exposure and absorption are modifiable factors; however, studies of dietary interventions to reduce Pi load seeking to modify FGF23 levels in patients with moderate CKD have shown conflicting results. Some demonstrated a positive effect, whereas others failed to show any influence; hence, controlling circulating FGF23 levels by manipulating dietary Pi intake remains an unmet clinical challenge [28]. The herein observed physiological adjustments are in agreement with the original seminal observations highlighting the remarkable ability of the failing kidneys to maintain Na and Pi homeostasis in uremic patients [14, 27]. The lower the GFR, the greater must be the fraction of solute excreted in a relationship such that for every 50% reduction in GFR, FENa increased 2-fold while on two different salt intake regimens [14]. We noted a similar trend in patients with eGFR <60 mL/min/1.73 m2 having FENa values about 3-fold the mean FENa of 0.5% of those with normal eGFR (Figure 2). Although in the initial bivariate analysis FENa correlated significantly with several physiological variables such as FGF23 levels, log10FGF23 values, FEPi, PTH concentrations and eGFR, the subsequent multivariable regression analysis revealed that the strongest determinant of increased FENa was the decline in GFR. The increased FENa in patients with incipient CKD does not reflect increased tubular Na reabsorption but, on the contrary, it is in agreement with experimental studies showing that in moderate renal insufficiency, the main adaptive changes take place in the distal portions of the nephron resulting in augmented fractional urinary Na excretion [29]. Na reabsorption in the distal nephron is highly regulated by the thiazide-sensitive NCC and by the amiloride-sensitive epithelial Na channel (ENaC). Regulation of the NCC and the ENaC is complex, and involves a number of stimulatory and inhibitory factors including aldosterone, angiotensin-II, arginine vasopressin and atrial natriuretic peptide, as well as other hormones such as insulin and endothelin [30]. FGF23 signaling could be yet an additional factor that leads to activation of with-no-lysine kinase 4 (WNK4) in distal renal tubules acting through the FGFR/Klotho receptor complex leading to increased NCC expression resulting in renal Na retention and plasma expansion, with subsequent increased BP [13].
According to this formulation, FGF23-induced Na retention would result in increased plasma volume during the earlier stages of CKD. A priori changes of plasma volume and Na homeostasis are usually accompanied by changes in the RAAS [31], resulting in changes of PRA since the assay quantifies the production of angiotensin-I, a functional measurement of renin levels [31]. While volume contraction or decreased effective circulating volume results in strong Na retention with reduced FENa and activation of the RAAS with elevations of PRA, volume expansion is accompanied by natriuresis, increased FENa and reduced PRA [32]. Similar changes were demonstrated in patients with CKD following high Na loads [16]. Of relevance to the present discussion, patients with Gordon syndrome or pseudohypoaldosteronism type II, a Mendelian syndrome caused by mutations in the WNK4-coding gene that results in NCC overactivity, usually display increased Na reabsorption and hypertension with reduced PRA and aldosterone levels due to volume expansion [33]. None of our patients with reduced GFR had evidence of congestive heart failure or other causes of insufficient effective circulatory volume such as acute nephrotic syndrome, and as a group they showed significantly higher values of PRA compared with those with normal GFR. Although a few patients were receiving angiotensin-converting enzyme inhibitor (ACEI) therapy, which may have contributed to elevate PRA values, more importantly none of the values was <25th percentile of the reference group and thus do not conform to a state of volume expansion. Previous studies evaluating PRA levels in patients with CKD but without congestive heart failure are limited, and have consistently shown progressively higher PRA values with declining GFR levels [34] and a markedly increased ratio of PRA to the simultaneously measured GFR compared with normal subjects [35]. These findings suggest activation of the RAAS as a result of the loss of functioning nephrons. Progressive elevations of PRA levels have also been observed in patients with heart failure and reduced renal function even without ACEI or angiotensin-receptor blockers treatment [36], and who concomitantly displayed higher FGF23 levels [37]. These observations raise the possibility that as a result of the renal dysfunction, FGF23 could contribute to RAAS activation [10] with subsequent increase in PRA [34, 35], and activation of the intrarenal RAAS [38] may contribute to the progression of CKD and hypertension. Our patients with reduced GFR demonstrated significantly higher FGF23 and PRA levels, concurrent with lower concentrations of 1,25(OH)2D, underscoring the complex relationships between FGF23, vitamin D and the RAAS independent of overt volume changes, as observed in uremic animals [39, 40] and in patients with genetically deficient 1,25(OH)2D secretion who display low or undetectable 1,25(OH)2D levels, elevated FGF23 and increased PRA values [22]. Whether a similar intricate cross-talk among the RAAS, vitamin D and FGF23 at the tissue level in the kidney and cardiomyocytes [39–41], may also operate at a systemic level involving the peripheral circulating RAAS and FGF23 independent of plasma volume changes in patients with CKD has been suggested [42] and will require additional investigations.
We documented overall systolic hypertension in about one-third of the patients, similar to previous cohorts of young patients with moderate CKD [43]. The relationship between elevated BP and the development of left ventricular hypertrophy (LVH) and other outcomes is not clear in young patients with moderate CKD, some showing no significant associations [44], and others reporting strong associations [45]. Whether the associations of FGF23 levels with LVH in patients of all ages with [6–8] or without known CKD [46, 47] are mediated through predominantly direct effects of FGF23 on the myocardium independent of hypertension [9] and renal impairment [12], or indirectly through BP changes, remains debatable. Most studies reported weak or no independent associations between FGF23 and BP [11] although recently higher FGF23 levels were independently associated with incident hypertension in younger adults without CKD [48]. We did not elicit significant associations between FGF23 levels and either systolic or diastolic BP measurements. Furthermore, none of the other traditional variables capable of modulating BP including BMI, eGFR, blood concentrations of Na, PTH, 1,25(OH)2D, Pi and PRA or tubular handling of Pi showed significant correlations with BP percentiles in the group with reduced eGFR.
Several observations suggest alternative FGF23-dependent or -independent mechanisms that may lead to adverse cardiovascular outcomes: improved cardiac hypertrophy was observed in young dialysis patients treated with paricalcitol despite further elevations of FGF23 and persistently high BPs [39]; LVH may progress even when BP is optimally controlled in patients with moderate pre-dialysis CKD [49]; and comparable BP reduction was observed in patients with CKD Stage 1 treated with amlodipine or ramipril, although FGF23 levels declined only in those given ramipril [50]. FGF23 also suppresses renal expression of α-Klotho, and its deficiency is linked to uremic cardiomyopathy through FGF23-independent mechanisms [51] and Klotho participates in the tubular reabsorption of Na [13].
Of additional interest is the potential role of Pi as a possible independent cause of hypertension. In healthy young adults, high Pi diets increased Pi, FGF23 levels and BP, with persistent hypertension after normalization of FGF23; the significant increases of urinary metanephrine and normetanephrine indicated Pi-mediated sympathetic activation as a contributing mechanism of hypertension independent of FGF23 [52]. Of note, in this latter study, changes of BP following the different phosphate diets occurred without changes in urinary Na, PRA or aldosterone levels, suggesting that factors other than FGF23 may participate in the modulation of BP and myocardial hypertrophy. Additional supporting evidence for the role of Pi as an essential cause of the cardiovascular toxicity that accompanies FGF23 in more advanced CKD comes from the previously mentioned experimental murine hypophosphatemic model mimicking human X-linked hypophosphatemic rickets showing absence of hypertension, LVH and cardiac dysfunction in this non-uremic hypophosphatemic model [12]. In our cohort, serum Pi levels were normal and neither Pi, FEPi nor TP/GFR associated with BP.
The present study has strengths and limitations. It is the first systematic evaluation of renal tubular handling of Na and Pi with simultaneously measured FGF23 levels and biomarkers of volume overload and their relationships with hypertension in young CKD patients. The studied cohort had little or no long-term exposure to traditional cardiovascular risk factors, and all patients were naïve to diuretic therapy, an important confounder of renal Na handling. Since it is an observational cross-sectional analysis, it does not allow us to draw conclusions about causation between FGF23, FENa and hypertension, and the observational nature of the study cannot rule out the possibility that FGF23 may increase tubular Na reabsorption in other non-uremic clinical conditions similar to those described in the aforementioned experimental model [13]. While recombinant FGF23 injection directly upregulated distal tubular NCC and reduced urinary Na excretion in the murine model, similar studies are not feasible in humans. The number of patients studied is not large, and our findings will require confirmatory and longitudinal studies with a larger number of participants including patients with more advanced stages of CKD and the use of novel noninvasive body volume measurements and biomarkers reflecting renal NCC expression [53] to help clarify the discrepancies between the experimental studies linking FGF23 and the NCC to Na retention and the findings in the current study. Such studies may conceivably contribute to substantiate the use of thiazides in patients with CKD [18].
In conclusion, the elevated FGF23, FENa and PRA and the positive association of FGF23 with urinary FENa values reported in young patients with incipient CKD in the present study do not support experimental findings bestowing FGF23 with tubular Na-retentive properties.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 2. Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 2012; 23: 578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peterson JC, Adler S, Burkart JM. et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123: 754–762 [DOI] [PubMed] [Google Scholar]
- 4. Mitsnefes M, Ho PL, McEnery PT.. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol 2003; 14: 2618–2622 [DOI] [PubMed] [Google Scholar]
- 5. Isakova T1, Xie H, Yang W, Xie D. et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutiérrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seeherunvong W, Abitbol CL, Chandar J. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol 2012; 27: 2129–2136 [DOI] [PubMed] [Google Scholar]
- 8. Mitsnefes MM, Betoko A, Schneider MF. et al. FGF23 and left ventricular hypertrophy in children with CKD. Clin J Am Soc Nephrol 2018; 13: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pi M, Ye R, Han X. et al. Cardiovascular interactions between fibroblast growth factor-23 and angiotensin II. Sci Rep 2018; 8: 12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fyfe-Johnson AL, Alonso A, Selvin E. et al. Serum fibroblast growth factor-23 and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. J Hypertens 2016; 34: 1266–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pastor-Arroyo EM, Gehring N, Krudewig C. et al. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int 2018; 94: 49–59 [DOI] [PubMed] [Google Scholar]
- 13. Andrukhova O, Slavic S, Smorodchenko A. et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 2014; 6: 744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Slatopolsky E, Elkan IO, Weerts C. et al. Studies on the characteristics of the control system governing sodium excretion in uremic man. J Clin Invest 1968; 47: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitch WE, Wilcox CS.. Disorders of body fluids, sodium and potassium in chronic renal failure. Am J Med 1982;72: 536–550 [DOI] [PubMed] [Google Scholar]
- 16. Koomans HA, Roos JC, Dorhout Mees EJ. et al. Sodium balance in renal failure. A comparison of patients with normal subjects under extremes of sodium intake. Hypertension 1985; 7: 714–721 [DOI] [PubMed] [Google Scholar]
- 17. Agarwal R, Sinha AD.. Thiazide diuretics in advanced chronic kidney disease. J Am Soc Hypertens 2012; 6: 299–308 [DOI] [PubMed] [Google Scholar]
- 18. Sinha AD, Agarwal R.. Thiazide diuretics in chronic kidney disease. Curr Hypertens Rep 2015; 17: 13. [DOI] [PubMed] [Google Scholar]
- 19. Srivastava T, Warady BA. Overview of the Management of Chronic Kidney Disease in Children. https://www.uptodate.com/contents/overview-of-the-management-of-chronic-kidney-disease-in-children (24 January 2019, date last accessed)
- 20. Xu H, Hashem A, Witasp A. et al. Fibroblast growth factor 23 is associated with fractional excretion of sodium in patients with chronic kidney disease. Nephrol Dial Transplant 2018; doi:10.1093/ndt/gfy315 [DOI] [PubMed] [Google Scholar]
- 21.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114: 555–576 [PubMed] [Google Scholar]
- 22. Cuervo C, Abitbol CL, Zilleruelo GE. et al. Fibroblast growth factor-23 and renin-angiotensin system levels in vitamin-D-dependent rickets type I. Pediatr Nephrol 2016; 31: 1189–1193 [DOI] [PubMed] [Google Scholar]
- 23. Stark H, Eisenstein B, Tieder M. et al. Direct measurement of TP/GFR: a simple and reliable parameter of renal phosphate handling. Nephron 1986; 44: 125–128 [DOI] [PubMed] [Google Scholar]
- 24. Shimada T, Hasegawa H, Yamazaki Y. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2003; 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 25. Isakova T, Wahl P, Vargas GS. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011; 79: 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Portale AA, Wolf M, Jüppner H. et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 2014; 9: 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slatopolsky E, Robson AM, Elkan I. et al. Control of phosphate excretion in uremic man. J Clin Invest 1968; 47: 1865–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christov M, Jüppner H.. Dietary phosphate: the challenges of exploring its role in FGF23 regulation. Kidney Int 2013; 84: 639–641 [DOI] [PubMed] [Google Scholar]
- 29. Meyer TW, Scholey JW, Brenner BM.. Nephron adaptation to renal injury In: Brenner BM, Rector FC (eds). The Kidney. Philadelphia, PA: W.B. Saunders Company, 1991, 1862–1864 [Google Scholar]
- 30. Verouti SN, Boscardin E, Hummler E. et al. Regulation of blood pressure and renal function by NCC and ENaC: lessons from genetically engineered mice. Curr Opin Pharmacol 2015; 21: 60–72 [DOI] [PubMed] [Google Scholar]
- 31. Laragh JH, Baer L, Brunner HR. et al. Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med 1972; 5: 633–652 [DOI] [PubMed] [Google Scholar]
- 32. Vande Walle JG, Donckerwolcke RA, Koomans HA.. Pathophysiology of edema formation in children with nephrotic syndrome not due to minimal change disease. J Am Soc Nephrol 1999; 10: 323–331 [DOI] [PubMed] [Google Scholar]
- 33. Flatman PW. Cotransporters, WNKs and hypertension: an update. Curr Opin Nephrol Hypertens 2008; 17: 186–189 [DOI] [PubMed] [Google Scholar]
- 34. Sim JJ, Shi J, Calara F. et al. Association of plasma renin activity and aldosterone-renin ratio with prevalence of chronic kidney disease: the Kaiser Permanente Southern California cohort. J Hypertens 2011; 29: 2226–2235 [DOI] [PubMed] [Google Scholar]
- 35. Yeyati NL, Adrogué HJ.. Inappropriately high plasma renin activity accompanies chronic loss of renal function. Am J Nephrol 1996; 16: 471–477 [DOI] [PubMed] [Google Scholar]
- 36. Poletti R, Vergaro G, Zyw L. et al. Prognostic value of plasma renin activity in heart failure patients with chronic kidney disease. Int J Cardiol 2013; 167: 711–715 [DOI] [PubMed] [Google Scholar]
- 37. Ter Maaten JM, Voors AA, Damman K. et al. Fibroblast growth factor 23 is related to profiles indicating volume overload, poor therapy optimization and prognosis in patients with new-onset and worsening heart failure. Int J Cardiol 2018; 253: 84–90 [DOI] [PubMed] [Google Scholar]
- 38. Kovesdy CP, Quarles LD.. FGF23 from bench to bedside. Am J Physiol Renal Physiol 2016; 310: F1168–F1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Czaya B, Seeherunvong W, Singh S. et al. Cardioprotective effects of paricalcitol alone and in combination with FGF23 receptor inhibition in chronic renal failure: experimental and clinical studies. Am J Hypertens 2019; 32: 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leifheit-Nestler M, Kirchhoff F, Nespor J. et al. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant 2018; 33: 1722–1734 [DOI] [PubMed] [Google Scholar]
- 41. Freundlich M, Quiroz Y, Zhang Z. et al. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 2008; 74: 1394–1402 [DOI] [PubMed] [Google Scholar]
- 42. de Borst MH, Vervloet MG, ter Wee PM. et al. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011; 22: 1603–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flynn JT, Mitsnefes M, Pierce C. et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 2008; 52: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matteucci MC, Wühl E, Picca S. et al. Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 2006; 17: 218–226 [DOI] [PubMed] [Google Scholar]
- 45. Mitsnefes M, Flynn J, Cohn S. et al. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 2010; 21: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ix JH, Katz R, Kestenbaum BR. et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 2012; 60: 200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ali FN, Falkner B, Gidding SS. et al. Fibroblast growth factor-23 in obese, normotensive adolescents is associated with adverse cardiac structure. J Pediatr 2014; 165: 738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Akhabue E, Montag S, Reis JP. et al. FGF23 (fibroblast growth factor-23) and incident hypertension in young and middle-aged adults: The CARDIA Study. Hypertension 2018; 72: 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seifert ME, de Las Fuentes L, Ginsberg C. et al. Left ventricular mass progression despite stable blood pressure and kidney function in stage 3 chronic kidney disease. Am J Nephrol 2014; 39: 392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yilmaz MI, Sonmez A, Saglam M. et al. Ramipril lowers plasma FGF-23 in patients with diabetic nephropathy. Am J Nephrol 2014; 40: 208–214 [DOI] [PubMed] [Google Scholar]
- 51. Xie J, Yoon J, An SW. et al. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 2015; 26: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohammad J, Scanni R, Bestmann L. et al. A controlled increase in dietary phosphate elevates BP in healthy human subjects. J Am Soc Nephrol 2018; 29: 2089–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Channavajjhala SK, Bramley R, Peltz T. et al. Urinary extracellular vesicle protein profiling and endogenous lithium clearance support excessive renal sodium wasting and water reabsorption in thiazide-induced hyponatremia. Kidney Int Rep 2018; 4: 139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]