Abstract
Osteoporosis characterized by low bone mineral density (BMD) as assessed by dual-energy X-ray absorptiometry (DXA) is common among end-stage renal disease (ESRD) patients and associates with high fracture incidence and high all-cause mortality. This is because chronic kidney disease-mineral bone disorders (CKD-MBDs) promote not only bone disease (osteoporosis and renal dystrophy) but also vascular calcification and cardiovascular disease. The disturbed bone metabolism in ESRD leads to ‘loss of cortical bone’ with increased cortical porosity and thinning of cortical bone rather than to loss of trabecular bone. Low BMD, especially at cortical-rich bone sites, is closely linked to CKD-MBD, vascular calcification and poor cardiovascular outcomes. These effects appear to be largely mediated by shared mechanistic pathways via the ‘bone–vascular axis’ through which impaired bone status associates with changes in the vascular wall. Thus, bone is more than just the scaffolding that holds the body together and protects organs from external forces but is—in addition to its physical supportive function—also an active endocrine organ that interacts with the vasculature by paracrine and endocrine factors through pathways including Wnt signalling, osteoprotegerin (OPG)/receptor activator of nuclear factor-κB (RANK)/RANK ligand system and the Galectin-3/receptor of advanced glycation end products axis. The insight that osteogenesis and vascular calcification share many similarities—and the knowledge that vascular calcification is a cell-mediated active rather than a passive mineralization process—suggest that low BMD and vascular calcification (‘vascular ossification’) to a large extent represent two sides of the same coin. Here, we briefly review changes of BMD in ESRD as observed using different DXA methods (central and whole-body DXA) at different bone sites for BMD measurements, and summarize recent knowledge regarding the relationships between ‘low BMD’ and ‘fracture incidence, vascular calcification and increased mortality’ in ESRD patients, as well as potential ‘molecular mechanisms’ underlying these associations.
Keywords: bone mineral density, bone–vascular axis, end-stage renal disease, mortality, osteoporosis, vascular calcification
This Review has been written in collaboration with NDT Educational.

INTRODUCTION
The kidneys play an important role in the systemic regulation of mineral metabolism. The decline in renal function in patients with chronic kidney disease (CKD) leads to the systemic syndrome of CKD-mineral bone disorders (CKD-MBDs) that feature, on one hand, impaired bone health caused by renal osteodystrophy and osteoporosis, and, on the other hand, cardiovascular disease (CVD) with arteriosclerosis and generalized vascular calcification including coronary artery calcification (CAC). These common interlinked features of CKD-MBD contribute to premature ageing [1] with severe and seldom fatal complications leading to markedly increased morbidity and high mortality, especially in patients with end-stage renal disease (ESRD).
In addition to ‘age-related osteoporosis’, bone status in CKD patients is affected by ‘renal osteodystrophy’, a collective term for a heterogeneous group of metabolic bone diseases associated with CKD-MBDs that are characterized by alterations of bone morphology due to abnormal bone turnover rate (high and low bone turnover diseases), defective mineralization and volume [2]. Bone disease in ESRD is a mixture of reduced bone density and impaired bone quality due to microdamage and disorders of microarchitecture and collagen. It associates not only with increased risk of fractures but also with poor nutritional status with reduced muscle strength and low lean body mass, and increased vascular calcification involving both intimal calcification linked to atherosclerotic plaque formation and medial calcification linked to arteriosclerosis, vascular stiffening and vascular senescence [3]. Altogether these alterations increase the risk for CVD events and mortality [4–10].
In the general population, according to meta-analysis of prospective cohort studies, low bone mineral density (BMD) levels at ‘all investigated sites’ are associated with increased CVD-related and all-cause mortality [11]. In patients with ESRD, low BMD is even more strongly associated with poor outcomes due to alterations in the bone–vascular axis and metabolic and hormonal abnormalities linked to CKD-MBD such as disturbances in mineral metabolism, vitamin D deficiency, secondary hyperparathyroidism, and excess or deficiencies of molecules influencing bone formation [12–16]. Bone status in ESRD is therefore more closely linked to accelerated vascular calcification and premature cardiovascular events than in the general population. Accordingly, in ESRD patients, low BMD determined by dual-energy X-ray absorptiometry (DXA) associates with markedly increased CVD-related and all-cause mortality [17–20].
There are still many unexplored research territories in CKD-MBD including factors that may explain the links between low BMD and mortality in ESRD patients. For instance, few scientific reports have so far explored whether the association between low BMD and mortality depends on the sites of BMD measurement. The molecular mechanism of bone–vascular axis is another area that remains to be explored. In this review, we (i) present available data on associations of BMD measured by DXA at various bone sites with vascular calcification and mortality in ESRD and (ii) discuss possible mechanisms behind bone–vascular axis alterations that may explain these associations.
Bone physiology and pathophysiology
The association of impaired bone status with poor clinical outcomes may reflect one or more of the many functions of bone:
(i) physical: body support, facilitation of movement and protection of internal organs against external forces;
(ii) haematopoiesis: harbours bone marrow, producing blood cells;
(iii) nutritional: storage of minerals and fat, and indirectly of muscle protein through harbouring skeletal muscles;
(iv) metabolic: mineral metabolism and acid–base balance; and
(v) endocrine and paracrine: bone–vascular axis pathways including Wnt signalling, the OPG/receptor activator of nuclear factor-κB (RANK)/RANK ligand (RANKL) system and the Galectin-3/receptor of advanced glycation end products (RAGE) axis.
In humans, 80% of the skeleton is composed of cortical bone and 20% of trabecular bone [21]. Cortical bone is much harder and denser than trabecular bone to protect soft tissues, while trabecular bone is a honeycomb-like network consisting of myriads of highly interconnected bony trabeculae [21]. The proportions of cortical to trabecular bone vary between different skeletal sites: vertebrae are trabecular rich sites, and contain >40% of trabecular tissue [22, 23]. In long bones, the diaphysis consists of mainly cortical bone, while the metaphysis and epiphysis are a mixture of cortical bone and trabecular bone, and the proximal femur and mid-radius are cortical rich sites [22]. Due to the differences in the ratio of cortical to trabecular content, the bone turnover rate differs between different bones and between different parts of the long bones, and the proportion of cortical to trabecular bone and bone turnover, are largely affected by age, sex, metabolic diseases and drugs. For example, bone turnover rate is generally lower in the peripheral skeleton than in the central skeleton, and the mean iliac trabecular bone turnover in post-menopausal women is twice that of cortical bone (18% versus 8%/year) [24]. In post-menopausal as compared with pre-menopausal women, bone remodelling rates assessed by iliac biopsy are 2-fold increased [25], the cortical bone becomes thin and porous and there is loss of trabecular bone and trabecular connectivity [26]. These changes in bone microarchitecture, which lead to fragile bones and increased risk for fractures following external forces such as falls, are magnified by hyperparathyroidism, which increases bone turnover rate [27–29].
Key points:
Cortical and trabecular bone differ as regards their functions, metabolic activity and implications for clinical outcomes.
The location of cortical and trabecular bone should be considered in bone status assessments.
Changes of cortical and trabecular bone in CKD
While this review focuses on BMD measures by DXA, there are several other options to assess changes in bone status in CKD, such as bone biopsy, quantitative ultrasound (QUS), digital X-ray radiogrammetry and peripheral quantitative computed tomography (pQCT) including high-resolution pQCT (HRpQCT). However, these techniques have drawbacks, such as invasiveness (biopsy), lower reproducibility (QUS and CT) and less documentation (all), and they are still not generally used for diagnosis and for monitoring effects of treatment.
Bone biopsy is considered as a gold standard tool for the classification of turnover, mineralization and volume defects in CKD patients; however, findings may vary due to differences in age, race, ethnicity, cause of ESRD, dialysis vintage and sample size between studies [30–33]. Nevertheless, histomorphometric measurements show that bone morphology might improve following therapeutic interventions. For example, after 12 months of treatment with cinacalcet, a calcimimetic agent, trans-iliac bone biopsies showed normal bone histology in 20 out of 77 haemodialysis (HD) patients, in parallel with normalizing serum parathyroid hormone (PTH) levels [29].
Changes of trabecular and cortical bone appear to be different in CKD as compared with the general population. Trabecular bone, which makes up two-thirds of total bone surfaces, shows greater metabolic activity than cortical bone [24]. Trabecular bone receives blood supply through the surrounding bone marrow and the metabolic activity is regulated by hormones and growth factors through this blood supply [34]. Trabecular bone is more rapidly lost than cortical bone in postmenopausal women in early phase [35, 36], and, based on results by QCT in a community-based study, this may explain, in large part, why fragility fractures are more common in elderly women than in elderly men [37]. However, in contrast, while CKD patients had rapid cortical bone loss during 1.5 years of follow-up, there was no trabecular bone loss as assessed by HRpQCT; there was even an increase of trabecular area [38]. This was supported by another study in CKD patients showing better preservation of trabecular bone than cortical bone as assessed by bone biopsy [28]. A possible explanation for this difference in bone loss pattern between the general population and CKD patients could be that trabecular bone loss is attenuated in CKD patients because of increased levels of PTH, a hormone that has anabolic effects on trabecular bone and catabolic effects on cortical bone [39]. In CKD patients, cortical bone loss associates with increased cortical porosity and decreased cortical thickness [38]. These changes, which may be partially explained by secondary hyperparathyroidism, are in general more severe in patients undergoing dialysis compared with patients without dialysis therapy [38, 40].
Key points:
In contrast to the general population, loss of cortical bone is more severe than loss of trabecular bone in patients with CKD.
DXA
Bone densitometry by DXA was developed and eventually approved by US Food and Drug Administration in 1980s, and DXA of the hip is the dominating technique to diagnose osteoporosis. DXA is a machine that generates two X-ray beams with different levels of energy, 45 and 100 keV, or ∼39 and 71 keV depending on the type of equipment. Because the tissues absorb (attenuate) the X-rays differently depending on their energy and the electron density of the tissue, the software of the DXA machine can calculate the tissue composition and the bone density. Though DXA is an X-ray-based imaging method, the radiation dose is very low and the risk from the exposure can be considered almost negligible. The effective radiation dose from a single whole-body DXA (<10 μSv) [41] is equivalent to <1 week of background radiation.
When BMD is assessed by ‘central DXA’, the DXA machine quantifies BMD of the central skeleton including lumbar spine and hip. According to the International Society for Clinical Densitometry, central DXA, and not whole-body DXA (see below), is recommended for diagnosis of osteoporosis in clinical practice [42], but for historical reasons DXA of the forearm is also accepted for making the diagnosis. Among central skeleton sites, BMD of the hip has the best prediction of fracture risk, but when monitoring response to treatment BMD of the spine is preferred [43, 44].
Another application of DXA, ‘whole-body DXA’, is increasingly used to assess skeletal mineral status of the whole body as well as for evaluating body composition (i.e. muscle and fat content). These applications have further promoted the use of whole-body DXA, not only in clinical practice, but also in clinical research. While the lower resolution of whole-body measurements makes the diagnostic performance not equivalent to that obtained by central DXA, BMD from central DXA of the hip, spine and distal forearm correlate closely with BMD at sub-regions obtained from whole-body DXA, especially in spine [45–47]. The validity of BMD using whole-body DXA was also confirmed by another study using pQCT [2]. However, as regards diagnosis of osteoporosis, prevalence estimations by sub-regions analysis of whole-body DXA were generally lower (i.e. underestimated) than corresponding central site-specific measurements of BMD [46].
Thus, as whole-body DXA and central DXA are not equivalent, the interpretation of BMD values yielded by these two methods may vary. The diagnostic criteria for osteoporosis by World Health Organization are based on femoral neck BMD by central DXA [44], and not by whole-body DXA, using data from the National Health and Nutrition Examination Survey III database [42]. Nevertheless, abundant data and clinical evidence support the usefulness of whole-body DXA in the day-to-day clinical practice in various disciplines of medical science.
Key points:
BMD data by central DXA and whole-body DXA differ.
Central DXA is the recommended method for diagnosis of osteoporosis.
Diagnostic methods in CKD-MBD
The pathogenesis and characteristics of bone disease linked to CKD-MBD in ESRD differ from primary osteoporosis. Bone biopsy has been recommended as the gold standard for the diagnosis and classification of renal osteodystrophy by Kidney Disease: Improving Global Outcomes [48] and ERA-EDTA [49]. Histological findings by bone biopsy are often different from estimations based on serum biomarkers, such as high levels of serum intact PTH (iPTH). However, in clinical practice, bone biopsy is rarely performed due to its invasive nature. In addition, it is expensive and time consuming, often requiring weeks to get a final histopathological report. Recently, Carvalho et al. [40] reported a significant association between BMD by DXA and bone histomorphometry data by bone biopsy, supporting the usefulness of DXA in CKD patients.
While DXA cannot provide details regarding the relative proportions of the cortical and trabecular bone or distinguish between different types of renal osteodystrophy, the potential of DXA to predict fracture risk not only in the general population but also in specific subgroups such as in CKD has gradually become clearer. Iimori et al. [50] reported that BMD in hip and femoral neck, but not in spine, was associated with any type of fracture in a prospective study of 485 dialysis patients. Similar results were replicated in another study of 384 incident fractures in 2754 individuals including 587 (21%) CKD patients [51].
Key points:
Bone biopsy is the gold standard but is not performed routinely in clinical practice.
DXA is increasingly used to analyse fracture risk in CKD patients.
Associations of low BMD with vascular calcification
As discussed below, mechanisms of vascular calcification and bone mineralization share several common pathways, which may explain why low BMD is strongly related to vascular calcification in the general population [52, 53] and in patients with ESRD [9, 54–58]. Not only low bone mass but also bone loss is an independent predictor for progression of vascular calcification [59, 60]. In a population-based cohort study with 25 years follow-up of older men and women, Kiel et al. [60] demonstrated that bone loss at the second metacarpal of the hand as assessed by simple plain radiographs is associated with increased aortic calcification index in women, but not in men. This suggests that endocrine factors such as oestrogen might play a role in the interplay between bone and vasculature. Low trabecular bone volume, as assessed by iliac bone biopsy, has been shown to correlate with the progression of calcification as assessed by CAC, and lower bone turnover (i.e. decreased bone resorption) was associated with less rapid CAC progression in HD patients [58]. These observations may imply that normalizing bone metabolism could represent an effective option to prevent vascular calcification; however, so far there is no strong clinical evidence confirming that such interventions will reduce established vascular calcification. Also, the crucial mechanism(s) of the bone–vascular axis are not fully understood.
Key points:
Low bone mass and bone loss are linked with vascular calcification.
Association of BMD with vascular calcification depends on bone site for BMD measurement
There is no consensus on which specific bone location should be used for measurement of BMD when studying relations between BMD and signs of vascular calcification. There are discrepancies between studies regarding the relation of vascular calcification, including CAC, with BMD measured at different anatomical bone sites. It is possible that such variations might be explained by the heterogeneity of study populations and the bone sites selected for BMD measurements. In the general population, an age-stratified random sample of 200 women showed that higher BMD of the spine but not of the hip was independently associated with less aortic calcification [53]. In postmenopausal women, carotid plaque score showed an inverse association with total body BMD, but not with lumbar spine BMD [61]. In another study in postmenopausal women, reduced BMD at the proximal femur is associated with aortic calcification [62]. In ESRD, the site of BMD measurements also appears to play a role. Thus, in HD patients, CAC score was negatively associated with BMD at hip site but not with lumbar spine BMD [20], while in another study greater bone loss at the ultra-distal (UD) radius is associated with significantly higher all-cause mortality [63].
The degree of atherosclerosis and hence the degree of intimal deposition of calcium, which may influence BMD measurements of the spine, varied between these studies. We have reported that low BMD of arms and legs—rather than BMD at other and more central locations—was associated with high CAC score in ESRD patients [64] and that BMD correlated inversely with CAC and histologically biopsy-verified epigastric arterial vascular calcification in extremities (arms and legs) but not in the pelvis, spine and femoral bone sites in ESRD patients undergoing living-donor kidney transplantation [65]. It is possible that these differences between different sites may be partially explained by absence of extensive arterial calcifications in the extremities, whereas BMD in spine could be overestimated due to aortic calcifications. Compared with central DXA, BMD measured at peripheral sites could represent a more appropriate method for analyses of relations between BMD and vascular calcification in individuals with substantial aortic calcification, such as ESRD patients.
Key points:
Spine BMD by DXA does not necessarily associate with vascular calcification due to interference by aortic calcifications.
Low BMD predicts increased all-cause and CVD mortality
Table 1 summarizes reported associations between BMD and all-cause and CVD mortality in ESRD. Compared with the general population where there is an abundance of data, the relationship between BMD and CVD mortality in ESRD is less clear due to the relatively small number of patients and publications in this field. Most previous reports regarding ESRD patients analysed the association between BMD and all-cause mortality, while only a few studies investigated the relationship between BMD and CVD mortality in ESRD patients [6, 7, 17–20, 66, 67] (Table 1).
Table 1.
Previous reports on association between BMD and mortality in ESRD patients
| References | Location | No. of patients | Sex | Age (years) (mean/ median) | Dialysis modality | Design | Follow-up (years) | BMD sites | Measurement method | Outcomes | Factors controlled for in multivariate analysis | Outcomes associated with |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taal et al. [18] | UK | 88 | F/M | 51–70 | HD | Prospective observational | 3.5 | Spine, hip | Central-DXA | All-cause mortality | Age, Ca × P and previous allograft | Hip |
| Matsubara et al. [7] | Sweden | 277 | F/M | 55 | HD/PD | Prospective observational | 5 | Total | Whole-DXA | All-cause and CVD mortality | Age, sex, DM, CVD, hsCRP, phosphate, Ca × P and SGA | Total |
| Park et al. [6] | Sweden | 361 | F/M | 53 | HD/PD | Prospective observational | 5 | Total | Whole-DXA | All-cause mortality | Age, fat body mass and SGA | Total |
| Disthabanchong et al. [20] | Thailand | 83 | F/M | 57 | HD | Prospective observational | 5 | Spine, hip | Central-DXA | All-cause mortality | Age, Davies score, dyslipidaemia, albumin and CAC score | Hip |
| Chen et al. [66] | Sweden | 231 | F/M | 56 | HD/PD | Prospective observational | 5 | Spine | Whole-DXA/CT | All-cause mortality | Age, sex, diabetes, CVD, hsCRP and SGA | Spine (VBD by CT) |
| Yap et al. [67] | Australia | 58 | F/M | 58–65 | HD/PD | Prospective observational | 6 | Spine/FN | Central-DXA/pQCT | All-cause mortality | Age, sex, transplant status, albumin, PTH, phosphate, dialysis duration, diabetes and smoking | FN |
| Orlic et al. [19] | Croatia | 134 | F/M | 59 | HD | Prospective observational | 4 | Spine, hip, forearm | Central-DXA | All-cause mortality | Age and sexa (used as a Z-score) | Forearm |
| Iseri et al. [17] | Sweden | 426 | F/M | 56 | HD/PD | Prospective observational | 5 | Total, head, arms, legs, trunk, hip, pelvis, spine | Whole-DXA | All-cause and CVD mortality | Framingham CVD risk score, SGA, %HGS, albumin, hsCRP, LBMI, recruitment year | Total, head, pelvis (and leg) |
DM, diabetes mellitus; FN, femoral neck; %HGS, percent handgrip strength; hsCRP, high-sensitivity C-reactive protein; LBMI, lean body mass index; SGA, subjective global assessment. aOutcomes significantly associated with.
In the study by Taal et al. [18], reduced total hip BMD measured by central DXA was significantly associated with poor outcomes after adjusting for age, calcium × phosphorus (Ca × P) product and previous allograft, whereas there was no significant association between reduced spine BMD and clinical outcome. In line with this, another study reported similar results after adjusting for age, Davies score, dyslipidaemia, albumin and CAC score [20]. Furthermore, another study reported that forearm sites in addition to femoral neck were associated with high all-cause mortality in univariate analysis [19]. Yap et al. [67] used pQCT in addition to DXA in a prospective study of 58 dialysis patients with 6 years follow-up and found that tibia cortical density by pQCT, in addition to BMD at femoral neck measured by DXA, was significantly associated with increased all-cause mortality.
Furthermore, also changes in bone mass were associated with increased mortality [63]. In a study of 269 men on chronic HD, 1-year bone reduction at UD radius was an independent predictor of all-cause mortality [63]. The authors speculated that high iPTH contributed to bone loss as well as to increased mortality.
While most studies in ESRD patients included HD patients, we investigated both peritoneal dialysis (PD) and HD patients and found that low total BMD measured by whole-body DXA was associated with increased all-cause mortality as well as increased CVD mortality [6, 7, 17]. As regards the impact of dialysis therapy on fracture events, some studies showed that compared with HD, PD was associated with lower risk of fracture events [68, 69], which in turn potentially could lead to lower subsequent mortality risk. A report from Taiwan National Database comprising 51 473 dialysis patients showed that HD patients had a 31% higher incidence of hip fractures than PD patients [Hazard ratio (HR) = 1.31, 95% CI 1.01–1.70] [68]. Similar results were reported in patients from the US Renal Data System, where HD patients had 1.6 times greater odds ratio of hip fracture risk than PD patients [69]. While the mechanism(s) for a potential beneficial effect of PD versus HD on fractures is not fully understood, better preservation of residual renal function in PD as compared with HD [69] may conceivably contribute to reduced fracture risk.
Key points:
Bone mass in ESRD patients is associated with all-cause mortality and CVD mortality.
The strength of this association may depend on treatment modality such as HD versus PD.
Association of BMD with mortality depends on bone site for BMD measurement and risk of fractures
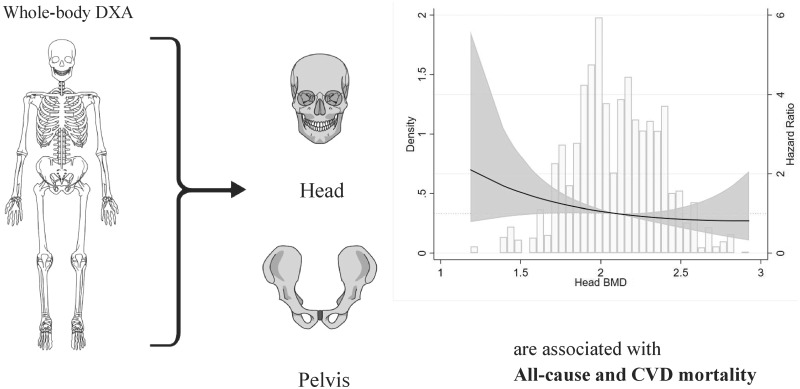
Even though methods were somewhat different between the reports discussed above, they all show that low BMD is related to poor survival in CKD. However, it is still unclear which BMD sites are more appropriate and suitable for predicting clinical outcomes. As compared with the general population, vascular calcification may be more important as a factor masking the ‘true’ value of BMD in ESRD. As mentioned above, BMD at spine was often similar, whereas BMD at hip and radius sites were generally lower in ESRD patients than in the general population. This suggests that existence of aortic vascular calcification could influence BMD measurements in ESRD [48]. To address this issue, we assessed the association between mortality and regional BMD, measured by whole-body DXA (head, arms, legs, trunk, hip, pelvis and spine), in a prospective study of 426 incident dialysis patients. Using multivariate competing risk analysis with renal transplantation as a competing risk, and adjusting for several confounders (including Framingham CVD risk score, nutritional status and inflammation), lower values of total BMD, head BMD and pelvis BMD were associated with increased all-cause and CVD mortality [17] (Figure 1). Leg BMD was associated with CVD mortality, while BMD at other sites (arms, trunk, hip and spine) was associated with mortality. To the best of our knowledge, our study [17] is the first to show the relationship between BMD at specific bone sites measured by whole-body DXA and clinical outcomes in ESRD patients.
FIGURE 1.

Low BMD as assessed by whole-body DXA at cortical-rich sites such as head and pelvis associates with increased mortality. The figure shows distribution of head BMD and restricted cubic spline curve with multivariable-adjusted sub-distribution hazard ratio (sHR) (95% CI) for all-cause mortality across values of head BMD (as continuous variable) in ESRD patients at initiation of dialysis therapy. Median value of head BMD was taken as reference. The results are adapted from a previous study [17] which concluded that cortical BMD appeared to have stronger association to survival in ESRD than trabecular BMD.
Vascular calcification including abdominal aortic calcification is highly prevalent in CKD [70, 71] and may affect measurements of the ‘true’ BMD, especially of spine BMD, as artefacts and local structural changes influence DXA measurements [72]. Besides, extensive osteophyte formation and degenerative changes are often seen in elderly patients. This might explain why several studies did not observe the association between BMD at spine by DXA and all-cause mortality [17–20, 65].
In a prospective study of 231 ESRD patients comprising incident dialysis patients (mostly PD patients and recipients of living-donor kidney transplant), we measured vertebral bone density (VBD) by cardiac computed tomography (CT) (64-channel detector scanner), which is not influenced by the presence of aortic calcification, and analysed the association between VBD and all-cause mortality [66]. Low VBD [receiver operating characteristics curve (ROC) = 0.72] was more strongly associated with increased all-cause mortality than total BMD by whole-body DXA (ROC = 0.56). VBD (ROC = 0.75) was more strongly associated with higher CAC score (CAC >100 AUs) compared with total BMD by whole-body DXA (ROC = 0.57). This study suggested that bone condition was strongly linked to cardiac calcification as assessed by CAC score in ESRD, a finding supported by the inverse relationship between CAC and BMD by DXA in dialysis patients [8]. Considering the significant association between increased CAC and increased CVD-related and all-cause mortality in CKD and dialysis patients [71, 73, 74], we assume that low BMD by DXA is linked to increased cardiac calcification, which in turn is associated with increased mortality. Although spine BMD by DXA may not reflect the ‘true’ BMD (as measured by CT), it appears to be a valid predictor of the mortality risk. However, our study based on VBD [66] suggests that one may measure the ‘true’ BMD value at the spine site—avoiding the influence of calcification, osteosclerosis and bone spur areas.
One possible mechanism for the link between BMD by DXA and mortality is that reduced BMD may increase incidence of fractures and thereby lead to fatal outcomes associated with fractures. Fractures are common, and increased mortality following fractures were reported in the general population [75, 76], CKD patients [77] and ESRD patients on HD [78]. Specifically, reduced cortical thickness and thin cortical envelope—which is overrepresented in CKD patients—had great impact on the risk of fracture incidence [79, 80]. In non-dialysed CKD patients, the risk of fracture was increased especially in patients with estimated glomerular filtration rate <30 mL/min [81]. Among fracture types, there was a larger effect of GFR decline on hip fracture risk than for other types of fractures, and the incidence of fractures was significantly associated with mortality and major adverse cardiac events. Tentori et al. [78] found higher fracture incidence in HD patients compared with general population, with hip fracture (44%) being an especially common fracture location in data from the Dialysis Outcomes and Practice Patterns Study. Substantial increase of subsequent morbidity and mortality following hip fracture was reported in the elderly [82], as well as in HD patients [66]. Long hospitalization and excessive mortality rate following fracture events in HD patients, especially in the first month following the fracture incident, may be related to the high frequency of a frail status in dialysis patients [78].
Another possible explanation, and most likely a much more important factor for the association between low BMD and increased mortality, involves the bone–vascular axis, whereby an impaired bone status may reflect progression of vascular calcification. This will be discussed below, first in relation to different bone sites and then as regards possible molecular mechanisms for the altered bone–vascular axis in ESRD.
Key points:
Associations of low BMD with mortality depend on bone site for BMD measurement and risk of fractures.
Importance of cortical bone in ESRD patients
Among several sites measured by whole-body DXA, the head site stands out due to its different characteristics as compared with other bone sites. In a recent study of 704 amateur sportsmen, physical activity was associated with total body BMD and regional BMD by whole-body DXA but not with head BMD [83], conceivably because the head is not a weight-bearing site and not usually involved in physical exercises. Furthermore, head BMD was not influenced by activity or disease condition in children and adolescents [84], unlike the total body mass. As regards assessment of bone fragility in children, a clinical guidance paper suggested that ‘cranium should be excluded from the total body scan analysis’ because of head BMD having a large proportion of the total body in spite of not reflecting physical activity or disease stage [84]. The head seems to be affected by age and body mass index to a different extent than other parts of the body [85]. Thus, in studies on adult ESRD patients, BMD of the head might be of interest as an indicator of bone health that is not necessarily influenced by physical activity. In a previous study in ESRD patients, there was a strong negative correlation between head BMD and iPTH and dialysis vintage [86]. We observed that high age, low hand grip strength, low lean body mass index and high serum iPTH were significantly associated with reduced head BMD, even after adjusting for several confounders [17]. Furthermore, in terms of disease conditions, those of our patients with low head BMD had high Framingham’s score (FRS) predicting 10-year risk of CVD, whereas there was no significant association between other sub-regional BMDs, or total BMD, and FRS [analysis of variance (ANOVA), P < 0.001] (Figure 2). Considering the fact that cortical bone is largely aggravated by progression of CKD and increase of serum PTH [87], cortical rich sites, such as the skull, may be preferred sites to capture the changes of the patient’s bone condition. However, skull BMD may be affected by hyperostosis cranii, an asymptomatic condition that has been reported to affect ∼22% of postmenopausal women [88].
FIGURE 2.
Association between tertiles of BMD head and total BMD (assessed by whole-body DXA; median and 10–90th percentiles) and Framingham’s cardiovascular risk score (adapted from a previous study [17]). Comparisons between tertiles were assessed with non-parametric ANOVA Kruskal–Wallis test followed by Dunn’s test for continuous variables. The figure illustrates that a possible reason why a cortical-rich bone site like the head appears to be suitable for analysing relations with survival in ESRD is that it associates with cardiovascular risk factors.
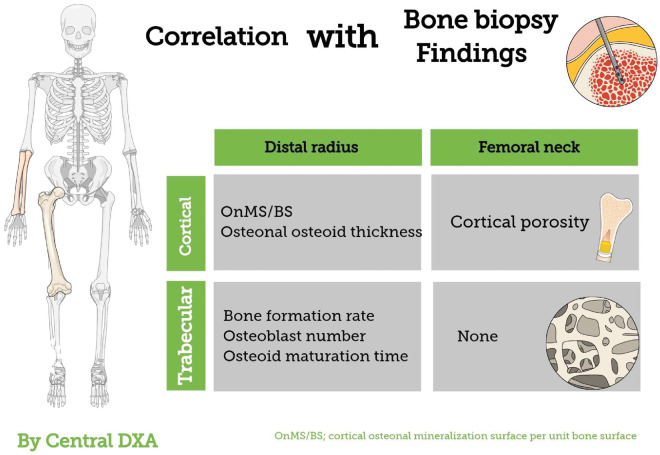
The femoral neck is another cortical-rich site; femoral neck BMD by central DXA had highly significant inverse association with cortical porosity assessed by iliac bone biopsy [89] (Figure 3). Recently, with the use of advanced hip analysis software allowing for the assessment of cortical parameters at femoral neck in DXA, it was shown that bone cortical thickness was reduced in ESRD patients as compared with healthy controls, and that this was associated with increased prevalence of fractures [90]. Thus, DXA may partially represent changes in bone histomorphology. Given the importance of cortical bone in CKD, impaired bone status measured by DXA may reflect not only bone mass but also, partially, bone quality. Cortical-rich sites may be more valuable locations for assessing metabolic alterations linked to CKD-MBD than trabecular-rich sites in CKD patients. These sites may differ depending on different measurement methods. For example, whole-body DXA is not able to divide long bone into portions [i.e. in forearm measurement of UD, mid-diaphysis , one-third distal radius of distal radius], whereas this can be accomplished by using central DXA.
FIGURE 3.
Associations between BMD by central DXA and histological findings by bone biopsy as reported in previous studies [40, 89].
Key points:
Bone mass at cortical-rich sites such as the head and femoral neck is more affected than trabecular bone and appears to be useful sites for predicting clinical outcomes in ESRD patients.
Mechanistic insights into the bone–vascular axis in CKD
The observation that low BMD, i.e. less mineralization of bone, associated with increased risk of ectopic calcification in blood vessels and its clinical sequelae [4, 55, 60, 91–94], referred to as the ‘calcification paradox’ [95], is supported by novel mechanistic insights into the bone–vascular axis. Vascular calcification with ‘ossification’ of vessels may be a consequence of impaired bone remodelling [96] and osteogenic processes driven by osteogenic transcriptional factors, such as runt-related transcription factor 2 (RUNX2) and Msh homoeobox 2 in cells surrounding calcified arteries [97–99]. Other bone regulatory factors including osteonectin, osteopontin, bone sialoprotein, type I collagen, alkaline phosphatase and matrix-Gla protein (MGP) are also present in extra-skeletal calcification sites [100, 101]. In mouse models, deletion of bone-related genes including OPG, MGP and the fibroblast growth factor-23/klotho axis result in extensive vascular calcification illustrating this ‘calcification paradox’ [96, 102, 103]. Likewise, in vitro studies demonstrated that vascular smooth muscle cells (VSMCs) and vascular pericytes can undergo osteogenic differentiation and produce bone forming transcription factors and proteins in response to high concentrations of phosphate, calcium, glucose, oxidized lipids, inflammatory cytokines and various toxins in uraemic serum [104]. The similarity of cellular and molecular signalling in osteogenesis and vascular calcification has led to the insight that vascular calcification is a cell-mediated active process rather than a passive mineralization routine.
In CKD, the synergism of oxidative stress, hyperphosphataemia, hypercalcaemia, hyperparathyroidism, medication with calcitriol, calcium carbonate and warfarin, and deficient anti-ageing processes provides a ‘perfect storm’ for bone loss and vascular calcification [105, 106]. Though this paradox—the coexistence of bone loss/osteoporosis and vascular calcification—is well-established among patients with CKD, the underlying mechanism(s) behind such a scenario has not yet been fully elucidated. Below we describe some signalling pathways that potentially could provide mechanistic links between bone loss and vascular calcification.
Wnt signalling
Wnt glycoproteins act as extracellular signalling ligands [107] in processes influencing bone [108] as well as the vasculature [109, 110]. Activation of the predominant signalling pathway, Wnt/β-catenin-dependent signalling, results in increased osteoblastogenesis and downregulated osteoclastogenesis, leading to enhanced bone formation and reduced bone resorption [111]. Genetic alterations in Wnt signalling components can result in either increased BMD or bone loss [112].
Sclerostin, encoded by the sclerostin (SOST) gene, is expressed by osteocytes and acts as a soluble inhibitor of wingless and Int-1 (Wnt) signalling to reduce bone formation; however, high serum sclerostin levels are associated with increased vascular calcification [113]. While mutations in SOST may increase bone mass [114, 115], overexpression of Dickkopf-related protein 1 (DKK1), another inhibitor of Wnt signalling, causes osteopaenia, whereas DKK1 insufficiency increases bone formation [116]. Blockade of sclerostin and DKK1 using neutralizing antibodies may increase BMD, trabecular bone volume, osteoblast number and bone formation, suggesting that downregulation of these two proteins potentially could be of value in future treatment strategies to ameliorate osteoporosis and bone loss [117, 118].
The expression of sclerostin and DKK1 is not restricted to bone. DKK1 is expressed in skin, placenta, prostate, kidney and platelets, and, to some extent, in endothelium [119]. Sclerostin is expressed in calcified VSMCs and aortic valves, as well as in the kidney and in chondrocytes [120]. Aside from affecting bone health, the Wnt/β-catenin signalling pathway may promote atherogenesis and vascular calcification [110]. In vitro studies indicate that DKK1 could attenuate transcriptional factor RUNX2 expression and thereby inhibit osteogenic differentiation of VSMC [121–123]. The observed expression of Wnt antagonists (sclerostin, DKK1 and Frizzled-related proteins) in calcified VSMCs [120, 124, 125] may represent a counteractive response to prevent progressive vascular calcification, potentially explaining the paradoxical inverse correlations of circulating levels of DKK1 and sclerostin with the extent of vascular calcification. In addition, increased expression of DKK1 in macrophages and endothelial cells may promote matrix metalloproteinase (MMP) activity in calcifying vascular cells, contributing to the instability of the atherosclerotic plaque [126, 127]. Inversely, a low expression of DKK1 hampers the adhesion of macrophages to endothelial cells and thus attenuates inflammation as well as the risk of plaque rupture [128]. It is thus tempting to speculate that Wnt/β-catenin signalling, involving particularly the antagonists DKK1 and sclerostin, affects the interplay between bone and vasculature.
OPG/RANK/RANKL axis
The triad of OPG, RANK and the RANKL are key proteins involved in bone metabolism [129]. RANKL, which is expressed by osteoblasts, stimulates its receptor RANK on cell surfaces including osteoclast cells. OPG, a secreted glycoprotein and a member of the tumour necrosis factor (TNF) receptor superfamily, is expressed in most tissues, including bone and vasculature, and acts as a soluble decoy receptor for RANKL and thus blocks the molecular effects of RANKL [130, 131]. Of note, since OPG can directly counter RANKL-mediated actions through RANK, the OPG/RANK/RANKL axis may influence bone remodelling processes and thus bone mass.
Dysfunction of OPG/RANK/RANKL system is of importance for skeletal disorders manifested either as excessive bone loss or excessive bone formation. Aside from RANKL, OPG also binds the pro-apoptotic factor—TNF-related apoptosis-inducing ligand (TRAIL) (produced by immune cells), and initiates cell death through TRAIL receptors expressed primarily on tumour or transdifferentiated cells [132]. OPG−/− mice exhibit osteoporosis due to excessive RANKL activation with enhanced bone resorption and diminished bone formation [133].
Compared with the relatively well-established role of OPG/RANK/RANKL in bone metabolism, the role of this triad in vasculature, particularly the effector OPG, is less clear. However, while OPG may counteract vascular calcification by suppressing bone resorption and by inhibiting VSMCs osteogenesis thus serving as a local vascular calcification inhibitor, OPG−/− mice develop severe vascular calcification accompanied by formation of bone matrix in the vasculature [96]. Moreover, OPG treatment rescues warfarin-induced vascular calcification in rodent models when administrated simultaneously with warfarin [134]. On the other hand, in CKD, increased circulating OPG levels are associated with increased severity of media vascular calcification [135, 136]. Furthermore, OPG is expressed along the margins of calcified areas [131, 137], and OPG expression is 2- to 4-fold higher in carotid endarterectomy specimens from symptomatic patients as compared with asymptomatic patients [138].
Several potential insights might explain such contradictive findings. First, RANKL stimulates the secretion of inflammatory cytokines including monocyte chemotactic protein-1, TNF-α and interleukin-1, as well as MMPs, which could induce OPG expression in VSMCs [139]. Since OPG is a soluble decoy receptor for RANKL, an elevated OPG level could thus inhibit such pro-inflammatory process as a compensatory and atheroprotective effect. Secondly, the cellular effect of OPG can be independent of RANK/RANKL system as it can also directly bind TRAIL and inhibit TRAIL receptors. This is especially crucial in the development of atherosclerotic plaques as apoptosis is tightly associated with plaque destabilization [140]. Hence, increased OPG expression in plaques could indicate risk of rupture-prone phenotype. The theoretic role of OPG, acting as a compensatory mechanism in response to various harmful stimuli, could partially contribute to the complexity of vascular calcification; yet, it is worth noting that under certain conditions, OPG may also amplify unfavourable capacities, e.g. inducing apoptosis of VSMCs, stimulating secretion of inflammatory cytokines and MMPs [131, 141]. Whether increased OPG has a counteractive effect aiming at preventing the vascular calcification process (either via localized inhibition of inflammatory and atherogenic effects induced by RANKL and TRAIL or via systemic inhibition of bone absorption)—or merely represents progressive plaque development with osteogenesis of VSMCs in the vasculature—remains to be determined.
Galectin-3/RAGE axis
Another novel insight into the ‘calcification paradox’ is the role of galectin-3 and the RAGEs in bone remodelling and vascular osteogenesis. Galectin-3 is a protein of the lectin family, encoded by the Galactin-3 (LGALS3) gene that influences growth, differentiation and apoptosis of cells in tissues such as bone and vasculature [142, 143], and is another key regulator of the Wnt/β-catenin signalling pathway [144]. Galectin-3 also acts as a receptor of advanced glycation end products (AGEs). Similar to other RAGEs it removes advanced glycation end products (AGEs), molecules that exert detrimental cellular effects and cause tissue injury [145], by binding of AGEs to RAGE [146]. With regards to bone metabolism, galectin-3 is a marker of chondrogenic and osteogenic cell lineages [147] and a critical factor in different stages of bone biology and function, and its expression is mediated by the bone growth regulator RUNX2 [148, 149]. On the other hand, galectin-3 has a role in atherogenesis [150] and thus participates in the bone-vascular interplay.
Both galectin-3 and RAGE are expressed in osteoblastic cell lines [151, 152]; while galectin-3 is critical for osteoblast differentiation by promoting osteoblastogenesis and suppressing osteoclastogenesis, RAGE is essential for osteoclast maturation and function [152]. Galectin-3-deficient (Lgals3−/−) mice develop a bone phenotype characterized by reduced trabecular bone compared with wild-type mice [153] and with VSMCs showing decreased activation of Wnt/β-catenin signalling, suggesting that galectin-3 is capable of inducing osteogenesis [149]. The impaired osteogenesis of Lgals3−/− VSMCs is accompanied by RAGE up-regulation, corroborating the counteractive roles of galectin-3 and RAGE in osteoblast-like phenotype differentiation [149]. Galectin-3 and RAGE are also involved in vascular calcification. Galectin-3 may transform VSMCs into osteoblast-like cells via Wnt/β-catenin signalling, contributing to sheet-like calcifications, a process potentially enhanced by its anti-inflammatory effects [154]. In contrast, RAGE favours deposition of spotty or granular calcification by promoting and aggravating inflammation and by counteracting the osteoblastogenic effect of galectin-3, potentially via inhibition of β-catenin activation [155]. In Apo-E null mice, VSMC-targeted expression of RAGE ligand S100A12 results in plaque instability with increased numbers of spotty calcified nodules [156]. The galectin-3/RAGE axis represents a novel link between bone and vasculature; however, its role in bone loss and vascular calcification in the context of CKD needs to be further studied.
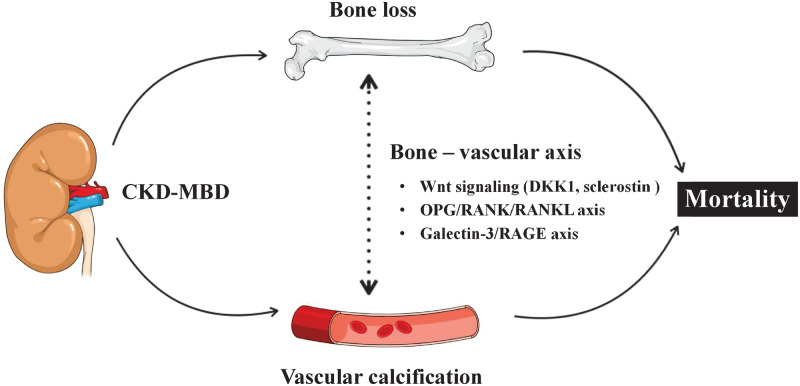
The mechanistic molecular framework of the bone–vascular axis—through which impaired bone status associated with changes in the vascular wall via pathways such as Wnt signalling, OPG/RANK/RANKL system and the Galectin-3/RAGE axis—illustrates that osteogenesis and vascular calcification in fact share many similarities and that low BMD and vascular calcification therefore may represent two sides of the same coin. These insights may be of potential value for future preventive and therapeutic strategies targeting low BMD and its links with increased mortality in ESRD patients.
Key points:
The bone–vascular axis including pathways such as Wnt signalling, OPG/RANK/RANKL system and the galectin-3/RAGE axis may influence concomitantly both osteogenesis and vascular calcification.
SUMMARY AND CONCLUSION
ESRD is associated with profound metabolic and nutritional alterations that affect essentially all organ and tissues, and bone is no exception. Osteoporosis and renal osteodystrophy with reduced density and impaired quality of bone are common manifestations of CKD-MBD that associate not only with increased risk of fractures but also with vascular calcification, arteriosclerosis and increased mortality. Among various measurement methods to assess bone condition, DXA is the one with the strongest documentation of predicting risk of fracture and recent studies show that low BMD as assessed by DXA is also associated with the presence and severity of vascular calcification and increased mortality risk in ESRD patients. BMD measurements by DXA provide important prognostic information of bone status and degree of osteoporosis but have different meaning depending on which bone sites are investigated. Recent evidence regarding bone changes caused by CKD-MBD suggests that cortical-rich bone sites might be more important than trabecular bone sites when assessing prognosis; however, further studies are needed. More importantly, changes of bone status, and in particular cortical-rich sites, reflect pathological conditions linked to vascular calcification, and are strong predictors of worse outcome in ESRD. The association between low BMD and poor clinical outcomes is to a large extent explained by the underlying alterations of the bone–vascular axis as processes involved in bone loss are also involved in ectopic calcification. Thus, low BMD and vascular calcification in ESRD may represent two sides of the same coin (Figure 4). Emerging evidence has indicated the important role of bone–vascular cross-talk involving pathways such as Wnt signalling, OPG/RANK/RANKL axis and galectin-3/RAGE axis, both in the aetiology of vascular calcification and in pathological changes of bone. The translation of these novel findings into new methods for preventive and therapeutic interventions would represent an important step towards improving the poor outcomes in the ESRD population that to a large extent appear to be linked to processes leading to bone disease and vascular calcification. Meanwhile, clinical and epidemiological studies are needed to link observed changes in bone status to biomarkers reflecting pathophysiological pathways including Wnt signalling, OPG/RANK/RANKL axis and galectin-3/RAGE axis. Further investigations of BMD and its relation to fractures, vascular calcification and mortality in ESRD patients receiving different nutritional, pharmacological and renal replacement therapies are warranted to establish optimal methods for DXA assessments, including selection of the ‘best’ sites for BMD measurements.
FIGURE 4.
Possible mechanisms of bone–vascular interplay in ESRD. CKD-MBDs involve activation of shared molecular signalling pathways that concomitantly may promote impaired bone status with loss of bone mass and vascular calcification.
FUNDING
Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. We acknowledge generous support of Karolinska Institutet Diabetes Theme Center (P.S.), European Union’s Horizon 2020 research and innovation Programme under the Marie Skłodowska-Curie grant agreement No 722609, Heart and Lung Foundation (P.S.), Njurfonden (P.S.) and Showa University Research Fund (K.I.). We thank Dr Magdalena Jankowska for revising the manuscript.
CONFLICT OF INTEREST STATEMENT
B.L. is employed by Baxter Healthcare Corporation. P.S. is on scientific advisory boards of REATA and Baxter. None of the other authors declare any conflict of interest.
REFERENCES
- 1. Kooman JP, Kotanko P, Schols A. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 [DOI] [PubMed] [Google Scholar]
- 2. Briggs AM, Perilli E, Parkinson IH. et al. Measurement of subregional vertebral bone mineral density in vitro using lateral projection dual-energy X-ray absorptiometry: validation with peripheral quantitative computed tomography. J Bone Miner Metab 2012; 30: 222–231 [DOI] [PubMed] [Google Scholar]
- 3. Hobson S, Arefin S, Kublickiene K. et al. Senescent cells in early vascular ageing and bone disease of chronic kidney disease—a novel target for treatment. Toxins (Basel) 2019; 11: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. London GM, Marchais SJ, Guérin AP. et al. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 2008; 19: 1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuoka M, Iseki K, Tamashiro M. et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol 2004; 8: 54–58 [DOI] [PubMed] [Google Scholar]
- 6. Park S-H, Jia T, Qureshi AR. et al. Determinants and survival implications of low bone mineral density in end-stage renal disease patients. J Nephrol 2013; 26: 485–494 [DOI] [PubMed] [Google Scholar]
- 7. Matsubara K, Suliman ME, Qureshi AR. et al. Bone mineral density in end-stage renal disease patients: association with wasting, cardiovascular disease and mortality. Blood Purif 2008; 26: 284–290 [DOI] [PubMed] [Google Scholar]
- 8. Malluche HH, Blomquist G, Monier-Faugere M-C. et al. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol 2015; 26: 2534–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkpantur A, Altun B, Hazirolan T. et al. Association among serum fetuin-A level, coronary artery calcification, and bone mineral densitometry in maintenance hemodialysis patients. Artif Organs 2009; 33: 844–854 [DOI] [PubMed] [Google Scholar]
- 10. Aoki A, Kojima F, Uchida K. et al. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic hemodialysis patients. Geriatr Gerontol Int 2009; 9: 246–252 [DOI] [PubMed] [Google Scholar]
- 11. Qu X, Huang X, Jin F. et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 166: 385–393 [DOI] [PubMed] [Google Scholar]
- 12. London GM. Bone-vascular axis in chronic kidney disease: a reality? Clin J Am Soc Nephrol 2009; 4: 254–257 [DOI] [PubMed] [Google Scholar]
- 13. Evenepoel P, D'Haese P, Brandenburg V.. Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 2015; 88: 235–240 [DOI] [PubMed] [Google Scholar]
- 14. Demer L, Tintut Y.. The bone-vascular axis in chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19: 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson B, Towler DA.. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol 2012; 8: 529–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vervloet MG, Massy ZA, Brandenburg VM. et al. Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2014; 2: 427–436 [DOI] [PubMed] [Google Scholar]
- 17. Iseri K, Qureshi AR, Dai L. et al. Bone mineral density at different sites and 5 years mortality in end-stage renal disease patients: a cohort study. Bone 2020; 130: 115075. [DOI] [PubMed] [Google Scholar]
- 18. Taal MW, Roe S, Masud T. et al. Total hip bone mass predicts survival in chronic hemodialysis patients. Kidney Int 2003; 63: 1116–1120 [DOI] [PubMed] [Google Scholar]
- 19. Orlic L, Mikolasevic I, Crncevic-Orlic Z. et al. Forearm bone mass predicts mortality in chronic hemodialysis patients. J Bone Miner Metab 2017; 35: 396–404 [DOI] [PubMed] [Google Scholar]
- 20. Disthabanchong S, Jongjirasiri S, Adirekkiat S. et al. Low hip bone mineral density predicts mortality in maintenance hemodialysis patients: a five-year follow-up study. Blood Purif 2014; 37: 33–38 [DOI] [PubMed] [Google Scholar]
- 21. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 2008; 3: S131–S139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ott SM. Review article: bone density in patients with chronic kidney disease stages 4–5. Nephrology (Carlton) 2009; 14: 395–403 [DOI] [PubMed] [Google Scholar]
- 23. Nottestad SY, Baumel JJ, Kimmel DB. et al. The proportion of trabecular bone in human vertebrae. J Bone Miner Res 2009; 2: 221–229 [DOI] [PubMed] [Google Scholar]
- 24. Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone 2002; 30: 807–809 [DOI] [PubMed] [Google Scholar]
- 25. Recker R, Lappe J, Davies KM. et al. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res 2004; 19: 1628–1633 [DOI] [PubMed] [Google Scholar]
- 26. Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci 2013; 68: 1218–1225 [DOI] [PubMed] [Google Scholar]
- 27. Silverberg SJ, Shane E, de la Cruz L. et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 2009; 4: 283–291 [DOI] [PubMed] [Google Scholar]
- 28. Sharma AK, Toussaint ND, Masterson R. et al. Deterioration of cortical bone microarchitecture: critical component of renal osteodystrophy evaluation. Am J Nephrol 2018; 47: 376–384 [DOI] [PubMed] [Google Scholar]
- 29. Behets GJ, Spasovski G, Sterling LR. et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 2015; 87: 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drüeke TB, Evenepoel P.. The bone after kidney transplantation. Clin J Am Soc Nephrol 2019; 14: 795–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evenepoel P, Behets GJ, Viaene L. et al. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int 2017; 91: 469–476 [DOI] [PubMed] [Google Scholar]
- 32. Keronen S, Martola L, Finne P. et al. Changes in bone histomorphometry after kidney transplantation. Clin J Am Soc Nephrol 2019; 14: 894–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malluche HH, Mawad HW, Monier-Faugere MC.. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 2011; 26: 1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filipowska J, Tomaszewski KA, Niedźwiedzki Ł. et al. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017; 20: 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clarke BL, Khosla S.. Physiology of bone loss. Radiol Clin North Am 2010; 48: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osterhoff G, Morgan EF, Shefelbine SJ. et al. Bone mechanical properties and changes with osteoporosis. Injury 2016; 47: S11–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riggs BL, Melton LJ, Robb RA. et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 2004; 19: 1945–1954 [DOI] [PubMed] [Google Scholar]
- 38. Nickolas TL, Stein EM, Dworakowski E. et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 2013; 28: 1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva BC, Costa AG, Cusano NE. et al. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest 2011; 34: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carvalho C, Magalhães J, Neto R. et al. Cortical bone analysis in a predialysis population: a comparison with a dialysis population. J Bone Miner Metab 2017; 35: 513–521 [DOI] [PubMed] [Google Scholar]
- 41. Bazzocchi A, Ponti F, Albisinni U. et al. DXA: Technical aspects and application. Eur J Radiol 2016; 85: 1481–1492 [DOI] [PubMed] [Google Scholar]
- 42. Shuhart CR, Yeap SS, Anderson PA. et al. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, periprosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom 2019; 22: 453–471 [DOI] [PubMed] [Google Scholar]
- 43. Stone KL, Seeley DG, Lui LY. et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 2003; 18: 1947–1954 [DOI] [PubMed] [Google Scholar]
- 44. Kanis JA, Melton LJ, Christiansen C. et al. The diagnosis of osteoporosis. J Bone Miner Res 2009; 9: 1137–1141 [DOI] [PubMed] [Google Scholar]
- 45. Boyanov M. Estimation of lumbar spine bone mineral density by dual-energy X-ray absorptiometry: standard anteroposterior scans vs sub-regional analyses of whole-body scans. Br J Radiol 2008; 81: 637–642 [DOI] [PubMed] [Google Scholar]
- 46. Melton LJ, Looker AC, Shepherd JA. et al. Osteoporosis assessment by whole body region vs. site-specific DXA. Osteoporos Int 2005; 16: 1558–1564 [DOI] [PubMed] [Google Scholar]
- 47. Graat-Verboom L, Spruit MA, van den Borne B. et al. Whole-body versus local DXA-scan for the diagnosis of osteoporosis in COPD patients. J Osteoporos 2010; 2010: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. Erratum: Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017; 7: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evenepoel P, D’Haese P, Bacchetta J. et al. ; ERA-EDTA Working Group on CKD-MBD. Bone biopsy practice patterns across Europe: the European renal osteodystrophy initiative—a position paper. Nephrol Dial Transplant 2017; 32: 1608–1613 [DOI] [PubMed] [Google Scholar]
- 50. Iimori S, Mori Y, Akita W. et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients-a single-center cohort study. Nephrol Dial Transplant 2012; 27: 345–351 [DOI] [PubMed] [Google Scholar]
- 51. Yenchek RH, Ix JH, Shlipak MG. et al. ; for the Health, Aging, and Body Composition Study. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 2012; 7: 1130–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barengolts EI, Berman M, Kukreja SC. et al. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int 1998; 62: 209–213 [DOI] [PubMed] [Google Scholar]
- 53. Frye MA, Melton LJ, Bryant SC. et al. Osteoporosis and calcification of the aorta. Bone Miner 1992; 19: 185–194 [DOI] [PubMed] [Google Scholar]
- 54. Braun J, Oldendorf M, Moshage W. et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 1996; 27: 394–401 [DOI] [PubMed] [Google Scholar]
- 55. Toussaint ND, Lau KK, Strauss BJ. et al. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 2007; 23: 586–593 [DOI] [PubMed] [Google Scholar]
- 56. Vogt MTS, Valentin R, Forrest KY. et al. Bone mineral density and aortic calcification: the Study of Osteoporotic Fractures. J Am Geriatr Soc 1997; 45: 140–145 [DOI] [PubMed] [Google Scholar]
- 57. Farhat GN, Cauley JA, Matthews KA. et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health across the Nation. J Bone Miner Res 2006; 21: 1839–1846 [DOI] [PubMed] [Google Scholar]
- 58. Barreto DV, Barreto FDC, Carvalho AD. et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis 2008; 52: 1139–1150 [DOI] [PubMed] [Google Scholar]
- 59. Tankò LB, Bagger YZ, Christiansen C.. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int 2003; 73: 15–20 [DOI] [PubMed] [Google Scholar]
- 60. Kiel DP, Kauppila LI, Cupples LA. et al. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int 2001; 68: 271–276 [DOI] [PubMed] [Google Scholar]
- 61. Uyama O, Yoshimoto Y, Yamamoto Y. et al. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke 1997; 28: 1730–1732 [DOI] [PubMed] [Google Scholar]
- 62. Banks LM, Lees B, MacSweeney JE. et al. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: links between osteoporosis and cardiovascular disease? Eur J Clin Invest 1994; 24: 813–817 [DOI] [PubMed] [Google Scholar]
- 63. Kohno K, Inaba M, Okuno S. et al. Association of reduction in bone mineral density with mortality in male hemodialysis patients. Calcif Tissue Int 2009; 84: 180–185 [DOI] [PubMed] [Google Scholar]
- 64. Chen Z, Qureshi AR, Brismar TB. et al. Differences in association of lower bone mineral density with higher coronary calcification in female and male end-stage renal disease patients. BMC Nephrol 2019; 20: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen Z, Sun J, Haarhaus M. et al. Bone mineral density of extremities is associated with coronary calcification and biopsy-verified vascular calcification in living-donor renal transplant recipients. J Bone Miner Metab 2017; 35: 536–543 [DOI] [PubMed] [Google Scholar]
- 66. Chen Z, Qureshi AR, Ripsweden J. et al. Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone 2016; 92: 50–57 [DOI] [PubMed] [Google Scholar]
- 67. Yap N, Wong P, McGinn S. et al. Femoral neck X-ray absorptiometry parameters and peripheral quantitative computer tomography tibial cortical density predict survival in dialysis patients. Nephron 2017; 136: 183–192 [DOI] [PubMed] [Google Scholar]
- 68. Lin ZZ, Wang JJ, Chung CR. et al. Epidemiology and mortality of hip fracture among patients on dialysis: Taiwan National Cohort Study. Bone 2014; 64: 235–239 [DOI] [PubMed] [Google Scholar]
- 69. Mathew AT, Hazzan A, Jhaveri KD. et al. Increasing hip fractures in patients receiving hemodialysis and peritoneal dialysis. Am J Nephrol 2014; 40: 451–457 [DOI] [PubMed] [Google Scholar]
- 70. Palit S, Kendrick J.. Vascular calcification in chronic kidney disease: role of disordered mineral metabolism. Curr Pharm Des 2014; 20: 5829–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ureña-Torres P, D’Marco L, Raggi P. et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant 2019; 2: 635–643 [DOI] [PubMed] [Google Scholar]
- 72. El Maghraoui A, Roux C.. DXA scanning in clinical practice. QJM 2008; 101: 605–617 [DOI] [PubMed] [Google Scholar]
- 73. Chen J, Budoff MJ, Reilly MP. et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2017; 2: 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mukai H, Dai L, Chen Z. et al. Inverse J-shaped relation between coronary arterial calcium density and mortality in advanced chronic kidney disease. Nephrol Dial Transplant 2018; 1–9 [DOI] [PubMed] [Google Scholar]
- 75. Brit BS, Forsén L, Omsland TK. et al. Does the association of comorbidity with 1-year mortality after hip fracture differ according to gender? The Norwegian Epidemiologic Osteoporosis Studies (NOREPOS). J Am Geriatr Soc 2018; 66: 553–558 [DOI] [PubMed] [Google Scholar]
- 76. Omsland TK, Emaus N, Tell GS. et al. ; A NOREPOS study. Mortality following the first hip fracture in Norwegian women and men (1999-2008). Bone 2014; 63: 81–86 [DOI] [PubMed] [Google Scholar]
- 77. Alem AM, Sherrard DJ, Gillen DL. et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000; 58: 396–399 [DOI] [PubMed] [Google Scholar]
- 78. Tentori F, Mccullough K, Kilpatrick RD. et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 2014; 85: 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Reeve J. Role of cortical bone in hip fracture. Bonekey Rep 2017; 6: 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kral R, Osima M, Borgen TT. et al. Increased cortical porosity and reduced cortical thickness of the proximal femur are associated with nonvertebral fracture independent of Fracture Risk Assessment Tool and Garvan estimates in postmenopausal women. PLoS One 2017; 12: e0185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Runesson B, Trevisan M, Iseri K. et al. Fractures and their sequelae in non-dialysis-dependent chronic kidney disease: the Stockholm CREAtinine Measurements project. Nephrol Dial Transplant 2019; 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Keene GS, Parker MJ, Pryor GA.. Mortality and morbidity after hip fractures. BMJ 1993; 307: 1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Morel J, Combe B, Francisco J. et al. Bone mineral density of 704 amateur sportsmen involved in different physical activities. Osteoporos Int 2001; 12: 152–157 [DOI] [PubMed] [Google Scholar]
- 84. Bachrach LK, Gordon CM, Section on Endocrinology. Bone densitometry in children and adolescents. Pediatrics 2016; 138: e20162398. [DOI] [PubMed] [Google Scholar]
- 85. Brismar TB, Ringertz H.. Effect of bone density of the head on total body dexa measurements in 100 healthy Swedish women. Acta Radiol 1996; 37: 101–106 [DOI] [PubMed] [Google Scholar]
- 86. Chan TM, Pun KK, Cheng I.. Total and regional bone densities in dialysis patients. Nephrol Dial Transplant 1992; 7: 835–839 [PubMed] [Google Scholar]
- 87. Klemetti E, Vainio P, Lassila V. et al. Cortical bone mineral density in the mandible and osteoporosis status in postmenopausal women. Eur J Oral Sci 1993; 101: 219–223 [DOI] [PubMed] [Google Scholar]
- 88. Salmi A, Voutilainen A, Holsti IR et al. Hyperostosis cranii in a normal population. Am J Roentgenol Radium Ther Nucl Med 1962; 87: 1032–1040 [PubMed] [Google Scholar]
- 89. Adragao T, Herberth J, Monier-Faugere MC. et al. Femoral bone mineral density reflects histologically determined cortical bone volume in hemodialysis patients. Osteoporos Int 2010; 21: 619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aleksova J, Milat F, Kotowicz MA. et al. Patients with end-stage kidney disease have markedly abnormal cortical hip parameters by dual-energy x-ray absorptiometry. Nephrol Dial Transplant 2019; 1–8 [DOI] [PubMed] [Google Scholar]
- 91. Von der Recke P, Hansen MA, Hassager C.. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med 1999; 106: 273–278 [DOI] [PubMed] [Google Scholar]
- 92. Lee SN, Cho JY, Eun YM. et al. Associations between osteoporosis and coronary artery disease in postmenopausal women. Climacteric 2016; 19: 458–462 [DOI] [PubMed] [Google Scholar]
- 93. Hyder JA, Allison MA, Wong N. et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA abdominal aortic calcium study. Am J Epidemiol 2008; 169: 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. London GM. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 2004; 15: 1943–1951 [DOI] [PubMed] [Google Scholar]
- 95. Persy V, D’Haese P.. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 2009; 15: 405–416 [DOI] [PubMed] [Google Scholar]
- 96. Bucay N, Sarosi I, Dunstan CR. et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998; 12: 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yamada S, Tatsumoto N, Tokumoto M. et al. Phosphate binders prevent phosphate-induced cellular senescence of vascular smooth muscle cells and vascular calcification in a modified, adenine-based uremic rat model. Calcif Tissue Int 2015; 96: 347–358 [DOI] [PubMed] [Google Scholar]
- 98. Sun Y, Byon CH, Yuan K. et al. Smooth muscle cell–specific runx2 deficiency inhibits vascular calcification. Circ Res 2012; 111: 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shao J, Cheng S, Pingsterhaus JM. et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 2005; 115: 1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Moe SM, Chen NX.. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res 2004; 95: 560–567 [DOI] [PubMed] [Google Scholar]
- 101. Boström K, Watson KE, Horn S. et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 1993; 91: 1800–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Luo G, Ducy P, McKee MD. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protien. Nature 1997; 386: 78–81 [DOI] [PubMed] [Google Scholar]
- 103. Razzaque MS, Lanske B.. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med 2006; 12: 298–305 [DOI] [PubMed] [Google Scholar]
- 104. Shanahan CM. Mechanisms of vascular calcification in CKD—evidence for premature ageing? Nat Rev Nephrol 2013; 9: 661–670 [DOI] [PubMed] [Google Scholar]
- 105. Dai L, Qureshi AR, Witasp A. et al. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J 2019; 17: 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Demer LL, Tintut Y.. Vascular calcification. Circulation 2008; 117: 2938–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gough NR. Focus issue: Wnt and β-catenin signaling in development and disease. Sci Signal 2012; 5: eg2. [DOI] [PubMed] [Google Scholar]
- 108. Monroe DG, McGee-Lawrence ME, Oursler MJ. et al. Update on Wnt signaling in bone cell biology and bone disease. Gene 2012; 492: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kobayashi Y, Uehara S, Udagawa N. et al. Regulation of bone metabolism by Wnt signals. J Biochem 2016; 159: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Foulquier S, Daskalopoulos EP, Lluri G. et al. WNT signaling in cardiac and vascular disease. Pharmacol Rev 2018; 70: 68–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Day TF, Guo X, Garrett-Beal L. et al. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005; 8: 739–750 [DOI] [PubMed] [Google Scholar]
- 112. Korvala J, Jüppner H, Mäkitie O. et al. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med Genet 2012; 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Qureshi AR, Olauson H, Witasp A. et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int 2015; 88: 1356–1364 [DOI] [PubMed] [Google Scholar]
- 114. Loots GG. deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 2005; 15: 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Balemans W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 2001; 10: 537–543 [DOI] [PubMed] [Google Scholar]
- 116. Li J, Sarosi I, Cattley RC. et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 2006; 39: 754–766 [DOI] [PubMed] [Google Scholar]
- 117. Glantschnig H, Hampton RA, Lu P. et al. Generation and selection of novel fully human monoclonal antibodies that neutralize dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 2010; 285: 40135–40147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li X, Ominsky MS, Warmington KS. et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 2009; 24: 578–588 [DOI] [PubMed] [Google Scholar]
- 119. Ke HZ, Richards WG, Li X. et al. Sclerostin and dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 2012; 33: 747–783 [DOI] [PubMed] [Google Scholar]
- 120. De Maré A, Maudsley S, Azmi A. et al. Sclerostin as regulatory molecule in vascular media calcification and the bone–vascular axis. Toxins (Basel) 2019; 11: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gaur T, Lengner CJ, Hovhannisyan H. et al. Canonical WNT signaling promotes osteogenesis by directly stimulating runx2 gene expression. J Biol Chem 2005; 280: 33132–33140 [DOI] [PubMed] [Google Scholar]
- 122. Cai T, Sun D, Duan Y. et al. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res 2016; 345: 206–217 [DOI] [PubMed] [Google Scholar]
- 123. Baetta R, Banfi C.. Dkk (Dickkopf) proteins. Arterioscler Thromb Vasc Biol 2019; 39: 1330–1342 [DOI] [PubMed] [Google Scholar]
- 124. Zhu D, Mackenzie NCW, Millán JL. et al. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One 2011; 6: e19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Román-García P, Carrillo-López N, Fernández-Martín JL. et al. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 2010; 46: 121–128 [DOI] [PubMed] [Google Scholar]
- 126. Zhang Y, Ge C, Wang L. et al. Induction of DKK1 by ox-LDL negatively regulates intracellular lipid accumulation in macrophages. FEBS Lett 2015; 589: 52–58 [DOI] [PubMed] [Google Scholar]
- 127. Li R, Mittelstein D, Lee J. et al. A dynamic model of calcific nodule destabilization in response to monocyte- and oxidized lipid-induced matrix metalloproteinases. Am J Physiol Physiol 2012; 302: C658–C665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li M, Liu X, Zhang Y. et al. Upregulation of Dickkopf1 by oscillatory shear stress accelerates atherogenesis. J Mol Med 2016; 94: 431–441 [DOI] [PubMed] [Google Scholar]
- 129. Teitelbaum SL. Bone resorption by osteoclasts. Science 2000; 289: 1504–1508 [DOI] [PubMed] [Google Scholar]
- 130. Simonet W, Lacey D, Dunstan C. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–319 [DOI] [PubMed] [Google Scholar]
- 131. Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 2004; 95: 1046–1057 [DOI] [PubMed] [Google Scholar]
- 132. LeBlanc HN, Ashkenazi A.. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ 2003; 10: 66–75 [DOI] [PubMed] [Google Scholar]
- 133. Ozaki Y, Koide M, Furuya Y. et al. Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis. PLoS One 2017; 12: e0184904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Price PA, June HH, Buckley JR. et al. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 2001; 21: 1610–1616 [DOI] [PubMed] [Google Scholar]
- 135. Kazama JJ, Shigematsu T, Yano K. et al. Increased circulating levels of osteoclastogenesis inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am J Kidney Dis 2002; 39: 525–532 [DOI] [PubMed] [Google Scholar]
- 136. Vattikuti R, Towler DA.. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Metab 2004; 286: E686–E696 [DOI] [PubMed] [Google Scholar]
- 137. Dhore CR, Cleutjens JPM, Lutgens E. et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2001; 21: 1998–2003 [DOI] [PubMed] [Google Scholar]
- 138. Golledge J, McCann M, Mangan S. et al. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004; 35: 1636–1641 [DOI] [PubMed] [Google Scholar]
- 139. Weitzmann MN. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica (Cairo) 2013; 2013: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol 2005; 25: 2255–2264 [DOI] [PubMed] [Google Scholar]
- 141. Sandberg WJ, Yndestad A, Øie E. et al. Enhanced T-cell expression of RANK ligand in acute coronary syndrome. Arterioscler Thromb Vasc Biol 2006; 26: 857–863 [DOI] [PubMed] [Google Scholar]
- 142. Colnot C, Sidhu SS, Poirier F, Balmain N.. Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell Mol Biol (Noisy-le-Grand) 1999; 45: 1191–1202 [PubMed] [Google Scholar]
- 143. Sádaba JR, Martínez ‐Martínez E, Arrieta V. et al. Role for galectin‐3 in calcific aortic valve stenosis. J Am Heart Assoc 2016; 5: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shimura T, Takenaka Y, Tsutsumi S. et al. Galectin-3, a novel binding partner of β-catenin. Cancer Res 2004; 64: 6363–6367 [DOI] [PubMed] [Google Scholar]
- 145. Pugliese G, Iacobini C, Pesce CM. et al. Galectin-3: an emerging all-out player in metabolic disorders and their complications. Glycobiology 2015; 25: 136–150 [DOI] [PubMed] [Google Scholar]
- 146. Schmidt AM, Yan S, Du Yan SF. et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001; 108: 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Aubin JE, Liu F, Malaval L. et al. Osteoblast and chondroblast differentiation. Bone 1995; 17: S77–S83 [DOI] [PubMed] [Google Scholar]
- 148. Stock M, Schäfer H, Stricker S. et al. Expression of galectin-3 in skeletal tissues is controlled by runx2. J Biol Chem 2003; 278: 17360–17367 [DOI] [PubMed] [Google Scholar]
- 149. Weilner S, Keider V, Winter M. et al. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging 2016; 8: 16–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Menini S, Iacobini C, Ricci C. et al. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc Res 2013; 100: 472–480 [DOI] [PubMed] [Google Scholar]
- 151. Li YJ, Kukita A, Teramachi J. et al. A possible suppressive role of galectin-3 in upregulated osteoclastogenesis accompanying adjuvant-induced arthritis in rats. Lab Invest 2009; 89: 26–37 [DOI] [PubMed] [Google Scholar]
- 152. Zhou Z, Immel D, Xi CX. et al. Regulation of osteoclast function and bone mass by RAGE. J Exp Med 2006; 203: 1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Simon D, Derer A, Andes FT. et al. Galectin-3 as a novel regulator of osteoblast-osteoclast interaction and bone homeostasis. Bone 2017; 105: 35–41 [DOI] [PubMed] [Google Scholar]
- 154. MacKinnon AC, Farnworth SL, Hodkinson PS. et al. Regulation of alternative macrophage activation by galectin-3. J Immunol 2008; 180: 2650–2658 [DOI] [PubMed] [Google Scholar]
- 155. Li G, Xu J, Li Z.. Receptor for advanced glycation end products inhibits proliferation in osteoblast through suppression of Wnt, PI3K and ERK signaling. Biochem Biophys Res Commun 2012; 423: 684–689 [DOI] [PubMed] [Google Scholar]
- 156. Hofmann Bowman MA, Gawdzik J, Bukhari U. et al. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E–null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol 2011; 31: 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]