Abstract
Background
Local anesthetic toxicity has been well-documented to cause neuronal injury, death, and dysfunction, particularly in a susceptible nerve.
Objective
To determine whether select local anesthetics affect neuron survival and/or functional recovery of an injured nerve.
Methods
This report describes 6 separate experiments that test immediate or delayed application of local anesthetics in 3 nerve injury models. Adult C57/black6 male mice underwent a facial nerve sham, transection, or crush injury. Local anesthetic or saline was applied to the facial nerve at the time of injury (immediate) or 1 day after injury (delayed). Average percent facial motoneuron (FMN) survival was evaluated four-weeks after injury. Facial nerve regeneration was estimated by observing functional recovery of eye blink reflex and vibrissae movement after facial nerve crush injury.
Results
FMN survival after: transection + immediate treatment with ropivacaine (54.8%), bupivacaine (63.2%), or tetracaine (66.9%) was lower than saline (85.5%) and liposomal bupivacaine (85.0%); crush + immediate treatment with bupivacaine (92.8%) was lower than saline (100.7%) and liposomal bupivacaine (99.3%); sham + delayed treatment with bupivacaine (89.9%) was lower than saline (96.6%) and lidocaine (99.5%); transection + delayed treatment with bupivacaine (67.3%) was lower than saline (78.4%) and liposomal bupivacaine (77.6%); crush + delayed treatment with bupivacaine (85.3%) was lower than saline (97.9%) and lidocaine (96.0%). The average post-operative time for mice to fully recover after: crush + immediate treatment with bupivacaine (12.83 days) was longer than saline (11.08 days) and lidocaine (10.92 days); crush + delayed treatment with bupivacaine (16.79 days) was longer than saline (12.73 days) and lidocaine (11.14 days).
Conclusions
Our data demonstrate that some local anesthetics, but not all, exacerbate motoneuron death and delay functional recovery after a peripheral nerve injury. These and future results may lead to clinical strategies that decrease the risk of neural deficit following peripheral nerve blocks with local anesthetics.
Keywords: Neurotoxicity, motoneuron survival, peripheral nerve block, local anesthetic toxicity, peripheral nerve injury, facial nerve injury, liposomal bupivacaine, regional anesthesia
1. Introduction
Local anesthetics have been used in the perioperative period to alleviate pain for more than 100 years. Local anesthetics exert their effects by reversibly binding to voltage-gated sodium channels on neurons to interrupt electrical impulse propagation (Nau & Wang, 2004). However, nerve damage can occur from peripheral nerve block procedures and lead to new or worsened functional impairment for patients. One mechanism of nerve damage is direct neurotoxicity of local anesthetics, however the pathways involved are incompletely understood (Hogan, 2008; Lirk et al., 2008; Selander, 1993). The question arises, therefore, whether nerves injured at the time of surgery are affected by direct exposure to local anesthetics either pre-, intra-, or post-procedure (i.e. after closing a wound). Tracking the incidence and recovery of perioperative nerve injury is challenging. Subjective sensory testing is fraught with uncertainties, and post-operative motor testing can be limited by apprehension, pain, dressings, patient expectations, etc. Even with objective electromyography studies, it is difficult to determine if a post-operative nerve deficit is related to a nerve block, iatrogenic surgical injury, or another mechanism. Because of these confounding factors, the incidence rates of peripheral nerve dysfunction range widely after peripheral nerve block reported in the literature (Liguori, 2004; Liu et al., 2009). Early transient postoperative neurologic symptoms are very common with some studies reporting up to 14% of patients complaining of dysesthesia, paresthesia, or mild pain in the immediate perioperative period, however long-term clinical implications of these symptoms are unknown (Brull, McCartney, Chan, & El-Beheiry, 2007; Liguori, 2004; Liu et al., 2009). We hypothesize that the effects of local anesthetic in the setting of nerve injury may result in worsening facial nerve function in a mouse nerve injury model. The objective of this study, therefore, was to determine if select local anesthetics can exacerbate neuron cell death and/or adversely delay functional recovery when applied to an injured facial nerve. For proof of concept, we have utilized the well described facial nerve injury model to evaluate the neurotoxic potential of commonly used local anesthetics.
2. Materials and methods
2.1. Animals
This manuscript adheres to the applicable EQUATOR guidelines (ARRIVE guidelines). 245 adult male C57B/6 mice (7–8 weeks old; 22g-26g; N = 5–12 per group) were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed and manipulated in accordance with institutional and National Institutes of Health guidelines and approved by the Animal Care and Use Committee at Edward Hines Jr., VA Hospital (Hines, IL) to conduct the following experiments. Three separate experimental injury models were studied; 1) facial nerve sham, 2) facial nerve transection, 3) facial nerve crush. Two separate local anesthetic application times were studied; 1) immediate local anesthetic treatment at the time of injury and 2) delayed local anesthetic treatment, 1 day after the initial injury. For each experiment in the immediate treatment study, the mice were randomly divided into one of eight treatment groups; vehicle (saline), lidocaine, 2,3-chloroprocaine, mepivacaine, ropivacaine, bupivacaine, tetracaine, or liposomal bupivacaine (Exparel; Pacira Pharmaceuticals, Parsippany, NJ). For each experiment in the delayed treatment study, the mice were randomly divided into one of four treatment groups; vehicle (saline), lidocaine, bupivacaine, or liposomal bupivacaine. The local anesthetic treatment groups were blinded to all investigators throughout the study until after data analysis was completed. Animal groups were coded by one investigator and subsequently analyzed under “blind” conditions by a second and third investigator, who were unaware of the injury and treatment group divisions.
For all surgical procedures, mice were fully anesthetized using 2% inhaled isoflurane and aseptic technique followed. In all 3 injury models, an incision was made behind the right ear and the right facial nerve was exposed at its exit from the stylomastoid foramen. For the sham surgery, no nerve injury was performed. For the transection injury, the right facial nerve was completely transected at the stylomastoid foramen using iridectomy scissors. The proximal and distal nerve stumps were manually displaced to prevent reconnection. Animals in the sham and transection groups were observed biweekly to confirm presence or absence of facial nerve function by evaluating eye blink or vibrissae movement. For the crush injury, the facial nerve was crushed twice for 30 seconds each, using Dumont #5/45 forceps held at different orientations to the nerve. All wounds were closed with 5–0 prolene monofilament suture. In the delayed local anesthetic treatment groups, mice underwent a second surgery to re-expose the intact (sham) or injured (transection or crush) nerves one day after injury, for local anesthetic application.
2.2. Local anesthetic treatment
2.2.1. Immediate local anesthetic application
At the time of facial nerve injury, one of seven commonly used local anesthetics (2% lidocaine [7 mg/kg], 3% 2, 3-chloroprocaine [15 mg/kg], 1% mepivacaine [7 mg/kg], 0.25% ropivacaine [5 mg/kg], 0.25% bupivacaine [5 mg/kg], 1% tetracaine [2 mg/kg], or 1.3% liposomal bupivacaine [5 mg/kg]), or vehicle (0.9% normal saline), was applied to the facial nerve in a total volume of 50μl via impregnated Gelfoam (Pfizer; New York, New York). Gelfoam was chosen in an attempt to sequester the local anesthetic to the site of injury and potentially avoid run-off into the surrounding tissue. The total dose for each local anesthetic was based on the maximal suggested dose used clinically by weight.
2.2.2. Delayed local anesthetic application
One day after facial nerve injury, one of three commonly used local anesthetics (2% lidocaine [7 mg/kg], 0.25% bupivacaine [5 mg/kg], or 1.3% liposomal bupivacaine [5 mg/kg]), or vehicle (0.9% normal saline), was applied directly to the facial nerve injury site via syringe in a total volume of 50μl. Of note, Gelfoam was not used in these studies to avoid its potential to confound the pharmacokinetics and pharmacodynamics of treatment. See Discussion for details. The total dose for each local anesthetic was based on the maximal suggested dose used clinically by weight.
2.3. Data collection
2.3.1. Facial motoneuron survival
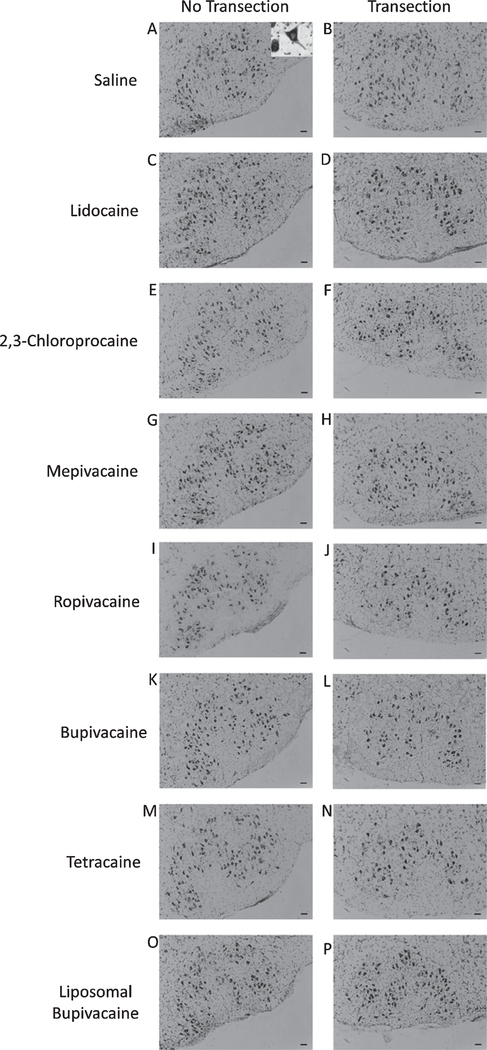
Four weeks post-operatively all animals were euthanized via isoflurane overdose followed rapidly by decapitation and the brains were removed and promptly frozen in a solution of butyl-bromide and 2-methylbutane cooled with dry ice to below —40°C. Twenty-five μm thick sections were collected throughout the facial nucleus, fixed in 4% paraformaldehyde and stained with thionin to reveal motoneuron cell bodies (Fig. 1). One investigator coded all slides and animal groups and a second and third investigator determined surviving FMN counts in each section using light microscopy. The abducens nuclei and internal genu of the facial nerves were used to precisely match the sections of the left (uninjured) and right (injured) sides. Using l00× magnification, surviving FMNs were easily identified by morphologic recognizability as nucleated multipolar cells with somatic cell size ~25—40 μm and only motoneurons containing a clear nucleus (see inset Fig. 1 A) were counted and compared on a minimum of 15 location-matched sections per animal. The percentage change between the uninjured and injured sides was calculated and the average percent survival recorded for each animal.
Fig. 1.
Thionin-stained facial motoneurons. Representative photomicrographs of thionin-stained facial motoneurons in the uninjured (no transection) and injured (transection) facial nuclei of mice treated with saline (A and B), lidocaine (C and D), 2,3chloroprocaine (E and F), mepivacaine (G and H), ropivacaine (I and J), bupivacaine (K and L), tetracaine (M and N), and liposomal bupivacaine (O and P) at the time of injury (immediate treatment). Scale bar - 50 μm. Inset of 1a is a magnified example motoneuron, demonstrating characteristic morphology and presence of distinct nucleus used for cell counting.
2.3.2. Functional recovery assessments
Facial nerve crush injury results in loss of eye blink reflex, vibrissae movement and abnormal vibrissae orientation with fibers flattened in a posterior direction against the head (Kujawa, Kinderman, & Jones, 1989). Recovery post-crush injury is considered complete when all three parameters are equal in magnitude and orientation relative to the uninjured (control) side. Animals in the nerve crush injury study were observed twice daily by 2 observers that were blinded to the treatment group. The eye blink reflex on the injured side was observed in comparison to the uninjured side, by carefully exhaling a gentle puff of air directed at the animal’s corneas. Eye blink was assessed utilizing a 4-point scale; 1-no blink, 2-slight blinking movement, 3-robust eye blink but not matching the control side, and 4-full blink, matching the control side. Evidence of bulbar retraction with passive eyelid closing was carefully observed to avoid over-scoring. Vibrissae movement was assessed utilizing a 4-point scale; 1-no movement, 2-slight movement, 3-robust movement but not equal to the control side, and 4-full movement matching and coordinated with the control side. Vibrissae orientation was assessed utilizing a 3-point scale; 1-posterior or “flattened” against the face, 2-away from face but not matching the control side, and 3-fully anterior, matching the control side. Careful consideration of contralateral intact vibrissae pad movement was taken to ensure accurate assessments. In the event whiskers were groomed off an animal by a cage mate and observers were unable to make reasonable behavioral assessments, the animals were removed from the behavioral analysis.
2.4. Statistical analysis
For FMN survival data, in accordance with our previously published work, the Abercrombie correction factor (N = n×T/T+D), where N is the actual number of cells, n is the number of nuclear profiles, T is the section thickness (25 μm), and D is the average diameter of nuclei (5 μm) (Byram, Byram, Miller, & Fargo, 2017; Coggeshall, 1992), was used to compensate for double counting in adjacent sections. The percent change in the number of FMNs between the uninjured and injured facial motor nuclei was determined for each animal and then the average percent survival and standard error of the mean (SEM) determined for each experimental group.
For animals receiving a crush injury, the average time in post-operative days until the onset of complete recovery of vibrissae orientation, eyeblink reflex, and vibrissae movement, as well as the onset of complete recovery of all three of these facial nerve functions, was determined for each animal. The average number of days and SEM was then determined for each experimental group of the crush injured animals.
A one-way analysis of variance (ANOVA) was conducted to compare the effects of select local anesthetics on FMN survival and time to complete recovery. Post-hoc comparisons using the Tukey’s multiple comparison test was done to identify local anesthetics that exert significant effects on the outcome measures (GraphPad Prism 8).
3. Results
3.1. FMN Survival
3.1.1. Immediate local anesthetic application
To determine if commonly used local anesthetics affect neuronal survival after a peripheral nerve sham, transection, or crush injury, we applied saline, lidocaine, 2,3-chloroprocaine, mepivacaine, ropivacaine, bupivacaine, tetracaine, or liposomal bupivacaine to the facial nerve immediately at the time of injury.
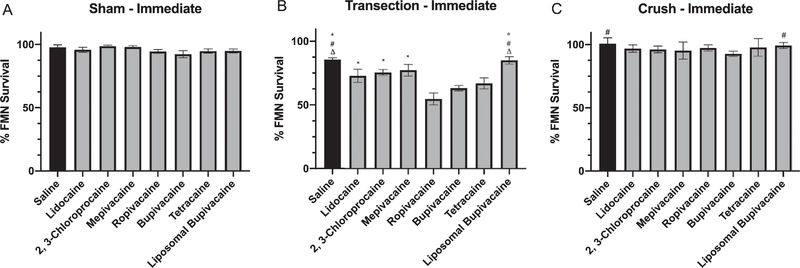
Four weeks post sham injury and immediate local anesthetic treatment, the average percent FMN survival in mice was determined. A one-way ANOVA conducted to compare the effect of 7 local anesthetics on FMN survival after facial nerve sham injury revealed no statistical difference for treatment with saline (97.8% ± 1.9; n = 6), lidocaine (95.8% ± 2.0; n = 6), 2, 3-chloroprocaine (98.7% ± 0.9; n = 5), mepivacaine (98.1% ± 1.2; n = 6), ropivacaine (94.5% ± 1.6; n = 6), bupivacaine (92.4% ± 2.9; n = 6), tetracaine (94.8% ± 1.8, n = 6), or liposomal bupivacaine (94.9% ± 1.7; n = 6) [F (7, 39)= 1.304, P = 0.27] (Fig. 2A).
Fig. 2.
Average percent facial motoneuron survival after immediate local anesthetic treatment. Bar graph demonstrating average percentage of facial motoneuron survival at 4 weeks after facial nerve injury and immediate treatment with saline, lidocaine, 2,3-chloroprocaine, mepivacaine, ropivacaine, bupivacaine, tetracaine, and liposomal bupivacaine. A - Sham injury, B - Transection injury, C - Crush injury. * denotes statistical difference compared to ropivacaine (P<0.05). # denotes statistical difference compared to bupivacaine (P<0.05). Δ denotes statistical difference compared to tetracaine (P<0.05).
Four weeks post transection injury and immediate local anesthetic treatment, the average percent FMN survival in mice was determined. A one-way ANOVA conducted to compare the effect of 7 local anesthetics on FMN survival after facial nerve transection revealed a statistically significant treatment effect [F (7, 51) = 8.039, P < 0.000l] (Fig. 1 and Fig. 2B). Post-hoc comparisons revealed that the average percent FMN survival after transection in mice immediately treated with ropivacaine (54.8% ± 4.7, P < 0.05; n = 7) was significantly decreased compared to saline (85.5% ± 1.5; n = 6), lidocaine (72.9% ± 5.2; n = 6), 2, 3-chloroprocaine (75.6% ± 2.4; n = 8), mepivacaine (77.3% ± 4.6; n = 8), and liposomal bupivacaine (85.0% ± 2.9; n = 8). The average percent FMN survival after transection in mice immediately treated with bupivacaine (63.2% ± 2.3, P < 0.05; n = 8) was significantly decreased compared to saline and liposomal bupivacaine. And the average percent FMN survival after transection in mice immediately treated with tetracaine (66.9% ± 4.3, P <0.05; n = 8) was significantly decreased compared to saline and liposomal bupivacaine.
Four weeks post crush injury and immediate local anesthetic treatment, the average percent FMN survival in mice was determined. A one-way ANOVA conducted to compare the effect of 7 local anesthetics on FMN survival after facial nerve crush revealed a statistically significant treatment effect [F (7,53) = 2.633,P = 0.02] (Fig. 2C). Post-hoc comparisons revealed that the average percent FMN survival after crush in mice immediately treated with bupivacaine (92.8% ± 0.9, P < 0.05; n = 9) was significantly decreased compared to saline (100.7% ± 1.8; n = 6) and liposomal bupivacaine (99.3% ± 1.0; n = 8). However, there were no statistically significant differences between lidocaine (96.9% ± 1.3; n = 12), 2, 3-chloroprocaine (96.2% ± 1.1; n = 7), mepivacaine (95.3% ± 2.6; n = 6), ropivacaine (97.4% ± 1.1; n = 8), or tetracaine (97.8% ± 2.5; n = 5).
3.1.2. Delayed local anesthetic application
To determine if delayed local anesthetic administration affects neuronal survival after a peripheral nerve sham, transection, or crush injury, we applied saline, lidocaine, bupivacaine, or liposomal bupivacaine to the facial nerve 1 day after facial nerve injury. Of note, based on clinical trends and the prior experiments, fewer local anesthetics were evaluated in the delayed-treatment experiments. Furthermore, to avoid the potential confounding treatment effects of Gelfoam, local anesthetic was administered as a solution via syringe directly to the nerve.
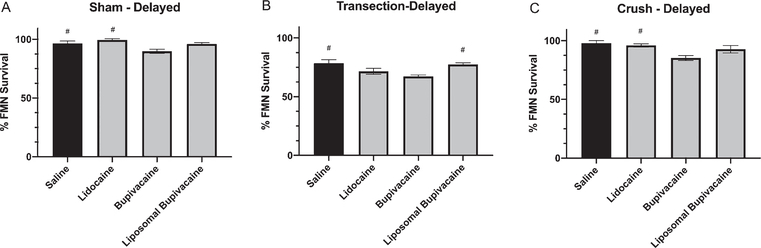
Four weeks post sham injury and delayed local anesthetic treatment, the average percent FMN survival in mice was determined (Fig. 3A). A one-way ANOVA conducted to compare the effect of 3 local anesthetics on FMN survival after facial nerve sham injury revealed a statistically significant treatment effect [F (3, 17) = 6.939, P = 0.003]. Post-hoc comparisons revealed that the average percent FMN survival after sham-injury in mice delay-treated with bupivacaine (89.9% ± 1.8, P < 0.05; n = 6), was significantly decreased compared to saline (96.6% ± 2.0; n = 5) and lidocaine (99.5% ± 1.1; n = 5). However, there were no statistically significant differences compared to liposomal bupivacaine (96.0% ± 1.2; n = 5).
Fig. 3.
Average percent facial motoneuron survival after delayed local anesthetic treatment. Bar graph demonstrating average percentage of facial motoneuron survival at 4 weeks after facial nerve injury and delayed treatment with saline, lidocaine, bupivacaine, and liposomal bupivacaine. A - Sham injury, B - Transection injury, C - Crush injury. # denotes statistical difference compared to bupivacaine (P < 0.05).
Four weeks post transection and delayed local anesthetic treatment, the average percent FMN survival in mice was determined (Fig. 3B). A one-way ANOVA conducted to compare the effect of 3 local anesthetics on FMN survival after facial nerve transection injury revealed a statistically significant treatment effect [F (3, 20) = 6.53, P = 0.003]. Post-hoc comparisons revealed that the average percent FMN survival after transection in mice delay-treated with bupivacaine (67.3% ± 1.2, P < 0.05; n = 7) was significantly decreased compared to saline (78.4% ± 3.1; n = 6) and liposomal bupivacaine (77.6% ± 1.3; n = 6). However, there were no statistically significant differences compared to lidocaine (71.6% ± 2.6; n = 5).
Four weeks post crush injury and delayed local anesthetic treatment, the average percent FMN survival in mice was determined (Fig. 3C). A one-way ANOVA conducted to compare the effect of 3 local anesthetics on FMN survival after facial nerve crush injury revealed a statistically significant treatment effect [F (3, 21) = 6.068, P = 0.003]. Post-hoc comparisons revealed that the average percent FMN survival after crush injury in mice delay-treated with bupivacaine (85.3% ± 2.2, P < 0.05; n = 5) was significantly decreased compared to saline (97.9% ± 2.2; n = 7) and lidocaine (96.0% ± 1.4; n = 8). However there were no statistically significant differences compared to liposomal bupivacaine (92.8% ± 3.2; n = 5).
3.2. Functional recovery
3.2.1. Immediate local anesthetic application
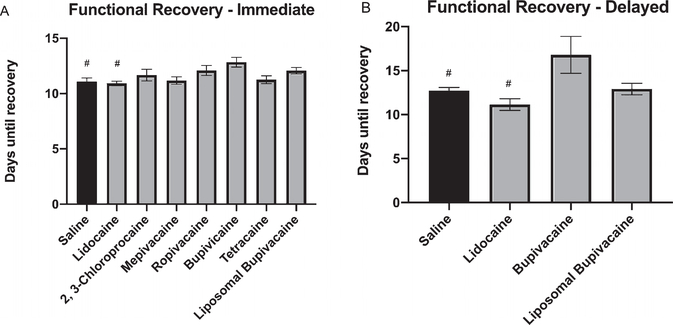
To determine if commonly used local anesthetics affect functional recovery after a peripheral nerve crush injury, we applied saline, lidocaine, 2, 3-chloroprocaine, mepivacaine, ropivacaine, bupivacaine, tetracaine, or liposomal bupivacaine to the facial nerve immediately at the time of injury. The average post-operative time for mice to fully recover, defined as full recovery of all three functional parameters (eye blink, vibrissea orientation, vibrissae movement), was determined (Fig. 4A). A one-way ANOVA conducted to compare the effect of 7 local anesthetics on functional recovery after facial nerve crush revealed a statistically significant treatment effect [F (7, 42) = 3.008, P = 0.01]. Post-hoc comparisons revealed that the average post-operative time for mice to fully recover after crush injury and immediate treatment with bupivacaine (12.83 ± 0.44 days, P < 0.05; n = 6) was significantly delayed compared to saline (11.08 ± 0.33 days; n = 6) and lidocaine (10.92 ± 0.20 days; n = 6). However there were no statistically significant differences compared with 2, 3-chloroprocaine (11.67 ± 0.53 days; n = 6), mepivacaine (11.17 ± 0.33 days; n = 6), ropivacaine (12.08 ± 0.45 days; n = 6), tetracaine (11.25 ± 0.36 days; n = 6), or liposomal bupivacaine (12.06 ± 0.29 days; n = 8).
Fig. 4.
Functional recovery after crush injury and local anesthetic treatment. Bar graph demonstrating average post-operative time in days until animals in crush injury groups achieved complete recovery of vibrissae orientation, vibrissae movement, and eyeblink. A - Immediate local anesthetic treatment, B - Delayed local anesthetic treatment. # denotes statistical difference compared to bupivacaine (P<0.05).
3.2.2. Delayed local anesthetic application
To determine if delayed treatment with commonly used local anesthetics affect functional recovery after a peripheral nerve crush injury, we applied saline, lidocaine, bupivacaine, or liposomal bupivacaine to the facial nerve 1-day after injury. The average post-operative time for mice to fully recover was determined (Fig. 4B). A one-way ANOVA conducted to compare the effect of delayed treatment with 3 local anesthetics on functional recovery after facial nerve crush revealed a statistically significant treatment effect [F (3, 27) = 4.743, P = 0.008]. Post-hoc comparisons revealed that the average post-operative time for mice to fully recover after crush injury and delayed treatment with bupivacaine (16.79 ± 2.10 days, P < 0.05; n = 7) was significantly delayed compared to saline (12.73 ± 0.37 days; n = 11) and lidocaine (11.14 ± 0.66 days; n = 7). However there were no statistically significant differences compared with liposomal bupivacaine (12.92 ± 0.65 days; n = 6).
4. Discussion
The benefits of providing local anesthetic and nerve blocks for patients undergoing surgery have been well established however it is less clear whether individuals with preexisting neurologic deficits also benefit from regional anesthesia or are they at increased risk of new or worsening nerve damage. Similarly, the questions arise as to whether all local anesthetics display a similar neurotoxic profile in all patients. At present, analgesia can be adapted to a clinical scenario with the availability of several local anesthetics with varying properties, such as speed of onset, potency, and/or duration of action. With the ever-expanding use of regional anesthesia and push for improved pain control in all patients, local anesthetics are frequently applied to peripheral nerves that may already be diseased (i.e.: diabetic neuropathy), injured, or at risk of injury during surgery (i.e. mechanical, stretch, etc.). It has been well documented in vitro that all local anesthetics are toxic to neurons and their support cells as a function of potency, concentration, dose, and duration of exposure (Boselli et al., 2003; Epstein-Barash et al., 2009; Kalichman, Powell, & Myers, 1989; Lambert, Lambert, & Strichartz, 1994; Ohtake et al., 2000; Perez-Castro et al., 2009; Radwan, Saito, & Goto, 2002; Sakura, Bollen, Ciriales, & Drasner, 1995; Werdehausen et al., 2009; Yang, Abrahams, Hum, Grafe, & Kirsch, 2011). Despite their neurotoxic potential, current opinion holds that local anesthetic concentrations at doses used clinically are likely safe for all patients, despite a paucity of clinical studies comparing dose or concentration between patients. However, the American Society of Regional Anesthesia has suggested careful consideration be taken in patients with pre-existing neurologic deficits for regional anesthesia due to the increased risk of exacerbating their underlying nerve dysfunction, and suggests limiting concentration, dose and avoidance of adjuvants in at-risk patients (Neal et al., 2015). For the present study, we employed the well-described mouse facial nerve injury model to evaluate the neurotoxic potential of commonly used local anesthetics. We hypothesized that previously injured neurons will be more susceptible to the toxicity of certain commonly used local anesthetics and will result in increased motoneuron cell death and possibly worsened or delayed functional recovery.
Our present data demonstrate differential toxicity between local anesthetics on an injured nerve despite a common mechanism of action between all local anesthetics. In a previously published study, we demonstrated that bupivacaine, but not lidocaine, exacerbated FMN death after a facial nerve transection (Byram et al., 2017). Others have also demonstrated the neurotoxicity of bupivacaine in vitro (Lirk et al., 2008; Werdehausen et al., 2009; Yamashita et al., 2003; Yang et al., 2011). We proposed that the preferential toxicity of bupivacaine over lidocaine was related to the longer duration of action (Byram et al., 2017). Therefore, in the present study, we expanded our investigation to evaluate 7 local anesthetics with varying pharmacologic characteristics (including variable durations of action) in 3 different injury models (sham, transection, crush). Furthermore, since the application of a local anesthetic immediately/simultaneously at the time of a nerve injury in clinical practice is likely a rare occurrence and may expose the intracellular environment to toxic levels of local anesthetic while the axolemma is open, we repeated our experiments for select local anesthetics using a delayed application model (1 -day) to allow the axolemma to seal and neuron survival and regenerative processes to initiate prior to local anesthetic exposure.
Similar to our previous findings, bupivacaine increased FMN cell death in 5 of the 6 experiments (immediate-transection, immediate-crush, delayed-sham, delayed-transection, delayed-crush) when compared to a saline control. And while bupivacaine did cause the most FMN death in the immediate-sham experiment, its effects did not reach statistical significance. Unexpectedly, delayed bupivacaine application after a sham injury increased FMN death compared to saline, suggesting toxicity of bupivacaine even in the setting of an uninjured nerve. It is unknown if the difference between immediate and delayed experiments is due to application method (Gelfoam vs infiltration), timing of application (immediate vs delayed), or another reason. (See discussion on Gelfoam below). Future studies will investigate differences in local anesthetic toxicity based on application timing from immediate to variable lengths of delay.
After immediate local anesthetic application at the time of facial nerve transection, ropivacaine, bupivacaine, and tetracaine caused increased FMN death compared to saline. Similarly, after delayed local anesthetic application after facial nerve transection, bupivacaine caused increased FMN death compared to saline (ropivacaine and tetracaine were not studied in the delayed experiments). Following a facial nerve crush injury, only bupivacaine caused increased FMN death and delayed functional recovery when applied immediately or delayed as compared to saline. The short and intermediate acting local anesthetics (lidocaine, 2, 3-chloroprocaine, mepivacaine) did not exacerbate FMN death nor delay functional recovery in any injury model when applied immediately or delayed. Of note, our prior publication reports FMN survival of 35% after facial nerve transection and immediate treatment with bupivacaine, while the present study reports FMN survival of 63% (Byram et al., 2017). There are two major differences between these studies: 1) Our prior study used a much higher concentration of bupivacaine (0.75%) compared to the present study (0.25%) and 2) our prior study did not use Gelfoam for application. We suspect the higher concentration of bupivacaine and/or the application technique of infiltration versus Gelfoam, may account for the difference in FMN survival between studies. (See discussion on Gelfoam below).
Unexpectedly, treatment with bupivacaine increased FMN death compared to liposomal bupivacaine after transection injury in both immediate and delayed application studies, as well as after crush injury and immediate application. 1.3% liposomal bupivacaine is a higher concentration bupivacaine formulation (compared to 0.25% free bupivacaine) and is the longest-acting local anesthetic tested, however it did not exacerbate FMN death nor delay functional recovery in any injury model tested. Thus, the idea that bupivacaine itself, total concentration, dose, or duration of exposure are responsible for the neurotoxic effects described is not entirely true for liposomal bupivacaine. Others have similarly demonstrated the lack of nerve toxicity with liposomal bupivacaine as compared to free bupivacaine (Damjanovska et al., 2015; McAlvin et al., 2014). Perhaps the slow release of bupivacaine from a liposomal formulation exposes nerves to lower concentrations and doses in a given time, resulting in less neurotoxicity. This finding is noteworthy, needs further study, and may have important implications in clinical practice.
The mechanisms responsible for local anesthetic neurotoxicity are not entirely understood and will be the focus of future studies. Local anesthetics not only target voltage-gated sodium channels, but also interact with a variety of other receptors which may mediate their neurotoxic effects (Lirk et al., 2014). For example, in vitro models of local anesthetic induced neurotoxicity have described effects on caspase-, PI3k-, and MAPK-pathways (Haller et al., 2006; Ma et al., 2010; Verlinde et al., 2016) Werdehausen et al., 2007). However, these studies do not evaluate the effects of local anesthetic application to an axon, but rather to a cell body in culture. Here, we have combined the application of local anesthetics to a peripheral nerve injury model, and therefore separate mechanisms of neurotoxicity may be responsible for the findings described. Under usual conditions, peripheral nerve transection results in increased expression of injury and regeneration associated genes in the cell body (Al-Majed, Brushart, & Gordon, 2000; Kiryu-Seo & Kiyama, 2011). Interestingly, several studies have demonstrated an increase in injury and regeneration associated gene expression induced by application of brief electrical stimulation proximal to axotomy, that is abolished by blocking action potentials with tetrodotoxin (Al-Majed, Brushart, et al., 2000; Al-Majed, Neumann, Brushart, & Gordon, 2000; Geremia, Gordon, Brushart, Al-Majed, & Verge, 2007). Thus, one possible mechanism of local anesthetic toxicity in the setting of nerve injury may be that blocking nerve transmission after injury impairs propagation of action potentials to the cell body and thus negatively affects the usual cell body response to injury. However, given that all local anesthetics tested are known to block action potentials, it is intriguing that some, but not all local anesthetics resulted in decreased FMN survival and delayed functional recovery in our study. Thus, another mechanism is likely responsible. Future studies will evaluate possible mechanisms causing variable neurotoxicity of local anesthetics.
Our studies have some important limitations. First, in clinical practice, the facial nerve is not commonly a target for nerve blocks, however branches of the facial nerve can be incidentally affected by local anesthetics during local infiltration for dental, facial reconstructive, or otolaryngology procedures (Tzermpos, Cocos, Kleftogiannis, Zarakas, & Iatrou, 2012). However, the facial nerve injury model is a simple and reproducible peripheral nerve injury model that has been extensively studied for decades to evaluate mechanisms related to nerve injury, survival, and regeneration in addition to the evaluation of neurotoxins and neurotrophic factors (Moran & Graeber, 2004). Second, to ensure an effective double-crush nerve injury model, we compared the neurotoxicity of local anesthetics after a complete nerve transection, which is an admittedly uncommon clinical scenario. Furthermore, the clinical or behavioral significance of neuron death after local anesthetic administration is unknown. Therefore, we also utilized a more commonly encountered nerve crush injury. The facial nerve crush model is useful for determining both neuron death and to evaluate axonal regeneration by observing the recovery of facial motor functions. Here, we utilized a subjective observational method with incremental scales to detect changes in functional recovery of vibrissae orientation, movement, and eye blink reflex. We endeavored to reduce observer bias by using 2 separate observers, and careful consideration of confounding influences such as contralateral intact vibrissae pad movement and bulbar retraction with passive eyelid closing were taken. This methodology does not provide a detailed or quantitative biometric analysis of motor recovery as described by others, which will be the goal for future studies (Guntinas-Lichius et al., 2001). Despite these drawbacks, the present study does demonstrate that after a nerve crush injury, bupivacaine causes facial neuron death as well as delay in functional recovery.
Clinically, local anesthetics are applied via injection into the perineuronal space which is typically bounded by fascial planes. In our surgical model, the fascia has been dissected away to perform a nerve injury. To avoid local anesthetic spilling out of the intended area we initially used local anesthetic impregnated Gelfoam for the immediate-application experiments. Gelfoam is a water-insoluble, porous sponge made from purified porcine skin and gelatin that can absorb and hold many times its weight of fluid (Pfizer, 2019). However, after recognizing that bupivacaine and liposomal bupivacaine had differential neurotoxicity, we appreciated that the delivery method, such as using liposomes or Gelfoam could skew our results. Sequestration of local anesthetic into the Gelfoam may affect the pharmacodynamics and/or pharmacokinetics of the local anesthetics. For example, Gelfoam sequestration may slow or delay release of local anesthetic and change the concentration or total dose interacting with the nerve at a given time, and thus inadvertently decrease the significance of our findings. Additionally, Gelfoam may affect the inflammatory state at the injury site, and the differential neurotoxicity reported here may be related to the differences each of the drugs has on inflammation or inflammation has on the action of the drug. Therefore, since Gelfoam is not used clinically during peripheral nerve blocks, we chose to remove it from our follow-up studies. Ultimately, despite the potentially confounding limitation of using Gelfoam in our immediate application studies, bupivacaine increased FMN death and delayed functional recovery after transection and/or crush injuries, validating the negative effects of bupivacaine.
There are ongoing questions regarding the effects of local anesthetics and regional techniques on injured or diseased peripheral nerves, and there are limited recommendations for these patients. Our current study has identified select local anesthetics to help focus forthcoming studies. We hope that an improved understanding of the effects of local anesthetics on injured peripheral nerves may allow us to prevent and/or minimize neural deficits following peripheral nerve blocks in at-risk patients.
Acknowledgement
Restorative Neurology and Neuroscience, Volume 38, Authors: Susanna C. Byram, Samantha E. Bialek, Vicki A. Husak, Daniel Balcarcel, James Park, Jacquelyn Dang and Eileen M. Foecking, Distinct neurotoxic effects of select local anesthetics on facial nerve injury and recovery, p173-183, Copyright (2020), with permission from IOS Press.
Funding/support
This work was supported in part (immediate local anesthetic administration) by The Department of Anesthesia at Loyola University Medical Center (Maywood, Illinois USA). This work was also supported in part (delayed local anesthetic administration) by Small Projects in Rehabilitation Research, Award # RX002228-01A1, from the United States Department of Veterans Affairs Rehabilitation Research & Development Service.
Role of the funder/sponsor
The sponsors had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript for publication. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Disclosures
Dr. Byram has received payment for consultation services from Pacira Pharmaceuticals, and ACI Clinical. These activities were unrelated to the research activities described here.
Access to data and data analysis
Drs. Byram and Foecking had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
References
- Al-Majed AA, Brushart TM, & Gordon T (2000). Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. European Journal of Neuroscience, 12(12), 4381–4390. [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, & Gordon T (2000). Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. Journal of Neuroscience, 20(7), 2602–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boselli E, Duflo F, Debon R, Allaouchiche B, Chassard D, Thomas L, & Portoukalian J (2003). The induction of apoptosis by local anesthetics: a comparison between lidocaine and ropivacaine. Anesthesia and Analgesia, 96(3), 755–756. [DOI] [PubMed] [Google Scholar]
- Brail R, McCartney CJ, Chan VW, & El-Beheiry H (2007). Neurological complications after regional anesthesia: contemporary estimates of risk. Anesthesia and Analgesia, 104(4), 965–974. 10.1213/01.ane.0000258740.17193.ec [DOI] [PubMed] [Google Scholar]
- Byram SC, Byram SW, Miller NM, & Fargo KN (2017). Bupivacaine increases the rate of motoneuron death following peripheral nerve injury. Restorative Neurology and Neuroscience, 35(1), 129–135. 10.3233/RNN-160692 [DOI] [PubMed] [Google Scholar]
- Coggeshall RE (1992). A consideration of neural counting methods. Trends in Neurosciences, 15(1), 9–13. [DOI] [PubMed] [Google Scholar]
- Damjanovska M, Cvetko E, Hadzic A, Seliskar A, Plavec T, Mis K,... Stopar Pintaric T (2015). Neurotoxicity of perineural vs intraneural-extrafascicular injection of liposomal bupivacaine in the porcine model of sciatic nerve block. Anaesthesia, 70(12), 1418–1426. 10.1111/anae.l3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, & Kohane DS (2009). Prolonged duration local anesthesia with minimal toxicity. Proceedings of the National Academy of Sciences of the United States of America, 106(17), 7125–7130. 10.1073/pnas.0900598106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Brushart TM, Al-Majed AA, & Verge VM (2007). Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Experimental Neurology, 205(2), 347–359. 10.1016/j.expneurol.2007.01.040 [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Angelov DN, Tomov TL, Dramiga J, Neiss WF, & Wewetzer K (2001). Transplantation of olfactory ensheathing cells stimulates the collateral sprouting from axotomized adult rat facial motoneurons. Experimental Neurology, 172(1), 70–80. 10.1006/exnr.2001.7774 [DOI] [PubMed] [Google Scholar]
- Haller I, Hausott B, Tomaselli B, Keller C, Klimaschewski L, Gerner P, & Lirk P (2006). Neurotoxicity of lidocaine involves specific activation of the p38 mitogen-activated protein kinase, but not extracellular signal-regulated or c-jun N-terminal kinases, and is mediated by arachidonic acid metabolites. Anesthesiology, 105(5), 1024–1033. 10.1097/00000542-200611000-00025 [DOI] [PubMed] [Google Scholar]
- Hogan QH (2008). Pathophysiology of peripheral nerve injury during regional anesthesia. Regional Anesthesia and Pain Medicine, 33(5), 435–441. 10.1016/j.rapm.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman MW, Powell HC, & Myers RR (1989). Quantitative histologic analysis of local anesthetic-induced injury to rat sciatic nerve. Journal of Pharmacology and Experimental Therapeutics, 250(1), 406–413. [PubMed] [Google Scholar]
- Kiryu-Seo S, & Kiyama H (2011). The nuclear events guiding successful nerve regeneration. Frontiers in Molecular Neuroscience, 4, 53 10.3389/fnmol.2011.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa KA, Kinderman NB, & Jones KJ. (1989). Testosterone-induced acceleration of recovery from facial paralysis following crash axotomy of the facial nerve in male hamsters. Experimental Neurology, 105( 1), 80–85. [DOI] [PubMed] [Google Scholar]
- Lambert LA, Lambert DH, & Strichartz GR (1994). Irreversible conduction block in isolated nerve by high concentrations of local anesthetics. Anesthesiology, 80(5), 1082–1093. [DOI] [PubMed] [Google Scholar]
- Liguori GA (2004). Complications of regional anesthesia: nerve injury and peripheral neural blockade. Journal of Neurosurgical Anesthesiology, 16( 1), 84–86. [DOI] [PubMed] [Google Scholar]
- Lirk P, Haller I, Colvin HP, Lang L, Tomaselli B, Klimaschewski L, & Gerner P (2008). In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesthesia and Analgesia, 106(5), 1456–1464, table of contents, 10.1213/ane.0b013e318168514b [DOI] [PubMed] [Google Scholar]
- Lirk P, Picardi S, & Hollmann MW (2014). Local anaesthetics: 10 essentials. European Journal of Anaesthesiology, 37(11), 575–585. 10.1097/EJA.0000000000000137 [DOI] [PubMed] [Google Scholar]
- Liu SS, Zayas VM, Gordon MA, Beathe JC, Maalouf DB, Paroli L,... Ya Deau JT (2009). A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesthesia and Analgesia, 109(1), 265–271. 10.1213/ane.0b013e3181a3272c [DOI] [PubMed] [Google Scholar]
- Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G,... Ding Z (2010). Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience, 167(2), 329–342.. 10.1016/j.neuroscience.2009.12.049 [DOI] [PubMed] [Google Scholar]
- McAlvin JB, Padera RF, Shankarappa SA, Reznor G, Kwon AH, Chiang HH,... Kohane DS (2014). Multivesicular liposomal bupivacaine at the sciatic nerve. Biomaterials, 35(15), 4557–4564. 10.1016/j.biomaterials.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LB, & Graeber MB (2004). The facial nerve axotomy model. Brain Research: Brain Research Reviews, 44(2–3), 154–178. 10.1016/j.brainresrev.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Nau C, & Wang GK (2004). Interactions of local anesthetics with voltage-gated Na+ channels. Journal of Membrane Biology, 201(1), 1–8. 10.1007/s00232-004-0702-y [DOI] [PubMed] [Google Scholar]
- Neal JM, Barrington MJ, Brull R, Hadzic A, Hebl JR, Horlocker TT,... Watson JC (2015). The Second ASRA Practice Advisory on Neurologic Complications Associated With Regional Anesthesia and Pain Medicine: Executive Summary 2015. Regional Anesthesia and Pain Medicine, 40(5), 401–430. 10.1097/AAP.0000000000000286 [DOI] [PubMed] [Google Scholar]
- Ohtake K, Matsumoto M, Wakamatsu H, Kawai K, Nakakimura K, & Sakabe T (2000). Glutamate release and neuronal injury after intrathecal injection of local anesthetics. Neuroreport, 11(5), 1105–1109. [DOI] [PubMed] [Google Scholar]
- Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J,... Xu F (2009). Cytotoxicity of local anesthetics in human neuronal cells. Anesthesia and Analgesia, 108(3), 997–1007. 10.1213/ane.0b013e31819385el [DOI] [PubMed] [Google Scholar]
- Pfizer. (2019). Gelfoam: absorbable gelatin sponge, USP. New York, NY: Pfizer. [Google Scholar]
- Radwan IA, Saito S, & Goto F (2002). The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesthesia and Analgesia, 94(2), 319–324, table of contents. [DOI] [PubMed] [Google Scholar]
- Sakura S, Bollen AW, Ciriales R, & Drasner K (1995). Focal anesthetic neurotoxicity does not result from blockade of voltage-gated sodium channels. Anesthesia and Analgesia, 81(2), 338–346. [DOI] [PubMed] [Google Scholar]
- Selander D (1993). Neurotoxicity of local anesthetics: animal data. Regional Anesthesia, 18(6 Suppl), 461–468. [PubMed] [Google Scholar]
- Tzermpos FH, Cocos A, Kleftogiannis M, Zarakas M, & Iatrou I (2012). Transient delayed facial nerve palsy after inferior alveolar nerve block anesthesia. Anesthesia Progress, 59(1), 22–27. 10.2344/11-03.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinde M, Hollmann MW, Stevens MF, Hermanns H, Werdehausen R, & Firk P (2016). Focal AnestheticInduced Neurotoxicity. International Journal of Molecular Sciences, 17(3), 339 10.3390/ijms17030339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdehausen R, Braun S, Essmann F, Schulze-Osthoff K, Walczak H, Lipfert P, & Stevens MF (2007). Fidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology, 107( 1), 136–143. 10.1097/0l.anes.0000268389.39436.66 [DOI] [PubMed] [Google Scholar]
- Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW,... Stevens MF (2009). Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. British Journal of Anaesthesia, 103(5), 711–718. 10.1093/bja/aep236 [DOI] [PubMed] [Google Scholar]
- Yamashita A, Matsumoto M, Matsumoto S, Itoh M, Kawai K, & Sakabe T (2003). A comparison of the neurotoxic effects on the spinal cord of tetracaine, lidocaine, bupivacaine, and ropivacaine administered intrathecally in rabbits. Anesthesia and Analgesia, 97(2), 512–519, table of contents. [DOI] [PubMed] [Google Scholar]
- Yang S, Abrahams MS, Hurn PD, Grafe MR, & Kirsch JR (2011). Focal anesthetic Schwann cell toxicity is time and concentration dependent. Regional Anesthesia and Pain Medicine, 36(5), 444–451. 10.1097/AAP.0b013e318228c835 [DOI] [PMC free article] [PubMed] [Google Scholar]