Abstract
Introduction
Sixteen percent of patients with gastric cancer will develop pulmonary metastases. Standard of care for these patients is systemic chemotherapy with a median survival of 6 months and a 5-year survival of only 2%. Our aim was to critically evaluate the published data on pulmonary resection for metastatic gastric cancer (MGC) and to analyze the potential rationale for surgical management to determine which patients may benefit from this approach.
Methods
The Pubmed and SCOPUS databases were queried for all studies reporting on pulmonary resections for MGC. All available clinicopathologic data were analyzed.
Results
Twenty-one studies from 1975 to 2008 reported 48 pulmonary resections in 43 patients including five repeat resections and four extrapulmonary metastasectomies. Eighty-two percent (34/43) of patients had solitary lesions with a median size of 24 mm (4–90 mm). Median time from gastrectomy to pulmonary resection was 35 months (0–120 months). At a median follow-up of 23 months, 15 of 43 (35%) patients were alive without disease, and two patients died without disease. Median survival was 29 months (3–84 months) after pulmonary metastasectomy and 65 months (5–180 months) after gastrectomy. Fifty-six percent (24/43) of patients had another recurrence at a median of 12 months (range: 6–48 months) after resection including 30% (13/43) of patients with pulmonary recurrences. Overall 5-year survival was 33%.
Conclusions
Pulmonary metastasectomy for MGC can potentially result in long-term survival in a highly selected group of patients and should be considered for those who present with small, isolated lesions after a prolonged disease-free interval.
Keywords: Gastric, Cancer, Lung, Pulmonary, Metastasectomy
Cancer is the second leading cause of death in the United States. Although the incidence and mortality of gastric cancer has decreased significantly since the 1930s, this malignancy is still the fourth most common cancer worldwide and the 14th most common in the United States.1 In 2009, there will be an estimated 21,130 new cases diagnosed (1.5% of all new cancers) and approximately 10,620 deaths (5.2% of all cancer deaths) from gastric cancer in the United States.1,2 The lifetime risk for development of gastric cancer is approximately 1 in 113 with an age-adjusted incidence of 8 in 100,000 and annual death rate of 4.1 per 100,000.3 The overall 5-year survival is 24.7%, ranging from 78% for localized disease to 2% for distant disease.4 Unfortunately, at diagnosis, only 25% of patients present with localized disease confined to the stomach itself; 30% present with regional nodal disease, and another 35% have distant metastases at presentation.5
Among patients who present with localized disease and undergo R0 resections, almost half will experience a recurrence. A recent retrospective review of 1172 patients who underwent R0 resections of gastric tumors with curative intent demonstrated a recurrence rate of 42%; 79% occurred in the first 2 years after gastric resection and rarely occurred after 4 years.6 More than half of patients with recurrences developed distant disease including 16% of patients who developed pulmonary metastases. Data from autopsy series of patients with gastric cancer demonstrate pulmonary metastases in 22 to 52% of patients.6,7 The incidence of clinically apparent pulmonary metastases ranges from 0.5 to 16%, and 0.3 to 6% of patients will have isolated pulmonary metastases from gastric cancer.6,8,9
Life expectancy from the time of pulmonary recurrence is poor with a median survival of 6 months, which is not significantly affected by systemic chemotherapy.10 Seventy percent of patients are dead within 1 year, and 89% are dead within 2 years of recurrence.6 A meta-analysis of patients with metastatic gastric cancer (MGC) demonstrated a 5-year survival of 0 to 7% with an overall 5-year survival of 2% with chemotherapy alone.11 No patients with pulmonary metastases treated with chemotherapy alone survived to the 5-year follow-up in any of these trials.
Once considered the sine qua non for surgically unresectable disease, advances in anesthesia, thoracic surgery, and postoperative care have made pulmonary metastasectomies for other intestinal malignancies such as colorectal cancer an essential part of the treatment of metastatic disease. A review of more than 5000 patients who underwent pulmonary resections for malignancies of various histologies showed that after complete metastasectomy, a median survival of 35 months with long-term survival of 36% at 5 years, 26% at 10 years, and 22% at 15 years is possible in selected patients and histologies.12
Pulmonary metastasectomy for metastatic colorectal cancer has become standard of care based on retrospective data alone and has never been subjected to a randomized controlled trial. Thus far, there have been no comprehensive analyses of the published data describing pulmonary metastasectomies for MGC. Furthermore, no studies comparing surgical resection to best alternative care of systemic chemotherapy have addressed this topic in a prospective randomized fashion.
It was our goal to review all published data for pulmonary resection of MGC and to attempt to identify potential patients who might benefit from this treatment modality.
MATERIALS AND METHODS
A comprehensive search of the Medline and SCOPUS databases was performed to identify all published reports of pulmonary resections for gastric cancer in three languages (English, German, and Japanese) using the following keywords: lung, pulmonary, metastasis, gastric, stomach, cancer, resection, and metastasectomy. All non-English publications were translated by an author fluent in the primary language of the publication. Data collected included the year of publication, name and country of institution, size of the series, patient demographics, pathologic details of primary gastric tumors including American Joint Committee on Cancer tumor, node, metastasis staging, site of first recurrence, timing of pulmonary metastasis and resection, type of operation, pathologic details of metastatic tumor, time to recurrence from metastasectomy, adjuvant and neoadjuvant chemotherapy and radiation therapy, follow-up, and overall survival (OS). Kaplan-Meier curves were generated for disease-free survival (DFS) and OS after gastrectomy and pulmonary metastasectomy using SigmaPlot software (Systat Software, San Jose, CA). Demographic and clinicopathological factors were used to generate separate Kaplan-Meier curves, and these were then evaluated for their association with DFS and OS. Statistical differences between curves were calculated by using the log-rank test. All p values are presented as two tailed without adjustment for multiple comparisons, and p values less than 0.05 were considered significant.
RESULTS
Patients
Twenty-one publications from 1975 to 2008 from 201 institutions in two countries were identified (Table 1).7–9,13–30 The majority of studies, 57% (12/21), were single case reports. These studies reported on 43 patients who underwent 48 pulmonary metastasectomies for MGC, including five patients who underwent repeat pulmonary resection. The median age of patients was 67 years (range: 42–84 years), and the majority (83%) were men. Most patients, 95% (41/ 43), were treated in Japan, and the other 5% (2/44) were treated in Germany.
TABLE 1.
Studies Reporting Pulmonary Resections for Gastric Cancer
| Study | Author | Institution | Year | Patients | Follow-Up (mo) | Status |
|---|---|---|---|---|---|---|
| 1 | Siono et al.13 | Tokushima University | 1975 | 1 | 12 | Alive |
| 2 | Mai et al.14 | Kanazawa University | 1984 | 1 | NR | Alive |
| 3 | Tomita et al.15 | Nagasaki University | 1988 | 1 | 9 | Dead |
| 4 | Raute and Trede16 | 1989 | 1 | NR | NR | |
| 5 | Umehara et al.8 | Hamamatsu University | 1989 | 2 | 79 | Alive |
| 65 | Dead | |||||
| 6 | Konno et al.17 | Hamatsu University | 1995 | 1 | 48 | Alive |
| 7 | Piltz et al.18 | 1996 | 1 | 8 | Dead | |
| 8 | Urabe et al.7 | Juntendo University | 1996 | 2 | 42 | Dead |
| 39 | Alive | |||||
| 9 | Kamiyoshihara et al.19 | Gunma University | 1998 | 3 | NR | Dead |
| Dead | ||||||
| Dead | ||||||
| 10 | Kanemitsu et al.9 | National Cancer Center | 1998 | 4 | 23 | Dead |
| 45 | Dead | |||||
| 10 | Dead | |||||
| 19 | Dead | |||||
| 11 | Tsumatori et al.20 | National Defense Medical College | 1998 | 5 | 60 | Alive |
| Dead | ||||||
| Dead | ||||||
| Dead | ||||||
| Dead | ||||||
| 12 | Tanaka et al.21 | Yamaguchi University | 2000 | 1 | 23 | Alive |
| 13 | Kobayashi et al.22 | Kanagawa Cencer center | 2001 | 1 | NR | NR |
| 14 | Tamura et al.23 | National Kinki-Chuo Hospital | 2002 | 4 | 12 | Dead |
| 19 | Dead | |||||
| 6 | Dead | |||||
| 18 | Dead | |||||
| 15 | Inoue et al.24 | Osaka Prefectural General Hospital | 2002 | 1 | 180 | Alive |
| 16 | Nakahashi et al.25 | National Cancer Center East | 2004 | 1 | 60 | Alive |
| 17 | Tsuboshima et al.26 | Shimane University | 2006 | 1 | 8 | NR |
| 18 | Takahiro et al.27 | Miyagi Cancer Center | 2006 | 2 | 26 | Alive |
| 21 | Alive | |||||
| 19 | Sakaguchi et al.28 | Tokyo Metropolitan Komagome Hospital | 2007 | 7 | 3 | Dead |
| 59 | Dead | |||||
| 76 | Alive | |||||
| 21 | Alive | |||||
| 21 | Alive | |||||
| 33 | Alive | |||||
| 8 | Alive | |||||
| 20 | Mikami et al.29 | Fukuoka University | 2007 | 2 | 10.2 | Dead |
| 10.2 | Dead | |||||
| 21 | Nakayama et al.30 | Kanagawa Cancer Center | 2008 | 1 | 65 | Dead |
NR, not reported.
Clinicopathologic Features of Primary Gastric Tumors
All patients had histopathologically confirmed metastatic gastric adenocarcinoma (Table 2). Staging data of the primary gastric tumors were available for 34 patients (79%) and included 38% (13/34) with stage I tumors (one unspecified, three IA, and nine IB), 15% (5/34) with stage II tumors, 32% (11/34) with stage III tumors (seven IIIA and four IIIB), and 15% (5/34) with stage IV tumors (four T4 tumors and one M1).
TABLE 2.
Clinicopathological Features of Primary Gastric Tumors
| n | Percentage | |
|---|---|---|
| Stage (n = 34), overall | ||
| I | 13 | 38 |
| II | 5 | 15 |
| III | 11 | 32 |
| IV | 5 | 15 |
| T | ||
| 1 | 5 | 15 |
| 2 | 16 | 47 |
| 3 | 8 | 24 |
| 4 | 5 | 15 |
| N | ||
| 0 | 12 | 35 |
| 1 | 13 | 38 |
| 2 | 5 | 15 |
| 3 | 4 | 12 |
| M | ||
| 0 | 33 | 97 |
| 1 | 1 | 3 |
| Gastrectomy (n = 37) | ||
| Total | 23 | 62 |
| Distal | 14 | 38 |
| Neoadjuvant therapy (n = 22) | ||
| Chemotherapy | 0 | 0 |
| Radiation therapy | 0 | 0 |
| Adjuvant therapy (n = 28) | ||
| Chemotherapy | 10 | 36 |
| Radiation therapy | 1 | 4 |
Fifteen percent (5/34) of patients had T1 tumors, 47% (16/34) had T2 tumors, 23% (8/34) had T3 tumors, and 15% (5/34) had T4 tumors. Thirty-five percent (12/34) of the patients had N0 disease, 38% (13/34) had N1 disease, 15% (5/34) had N2 disease, and 12% (4/34) had N3 disease.
Six tumors had evidence of lymphatic and/or vascular invasion on final pathology. For those with histologic descriptions, 53% (8/15) of tumors were well differentiated, 33% (5/15) were moderately differentiated, and 13% (2/15) were reported as poorly differentiated.
Data on the extent of gastric resections were available for 86% (37/43) of the patients. Sixty-two percent (23/37) of the patients underwent total gastrectomy, and 38% (14/37) of the patients underwent distal gastrectomy. Extent of lymph node dissection was not reported in any study. All patients were resected to no evidence of disease after gastrectomy. Data on neoadjuvant treatment before gastrectomy were available for 50% (22/44) of patients, none of whom received neoadjuvant chemotherapy or radiation before primary gastrectomy. Data on adjuvant therapy after gastrectomy were available for 65% (28/43) of the patients, 36% (10/28) of whom received adjuvant chemotherapy and one of whom also received adjuvant radiation therapy.
Prognostic Factors at Gastrectomy
Demographic and clinicopathologic factors were examined to determine any prognostic factors associated with DFS after gastrectomy (Table 3). There was a trend toward decreased DFS among patients with more advanced stage tumors (T3–4) compared with those with less advanced tumors (T1–2) (median DFS 24 months versus 34 months, p = 0.194); however, the only factor found to be statistically significant was type of gastrectomy with those patients undergoing distal gastrectomies having longer DFS than those undergoing total gastrectomy (42 months versus 24 months, p = 0.02). On further analysis, those patients who underwent total gastrectomy were found to have higher overall stage tumors than those who underwent distal gastrectomy. Patients in the total gastrectomy group had 24% and 76% stage I and stages II–IV tumors, respectively, compared with 62% and 38% among those undergoing distal gastrectomy. Similarly, T and N stages were higher among patients in the total gastrectomy group with 52% T1–2 and 48% T3–4 lesions with 19% N0 and 81% N1–3 tumors compared with 77% T1–2 and 23% T3–4 lesions with 62% N0 and 38% N1–3 tumors in the distal gastrectomy group. The association of adjuvant chemotherapy after gastrectomy on DFS was also examined, and there was no statistically significant difference in DFS between those who received adjuvant chemotherapy and those who did not (22 months versus 39 months, p= 0.139).
TABLE 3.
Prognostic Factors for Disease-Free Survival After Gastrectomy
| Feature | Group | Patients | Median DFS (mo) | p |
|---|---|---|---|---|
| Sex | Male | 6 | 20 | 0.791 |
| Female | 24 | 38 | ||
| Age | <66.5 yr | 15 | 33 | 0.186 |
| >66.5 yr | 16 | 45 | ||
| Overall stage | I and II | 16 | 34 | 0.194 |
| III and IV | 13 | 24 | ||
| T stage | T1–2 | 19 | 33 | 0.495 |
| T3–4 | 10 | 38 | ||
| N stage | N0 | 10 | 33 | 0.261 |
| N1–3 | 19 | 34 | ||
| Gastrectomy | Distal gastrectomy | 12 | 42 | 0.02 |
| Total gastrectomy | 20 | 24 | ||
DFS, disease-free survival
Timing of Pulmonary Metastases
Ninety-eight (42/43) percent of patients presented with metachronous pulmonary metastases and 2% (1/43) had a synchronous presentation. Of the patients presenting with metachronous pulmonary MGC, the majority of patients, 95% (40/42), had a pulmonary metastasis as the first site of metastatic disease. The median disease-free interval (DFI) was 35 months (0–108 months). Seven percent (3/43) had hepatic metastases before pulmonary metastases. The DFI before hepatic metastasis was 14 and 38 months in the two patients in which this data were available. Fifty-one percent (22/43) of patients had detailed information regarding the diagnosis of pulmonary metastases. All these patients had radiographic evidence of metastasis (chest x-ray or computed tomography) during routine follow-up, and two patients also had subsequent bronchoscopy. No tumors were pathologically confirmed to be MGC before resection. Twenty-three percent (5/22) of tumors were associated with abnormal laboratory values (three with elevated CEA and two with elevated AFP). Fourteen percent (3/22) of patients were symptomatic with cough or fever.
Of the 74% (32/43) of the patients for whom the preoperative workup for pulmonary metastases was described, 47% (15/32) of patients were thought to have MGC, 31% (10/32) of patients were thought to have primary lung cancer, and 22% (7/32) of the patients were thought to have either primary lung cancer or gastric metastases before resection. Therefore, the preoperative intent was to perform a metastasectomy in approximately half of the patients, whereas the remainder had incidentally discovered gastric cancer on pathologic examination.
Clinicopathologic Features of Resected Pulmonary Metastases
Eighteen studies reported details about the 48 pulmonary resections in the 43 patients included in our analysis (Table 4). Overall, pulmonary resections were performed at a median of 35 months (range: 0–120 months) after primary gastrectomy in the 19 studies for which information was available. Of the 88% (42/48) of the pulmonary resections for which detailed data were available, the majority of patients 81% (34/42) had one lesion, 17% (7/42) had two lesions, and 2% (1/42) had three lesions resected in the same operation. Lesion size was reported in 44% (22/48) of resections. The median size of the resected MGC in the lung was 24 mm (range: 4–90 mm). All patients were resected to no evidence of disease after pulmonary metastasectomy.
TABLE 4.
Clinicopathological Features of the Pulmonary Metastases
| n | Percentage | |
|---|---|---|
| Number of lesions (n = 42) | ||
| 1 | 34 | 81 |
| 2 | 7 | 17 |
| 3 | 1 | 2 |
| Location of lesion (n = 28) | ||
| RUL | 11 | 39 |
| RML | 3 | 11 |
| RLL | 5 | 18 |
| LUL | 4 | 14 |
| LLL | 5 | 18 |
| Type of resection (n = 38) | ||
| Pneumonectomy | 0 | |
| Lobectomy | 21 | |
| Segmentectomy | 10 | |
| Wedge | 5 | |
| Other | 2 | |
| Adjuvant therapy (n = 27) | ||
| Chemotherapy | 7 | 26 |
| Radiation therapy | 0 | 0 |
| Median (mm) | Range (mm) | |
| Size of lesion (n = 22) | 24 | 4–90 |
Seventeen right-sided resections, eight left-sided resections, and 23 nonspecified resections were performed. All the patients who had more than one lesion resected had disease that was confined to one hemithorax. For the seven patients with two lesions resected, five patients had unilobar ipsilateral disease, and two patients had disease in two lobes. The one patient with three lesions had disease in two lobes.
Of the pulmonary resections performed, 44% (21/48), 21% (10/48), 8% (4/48), 2% (1/48), and 2% (1/48) were lobectomies, segmentectomies, wedge resections, combined lobectomy and wedge resection, and sternal resection with en bloc wedge resection, respectively. Ten percent (5/48) were pulmonary resections that were not otherwise specified. The extent of thoracic lymphadenectomy was not reported in any study; however, 10 studies reported lymph node status on final pathology, and two of these patients had evidence of lymphatic involvement. Operative complications were reported in 10 studies; in nine, there were no morbidities reported, and in one there was a chylothorax. There were no perioperative mortalities.
Data on adjuvant chemotherapy or radiation therapy after pulmonary resection were reported for 63% (27/43) of the patients, 26% (7/27) of whom received chemotherapy, and none of whom received radiation therapy to the chest after their pulmonary resections. Five percent (2/43) of the patients subsequently underwent radiation therapy for intracranial metastases, which developed after pulmonary resection. Adjuvant chemotherapy after pulmonary metastasectomy was not associated with a statistically significant difference in DFS (median DFS: 36 months versus not yet reached, p = 0.561) or OS (median OS: 39 months versus 21 months p = 0.707) compared with no chemotherapy.
Prognostic Factors at Pulmonary Metastasectomy
Demographic and clinicopathological data were analyzed to evaluate for prognostic factors for DFS and OS after pulmonary metastasectomy (Tables 5 and 6). There were no statistically significant prognostic factors for DFS after pulmonary metastasectomy. There were no statistically significant prognostic factors for OS; however, there was a trend toward increased OS among patients who had longer than the historical DFI of 24 months (median OS: 65 months versus 19 months, p = 0.119) and who had less extensive resections compared with lobectomy (median OS: not reached versus 45 months, p = 0.081).
TABLE 5.
Prognostic Factors for Disease-Free Survival After Pulmonary Resection
| Feature | Group | Patients | Median DFS (mo) | p |
|---|---|---|---|---|
| Sex | Male | 23 | 36 | 0.303 |
| Female | 5 | NR | ||
| Age | <66.5 yr | 14 | 36 | 0.108 |
| >66.5 yr | 15 | NR | ||
| Side of resection | R | 10 | 36 | 0.407 |
| L | 4 | NR | ||
| Number of | 1 | 24 | 36 | 0.398 |
| pulmonary lesions | >1 | 5 | 48 | |
| Size of | <23.5 mm | 9 | 36 | 0.451 |
| pulmonary lesions | >23.5 mm | 8 | NR | |
| Type of | Lobectomy | 15 | 36 | 0.496 |
| pulmonary resection | Other | 10 | NR | |
| DFI from | <35 mo | 15 | 48 | 0.427 |
| gastrectomy | >35 mo | 13 | 36 | |
| DFI from | <24 mo | 11 | 36 | 0.848 |
| gastrectomy | >24 mo | 17 | 48 | |
DFS, disease-free survival. DFI, disease-free interval.
TABLE 6.
Prognostic Factors for Overall Survival After Pulmonary Resection
| Feature | Group | Patients | Median OS (mo) | p |
|---|---|---|---|---|
| Sex | Male | 24 | 29 | 0.396 |
| Female | 5 | 59 | ||
| Age | <66.5 yr | 16 | 42 | 0.221 |
| >66.5 yr | 14 | NR | ||
| Number of pulmonary | 1 | 26 | 45 | 0.901 |
| lesions | >1 | 5 | 42 | |
| Size of pulmonary | <23.5 mm | 8 | NR | 0.367 |
| lesions | >23.5 mm | 9 | 65 | |
| Type of pulmonary | Lobectomy | 17 | 45 | 0.081 |
| resection | Other | 10 | NR | |
| DFI from gastrectomy | <35 mo | 18 | 42 | 0.527 |
| >35 mo | 13 | 45 | ||
| DFI from gastrectomy | <24 mo | 14 | 19 | 0.119 |
| >24 mo | 17 | 65 | ||
OS, overall survival. DFI, disease-free interval.
Repeat Pulmonary Resections
Five patients (12%) underwent repeat pulmonary resections.
Umehara et al.8 reported on a 77-year-old patient who underwent total gastrectomy for stage IV (T4N2M0) adenocarcinoma. He subsequently had a left lower lobe segmentectomy 12 months after gastrectomy and then had a left upper lobe segmentectomy for pulmonary recurrence 23 months after gastrectomy. This patient was alive without evidence of disease at 79 months postgastrectomy and 67 months after his first pulmonary metastasectomy. The second patient in this series was a 58-year-old woman who underwent a distal gastrectomy for stage IV (T4N3M0) adenocarcinoma. She had an initial recurrence in the liver 14 months after gastrectomy and underwent liver resection, followed by right upper and lower lung segmentectomies 36 months after gastrectomy and then a repeat right lower segmentectomy 44 months after gastrectomy for pulmonary metastases. This patient developed brain metastases and died of disease 65 months after gastrectomy and 29 months after her first pulmonary resection.
Inoue et al.24 described a 68-year-old patient who underwent a distal gastrectomy for stage I (T1N0M0) gastric adenocarcinoma who underwent right upper lobectomy 96 months after gastrectomy and then underwent left upper and lower lobe wedge resections at 108 months after gastrectomy. This patient was alive without evidence of disease 180 months after gastrectomy and 84 months after initial pulmonary metastasectomy.
Nakahashi et al.25 reported on a 59-year-old patient who underwent total gastrectomy for stage IIIA (T3N1M0) adenocarcinoma who experienced a recurrence and underwent right middle lobectomy 24 months after gastrectomy. During the next 18 months, the patient underwent right adrenal and retroperitoneal metastasectomies in two separate procedures. Sixty months after gastrectomy, the patient underwent right upper lobe wedge resection for an additional pulmonary metastasis and was alive without evidence of disease 84 months after gastrectomy and 60 months after the last pulmonary resection.
Nakayama et al.30 described a 59-year-old patient who underwent a distal gastrectomy for stage II (T2N1) adenocarcinoma who then underwent right middle lobectomy for two lesions 34 months after gastrectomy and then underwent left lower lobectomy 82 months after gastrectomy. He died of a cerebral infarct 99 months after gastrectomy and 65 months after the last pulmonary resection with no evidence of disease.
For patients with pulmonary recurrences after initial resection, repeat metastasectomy was associated with significant increase in OS compared with those who did not undergo repeat resection of their pulmonary recurrence (median OS: not yet reached versus 18 months, p = 0.008). When comparing these five patients who underwent repeat resection with the rest of the patients who only underwent a single resection, there was a trend toward increased OS among those who underwent repeat resection, but this was not statistically significant (median OS not yet reached versus 22 months, p = 0.053).
Extrapulmonary Resections
Nine percent (4/43) of the patients had isolated extrapulmonary metastases that were resected in addition to pulmonary metastasectomy. Three patients had hepatic metastases that were resected at 14 months, 38 months, and an unspecified time after gastrectomy, and subsequently underwent pulmonary resections at 36 months, 71 months, and 16 months after gastrectomy, respectively. One patient had a pulmonary metastasectomy at 24 months after gastrectomy, followed by resection of a right adrenal metastasis at 30 months, resection of a retroperitoneal recurrence at 42 months, and a second pulmonary metastasectomy at 50 months after gastrectomy. There was no statistical difference in OS after pulmonary metastasectomy among those patients who underwent extrapulmonary metastasectomies compared with those who did not (median OS: 29 months versus 23 months, p = 0.635).
Follow-Up
Follow-up data were available for 91% (39/43) of the patients at a median follow-up of 23 months (range: 3–180 months) (Table 7). Median DFS after pulmonary metastasectomy was 36 months (range: 3–76 months) in the 33 patients in which this information was available.
TABLE 7.
Follow-Up
| Median (mo) | Range (mo) | |
|---|---|---|
| Follow-up (n = 39) | 23 | 3–180 |
| Time to recurrence (n = 39) | 11 | 5–48 |
| n | Percentage | |
| Recurrence (n = 39) | ||
| No recurrence | 15 | 38 |
| Recurrence | 24 | 62 |
| Brain | 4 | 17 |
| Bone | 3 | 13 |
| Liver | 2 | 8 |
| Pulmonary | 13 | 54 |
| Ipsilateral hemithorax | 3 | 23 |
| Contralateral hemithorax | 3 | 23 |
| Unspecified | 7 | 54 |
Thirty-eight percent (15/39) of patients had no recurrences at any site after their pulmonary resections, and another 10% (4/39) had no recurrences after a resection of a second pulmonary metastasis in a separate anatomic lobe. Those patients without recurrence had a significantly longer survival compared with those with further recurrences (median OS not yet reached versus 22 months, p = 0.005).
Of the 56% (24/43) of patients with recurrences, 54% (13/24) had recurrences in the lung after pulmonary resection. One was in the same lobe, two were in the ipsilateral hemithorax in a different lobe, three were in the contralateral hemithorax, and seven were unspecified. Thirty-eight percent (5/13) of these patients underwent repeat pulmonary resection, and all five had no further pulmonary or other recurrences, one of these patients developed a cerebral metastasis. Seventeen percent (4/24) of the patients had intracranial metastases, 13% (3/24) of the patients had bone metastases, and 8% (2/24) had liver metastases after pulmonary metastasectomy. Median time to recurrence after pulmonary resection was 12 months (6–48 months).
Follow-up for recurrences was measured by the authors at the time of publication of the original article. At the end of this period, 37% (16/43) patients were alive, and all were without evidence of disease. During follow-up, 24 deaths occurred with 63% (15/24) attributable to disease progression. Two patients had no evidence of disease at the time of death from a cerebrovascular accident and pneumonia. Seven patients had no listed cause of death. The status of three patients was not reported.
Survival
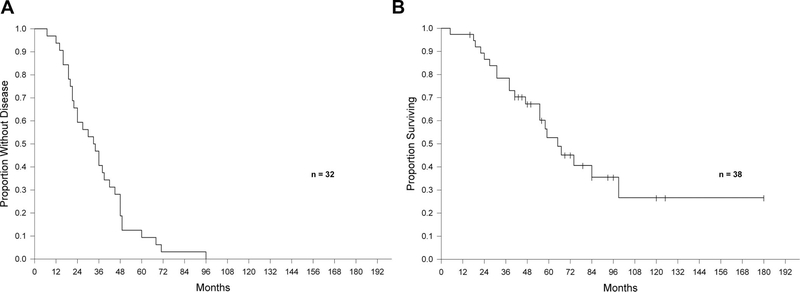
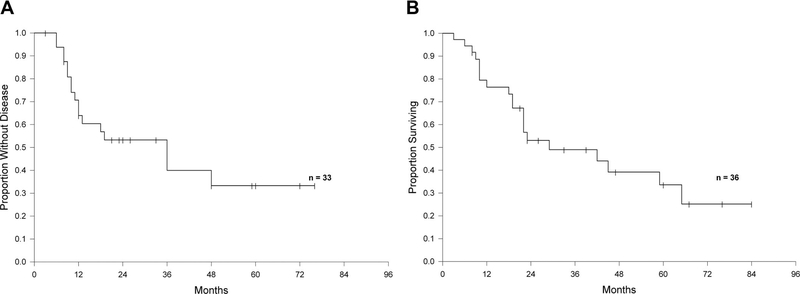
Median survival after pulmonary metastasectomy was 29 months (range: 3–84 months) for the 36 patients in which this information was available. The median survival from gastrectomy was 65 months (range: 5–180 months) in the 38 patients in which this information was available. Kaplan-Meier survival analyses are presented in Figures 1 and 2.
FIGURE 1.
A, Disease-free survival after gastrectomy. B, Overall survival after gastrectomy.
FIGURE 2.
A, Disease-free survival after pulmonary metastasectomy. B, Overall survival after pulmonary metastasectomy.
Thirty-one percent (11/36) of patients survived for longer than 3 years after pulmonary resection, including 16% (6/36) who survived for longer than 5 years, 6% (2/36) of whom were alive at 76 and 84 months after metastasectomy without evidence of disease with an OS of up to 15 years after gastrectomy (Table 8). Nearly all these patients (5/6) were alive and without any evidence of disease, and an additional patient was deceased from other causes without evidence of disease.
TABLE 8.
Patients with Long-Term Survival
| Study | Age (yr) | Stage (TNM) | Gastrectomy | Lesions (No.) | Location | Size (mm) | Operation | Time of Pulmonary Resection from Gastrectomy (mo) | Survival from Gastrectomy (mo) | Survival from Pulmonary Resection (mo)a | Overall Status | Disease Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5b | 77 | T4N2M0 (IV) | Total | 1 | LLL | 20 | Segmentectomy | 12 | ||||
| 1 | LUL | 10 | Segmentectomy | 23 | 79 | 67 | Alive | NED | ||||
| 11 | 71 | T4N1M0 (IV) | Distal | 1 | RUL | 30 | Segmentectomy | NR | 48 | 60 | Alive | NED |
| 15b | 42 | T1N0M0 (IA) | Distal | 1 | RUL | 30 | Lobectomy | 96 | ||||
| 2 | LUL/LLL | 10, 5 | Wedge resections | 108 | 180 | 84 | Alive | NED | ||||
| 16b | 59 | T3N1M0 (IIIA) | Total | 1 | RML | 15 | Lobectomy | 24 | ||||
| 1 | RUL | 20 | Wedge resection | 60 | 84 | 60 | Alive | NED | ||||
| 19 | 67 | T4N2M0 (IV) | Total | 1 | NR | 16 | Segmentectomy | 49 | 125 | 76 | Alive | NED |
| 21b | 59 | T2N1M0 (II) | Distal | 2 | RML | 36, 4 | Lobectomy | 34 | ||||
| 1 | LLL | 24 | Lobectomy | 82 | 99 | 65 | Dead | NED | ||||
Survival from first pulmonary resection for patients who underwent repeat pulmonary resections.
Patients who underwent repeat resections.
NED, no evidence of disease; TNM, tumor, node, metastasis.
DISCUSSION
More than 60,000 patients with a history of gastric cancer are currently alive in the United States.31 Given the fact that up to one in seven patients will develop a pulmonary recurrence and up to 1 in 16 patients will have isolated pulmonary metastases, our study is potentially applicable to a significant number of patients who have disease that is amenable to surgical therapy.7–9 Patients with pulmonary metastases from gastric cancer are considered nonsurgical candidates, but those treated with chemotherapy alone have poor survival that is measured on the order of several months.10 We undertook an analysis of all published data of patients who underwent pulmonary resections for MGC in an attempt to identify potential patients who may benefit from an aggressive surgical approach and to determine selection criteria that can be used in a prospective study.
We report on 43 patients who underwent pulmonary metastasectomy for MGC. The median OS of this group of patients was 29 months (Figure 2B), and 5-year survival was 33% after pulmonary metastasectomy. Despite the obvious limitations and biases, our data show that survival in a small selected group of patients who underwent surgical removal of pulmonary metastases from MGC can be potentially longer compared with historical patients treated with standard chemotherapy.
The majority of published reports were case reports and few small case series from larger tertiary centers, which is understandable given the fact that resection of MGC from any site is not common. Additionally, almost all reports were from Japan, which is not surprising as this disease is more prevalent in the East than in the West. The small number and the nature of these publications indicate that a possible publication bias exists. It is unreasonable to assume that “bad outcomes” for this controversial procedure would be published.
Pathologic features of the primary tumors were evaluated in an attempt to discern which patients might benefit from metastasectomy. Eighty-five percent of primary tumors invaded at least into the muscularis (T2) including 39% into and through the serosa (T3–T4). Sixty-five percent had nodal involvement (N1–N3), and 47% of the patients presented in advanced stage (stages III–IV). Nearly half of all tumors were moderately or poorly differentiated, and several had evidence of lymphatic and/or vascular invasion, all of which are negative prognostic factors and could have contributed to the development of metastases. These tumor characteristics are to be expected given that our patient population was defined by those who had pulmonary metastases. Not surprisingly, there was a trend toward decreased DFS after gastrectomy among patients with more advanced tumors.
More than 40% of patients with gastric cancer will develop recurrences after curative gastrectomy. The majority will recur within the first 2 years and nearly all within 4 years of the initial operation.6 The patients in our report had a median DFI of 35 months, which is longer than in historical reports and suggests that there may be selection bias among these patients who have a longer than average DFI after gastrectomy. Approximately half of patients will recur distantly with metastasis to the liver 3 times as often as the lung.6 As our patient population was selected from patients who underwent pulmonary metastasectomy, not surprisingly, nearly all patients (98%) recurred in the lung as the initial site of metastatic disease. The next most common place for initial recurrence was the liver; the two patients in our report had DFI of 14 and 38 months, similar to that published in the literature.
Nearly all patients in our study had metachronous pulmonary metastases, the majority of which were unilateral, single, and small. Because pulmonary resections of MGC are not yet routine, it would be expected that only the most favorable cases would be selected for resection as these would be expected to lead to the best outcomes, and this introduces another potential selection bias to this cohort.
Of note, nearly one third of patients were thought to have primary lung cancer before resection of their pulmonary metastasectomy. The frequency of primary lung adenocarcinoma in patients with existing gastric carcinoma has been studied in the Japanese population in two studies, which report a 0.5 to 1.7% incidence of primary lung cancer in these patients.32,33 Although not common, the possibility of primary lung cancer must be entertained in a patient with pulmonary nodules who has a history of resected gastric cancer. Modern immunohistochemistry studies using special markers, such as thyroid transcription factor 1 (TTF-1), can help make this distinction between primary pulmonary adenocarcinoma and metastastic adenocarcinoma from another site.
More than half of patients who were thought to have primary lung cancer, or in which the definitive preoperative diagnosis between primary lung cancer and MGC was not clear, were operated in the modern era where TTF-1 and other immunohistochemical techniques were used to definitively diagnose these lesions as MGC and not primary lung cancer. For the other patients who underwent resection before the widespread use of TTF-1, it is possible that these lesions could represent primary lung cancer; however, all clinicopathological data available at the time of resection and subsequent report in the literature identified these lesions as MGC with the best certainty of available techniques.
A wide variety of operations were performed from nonanatomic wedge resections to formal lobectomies. As the primary objective for metastasectomy is complete removal of tumor with grossly and microscopically negative margins, many options for pulmonary resection of MGC are possible. Few of these studies reported morbidity, but as pulmonary metastasectomies for other histologies have become commonplace, it is expected that rates of complications after pulmonary resections for MGC would be similar and acceptable.
In this report, five patients underwent repeat pulmonary resections and three underwent hepatic resections before pulmonary metastasectomy. Patients who undergo pulmonary resection of colorectal metastases have a 30 to 45% 5-year survival, and those who undergo repeat pulmonary metastasectomies show similar long-term survivals to those with only one resection,34–37 which was also seen among our five patients who had repeat resections. In addition, pulmonary resections after hepatic resections for colorectal metastases are performed and can result in long-term survival with 60% of the patients alive 3 years after pulmonary resection, similar rates to those who underwent resection of either pulmonary or hepatic metastases alone.37 In our report, patients who underwent liver resection and subsequent pulmonary metastasectomy for MGC had similar survival compared with those who only had one pulmonary resection. It is not possible to draw conclusions from these positive outcomes of few highly selected patients. However, given the precedent for repeat and multivisceral resections for metastatic colorectal cancer, one can hypothesize that similar outcomes could be achieved in MGC. This hypothesis should be explored in a scientific manner in a randomized controlled trial.
Although surgery remains the mainstay in the treatment of primary gastric cancer and remains the only curative modality for this disease, the standard of care treatment for MGC is systemic therapy. However, even with the modern addition of perioperative chemotherapy and/or radiation therapy, the prognosis of MGC has been only modestly improved. The best reported results came from two phase II trials using 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) for patients with metastatic or recurrent gastric adenocarcinoma. The median survival was 11.9 to 14.8 months and time to progression of 7.3 to 9.6 months; however, this regimen has yet to be tested in a randomized fashion or in combination with surgical resections.38,39 In our report, only one quarter of the patients received chemotherapy in a neoadjuvant or adjuvant manner for their pulmonary resection. This most likely reflects the fact that these studies are from a wide range of time periods. As the prospect of promising new chemotherapeutic regimens are on the horizon, it is possible that in the future the combination of resection of MGC with chemotherapy may result in longer, more durable responses than either modality alone.
The median 5-year survival for pulmonary metastasectomy, all histologies combined, is 43% for single lesions and drops to 27% for four or more lesions.12 Among our patients, of whom the majority had a single lesion, 5-year survival after lung resection was 33%, leading to a median survival of 29 months from metastasectomy and a median survival of more than 5 years from gastrectomy. Almost one quarter of patients survived longer than 3 years including nearly 10% who were alive longer than 5 years. These results are comparable with that which has been published previously for all histologies combined, and although promising, is representative of a small group of highly selected patients. Nevertheless, these retrospective data are encouraging and suggest that pulmonary metastasectomy may contribute to survival of patients with MGC in a meaningful way. The hypothesis that pulmonary metastasectomy for MGC prolong survival should be tested in prospective randomized trial.
CONCLUSION
We report on a small and highly selected group of patients where pulmonary metastasectomy for MGC resulted in a median survival of 29 months. In contrast, current standard of care options for these patients results in limited survival measured in few months. Despite the selection and publication biases and the retrospective nature of our analysis, it is reasonable to consider pulmonary metastasectomy for MGC in patients with single, small lesions and a long DFI. To better address the question as to whether systemic therapy in combination with resection of oligometastatic disease prolong survival, we are currently accruing patients for the Gastrectomy with Metastasectomy Plus Systemic Chemotherapy Versus Systemic Chemotherapy Alone (GYMSSA) trial, which will compare gastric resection and metastasectomy plus FOLFOXIRI compared with FOLFOXIRI alone for patients with metastatic gastric cancer (ClinicalTrials.gov ID. NCT00941655).40
Footnotes
Disclosure: The authors declare no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2009. Atlanta: American Cancer Society, 2009. [Google Scholar]
- 3.SEER Stat Fact Sheets. Available at: http://seer.cancer.gov/statfacts/html/stomach.html Accessed February 15, 2010. [Google Scholar]
- 4.Hundahl SA, Philips JL, Mench HR. The National Cancer Database report on survival of U.S. gastric carcinoma patients treated with gastrectomy. Cancer 2000;88:921–932. [PubMed] [Google Scholar]
- 5.Greene FL, Fritz AG, Balch CM, et al. AJCC Cancer Staging Handbook, 6th Edition NY: Springer-Verlag, 2002. [Google Scholar]
- 6.D’Angelica MD, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urabe M, Sakakibara T, Daibo M, et al. Two cases of recurrent pulmonary metastasis resected after operation for gastric cancer. Arch Jpn Chir 1996;65:22–29. [PubMed] [Google Scholar]
- 8.Umehara Y, Miyahara T, Yoshida M, et al. Lung metastasis of stomach cancer-clinical and pathological assessment. Jpn J Gastrointestinal Surg 1989;22:2772–2777. [Google Scholar]
- 9.Kanemitsu Y, Kondo H, Katai H, et al. Surgical resection of pulmonary metastases from gastric cancer. J Surg Oncol 1998;69:147–150. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi O, Kanari M, Yoshikawa T, et al. Prognosis of pulmonary metastases after curative gastrectomy. Proc Am Soc Clin Oncol 2001; 20:2260. [Google Scholar]
- 11.Yoshida M, Ohtsu A, Boku N, et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol 2004;34:654–659. [DOI] [PubMed] [Google Scholar]
- 12.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37–49. [DOI] [PubMed] [Google Scholar]
- 13.Siono Y, Tsunoda E, Kusunose K. A case of solitary pulmonary metastasis of gastric carcinoma treated with pulmonary resection. J Clin Surg 1975;30:605–608. [Google Scholar]
- 14.Mai M, Kitagawa I, Ueno M, et al. Multiple early gastric cancer metastasized to the lung, report of a case: confirmed by lobectomy of right lung one year later after total gastrectomy following partial gastrectomy for three years. Stomach Intestine 1984;19:825–830. [Google Scholar]
- 15.Tomita M, Shimzu T, Honda H, et al. Surgery for postoperative recurrence of gastric cancer. Acta Medica Nagasaki 1988;33:163–166. [Google Scholar]
- 16.Raute M, Trede M. Die resection von leber- und lungenmetastasen. Dt Artztebl 1989;86:B-2703–B-2708. [Google Scholar]
- 17.Konno H, Baba M, Nakamura S, Baba S. A long surviving case of gastric cancer with metachronous liver and lung metastases. Am J Gastroenterol 1995;90:2267–2268. [PubMed] [Google Scholar]
- 18.Piltz S, Dienemann H, Jauch KW, et al. Results of operative treatment of sequential liver and lung metastases. Langenbecks Arch Chir Suppl Kongressbd 1996;113:225–228. [PubMed] [Google Scholar]
- 19.Kamiyoshihara M, Hirai T, Kawashima O, et al. The surgical treatment of metastatic tumors in the lung: is lobectomy with mediastinal lymph node dissection suitable treatment? Oncol Rep 1998;5:453–457. [DOI] [PubMed] [Google Scholar]
- 20.Tsumatori G, Ozeki Y, Aoki T, et al. Five cases of surgically resected pulmonary metastases from gastric cancer: relationship between the expression of carbohydrate antigen and metastasis. Jpn J Chest Surg 1998;12:85–91. [Google Scholar]
- 21.Tanaka T, Kaneda Y, Fujita N, et al. A case of simultaneous resection of primary lung cancer and pulmonary metastasis from gastric cancer. J Jpn Assoc Chest Surg 2000;823–826. [Google Scholar]
- 22.Kobayashi O, Kanari M, Yoshikawa T, et al. Significance of extirpation to recurrent gastric cancer as assessed by survival after recurrence. Jpn J Gastroenterol Surg 2001;34:1501–1505. [Google Scholar]
- 23.Tamura M, Hiroshima K, Sugita K, et al. Four cases of resected pulmonary tumor metastastic from gastric cancer. Jpn J Lung Cancer 2002;42:611–613. [Google Scholar]
- 24.Inoue Y, Kido T, Tanaka Y, et al. A long-term survivor after early gastric cancer with two times of lung metastases which were successfully resected. Nippon Rinshogeka Gakkai Zasshi 2002;63:52–55. [Google Scholar]
- 25.Nakahashi C, Kinoshita T, Konishi M, et al. Long term survival achieved by repeated resections of metachronous pulmonary and adrenal metastases of alpha-fetoprotein producing gastric cancer: report of a case. Surg Today 2004;34:784–787. [DOI] [PubMed] [Google Scholar]
- 26.Tsuboshima K, Nishio W, Wakahara T, Kikuchi K. Surgical resection for sternal metastasis from gastric cancer; report of a case. Jpn J Thorac Surg 2006;59:251–254. [PubMed] [Google Scholar]
- 27.Takahiro S, Kaoru K, Masami S, et al. Two cases of resected metastatic lung tumor from gastric cancer. J Jpn Assoc Chest Surg 2006;20:686–693. [Google Scholar]
- 28.Sakaguchi K, Yamamoto M, Horio H. Resection of solitary pulmonary metastasis from gastric cancer. Jpn J Lung Cancer 2007;47:323–326. [Google Scholar]
- 29.Mikami K, Yamashita Y. Maekawa T, et al. Surveillance program for recurrence after curative gastric cancer surgery. Chirurgische Gastroenterol 2007;23:392–398. [Google Scholar]
- 30.Nakayama H, Ichinose S, Kato Y, et al. Long term survival after a surgical resection of pulmonary metastases from gastric cancer: report of a case. Surg Today 2008;38:150–153. [DOI] [PubMed] [Google Scholar]
- 31.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute, 2009. Available at: http://seer.cancer.gov/csr/1975_2006/ Accessed February 15, 2010. [Google Scholar]
- 32.Kaibara N, Maeta M, Ikeguchi M. Patients with multiple primary gastric cancers tend to develop second primaries in organs other than the stomach. Surg Today 1993;23:186–188. [DOI] [PubMed] [Google Scholar]
- 33.Ikeguchi M, Ohfuji S, Oka A, et al. Synchronous and metachronous primary malignancies in organs other than the stomach in patients with early gastric cancer. Hepatogastroenterology 1995;42:672–676. [PubMed] [Google Scholar]
- 34.Saito Y, Omiya H, Kohno K, et al. Pulmonary metastectomy for 165 patients with colorectal carcinoma: a prognostic assessment. J Thorac Cardiovasc Surg 2002;24:1007–1013. [DOI] [PubMed] [Google Scholar]
- 35.Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238–244. [DOI] [PubMed] [Google Scholar]
- 36.Iizasa T, Suzuki M, Yoshia S, et al. Prediction of prognosis and surgical indications for pulmonary metastectomy from colorecal cancer. Ann Thorac Surg 2006;82:254–260. [DOI] [PubMed] [Google Scholar]
- 37.Rozk NP, Downey RJ. Resection of pulmonary metastases from colorectal cancer. Semin Thorac Cardiovasc Surg 2002;14:29–34. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Kang WK, Kwon JM, et al. Phase II trial of irinotecan plus oxaliplatin in patients with untreated metastatic gastric adenocarcinoma. Ann Oncol 2007;18:88–92. [DOI] [PubMed] [Google Scholar]
- 39.Cao W, Yang W, Lou G, et al. Phase II trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) as first-line treatment for advanced gastric cancer. Anticancer Drugs 2009;20:287–293. [DOI] [PubMed] [Google Scholar]
- 40.Kerkar S, Kemp CD, Duffy A, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials 2009;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]