Abstract
Background
End-stage renal disease (ESRD) is associated with a broad spectrum of morphological and functional thyroid disorders. Recent studies have shown that low free triiodothyronine (fT3) levels are related to inflammatory status and endothelial activation in ESRD patients on haemodialysis (HD). Limited data exist about a possible relationship between dialysis regimen, namely long nocturnal haemodialysis (LNHD), and thyroid function parameters. The aim of this study was to evaluate the relationship between dialysis regimen and thyroid function, and consequently with the main patient outcomes.
Methods
To this purpose, we performed a retrospective, single-centre cohort study including 220 incident chronic HD patients treated during an 8-year period (from January 2010 to December 2017). The main clinical and haematochemical parameters, including thyroid function, were evaluated and related to the main patient outcomes.
Results
Patients with low fT3 levels (<3.05 ng/mL) showed significantly lower survival rates than patients with normal fT3 levels (>3.05 ng/mL) (P < 0.001), although there were no substantial differences in the demographic and clinical characteristics between the two groups. After propensity score 1:3 matching of 25 patients treated with nocturnal HD to 75 patients treated with diurnal HD, LNHD patients showed significantly higher survival rates (88.0% versus 61.3%, P = 0.001) and lower incidence of cardiovascular events than patients on diurnal dialysis (8.0% versus 40.0%, P = 0.001). Moreover, an 8-year time-dependent analysis showed that at any time, except for baseline, the rate of patients with fT3 levels >3.05 ng/mL was significantly higher in LNHD patients than in patients treated with diurnal dialysis.
Conclusions
Our data suggest that the application of alternative dialysis regimens, also reducing the frequency of low T3, could ameliorate outcomes and therefore reduce the incidence of cardiovascular events in HD patients.
Keywords: cardiovascular risk, haemodialysis, long nocturnal haemodialysis, low fT3 serum levels
INTRODUCTION
Morbidity and mortality in patients with end-stage renal disease (ESRD) treated with haemodialysis (HD) is unacceptably high, mainly due to cardiovascular disease (CVD) [1]. Several factors negatively influence the life expectancy of patients on renal replacement therapy, including the uraemic milieu, anaemia, bone–mineral disorders and hyper-homocysteinaemia [2]. Inflammation and endothelial dysfunction are key risk factors for morbidity and mortality in these patients [3, 4]. So far, there are no reliable markers of disease severity in ESRD that are able to guide treatment and/or to predict morbidity and mortality in these patients [5].
ESRD is associated with a broad spectrum of thyroid disorders, both functional and morphologic [6]. Patients with severe diseases, both acute and chronic, including patients with ESRD, can present with changes in the circulating thyroid hormones, usually a decrease in serum free triiodothyronine (fT3) concentrations in the absence of an intrinsic thyroid disorder (so-called non-thyroidal illness) [7]. Non-thyroidal illness syndrome, also known as euthyroid sick syndrome, refers to patients with severe chronic illnesses like malnutrition, infections, ESRD and ischaemic heart diseases, with a decrease in serum thyroid hormone levels without identifiable primary thyroid disease. The decrease in fT3 levels commonly observed in ESRD patients is significantly associated with systemic acidosis, duration of dialysis therapy, and some markers of endothelial damage and inflammation [8]. Low fT3 has emerged as a potent biomarker in ESRD in several studies and represents a highly prevalent finding in renal disease [9–11]. The pathogenic mechanisms of low fT3 status are not yet fully elucidated, but iodine retention, alterations of protein binding, derangements of deiodinases activity and dysregulation of the hypothalamic control all seem to play a role [12].
Nocturnal HD is characterized by a long dialysis session, usually between 8 and 10 h, performed 3–7 times/week during the night. This approach extends the effective duration of dialysis without affecting the patient’s activities, making it a valid alternative to diurnal dialysis. Several publications in this field have reported significant improvements in clinical outcomes including a reduction in left ventricular hypertrophy and an improved control of blood pressure, anaemia, calcium-phosphate homoeostasis, and fluid and electrolyte balance [13], leading to an improvement in quality of life [14].
Due to the relationship between the onset of low fT3 serum levels and the systemic inflammation, a potential beneficial role of alternative dialysis schedules could be expected, both in terms of ameliorating thyroidal status and the patient's clinical outcomes, but it has not yet been assessed.
Thus, the aim of the present observational, single-centre and retrospective cohort study was to evaluate the impact of thyroid function on the survival rate in a cohort of haemodialysed patients, comparing diurnal and nocturnal dialysis regimens.
MATERIALS AND METHODS
Participants
This retrospective, single-centre, observational and cohort study was performed including 220 incident patients with ESRD undergoing replacement therapy with HD at the Nephrology Dialysis and Transplantation Unit of the University Hospital "Ospedali Riuniti", Foggia (Italy), between January 2010 and December 2017.
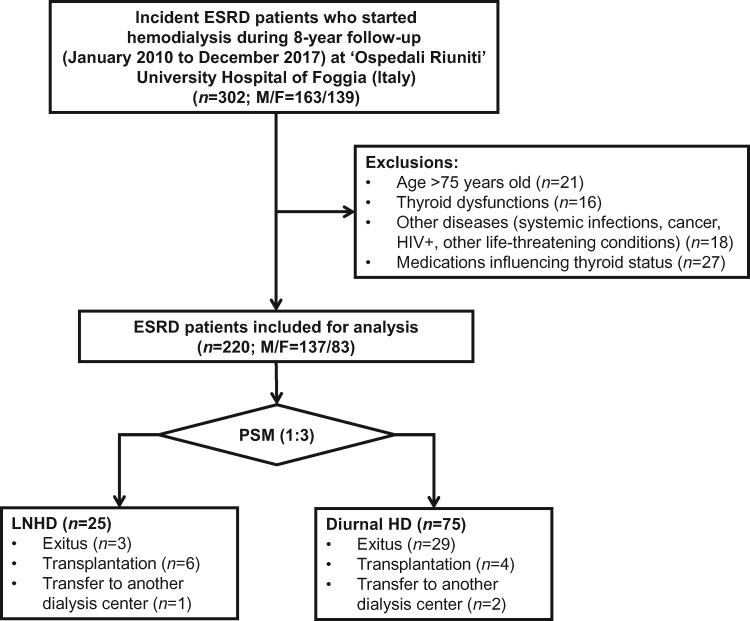
All patients starting HD with an age <75 years were included. Exclusion criteria were systemic infections, cancer, HIV positivity or other life-threatening conditions with life expectancy <6 months. Furthermore, patients with known thyroid dysfunction or taking medication potentially influencing thyroid hormone values or impairing the conversion of T4 to T3 (amiodarone, lithium, glucocorticoids, contrast agents—e.g. iopanoic acid—propylthiouracil, propranolol and nadolol) were excluded from the study, as shown in Figure 1 [15].
FIGURE 1.
Study design flow chart. PSM, propensity score matching.
All patients underwent monthly measurements of the main laboratory parameters, while fT3, free thyroxine (fT4) and thyroid-stimulating hormone (TSH) were measured every 3 months.
All patients included in the study were treated according to the Kidney Disease Outcomes Quality Initiative guidelines [16]. All patients were on a 4-h, thrice-weekly HD schedule (n = 195) or on an 8-h, thrice-weekly long nocturnal HD (LNHD) (n = 25). The dialysis modalities were online haemodiafiltration (n = 32) or conventional HD (n = 188). The dialysis solution consisted of standard bicarbonate preparations (HCO3−: 32–35 mmol/L, Na+: 138 mmol/L, K+: 1–3 mmol/L, Mg2+: 0.5–0.75 mmol/L, Ca2+: 1.25–1.75 mmol/L). The dialysis accesses were arteriovenous fistula or the central venous catheter.
Low-molecular weight or unfractionated heparin was used as standard anti-coagulation therapy. Dialysis prescription was guided aiming at a value of urea reduction rate ≥0.65 and a Kt/V ≥1.2. The above indices of dialysis adequacy were calculated according to the second-generation Daugirdas equation [17].
History of CVD was defined as previous myocardial infarction, coronary artery diseases (CAD) requiring revascularization [percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG)], or clinical signs of angina pectoris, stroke or transient ischaemic attack or peripheral vascular disease.
The two primary outcomes of the study were patients’ survival and the incidence of cardiovascular events.
De novo cardiovascular events were defined as onset of central or peripheral vasculopathy (intima-media thickness >0.9 or >75th percentile, detection of atheromatous plaques at femoral or carotid arteries, transient ischaemic attack, ischaemic stroke), CAD requiring revascularization (PTCA or CABG) or severe peripheral stenosis (stenosis >80% at ultrasound examination of femoral or carotid arteries), requiring treatment (PTCA).
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved both by the institutional review board and the ethics committee (Decision no. 147/CE/2014 of 23 July 2014; Ethical Committee at the University Hospital "Ospedali Riuniti" of Foggia). This was in accordance with the guidelines laid down by the Regional Ethics Committee on human experimentation.
Laboratory assessment
Serum concentrations of fT3 (normal range 2.5–5.2 pg/mL), fT4 (normal range 0.8–2.2 ng/mL) and TSH (normal range 0.3–4.5 μIU/mL) were measured using immuno-chemiluminescent assays by an automated analyser (Immulite 2000, DPC Cirrus, Los Angeles, CA, USA) employing commercially available kits (all from Diagnostic Products Corporation, Los Angeles, CA, USA).
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) 25.0 software (SPSS Inc., Evanston, IL, USA), as previously described [18–23]. Variable distribution was tested using Kolmogorov–Smirnov test. Serum parameters were compared between groups by Student’s t-test for unpaired data and Mann–Whitney U-test, as appropriate. A comparison of more than two group means was performed with one-way analysis of variance (ANOVA).
Frequencies were compared among groups by X2-test. Correlation between two variables was ascertained by Pearson’s or Spearman’s correlation tests, as appropriate.
A receiver operating characteristic (ROC) curve analysis was performed to validate the association of fT3 serum levels with patients’ survival, and an operational cut-off level was defined to differentiate haemodialysed patients at higher risk of death. Subsequently, all the patients were stratified according to fT3 cut-off level with the highest sensitivity and specificity for predicting mortality during the 8-year follow-up.
Kaplan–Meier estimates were used to generate both an overall survival curve and a cumulative incidence of cardiovascular events for HD patients, while differences among groups were assessed by log-rank test. The data were censored if a patient underwent renal transplantation or converted from HD to peritoneal dialysis during the study period. To test the independent effects of different variables on patient’s survival, univariate and multivariate Cox regression analysis was used and partial correlation coefficients were computed and presented as hazard ratio and 95% confidence intervals (HR; 95% CI). Covariates included in the Cox model were age, diagnosis of diabetes mellitus, haemoglobin, high-sensitivity C-reactive protein (hsCRP), albumin and fT3 serum levels. Additional variables were included in the multivariate analyses if they had a P-value <0.05 in the univariate analysis or if they were clinically relevant confounders.
To compare the LNHD cohort with the diurnal HD, a propensity score matching analysis was conducted in R using the MatchIt package with nearest neighbour 1:3 matching.
All the data are reported as mean ± SD or as percentage frequency, unless otherwise specified. A P-value <0.05 was considered statistically significant.
Ethics approval and consent to participate
This study involving human participants was approved by the local ethical committee (Decision no. 147/CE/2014 of 23 July 2014; Ethical Committee at the University Hospital “Ospedali Riuniti” of Foggia). All procedures performed in this study were in accordance with the ethical standards of the Declaration of Helsinki and all the enrolled patients provided informed consent to participate in this study.
RESULTS
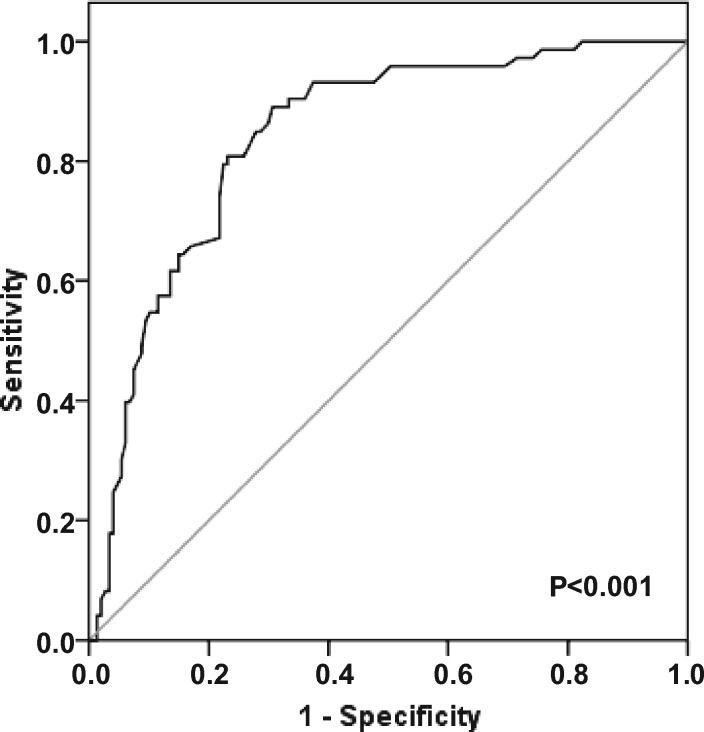
Mean values of serum fT3 levels, assessed every 3 months during the follow-up for each patient in the entire study group, were obtained and an ROC curve analysis was carried out to validate the possible role of low serum fT3 levels as predictors of mortality in haemodialysed patients and to define an operational cut-off value. The analysis showed mean serum fT3 levels were significantly associated with mortality during an 8-year follow-up and an fT3 cut-off value of 3.05 pg/mL was defined with an 80.8% specificity and a 76.9% sensitivity, a positive predictive value of 63.4% and a negative predictive value of 89.0% (Figure 2).
FIGURE 2.
ROC curve for serum fT3 levels and 8-year survival. ROC curve analysis to evaluate the predictive role of serum fT3 levels as risk factor affecting survival in haemodialysed patients during 8-year follow-up (area under curve = 0.841, 95% CI 0.787–0.895, P < 0.001).
The main clinical and laboratory features of all patients after stratification in two groups according to serum fT3 level above or below the cut-off value found in the whole study group (3.05 pg/mL) are shown in Table 1. Of note, 42% of patients from the entire cohort study (n = 220) were under the identified threshold of fT3.
Table 1.
Baseline clinical and laboratory characteristics of patients with fT3 level above and under the cut-off value (3.05 pg/mL)
| Total | fT3 <3.05 pg/mL | fT3 >3.05 pg/mL | P-value | |
|---|---|---|---|---|
| (n = 220) | (n = 93) | (n = 127) | ||
| Female gender, n (%) | 83 (37.7) | 40 (43.0) | 43 (33.9) | 0.038 |
| Age (years) | 58.1 ± 11.1 | 59.2 ± 9.9 | 57.3 ± 11.8 | 0.407 |
| Follow-up (months) | 41.4 ± 29.9 | 33.7 ± 26.4 | 47.0 ± 31.2 | 0.001 |
| Type of HD (HDF), n (%) | 32 (14.5) | 13 (14.0) | 19 (15.0) | 0.838 |
| Type of dialysis access (CVC), n (%) | 53 (24.1) | 25 (26.8) | 28 (22.0) | 0.407 |
| Diabetes mellitus (yes), n (%) | 53 (24.1) | 30 (32.6) | 23 (18.1) | 0.015 |
| Previous CAD (PTCA or CABG), n (%) | 13 (5.9) | 7 (7.5) | 6 (4.7) | 0.384 |
| Previous peripheral vasculopathy, n (%) | 51 (23.2) | 22 (23.7) | 29 (22.8) | 0.887 |
| Severe peripheral arterial stenosis, n (%) | 7 (3.2) | 4 (4.3) | 3 (2.4) | 0.418 |
| Treatment of peripheral arterial stenosis (PTCA), n (%) | 5 (2.3) | 2 (2.2) | 3 (2.2) | 0.971 |
| Kt/Vurea | 1.48 ± 0.68 | 1.40 ± 0.42 | 1.62 ± 0.36 | 0.412 |
| hsCRP (mg/dL) | 15.1 ± 15.0 | 19.7 ± 12.4 | 14.8 ± 11.6 | < 0.001 |
| Albumin (g/dL) | 4.0 ± 0.8 | 3.9 ± 0.4 | 4.5 ± 2.4 | 0.006 |
| Haemoglobin (g/dL) | 10.9 ± 0.7 | 10.6 ± 0.7 | 11.2 ± 0.7 | <0.001 |
| Calcium (mg/dL) | 8.9 ± 0.5 | 8.9 ± 0.5 | 9.0 ± 0.6 | 0.293 |
| Phosphate (mg/dL) | 5.0 ± 0.9 | 5.0 ± 1.0 | 5.1 ± 0.9 | 0.810 |
| PTH (pg/mL) | 199.4 ± 145.6 | 207.2 ± 102.8 | 193.1 ± 98.2 | 0.371 |
| Ferritin (ng/mL) | 319.2 ± 276.4 | 332.5 ± 209.0 | 290.5 ± 224.2 | 0.098 |
| TSH (µIU/mL) | 2.00 (1.40–2.50) | 2.00 (1.45–2.73) | 1.91 (1.37–2.40) | 0.139 |
| fT3 (pg/mL) | 3.18 ± 0.46 | 2.77 ± 0.23 | 3.48 ± 0.33 | < 0.001 |
| fT4 (ng/mL) | 1.10 ± 0.25 | 1.06 ± 0.26 | 1.12 ± 0.24 | 0.125 |
| Patients with NTIS (fT3 <2.50 pg/mL), n (%) | 12 (5.4) | 12 (12.9) | 0 (0) | <0.001 |
Values are expressed as mean ± SD for normally distributed variables, median and interquartile range for not normally distributed variables or number of cases and (percentage) for frequencies. A comparison between the main clinical and laboratory parameters of patients with fT3 <3.05 pg/mL and patients with fT3 >3.05 pg/mL is shown and a P-value is reported.
AVF, arteriovenous fistula; CVC, central venous catheter; HDF, haemodialfiltration; NTIS, non-thyroidal illness syndrome; PTH, parathyroid hormone.
Female gender and diagnosis of diabetes mellitus were more frequent in the group of patients with lower fT3 (43.0% versus 33.9% for female gender, P = 0.038; 32.6% versus 18.1% for diabetes mellitus, P = 0.015). Moreover, the length of follow-up was significantly shorter in patients with lower fT3 as compared with patients with normal fT3 (33.7 ± 26.4 months versus 47.0 ± 31.2 months, P < 0.001). No differences for mean age, or type of either dialysis treatment or dialysis access were observed between patients with high and low fT3. Moreover, patients with an fT3 level above or below the cut-off value did not differ for the incidence of previous CVD or peripheral vascular disease (Table 1).
The analysis of baseline laboratory parameters showed that patients with low fT3 level showed higher hsCRP, and lower serum albumin and haemoglobin (19.7 ± 12.4 mg/dL versus 14.8 ± 11.6 mg/dL for hsCRP, P < 0.001; 3.9 ± 0.4 g/dL versus 4.5 ± 2.4 g/dL for albumin, P = 0.006; 10.6 ± 0.7 g/dL versus 11.2 ± 0.7g/dL for haemoglobin, P < 0.001), as compared with patients with higher fT3 level (Table 1). No differences were observed in other clinical variables including dialysis adequacy and calcium/phosphate homoeostasis. Of note, no differences in TSH and fT4 levels were observed between patients with low versus normal fT3 levels (Table 1).
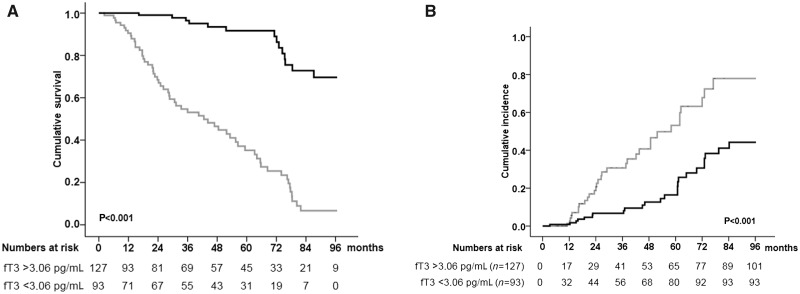
The 8-year overall survival rate in the whole study group was 66.8%. However, lifetime analysis performed after stratification of patients in two groups according to a serum fT3 level above or below the cut-off value (3.05 pg/mL) yielded significant differences. Indeed, patients displaying fT3 levels above the cut-off value showed a significantly higher 8-year survival rate as compared with those with serum fT3 <3.05 pg/mL (89.0% versus 36.6%, log-rank Mantel–Cox P < 0.001) (Figure 3A).
FIGURE 3.
Kaplan–Meier estimate of 8-year survival and cumulative incidence of cardiovascular events in haemodialysed patients according to serum fT3 level (>3.05 pg/mL or <3.05 pg/mL). (A) HD patients with fT3 level above the cut-off value (black line) showed significantly higher 8-year survival rate as compared with those with serum fT3 below the cut-off value (grey line) (89.0% versus 36.6%, Kaplan–Meier lifetime analysis and log-rank test P<0.001). (B) HD patients with fT3 level above the cut-off value (black line) showed significantly lower 8-year cumulative incidence of cardiovascular events as compared with those with serum fT3 below the cut-off value (grey line) (18.9% versus 34.4%, Kaplan–Meier lifetime analysis and log-rank test P<0.001). Patients with fT3 >3.05 pg/mL: black line; patients with fT3 <3.05 pg/mL: grey line.
Throughout the follow-up, a 25.5% overall cumulative incidence of de novo major cardiovascular events was observed in the entire cohort. Of note, patients with lower fT3 levels showed a significantly higher cumulative incidence of total cardiovascular events as compared with those with normal serum fT3 (34.4% versus 18.9%, Kaplan–Meier lifetime analysis and log-rank test P < 0.001) (Figure 3B). Of note, in haemodialysed patients with lower fT3 a higher incidence of de novo vasculopathy was recorded, as compared with patients with higher fT3 levels (34.4% versus 15.0%, P < 0.001). Instead, no differences were observed between patients with lower versus higher fT3 levels in the onset of de novo CAD requiring revascularization (PTCA or CABG) (17.2% versus 12.6%, P = 0.338) or severe peripheral stenosis (15.1% versus 9.4%, P = 0.203), even requiring treatment (PTCA) (12.9% versus 8.7%, P = 0.310).
To estimate the relative risk for a patient’s survival in patients showing serum fT3 level above or below the cut-off value (3.05 pg/mL), a Cox regression analysis was performed using patient’s death as dependent variable, and patient’s age, hsCRP, haemoglobin, albumin and fT3 levels as covariates (Table 2).
Table 2.
Univariate and multivariate regression analysis of factors affecting survival in 220 haemodialysed patients
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI |
95% CI |
|||||||
| Covariates | HR | Lower | Higher | P-value | HR | Lower | Higher | P-value |
| Age | 1.031 | 1.007 | 1.057 | 0.011 | 1.011 | 0.985 | 1.038 | 0.409 |
| Diabetes (yes/no) | 1.740 | 1.091 | 2.775 | 0.020 | 1.110 | 0.657 | 1.877 | 0.697 |
| hsCRP (mg/dL) | 1.020 | 1.012 | 1.028 | <0.001 | 1.010 | 1.002 | 1.018 | 0.020 |
| Haemoglobin (g/dL) | 0.205 | 0.141 | 0.296 | <0.001 | 0.343 | 0.215 | 0.559 | <0.001 |
| Albumin (g/dL) | 0.478 | 0.317 | 0.722 | 0.001 | 0.956 | 0.836 | 0.994 | 0.041 |
| fT3 <3.05 pg/mL (yes/no)a | 0.105 | 0.058 | 0.189 | <0.001 | 0.183 | 0.096 | 0.349 | <0.001 |
fT3 was entered as dichotomic variable (above or below the cut-off value of 3.05 pg/mL), while the other factors were entered as continuous variables.
Univariate analysis showed that the following covariates affected patient’s survival: age (HR 1.031, 95% CI 1.007–1.057, P = 0.011); diabetes (HR 1.740, 95% CI 1.091–2.775, P = 0.020); serum hsCRP (HR 1.020, 95% CI 1.012–1.028, P < 0.001); haemoglobin (HR 0.205, 95% CI 0.141–0.296, P < 0.001); serum albumin (HR 0.478, 95% CI 0.317–0.722, P = 0.001) and fT3 (HR 0.105, 95% CI 0.058–0.189, P < 0.001). As shown in Table 2, the results of the multivariate analysis confirmed a significant effect on patient survival of all of the above covariates, with the exception of the patient age and diabetes (HR 1.010, 95% CI 1.002–1.018, P = 0.020 for hsCRP; HR 0.343, 95% CI 0.215–0.559, P < 0.001 for haemoglobin; HR 0.956, 95% CI 0.836–0.994, P = 0.041 for albumin; HR 0.183, 95% CI 0.096–0.349, P < 0.001 for fT3).
Of note, fT3 levels in HD patients negatively correlated with hsCRP (r2 = −0.231, P = 0.001) and positively correlated with haemoglobin and serum albumin (r2 = 0.182, P = 0.007 and r2 = 0.379, P < 0.001, respectively).
To further evaluate the influence of dialysis therapy on patient’s survival and thyroid function, all patients were propensity score matched to two groups according to the HD schedule with nearest neighbour 1:3 matching (diurnal HD, n = 75 versus LNHD, n = 25). The resulted two groups did not differ for age and gender distribution and for prevalence of main comorbidity (diabetes, previous cardiovascular events) (Table 3), or for other relevant cardiovascular risk factors (smoking, hyperlipidaemia).
Table 3.
Baseline clinical and laboratory characteristics of haemodialysed patients (nocturnal versus diurnal) after propensity score matching
| Total | Nocturnal | Diurnal | P-value | |
|---|---|---|---|---|
| (n = 100) | (n = 25) | (n = 75) | ||
| Female gender, n (%) | 25 (25.0) | 3 (12.0) | 22 (20.0) | 0.367 |
| Age (years) | 58.5 ± 9.6 | 60.0 ± 10.0 | 58.0 ± 9.5 | 0.477 |
| Follow-up (months) | 47.8 ± 31.0 | 60.3 ± 31.5 | 43.7 ± 30.0 | 0.017 |
| Type of HD (HDF), n (%) | 14 (14.0) | 4 (16.0) | 10 (13.3) | 0.397 |
| Diabetes mellitus (yes), n (%) | 25 (25.0) | 5 (20.0) | 20 (31.0) | 0.304 |
| Previous CAD (PTCA or CABG), n (%) | 6 (6.0) | 2 (8.0) | 4 (5.3) | 0.627 |
| Previous peripheral vasculopathy, n (%) | 23 (23.0) | 5 (20.0) | 18 (24.0) | 0.681 |
| Severe peripheral arterial stenosis, n (%) | 3 (3.0) | 1 (4.0) | 2 (2.7) | 0.418 |
| Treatment of peripheral arterial stenosis (PTCA), n (%) | 2 (2.0) | 1 (4.0) | 1 (1.3) | 0.971 |
| Kt/Vurea | 1.48 ± 0.68 | 1.68 ± 0.39 | 1.41 ± 0.45 | 0.009 |
| hsCRP (mg/dL) | 14.4 ± 7.3 | 10.7 ± 7.8 | 15.7 ± 10.9 | 0.013 |
| Albumin (g/dL) | 4.1 ± 1.3 | 4.2 ± 0.5 | 4.0 ± 1.5 | 0.199 |
| Haemoglobin (g/dL) | 11.0 ± 0.6 | 11.2 ± 0.6 | 11.0 ± 0.7 | 0.066 |
| Calcium (mg/dL) | 9.0 ± 0.5 | 9.1 ± 0.4 | 8.9 ± 0.5 | 0.148 |
| Phosphate (mg/dL) | 5.0 ± 1.0 | 4.9 ± 0.9 | 5.0 ± 1.0 | 0.990 |
| PTH (pg/mL) | 199.4 ± 145.6 | 130.0 ± 106.7 | 204.7 ± 151.7 | 0.025 |
| Ferritin (ng/mL) | 319.2 ± 276.4 | 299.4 ± 101.8 | 325.0 ± 287.4 | 0.081 |
| TSH (µIU/mL) | 1.96 (1.47–2.39) | 1.88 (1.61–2.53) | 1.99 (1.41–2.30) | 0.443 |
| fT3 (pg/mL) | 3.25 ± 0.45 | 3.56 ± 0.49 | 3.14 ± 0.37 | <0.001 |
| fT4 (ng/mL) | 1.09 ± 0.23 | 1.15 ± 0.16 | 1.07 ± 0.24 | 0.150 |
| Patients with fT3 <3.05 pg/mL, n (%) | 37 (37.0) | 3 (12.0) | 34 (45.3) | 0.003 |
Values are expressed as mean ± SD for normally distributed variables, median and interquartile range for not normally distributed variables or number of cases and (percentage) for frequencies. A comparison between the main clinical and laboratory parameters of patients treated with diurnal versus LNHD is shown and a P-value is reported.
HDF, haemodialfiltration; PTH, parathyroid hormone.
As shown in Table 3, follow-up length was significantly shorter in diurnal HD patients as compared with LNHD patients (33.7 ± 26.4 versus 47.0 ± 31.2, P < 0.001). Furthermore, diurnal HD patients showed higher hsCRP (15.7 ± 10.9 mg/dL versus 10.7 ± 7.8 mg/dL for hsCRP, P = 0.013), as compared with LNHD patients (Table 3). Intriguingly, patients treated with diurnal HD showed lower fT3 serum levels as compared with those treated with nocturnal HD (LNHD) when assessed as either mean value (3.14 ± 0.38 pg/mL versus 3.56 ± 0.50 pg/mL; P < 0.001) or as percentage of subjects with fT3 <3.06 pg/mL (Table 3). No statistically significant differences were found for any other parameter.
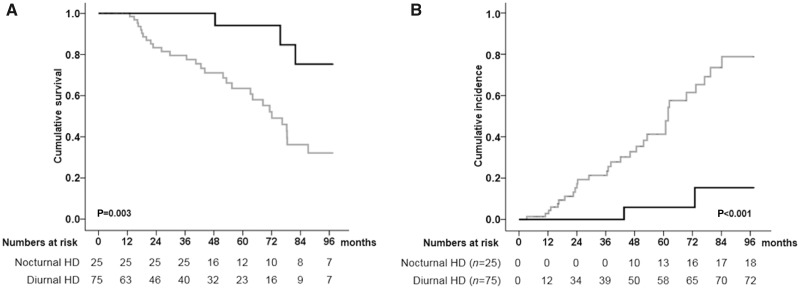
An 8-year lifetime analysis, performed after stratification of patients in two groups according to different dialysis schedule (LNHD and diurnal HD), showed a significantly higher survival rate in LNHD patients as compared with control group of diurnal HD patients (88.0% versus 61.3%, log-rank Mantel–Cox P = 0.003) (Figure 4A). Moreover, diurnal HD patients showed a significantly higher cumulative incidence of total cardiovascular events as compared with LNHD patients (40.0% versus 8.0%, Kaplan–Meier lifetime analysis and log-rank test P < 0.001) (Figure 4B). Of note, in HD patients a higher incidence of de novo vasculopathy, de novo CAD requiring revascularization and severe peripheral stenosis, even requiring treatment (PTCA), were recorded, as compared with LNHD patients, while not reaching the statistical significance (25.3% versus 8.0%, P = 0.065 for de novo vasculopathy; 16.0% versus 4.0%, P = 0.122 for de novo CAD requiring revascularization; 13.3% versus 4.0%, P = 0.196 for severe peripheral stenosis; 10.7% versus 4.0%, P = 0.313 for severe peripheral stenosis requiring treatment).
FIGURE 4.
Kaplan–Meier estimate of 8-year survival and cumulative incidence of cardiovascular events in haemodialysed patients according to different dialysis schedule (LNHD and diurnal HD) after propensity score matching. (A) Patients treated with nocturnal HD (LNHD) (black line) showed significantly higher 8-year survival rate as compared with treated with diurnal HD (grey line) (88.0% versus 61.3%, Kaplan–Meier lifetime analysis and log-rank test P=0.003). (B) Patients treated with nocturnal HD (LNHD) (black line) showed significantly lower 8-year cumulative incidence of cardiovascular events as compared with treated with diurnal HD (grey line) (8.0% versus 40.0%, Kaplan–Meier lifetime analysis and log-rank test P<0.001). Patients treated with nocturnal HD (LNHD): black line; patients treated with diurnal HD: grey line.
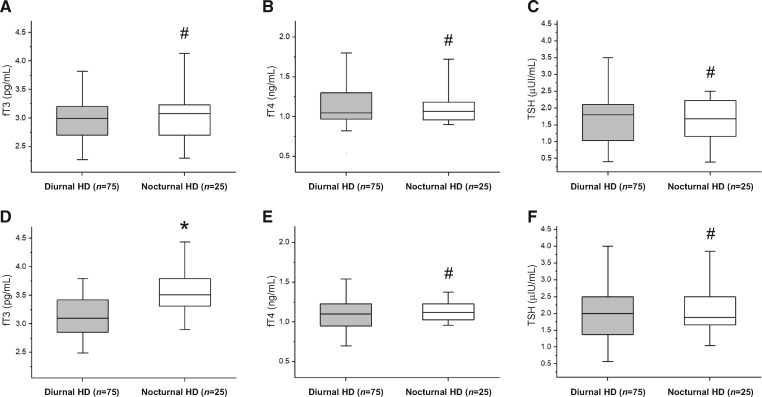
To elucidate the possible relationship between dialysis schedule and thyroidal status, thyroid function parameters assessed quarterly during an 8-year follow-up were compared between patients performing different dialysis schedule (diurnal HD versus LNHD) (Figure 5). Of note, while at baseline no differences were observed in thyroidal status among patients treated with different dialysis schedules [2.98 ± 0.45 pg/mL versus 3.11 ± 0.52 pg/mL, P = 0.280 for fT3 (Figure 5A); 1.11 ± 0.29 ng/mL versus 1.18 ± 0.39 ng/mL, P = 0.136 for fT4 (Figure 5B); 1.74 ± 0.92 µIU/mL versus 1.59 ± 0.97 µIU/mL, P = 0.307 for TSH (Figure 5C)], during an 8-year follow-up patients treated with diurnal HD showed lower mean fT3 serum levels as compared with those treated with nocturnal HD (LNHD) (Figure 5D) (3.14 ± 0.38 pg/mL versus 3.56 ± 0.50 pg/mL, P < 0.001), while fT4 [1.07 ± 0.24 ng/mL versus 1.15 ± 0.16 ng/mL, P = 0.150 (Figure 5E)] and TSH [2.16 ± 0.95 µIU/mL versus 2.00 ± 0.89 µIU/mL, P = 0.462 (Figure 5F)] did not differ between the two groups.
FIGURE 5.
Thyroidal status between diurnal and nocturnal HD patients assessed at baseline and during an 8-year follow-up. At baseline, patients treated with diurnal HD showed a thyroidal status comparable with patients treated with nocturnal HD (LNHD) [2.98 ± 0.45 pg/mL versus 3.11 ± 0.52 pg/mL, P = 0.280 for fT3 (A); 1.11 ± 0.29 ng/mL versus 1.18 ± 0.39 ng/mL, P = 0.136 for fT4 (B); 1.74 ± 0.92 µIU/mL versus 1.59 ± 0.97 µIU/mL, P = 0.307 for TSH (C)]. During an 8-year follow-up, patients treated with diurnal HD showed lower mean fT3 serum levels as compared with those treated with nocturnal HD (LNHD) (D) (3.14 ± 0.38 pg/mL versus 3.56 ± 0.50 pg/mL, P < 0.001). No differences in mean fT4 and TSH levels were observed between diurnal versus nocturnal patients in the same period (E and F) (1.07 ± 0.24 ng/mL versus 1.15 ± 0.16 ng/mL, P = 0.150 for fT4; 2.16 ± 0.95 µIU/mL versus 2.00 ± 0.89 µIU/mL, P = 0.462 for TSH). Mann–Whitney U-test for non-parametric data. Data in the graphs are expressed as median and 25th and 75th percentiles in boxes and 5th and 95th percentiles as whiskers. #P = Not significant (NS); *P < 0.001. Patients treated with nocturnal HD (LNHD): white box; patients treated with diurnal HD: grey box.
Finally, serum levels of fT3 were longitudinally evaluated in patients treated with diurnal and nocturnal HD at baseline and throughout the 8-year follow-up (Figure 6).
FIGURE 6:
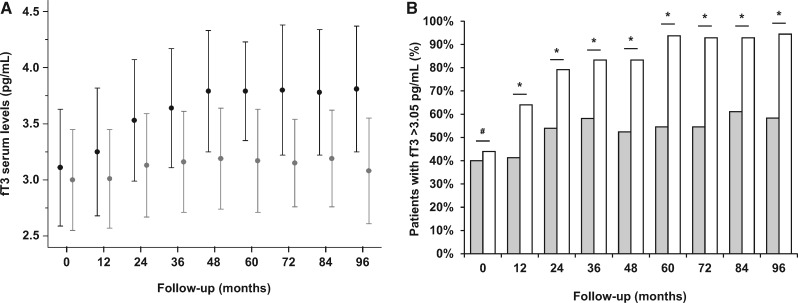
Variations of fT3 serum levels during an 8-year follow-up in patients treated with diurnal and nocturnal HD. Serum levels of fT3 at baseline did not differ between HD and LNHD patients (P = 0.609). In the following 8 years, serum fT3 levels increased in the entire study group (ANOVA F = 5.700, P < 0.001). After stratification of the patients to two groups according to the HD schedule, a significant increase in mean fT3 serum levels was observed in both groups (ANOVA F = 3.315, P = 0.002 for LNHD; ANOVA F = 2.386, P = 0.016 for HD (A). At baseline, the percentage of patients with fT3 serum levels >3.05 pg/mL cut-off among different dialysis schedules did not differ between LNHD patients as compared with HD patients (44.0% versus 40.0% for LNHD and HD, respectively; P = 0.725). However, during an 8-year follow-up this rate significantly increased in LNHD patients as compared with HD patients (64.0% versus 41.3% at 1st year, P = 0.049; 79.2% versus 54.0% at 2nd year, P = 0.031; 83.3% versus 58.1% at 3rd year, P = 0.035; 83.3% versus 52.4% at 4th year, P = 0.024; 93.8% versus 54.5% at 5th year, P = 0.006; 92.9% versus 58.3% at 6th year, P = 0.024; 92.9% versus 61.1% at 7th year, P = 0.040; 94.4% versus 58.3% at 8th year, P = 0.015 for LNHD and HD, respectively) (B). X2-test for comparison of frequencies. #P = NS; *P < 0.05. Data in the graphs are expressed as mean ± SD (A) or percentage (B). LNHD patients: black dot with confidence interval (A) and white columns (B); HD patients: grey dot with confidence interval (A) and grey columns (B).
Serum levels of fT3 at baseline did not differ between HD and LNHD patients (P = 0.280). In the following 8 years, serum fT3 levels increased in the entire study group (ANOVA F = 5.700, P < 0.001). After stratification of the patients to two groups according to the HD schedule, a significant increase in mean fT3 serum levels was observed in both groups (ANOVA F = 3.315, P = 0.002 for LNHD; ANOVA F = 2.386, P = 0.016 for HD; Figure 6A). However, if evaluating the percentage of patients with fT3 serum levels above the cut-off value (3.05 pg/mL) among different dialysis schedules, while no differences were found at baseline (44.0% versus 40.0% for LNHD and HD, respectively, P = 0.725), at any time during the follow-up, the rate of patients with an fT3 serum levels >3.05 pg/mL was found significantly increased in LNHD patients as compared with HD patients (64.0% versus 41.3% at 1st year, P = 0.049; 79.2% versus 54.0% at 2nd year, P = 0.031; 83.3% versus 58.1% at 3rd year, P = 0.035; 83.3% versus 52.4% at 4th year, P = 0.024; 93.8% versus 54.5% at 5th year, P = 0.006; 92.9% versus 58.3% at 6th year, P = 0.024; 92.9% versus 61.1% at 7th year, P = 0.040; 94.4% versus 58.3% at 8th year, P = 0.015 for LNHD and HD, respectively; Figure 6B).
DISCUSSION
This study was specifically aimed at evaluating the role of (i) the baseline levels of circulating fT3 in determining overall mortality and the incidence of cardiovascular events in patients with ESRD receiving dialytic treatment throughout an 8-year follow-up period; (ii) the impact of different types of dialysis (HD versus LNHD) on patients’ outcomes as well as in terms of changes in the circulating fT3 levels.
Growing evidence has shown that thyroid dysfunction was highly prevalent in chronic kidney disease patients. Subclinical hypothyroidism and low triiodothyronine syndrome are common features in patients with chronic kidney disease [24, 25]. Patients treated by both HD and peritoneal dialysis, as well as renal transplantation recipients, often exhibit thyroid hormone alterations, which may affect patient survival [26–30]. Low serum fT3 concentrations have been considered as an independent predictor of all-cause mortality in dialysis and graft failure in kidney transplant recipients [31, 32].
In our study, stratification of patients according to a fT3 serum levels above or below the cut-off (3.05 pg/mL) of baseline fT3 showed that patients with higher fT3 concentrations had a significantly higher long survival (89.0% versus 36.6%) and reduced rate of cardiovascular events (18.9% versus 34.4%) as compared with patients with lower fT3 levels.
Of note, low fT3 was an independent risk factor for higher mortality in our patients’ population even if entered as covariate in a multiple regression model including other major variables associated with malnutrition–inflammation syndrome (for instance, albumin, hsCRP, haemoglobin) previously proposed as possible predictors of death in haemodialysed patients [33].
Previous studies have widely investigated the prognostic value of the presence of non-thyroidal illness for long-term mortality in chronic HD patients and its potential association with low-grade inflammation, a common feature of uraemia [34]. In haemodialysed patients, serum T3 and fT3 levels have been inversely correlated with malnutrition and inflammation parameters (hsCRP, interleukin-6), because malnutrition–inflammation complex syndrome can affect serum concentrations of thyroid hormones [9, 10, 31, 35]. On the contrary, previous studies reported an association between a state of malnutrition–inflammation–atherosclerosis syndrome and poor dialysis outcome in ESRD patients [9, 36].
The possible link might rely on the pathogenic role of several pro-inflammatory cytokines, released in haemodialysed patients affected by malnutrition–inflammation syndrome, in the onset of endothelial activation and dysfunction, chronic microvascular inflammation and neuroendocrine derangement, which lead to development of non-thyroidal illness [37]. This is consistent with the view that non-thyroidal illness is an acute-phase response generated by activation of the cytokine network [38]. In line with these findings, in our cohort study, fT3 levels negatively correlated with hsCRP and positively correlated with haemoglobin and albumin, strengthening the pathogenic relation between low fT3 serum levels and the presence of a sub-clinical grade of inflammation and malnutrition in our patient population.
Moreover, stratification of patients according to whether they were above or below a cut-off level (3.05 pg/mL) of fT3 at baseline showed that, besides a higher prevalence of female gender, which is in agreement with what reported by Fragidis et al. [31], the prevalence of concurrent Type 2 diabetes was higher in patients with lower fT3 at baseline.
During the 8-year follow-up period, 33.2% of patients died, mostly from cardiovascular causes. Moreover, although de novo major cardiovascular events displayed a 25.5% overall cumulative incidence in the entire cohort, ESRD patients with lower serum fT3 showed significantly higher number of events compared with those with normal fT3 (34.4% versus 18.9%). Interestingly, in our study population, the effect of fT3 serum levels on both overall mortality and cardiovascular morbidity was independent from hsCRP levels, suggesting that although fT3 serum concentration is deeply influenced by the inflammatory milieu, a feature in HD patients, the effects on mortality and morbidity of low fT3 status and inflammation follow two distinct pathogenic pathways.
The major strength of this study is the comparative analysis between different dialysis schedules on patient survival and thyroid function. The presence of an LNHD programme (since 2006) at our centre allowed us to assign all the incident ESRD patients entered in the study into two groups according to the different dialysis regimens. After propensity score matching in two groups according to the HD schedule, the results indicate that patients treated with LNHD showed significantly higher survival rates (88.0% versus 61.3%) and lower incidence of cardiovascular events (8.0% versus 40.0%) as compared with those treated with diurnal HD. These findings are consistent with previous studies that report significantly improved patient outcomes in LNHD schedules. Beyond the significant improvement of several clinical and laboratory parameters (blood pressure, left ventricular hypertrophy, phosphataemia and anaemia management), 3-times weekly nocturnal HD gained a significant reduction both in cardiovascular events frequency and all-cause mortality as compared with conventional HD [39–47].
Despite the two groups displaying similar thyroid parameters at baseline, significant differences were recorded throughout the follow-up. Indeed, despite an increase in serum fT3 levels being observed throughout the follow-up in both groups, patients receiving LNHD showed a consistently higher rate of fT3 serum levels above the 3.05 pg/mL cut-off as compared with HD patients. It would seem reasonable to propose that this result stems from the beneficial effects of longer time of dialysis, which warrants better control of volume overload, improves the uraemic milieu and reduces inflammatory markers, thereby contributing to a reduced risk of CVDs [40, 47–50].
Taken together, these results underline the strong benefits of nocturnal HD on patient survival, probably due to lower micro-inflammation and cardiovascular risk, which deserve further investigation.
To this aim, serum fT3 level, assessed at baseline in incident ESRD patients, seems to act as predictor of cardiovascular events and mortality in this population. Moreover, the use of more biocompatible dialytic regime allows significant reduction of the prevalence of low fT3 status and reduction of the cardiovascular risk, and improvement in the overall survival in ESRD patients, although some organizational and reimbursement issues are posed by long or frequent dialysis schedules. Moreover, the retrospective design together with the rather limited sample size clearly means that further prospective multicentre studies will be required to confirm our observations.
In conclusion, this study underlines the potential role of low fT3 as significant predictor of mortality and cardiovascular risk in ESRD patients. The results reported here represent the first demonstration that LNHD is more effective in reducing the prevalence of low fT3 serum levels as compared with HD, thus likely improving patient outcomes.
ACKNOWLEDGEMENTS
The authors thank Mr Luigi Consagro, the nurse coordinator at the Dialysis Unit of the University Hospital “Ospedali Riuniti”, Foggia (Italy), for his invaluable collaboration.
AUTHORS’ CONTRIBUTIONS
G.S.N. conceived and designed the study, analysed the data and drafted the manuscript; A.D.L., D.P., A.T., M.S., F.S., B.I. and R.Perulli collected the clinical data and helped to interpret results of analysis; M.R., L.C., G.I. and F.F. analysed the data, interpreted results and prepared the figures; R.Prato, G.C. and L.G. helped to draft the manuscript; F.C., M.T.R., G.S., E.R. and G.G. edited and revised manuscript and approved the final version of manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Collins AJ, Foley RN, Chavers B. et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 2014; 63: A7. [DOI] [PubMed] [Google Scholar]
- 2. Stenvinkel P, Carrero JJ, Axelsson J. et al. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 2008; 3: 505–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Honda H, Qureshi AR, Heimbürger O. et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 2006; 47: 139–148 [DOI] [PubMed] [Google Scholar]
- 4. Carrero JJ, Stenvinkel P.. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol 2009; 4 (Suppl 1): S49–S55 [DOI] [PubMed] [Google Scholar]
- 5. Ortiz A, Massy ZA, Fliser D. et al. Clinical usefulness of novel prognostic biomarkers in patients on hemodialysis. Nat Rev Nephrol 2012; 8: 141–150 [DOI] [PubMed] [Google Scholar]
- 6. Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis 2001; 38 (4 Suppl 1): S80–S84 [DOI] [PubMed] [Google Scholar]
- 7. Abdel-Rahman EM, Mansour W, Holley JL. et al. Thyroid hormone abnormalities and frailty in elderly patients with chronic kidney disease: a hypothesis. Semin Dial 2010; 23: 317–323 [DOI] [PubMed] [Google Scholar]
- 8. Lo JC, Chertow GM, Go AS. et al. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 2005; 67: 1047–1052 [DOI] [PubMed] [Google Scholar]
- 9. Zoccali C, Tripepi G, Cutrupi S. et al. Low triiodothyronine: a new facet of inflammation in end-stage renal disease. J Am Soc Nephrol 2005; 16: 2789–2795 [DOI] [PubMed] [Google Scholar]
- 10. Meuwese CL, Dekker FW, Lindholm B. et al. Baseline levels and trimestral variation of triiodothyronine and thyroxine and their association with mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol 2012; 7: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zoccali C, Mallamaci F, Tripepi G. et al. Low triiodothyronine and survival in end-stage renal disease. Kidney Int 2006; 70: 523–528 [DOI] [PubMed] [Google Scholar]
- 12. St Germain DL, Galton VA, Hernandez AM.. Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology 2009; 150: 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tartaglia L, Infante B, Stallone G. et al. Nocturnal hemodialysis: an alternative treatment for a better quality of life. G Ital Nefrol 2008; 25: 702–707 [PubMed] [Google Scholar]
- 14. Wong B, Collister D, Muneer M. et al. In-center nocturnal hemodialysis versus conventional hemodialysis: a systematic review of the evidence. Am J Kidney Dis 2017; 70: 218–234 [DOI] [PubMed] [Google Scholar]
- 15. Trainer TD, Howard PL, Rock RC.. Thyroid function tests in thyroid and non thyroid disease. Crit Rev Clin Lab Sci 1983; 19: 135–171 [DOI] [PubMed] [Google Scholar]
- 16.Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 2006; 48 (Suppl 1): S2–S90 [DOI] [PubMed] [Google Scholar]
- 17. Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol 1993; 4: 1205–1213 [DOI] [PubMed] [Google Scholar]
- 18. Rotondi M, Netti GS, Lazzeri E. et al. High pretransplant serum levels of CXCL9 are associated with increased risk of acute rejection and graft failure in kidney graft recipients. Transpl Int 2010; 23: 465–475 [DOI] [PubMed] [Google Scholar]
- 19. Gigante M, Lucarelli G, Divella C. et al. Soluble serum αklotho is a potential predictive marker of disease progression in clear cell renal cell carcinoma. Medicine (Baltimore) 2015; 94: e1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Netti GS, Prattichizzo C, Montemurno E. et al. Exposure to low- vs iso-osmolar contrast agents reduces NADPH-dependent reactive oxygen species generation in a cellular model of renal injury. Free Radic Biol Med 2014; 68: 35–42 [DOI] [PubMed] [Google Scholar]
- 21. Santangelo L, Gigante M, Netti GS. et al. A novel SMARCAL1 mutation associated with a mild phenotype of Schimke immuno-osseous dysplasia (SIOD). BMC Nephrol 2014; 15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Martino M, Gigante M, Cormio L. et al. JAK3 in clear cell renal cell carcinoma: mutational screening and clinical implications. Urol Oncol 2013; 31: 930–937 [DOI] [PubMed] [Google Scholar]
- 23. Gigante M, d’Altilia M, Montemurno E. et al. Branchio-Oto-Renal Syndrome (BOR) associated with focal glomerulosclerosis in a patient with a novel EYA1 splice site mutation. BMC Nephrol 2013; 14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iglesias P, Bajo MA, Selgas R. et al. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord 2017; 18: 131–144 [DOI] [PubMed] [Google Scholar]
- 25. Song SH, Kwak IS, Lee DW. et al. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant 2009; 24: 1534–1538 [DOI] [PubMed] [Google Scholar]
- 26. Rhee CM, You AS, Nguyen DV. et al. Thyroid status and mortality in a prospective hemodialysis cohort. J Clin Endocrinol Metab 2017; 102: 1568–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Enia G, Panuccio V, Cutrupi S. et al. Subclinical hypothyroidism is linked to micro-inflammation and predicts death in continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2006; 22: 538–544 [DOI] [PubMed] [Google Scholar]
- 28. Rhee CM, Ravel VA, Streja E. et al. Thyroid functional disease and mortality in a national peritoneal dialysis cohort. J Clin Endocrinol Metab 2016; 101: 4054–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang TI, Nam JY, Shin SK. et al. Low triiodothyronine syndrome and long-term cardiovascular outcome in incident peritoneal dialysis patients. Clin J Am Soc Nephrol 2015; 10: 975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rotondi M, Leporati P, Lodigiani S. et al. Pretransplant positivity for circulating thyroid antibodies and graft survival in patients undergoing kidney transplant. Horm Res 2009; 71: 324–330 [DOI] [PubMed] [Google Scholar]
- 31. Fragidis S, Sombolos K, Thodis E. et al. Low T3 syndrome and long-term mortality in chronic hemodialysis patients. World J Nephrol 2015; 4: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rotondi M, Netti GS, Rosati A. et al. Pretransplant serum FT3 levels in kidney graft recipients are useful for identifying patients with higher risk for graft failure. Clin Endocrinol (Oxf) 2008; 68: 220–225 [DOI] [PubMed] [Google Scholar]
- 33. Zyga S, Christopoulou G, Malliarou M.. Malnutrition-inflammation-atherosclerosis syndrome in patients with end-stage renal disease. J Ren Care 2011; 37: 12–15 [DOI] [PubMed] [Google Scholar]
- 34. Pertosa G, Simone S, Ciccone M. et al. Serum fetuin A in hemodialysis: a link between derangement of calcium-phosphorus homeostasis and progression of atherosclerosis? Am J Kidney Dis 2009; 53: 467–474 [DOI] [PubMed] [Google Scholar]
- 35. Chávez Valencia V, Mejía Rodríguez O, Viveros Sandoval ME. et al. Prevalence of malnutrition-inflammation complex syndrome and its correlation with thyroid hormones in chronic haemodialysis patients. Nefrologia 2018; 38: 57–63 [DOI] [PubMed] [Google Scholar]
- 36. Tripepi G, Mallamaci F, Zoccali C.. In ammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 2005; 16 (Suppl 1): S83–S88 [DOI] [PubMed] [Google Scholar]
- 37. DeGroot LJ. Dangerous dogmas in medicine: the non thyroidal illness syndrome. J Clin Endocrinol Metab 1999; 84: 151–164 [DOI] [PubMed] [Google Scholar]
- 38. Boelen A, Platvoet-ter Schiphorst MC, Wiersinga WM.. Soluble cytokine receptors and the low 3,5,3′-triiodothyronine syndrome in patients with non-thyroidal disease. J Clin Endocrinol Metab 1995; 80: 971–976 [DOI] [PubMed] [Google Scholar]
- 39. Rocco MV, Lockridge RS Jr, Beck GJ. et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Culleton BF, Walsh M, Klarenbach SW. et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007; 298: 1291–1299 [DOI] [PubMed] [Google Scholar]
- 41. Weinreich T, De los Rios T, Gauly A. et al. Effects of an increase in time vs. frequency on cardiovascular parameters in chronic hemodialysis patients. Clin Nephrol 2006; 66: 433–439 [DOI] [PubMed] [Google Scholar]
- 42. Mahadevan K, Pellicano R, Reid A. et al. Comparison of biochemical, haematological and volume parameters in two treatment schedules of nocturnal home haemodialysis. Nephrology (Carlton )2006; 11: 413–418 [DOI] [PubMed] [Google Scholar]
- 43. Van Eps CL, Jeffries JK, Anderson JA. et al. Mineral metabolism, bone histomorphometry and vascular calcification in alternate night nocturnal haemodialysis. Nephrology (Carlton )2007; 12: 224–233 [DOI] [PubMed] [Google Scholar]
- 44. Schwartz DI, Pierratos A, Richardson RM. et al. Impact of nocturnal home hemodialysis on anemia management in patients with end-stage renal disease. Clin Nephrol 2005; 63: 202–208 [DOI] [PubMed] [Google Scholar]
- 45. Ting GO, Kjellstrand C, Freitas T. et al. Long-term study of high-comorbidity ESRD patients converted from conventional to short daily hemodialysis. Am J Kidney Dis 2003; 42: 1020–1035 [DOI] [PubMed] [Google Scholar]
- 46. Lacson E Jr, Xu J, Suri RS. et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol 2012; 23: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Netti GS, Sangregorio F, Spadaccino F. et al. LPS removal reduces CD80-mediated albuminuria in critically ill patients with Gram-negative sepsis. Am J Physiol Renal Physiol 2019; 316: F723–F731 [DOI] [PubMed] [Google Scholar]
- 48. Netti GS, Infante B, Spadaccino F. et al. Serum levels of BAFF and APRIL predict clinical response in anti-PLA2R-positive primary membranous nephropathy. J Immunol Res 2019; 2019: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. FHN Trial Group, Chertow GM, Levin NW. et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Demirci MS, Celik G, Ozkahya M. et al. Effects of thrice weekly nocturnal hemodialysis on arterial stiffness. Atherosclerosis 2012; 220: 477–485 [DOI] [PubMed] [Google Scholar]