Abstract
Background
In August 2019, the European Union licensed the first ever haemoperfusion device aimed to reduce pathogens in the blood. The core of the adsorber consists of ultra-high molecular weight polyethylene beads with endpoint-attached heparin. These beads utilize pathogen inherent adhesion mechanisms to reduce pathogen load. So far, it is unknown whether the device has an effect on anti-infective drug concentrations. The aim of this study was to investigate the in vitro adsorption of multiple anti-infective drugs from human plasma.
Methods
In this in vitro study, 18 anti-infective drugs were administered to human donor plasma and pumped through the heparin-coated pathogen adsorber (Seraph® 100 Microbind®Affinity Blood Filter; ExThera Medical Corp., Martinez, CA, USA) at a plasma flow rate of 250 mL/min for 60 min. Pre- and post-adsorber plasma samples were quantified after 5, 15, 30 and 60 min.
Results
We found a reduction ratio (RR) in anti-infective plasma levels between −1% and 62%. This decrease occurred mainly in the first 5 min of the experiment (RR0–5 −4 to 62%). Mean plasma clearance rates ranged between –11.93 mL/min (fluconazole) and 4.86 mL/min (clindamycin). The highest RRs were measured for aminoglycosides (tobramycin 62% and gentamycin 59%).
Conclusions
The elimination of anti-infective drugs by the Seraph is neglectable in all but 2 of 18 of the investigated substances. Aminoglycosides may be adsorbed by the device during their first pass.
Keywords: adsorber, antibiotics, in vitro, pharmacokinetics, sepsis
INTRODUCTION
Sepsis is a leading cause of mortality in critically ill patients. This applies in particular for infections with multidrug resistant (MDR) bacteria, as they are often faced with an insufficient initial anti-infective therapy regime [1]. Development of new antibiotic drugs and the increased usage of old antibiotics, which may preserve modern antibiotics and therefore avoid the spread of resistance, are practiced strategies to overcome this growing medical and economical issue [2].
To quickly reduce the blood pathogen load, even of MDR bacteria, extracorporeal methods removing bacteria have been developed by several companies, using different strategies such as filtration [3], magnetic nanoparticle separation [4], bendable polycrystalline nanowires/carbon foam [5] and polyethylene beads with endpoint-attached heparin [6]. The latter one uses covalently endpoint-attached heparin-coated ultrahigh molecular weight polyethylene that mimics heparan sulphate on cell surfaces so that pathogens bind to it. The capitalized mechanism is usually thought for bacteria to overcome cellular barriers by interactions on cell surfaces [7]. It has been shown to be safe and effective in haemodialysis patients with blood stream infections [8]. However, the usage of this non-selective mechanism may interfere with serum levels of anti-infective drugs, a key element in sepsis therapy, and thereby reduce the effectiveness of initiated therapy regimens [9]. As shown for other extracorporeal treatment methods like dialysis, this might necessitate special dosing recommendations [10]. To avoid such a knowledge gap, early in vitro studies for drug clearance (CL) of extracorporeal methods have been recommended [11].
The objective of this study was to determine a possible elimination of anti-infective drugs by the first approved pathogen adsorber in the European Union, the Seraph® 100 Microbind®Affinity Blood Filter (ExThera Medical Corp., Martinez, CA, USA).
MATERIALS AND METHODS
Patients and study protocol
Blood plasma from five voluntary donors (3 male/2 female, age ± standard deviation: 46 ± 15 years) was obtained during regular therapeutic plasma exchange (TPE) treatments due to various indications. None of the patients received one of the investigated anti-infective drugs. The removed plasma of a single TPE treatment was stored at 4°C and used within 48 h. The median collected plasma volume was 3200 mL and ranged from 2800 to 4000 mL. Anti-infective drugs were added to the plasma. Single doses of acyclovir (250 mg), amphothericin B (50 mg), ceftazidime (2000 mg), cefazolin (2000 mg), clindamycin (600 mg), daptomycin (350 mg), fluconazole (200 mg), fosfomycin (2000 mg), gentamicin (20 mg), levofloxacin (250 mg), linezolid (600 mg), meropenem (500 mg), moxifloxacin (400 mg), piperacillin (4000 mg), rifampicin (600 mg), tazobactam (500 mg), tobramycin (80 mg) and vancomycin (500 mg) were added to the plasma drain bag and mixed for 10 min. Visual controls were performed to detect possible drug precipitations. One litre of the plasma was separated into a compatible plasma bag, which could be connected to the tubing system. We primed a standard haemoperfusion blood tubing system (Meise Medizintechnik GmbH, Germany) as well as the Seraph® 100 Microbind®Affinity Blood Filter (ExThera Medical Corp., Martinez, CA, USA) with a total filling volume of ∼200 mL 0.9% saline and connected the plasma bag with the haemoperfusion tubing. The AFERsmart device (Meise Medizintechnik GmbH, Germany) was used to circle the plasma through the adsorber; a schematic overview of the experimental setup is given in Figure 1. Plasma flow was set to 250 mL/min and the plasma was continuously shaken during the procedure to ensure the mixture of the plasma. The experiment was repeated five times with different plasma bags from different donors.
FIGURE 1.

Schematic overview of the experimental setup. Plasma was circled through the Seraph cartridge by a roller pump. Plasma samples were obtained before and after passing through the Seraph device.
Sampling and analysis
A plasma sample of the whole plasma (C0) was obtained after mixing the anti-infective drugs and the plasma. One litre of the mixed plasma was separated into an empty dialysis flush bag. Pre (Cpre) and post (Cpost) adsorber plasma levels were obtained at 5 (C5), 15 (C15), 30 (C30) and 60 (C60) min into the procedure and stored at −80°C until further measurements.
Ethics
Written informed consent was obtained from the voluntary plasma donors. The study protocol was approved by the Hannover Medical School Ethics Committee and was performed in accordance with the Declaration of Helsinki and German federal guidelines.
Chemical assays
Plasma concentrations of anti-infective drugs were determined separately at the Limbach Group laboratory, Heidelberg, Germany or the Institute of Clinical Pharmacology at the Otto-von-Guericke-University Magdeburg, Germany. Liquid chromatography–mass spectrometry/mass spectrometry and cloned enzyme donor immunoassays were used to measure the plasma levels of the investigated anti-infective drugs.
Statistical analysis
The adsorber CL of every investigated drug 5, 15, 30 and 60 min into the procedure was calculated based on the plasma flow (Qe) and extraction ratio, using the equation:
Plasma drug reduction ratios (RRs) over the first 5 min of every experiment were calculated using the equation:
as well RRs from 5 min into the procedure to the end of the experiment, using the equation:
Calculations were performed with GraphPad Prism version 6 (San Diego, CA, USA).
RESULTS
All of the five in vitro treatments could be carried out over their planned length. Despite the fact that we did not use any anticoagulation, no procedure had to be prematurely ended or interrupted due to circuit failure. No visual detectable signs of clotting in the adsorber cartridge appeared in any of the runs.
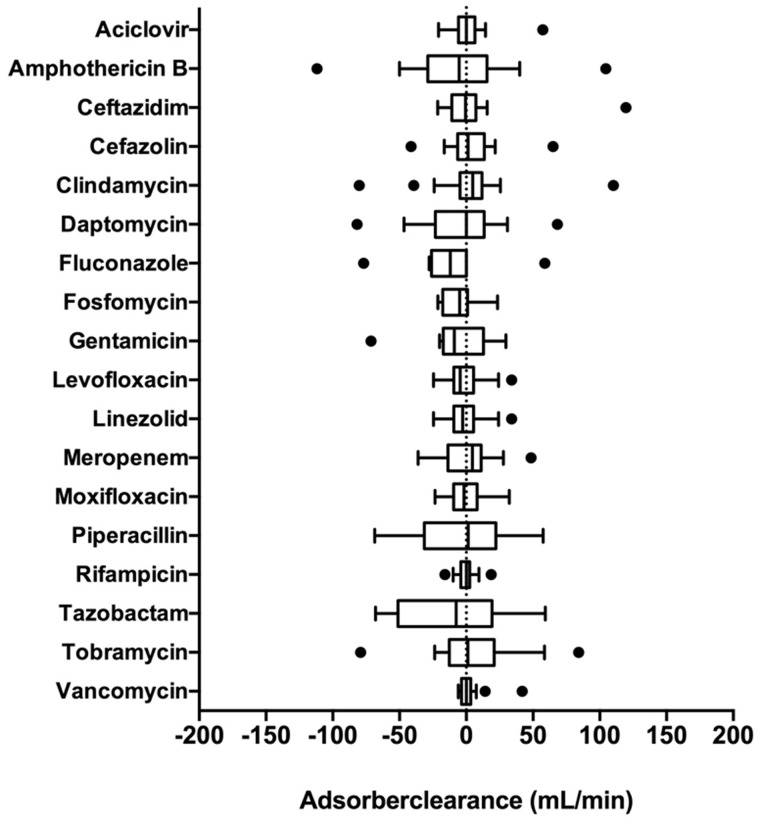
We found no significant drug plasma CL during the procedure. Mean CL values ranged from −11.93 mL/min (fluconazole) to 4.86 mL/min (clindamycin). Adsorber CL confidence intervals included zero in all investigated drugs.
We found a slight decrease in the drug plasma levels from the start of the procedure C0–C5 in all measured anti-infective drugs. This decrease was within the range of −4 to 20% for most of the investigated substances. Aminoglycosides showed higher RR0–5 of 54% for gentamycin and 62% for tobramycin. However, no additional decrease in drug plasma levels was observed during the experiment for any investigated drug. Additionally, plasma CL rates at 5 min into the experiment (CL5), as well as at the other measurement time points, were neglectable.
Our results are summarized in Table 1 and Figure 2.
Table 1.
Summary of the in vitro anti-infective drug elimination profile of the Seraph® 100
| Drug | C0 | CL5 | CL15 | CL30 | CL60 | RR0–5 | RR5–60 | RR0–60 |
|---|---|---|---|---|---|---|---|---|
| Aciclovir | 58.6 ± 8.3 | −2.12 | 1.07 | 10.73 | −2.3 | 20 | 0 | 22 |
| Amphotericin B | 13 ± 2.1 | 8.45 | 8.5 | −5.52 | −20.69 | 19 | 8 | 26 |
| Ceftazidime | 276 ± 22.8 | −2.07 | −7.23 | 29.09 | −4.12 | 14 | 2 | 11 |
| Cefazolin | 828.8 ± 86.7 | 0.52 | −2.03 | 17.78 | −2.35 | 20 | 0 | 15 |
| Clindamycin | 9.9 ± 3.3 | 3.53 | 5.51 | 17.77 | 3.19 | −1 | −2 | 20 |
| Daptomycin | 68.5 ± 3.5 | 17.32 | −22 | 0 | −2.17 | −4 | 19 | 15 |
| Fluconazole | 28.5 ± 4.9 | −5.35 | −20.41 | −40 | 99.05 | 19 | −25 | −1 |
| Fosfomycin | 953.9 ± 487.8 | −14.1 | −4.9 | 2 | −8 | 14 | −2 | 13 |
| Gentamycin | 6.5 ± 0.1 | 24.6 | −30.61 | −9.09 | −14.15 | 54 | 12 | 59 |
| Levofloxacin | 89.6 ± 37.5 | −1.51 | −2.08 | 9.11 | −13.21 | 9 | 3 | 9 |
| Linezolid | 109.4 ± 10.9 | −1.51 | −2.08 | 10.12 | −13.21 | 11 | 3 | 14 |
| Meropenem | 125.6 ± 17.9 | −3.48 | 11.36 | 12.39 | −6.05 | 13 | −2 | 15 |
| Moxifloxacin | 67.4 ± 7.1 | 0.8 | 2.48 | 9.65 | −11.78 | 9 | 3 | 11 |
| Piperacillin | 1364.3 ± 193 | 18.87 | 16.44 | 5.53 | −50.08 | 11 | 5 | 16 |
| Rifampicin | 109.4 ± 4.4 | −0.97 | −5 | 5.86 | 0.99 | 7 | 1 | 7 |
| Tazobactam | 142.3 ± 16.7 | 11.33 | 7.89 | 44.92 | −36.6 | 12 | 8 | 17 |
| Tobramycin | 18.8 ± 6.5 | 48.7 | −4.19 | −25.07 | −4.56 | 62 | 7 | 62 |
| Vancomycin | 179.8 ± 23.8 | 0.46 | −1.88 | 13.48 | −1.05 | 20 | 2 | 23 |
C0 = plasma bag concentration at the beginning of the experiment (mg/L). CL = mean drug plasma CL of the seraph adsorber at different investigated measure time points in millilitre per minute. RR (%) of the investigated drug during the first 5 min and during the rest of the experiment.
FIGURE 2.
Tukey boxplot of the Seraph® 100 plasma CL of the investigated anti-infective drugs during the procedure in millilitres per minute.
DISCUSSION
The Seraph® 100 Microbind®Affinity Blood Filter (ExThera Medical Corp., Martinez, CA, USA) is the first CE-approved extracorporeal device to filter pathogens from the blood. To our knowledge, this is the first in vitro measurement of the drug elimination characteristics of the Seraph® 100 using a life size adsorber and human plasma. Furthermore, a dialysis device and plasma flow rate used in the clinical setting were employed. Our work indicates that the Seraph® 100 does not affect plasma levels of the investigated anti-infective drugs to a clinically important degree. Therefore, we predict no interference, i.e. no removal of the investigated anti-infective drugs, thus avoiding the need for a dose adaption or an additional dose after treatment with the Seraph® 100.
This result is based on two different lines of evidence: (i) the lack of significant drug elimination in the investigated adsorber CL rates and (ii) the steady drug levels in the plasma during the whole procedure time. As expected, we measured a slight initial decrease in drug levels during the first 5 min of the procedure as compared with the baseline level (RR0–5) due to mixture of the 1 L volume plasma bag with the 200 mL 0.9% NaCl priming solution. The total filling volume of the plasma circuit and the adsorber itself corresponds to ∼20% of the plasma bag size. After the mixing of plasma and priming solution (all data after Minute 5 of the procedure), no further drug level decrease can be found.
However, the 5 min RR of aminoglycosides as gentamycin and tobramycin was considerably higher than the expected 20%; therefore, an adsorption of the drug during the first pass of the plasma through the device can be postulated. Similar findings have been shown in the usage of other devices [12].
We need to address several shortcomings of this in vitro study. First, the sample size is rather small, and the investigated drug medium was human plasma rather than whole blood. Therefore, interactions between cellular blood components, the Seraph® device and anti-infective drugs could not be investigated by this study. Human plasma was used due to ethical considerations, as it is available without further clinical risks for the donor after TPE. A realistic study approach with a continuous whole-blood stream through the adsorber may reveal such effects. Additionally, we did not perform drug level monitoring in the plasma that was not used in the experiment to determine drug stability effects.
FUNDING
This study was supported by an unrestricted grant from ExThera Medical Corp., Martinez, CA, USA and InfectoPharm Arzneimittel & Consilium GmbH, Heppenheim, Germany.
CONFLICT OF INTEREST STATEMENT
J.J.S., G.E. and J.T.K. received research funding for the first-in-man study with the device as well as travel support from ExThera Medical Corp., Martinez, CA, USA. All other authors: none to declare.
REFERENCES
- 1. Zilberberg MD, Shorr AF, Micek ST. et al. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18: 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cassir N, Rolain JM, Brouqui P.. A new strategy to fight antimicrobial resistance: the revival of old antibiotics. Front Microbiol 2014; 5: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kroll S, Brandes C, Wehling J. et al. Highly efficient enzyme-functionalized porous zirconia microtubes for bacteria filtration. Environ Sci Technol 2012; 46: 8739–8747 [DOI] [PubMed] [Google Scholar]
- 4. Lee JJ, Jeong KJ, Hashimoto M. et al. Synthetic ligand-coated magnetic nanoparticles for microfluidic bacterial separation from blood. Nano Lett 2014; 14: 1–5 [DOI] [PubMed] [Google Scholar]
- 5. Liu L, Chen S, Xue Z. et al. Bacterial capture efficiency in fluid bloodstream improved by bendable nanowires. Nat Commun 2018; 9: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCrea K, Ward R, LaRosa SP.. Removal of Carbapenem-Resistant Enterobacteriaceae (CRE) from blood by heparin-functional hemoperfusion media. PLoS One 2014; 9: e114242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fagan RP, Lambert MA, Smith S.. The hek outer membrane protein of Escherichia coli strain RS218 binds to proteoglycan and utilizes a single extracellular loop for adherence, invasion, and autoaggregation. Infect Immun 2008; 76: 1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eden G, Kümpers P, Ward R. et al. Sao040treatment of bacteremia during dialysis using a biomimetic sorbent hemoperfusion device - results of an ongoing first-in-human study. Nephrol Dial Transplant 2018; 33: i332–i333 [Google Scholar]
- 9. König C, Röhr AC, Frey OR. et al. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int J Artif Organs 2019; 42: 57–64 [DOI] [PubMed] [Google Scholar]
- 10. Schmidt JJ, Strunk AK, David S. et al. Single- and multiple-dose pharmacokinetics and total removal of colistin in critically ill patients with acute kidney injury undergoing prolonged intermittent renal replacement therapy. J Antimicrob Chemother 2019; 74: 997–1002 [DOI] [PubMed] [Google Scholar]
- 11. Matzke GR, Aronoff GR, Atkinson AJ. et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011; 80: 1122–1137 [DOI] [PubMed] [Google Scholar]
- 12. Tian Q, Gomersall CD, Ip M. et al. Effect of preexposure to aminoglycosides on in vitro adsorption of amikacin by polyacrylonitrile hemofilters. Antimicrob Agents Chemother 2011; 55: 3641–3642 [DOI] [PMC free article] [PubMed] [Google Scholar]